User login
Maternal oxygen in labor: False reassurance?
False reassurance?
CASE Heart rate tracing suggests fetal distress
Ms. M. presents for elective induction of labor at 39 weeks’ gestation. During the course of her labor, a Category II fetal heart rate (FHR) tracing is noted, and maternal oxygen is administered as part of the intrauterine resuscitative efforts. Her infant ultimately was delivered vaginally with an arterial cord blood pH of 7.1 and Apgar scores of 5 and 7.
Should intrauterine resuscitation include maternal oxygen administration?
It is a common sight on labor and delivery: An FHR monitoring strip is noted to be a Category II tracing. There may be fetal tachycardia, late decelerations, or perhaps decreased variability. The nurse or physician goes to the laboring mother’s room, checks cervical dilation, changes the patient’s position, and puts an oxygen mask over her face.
The American College of Obstetricians and Gynecologists (ACOG) lists maternal oxygen administration, most commonly at 10 L/min via a nonrebreather face mask, as an intrauterine resuscitative measure for Category II or Category III FHR tracings.1 Maternal oxygen is used to treat abnormal FHR tracings in approximately half of all births in the United States.2 Despite these recommendations and the frequency of its use, however, evidence is limited that maternal oxygenation improves neonatal outcome. In fact, there is emerging evidence of potential harm.
Why use oxygen?
The use of maternal oxygen supplementation intuitively makes sense. We know that certain abnormalities in FHR tracings can signal fetal hypoxia. Left untreated, the hypoxia could lead to fetal acidemia and associated neonatal sequelae. Theoretically, the administration of maternal oxygen should lead to improved fetal oxygenation and improved fetal outcome. This is supported by studies from the 1960s that demonstrate improved FHR tracings after maternal oxygen administration.3
This idea was further supported by studies that demonstrated an increase in fetal oxygen levels when maternal oxygen is administered. Haydon and colleagues evaluated the administration of maternal oxygen in women with nonreassuring FHR tracings.4 Their data showed that maternal oxygen administration increased fetal oxygen as measured by fetal pulse oximetry. The lower the initial fetal oxygen levels prior to oxygen administration, the greater the increase.
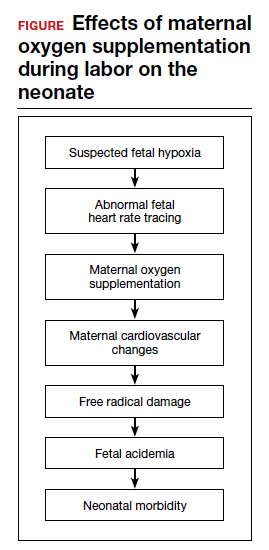
Despite these findings, evidence for improved neonatal outcomes is lacking.5 While heart rate tracings and fetal oxygen saturation may be improved with maternal oxygen supplementation, neonatal morbidity appears to remain unchanged (FIGURE). In fact, newer research suggests potential harm. Although an improved FHR tracing may be comforting to the clinician, the end result may be less so. Given these findings on maternal oxygen supplementation, it is time to break this practice habit.
Maternal cardiovascular effects
Most of the literature on maternal hyperoxygenation focuses on fetal response. Before examining the effects on the fetus, however, we must consider the effect on the mother. Cardiovascular changes occur during and after maternal oxygen administration that should be taken into account.
McHugh and colleagues measured the hemodynamic changes in 46 pregnant and 20 nonpregnant women before, immediately, and 10 minutes after a 30-minute period of high-flow oxygen administration.6 While there were no changes in the nonpregnant women’s parameters, in the pregnant women heart rate and stroke volume were decreased after oxygen administration. Additionally, systemic vascular resistance increased and did not return to baseline by 10 minutes postadministration.
Since the purpose of the maternal oxygen administration is to increase oxygen to the fetus, this decrease in cardiac output and increase in systemic vascular resistance is concerning. These results may negate the intended effect of increased oxygen delivery to the fetus.
Continue to: Maternal and fetal oxidative stress...
Maternal and fetal oxidative stress
Assuming that the abnormal FHR tracing in our case patient is actually due to fetal hypoxia, it would seem prudent to increase fetal oxygenation. However, fetal hyperoxygenation may lead to free radical damage that could worsen neonatal outcomes. Oxidative stress, which can be caused by both hypoxia and hyperoxia, can lead to endothelial and cell receptor damage. This is known to contribute to the cerebral damage of hypoxic-ischemic encephalopathy.
In a randomized trial, Khaw and colleagues measured lipid peroxidases as a “free radical footprint” in women undergoing elective cesarean delivery who were administered oxygen or room air.7 Maternal and fetal oxygen levels were higher in the oxygen-supplementation group, but lipid peroxidases also were elevated. This finding suggests that the excess oxygen results in free radical formation and potentially negative effects on the neonate.
Although maternal oxygen supplementation frequently is viewed as harmless, this research shows that free radical damage may occur in the mother as well.
Additional research shows that longer durations of oxygen administration are correlated with worsening neonatal outcomes. In a study of liberal versus indicated oxygen use, the average time was approximately 90 minutes.8 Use for longer than 176 minutes was associated with lower oxygen levels in fetal blood. A proposed mechanism for this response is placental vasoconstriction thought to protect the fetus from free radical damage.
Again, if the goal is to increase oxygenation, prolonged maternal oxygen supplementation appears to produce the opposite effect.
Fetal acidemia and neonatal morbidity
If a fetus with an abnormal FHR tracing is thought to be hypoxic or acidemic, adding the potentially harmful effects of free radicals could worsen this condition. This is exactly what Raghuraman and colleagues demonstrated in a large prospective cohort analysis.9 While there was no difference in neonatal morbidity between those receiving oxygen and those on room air, there was a significant difference among infants with acidemia and hyperoxia. Composite morbidity (mechanical ventilation, hypothermic therapy, meconium aspiration, and death) was significantly increased in neonates with both hyperoxia and acidemia compared with nonacidemic hyperoxic infants.9 This is further supported by reports of an increased need for neonatal resuscitation and a fourfold increase in umbilical cord pH of less than 7.2.10
While intrauterine and extrauterine life certainly differ, these findings align with the pediatric literature that supports neonatal resuscitation with room air rather than 100% oxygen.11 Additionally, the intrauterine environment is relatively hypoxic, which may make free radical damage more severe.
Continue to: Oxygen use during the COVID-19 pandemic...
Oxygen use during the COVID-19 pandemic
While high-flow oxygen by mask is not considered an aerosol-generating procedure according to the Centers for Disease Control and Prevention, data are limited regarding the cleaning and filtering of oxygen. It is unknown if high-flow oxygen by mask increases the risk of infectious disease transmission to care providers. Therefore, in the midst of the COVID-19 pandemic, ACOG currently recommends against using supplemental oxygen for Category II and Category III tracings, since the benefits are not well established and the possibility of harm to providers may be increased.12 Oxygen supplementation still should be used in mothers with hypoxia.
Other intrauterine resuscitation options
Maternal oxygen administration does not appear beneficial for neonatal outcomes, but other methods can be used. An intravenous fluid bolus and lateral positioning of the mother, for example, are both associated with increased fetal oxygenation. Reducing uterine activity by discontinuing oxytocin or cervical ripening agents or by administering a tocolytic also can improve FHR abnormalities. Oxygen use should be reserved for patients with maternal hypoxia.
The bottom line
The liberal use of maternal oxygenation for the management of abnormal FHR tracings should be stopped. Clear evidence of its benefit is lacking, and the real possibility of fetal and maternal harm remains. This may be especially true during the COVID-19 pandemic. ●
- American College of Obstetricians and Gynecologists. Practice bulletin No. 116. Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116:1232-1240.
- Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. Am J Obstet Gynecol. 2014;211:124-127.
- Althabe O, Schwarcz RL, Pose SV, et al. Effects on fetal heart rate and fetal pO2 of oxygen administration to the mother. Am J Obstet Gynecol. 1967;98:858-870.
- Haydon ML, Gorenberg DM, Nageotte MP, et al. The effect of maternal oxygen administration on fetal pulse oximetry during labor in fetuses with nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2006;195:735-738.
- Fawole B, Hofmeyr GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev. 2012;12:CD0000136.
- McHugh A, El-Khuffash A, Bussmann N, et al. Hyperoxygenation in pregnancy exerts a more profound effect on cardiovascular hemodynamics than is observed in the nonpregnant state. Am J Obstet Gynecol. 2019;220:397.e1-397.e8.
- Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18-23.
- Watkins VY, Martin S, Macones GA, et al. The duration of intrapartum supplemental oxygen administration and umbilical cord oxygen content. Am J Obstet Gynecol. 2020;223:440.e1-440.e7.
- Raghuraman N, Temming LA, Stout MJ, et al. Intrauterine hyperoxemia and risk of neonatal morbidity. Obstet Gynecol. 2017;129:676-682.
- Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172(2 pt 1):465-474.
- Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353-363.
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed October 15, 2020.
CASE Heart rate tracing suggests fetal distress
Ms. M. presents for elective induction of labor at 39 weeks’ gestation. During the course of her labor, a Category II fetal heart rate (FHR) tracing is noted, and maternal oxygen is administered as part of the intrauterine resuscitative efforts. Her infant ultimately was delivered vaginally with an arterial cord blood pH of 7.1 and Apgar scores of 5 and 7.
Should intrauterine resuscitation include maternal oxygen administration?
It is a common sight on labor and delivery: An FHR monitoring strip is noted to be a Category II tracing. There may be fetal tachycardia, late decelerations, or perhaps decreased variability. The nurse or physician goes to the laboring mother’s room, checks cervical dilation, changes the patient’s position, and puts an oxygen mask over her face.
The American College of Obstetricians and Gynecologists (ACOG) lists maternal oxygen administration, most commonly at 10 L/min via a nonrebreather face mask, as an intrauterine resuscitative measure for Category II or Category III FHR tracings.1 Maternal oxygen is used to treat abnormal FHR tracings in approximately half of all births in the United States.2 Despite these recommendations and the frequency of its use, however, evidence is limited that maternal oxygenation improves neonatal outcome. In fact, there is emerging evidence of potential harm.
Why use oxygen?
The use of maternal oxygen supplementation intuitively makes sense. We know that certain abnormalities in FHR tracings can signal fetal hypoxia. Left untreated, the hypoxia could lead to fetal acidemia and associated neonatal sequelae. Theoretically, the administration of maternal oxygen should lead to improved fetal oxygenation and improved fetal outcome. This is supported by studies from the 1960s that demonstrate improved FHR tracings after maternal oxygen administration.3
This idea was further supported by studies that demonstrated an increase in fetal oxygen levels when maternal oxygen is administered. Haydon and colleagues evaluated the administration of maternal oxygen in women with nonreassuring FHR tracings.4 Their data showed that maternal oxygen administration increased fetal oxygen as measured by fetal pulse oximetry. The lower the initial fetal oxygen levels prior to oxygen administration, the greater the increase.
Despite these findings, evidence for improved neonatal outcomes is lacking.5 While heart rate tracings and fetal oxygen saturation may be improved with maternal oxygen supplementation, neonatal morbidity appears to remain unchanged (FIGURE). In fact, newer research suggests potential harm. Although an improved FHR tracing may be comforting to the clinician, the end result may be less so. Given these findings on maternal oxygen supplementation, it is time to break this practice habit.

Maternal cardiovascular effects
Most of the literature on maternal hyperoxygenation focuses on fetal response. Before examining the effects on the fetus, however, we must consider the effect on the mother. Cardiovascular changes occur during and after maternal oxygen administration that should be taken into account.
McHugh and colleagues measured the hemodynamic changes in 46 pregnant and 20 nonpregnant women before, immediately, and 10 minutes after a 30-minute period of high-flow oxygen administration.6 While there were no changes in the nonpregnant women’s parameters, in the pregnant women heart rate and stroke volume were decreased after oxygen administration. Additionally, systemic vascular resistance increased and did not return to baseline by 10 minutes postadministration.
Since the purpose of the maternal oxygen administration is to increase oxygen to the fetus, this decrease in cardiac output and increase in systemic vascular resistance is concerning. These results may negate the intended effect of increased oxygen delivery to the fetus.
Continue to: Maternal and fetal oxidative stress...
Maternal and fetal oxidative stress
Assuming that the abnormal FHR tracing in our case patient is actually due to fetal hypoxia, it would seem prudent to increase fetal oxygenation. However, fetal hyperoxygenation may lead to free radical damage that could worsen neonatal outcomes. Oxidative stress, which can be caused by both hypoxia and hyperoxia, can lead to endothelial and cell receptor damage. This is known to contribute to the cerebral damage of hypoxic-ischemic encephalopathy.
In a randomized trial, Khaw and colleagues measured lipid peroxidases as a “free radical footprint” in women undergoing elective cesarean delivery who were administered oxygen or room air.7 Maternal and fetal oxygen levels were higher in the oxygen-supplementation group, but lipid peroxidases also were elevated. This finding suggests that the excess oxygen results in free radical formation and potentially negative effects on the neonate.
Although maternal oxygen supplementation frequently is viewed as harmless, this research shows that free radical damage may occur in the mother as well.
Additional research shows that longer durations of oxygen administration are correlated with worsening neonatal outcomes. In a study of liberal versus indicated oxygen use, the average time was approximately 90 minutes.8 Use for longer than 176 minutes was associated with lower oxygen levels in fetal blood. A proposed mechanism for this response is placental vasoconstriction thought to protect the fetus from free radical damage.
Again, if the goal is to increase oxygenation, prolonged maternal oxygen supplementation appears to produce the opposite effect.
Fetal acidemia and neonatal morbidity
If a fetus with an abnormal FHR tracing is thought to be hypoxic or acidemic, adding the potentially harmful effects of free radicals could worsen this condition. This is exactly what Raghuraman and colleagues demonstrated in a large prospective cohort analysis.9 While there was no difference in neonatal morbidity between those receiving oxygen and those on room air, there was a significant difference among infants with acidemia and hyperoxia. Composite morbidity (mechanical ventilation, hypothermic therapy, meconium aspiration, and death) was significantly increased in neonates with both hyperoxia and acidemia compared with nonacidemic hyperoxic infants.9 This is further supported by reports of an increased need for neonatal resuscitation and a fourfold increase in umbilical cord pH of less than 7.2.10
While intrauterine and extrauterine life certainly differ, these findings align with the pediatric literature that supports neonatal resuscitation with room air rather than 100% oxygen.11 Additionally, the intrauterine environment is relatively hypoxic, which may make free radical damage more severe.
Continue to: Oxygen use during the COVID-19 pandemic...
Oxygen use during the COVID-19 pandemic
While high-flow oxygen by mask is not considered an aerosol-generating procedure according to the Centers for Disease Control and Prevention, data are limited regarding the cleaning and filtering of oxygen. It is unknown if high-flow oxygen by mask increases the risk of infectious disease transmission to care providers. Therefore, in the midst of the COVID-19 pandemic, ACOG currently recommends against using supplemental oxygen for Category II and Category III tracings, since the benefits are not well established and the possibility of harm to providers may be increased.12 Oxygen supplementation still should be used in mothers with hypoxia.
Other intrauterine resuscitation options
Maternal oxygen administration does not appear beneficial for neonatal outcomes, but other methods can be used. An intravenous fluid bolus and lateral positioning of the mother, for example, are both associated with increased fetal oxygenation. Reducing uterine activity by discontinuing oxytocin or cervical ripening agents or by administering a tocolytic also can improve FHR abnormalities. Oxygen use should be reserved for patients with maternal hypoxia.
The bottom line
The liberal use of maternal oxygenation for the management of abnormal FHR tracings should be stopped. Clear evidence of its benefit is lacking, and the real possibility of fetal and maternal harm remains. This may be especially true during the COVID-19 pandemic. ●
CASE Heart rate tracing suggests fetal distress
Ms. M. presents for elective induction of labor at 39 weeks’ gestation. During the course of her labor, a Category II fetal heart rate (FHR) tracing is noted, and maternal oxygen is administered as part of the intrauterine resuscitative efforts. Her infant ultimately was delivered vaginally with an arterial cord blood pH of 7.1 and Apgar scores of 5 and 7.
Should intrauterine resuscitation include maternal oxygen administration?
It is a common sight on labor and delivery: An FHR monitoring strip is noted to be a Category II tracing. There may be fetal tachycardia, late decelerations, or perhaps decreased variability. The nurse or physician goes to the laboring mother’s room, checks cervical dilation, changes the patient’s position, and puts an oxygen mask over her face.
The American College of Obstetricians and Gynecologists (ACOG) lists maternal oxygen administration, most commonly at 10 L/min via a nonrebreather face mask, as an intrauterine resuscitative measure for Category II or Category III FHR tracings.1 Maternal oxygen is used to treat abnormal FHR tracings in approximately half of all births in the United States.2 Despite these recommendations and the frequency of its use, however, evidence is limited that maternal oxygenation improves neonatal outcome. In fact, there is emerging evidence of potential harm.
Why use oxygen?
The use of maternal oxygen supplementation intuitively makes sense. We know that certain abnormalities in FHR tracings can signal fetal hypoxia. Left untreated, the hypoxia could lead to fetal acidemia and associated neonatal sequelae. Theoretically, the administration of maternal oxygen should lead to improved fetal oxygenation and improved fetal outcome. This is supported by studies from the 1960s that demonstrate improved FHR tracings after maternal oxygen administration.3
This idea was further supported by studies that demonstrated an increase in fetal oxygen levels when maternal oxygen is administered. Haydon and colleagues evaluated the administration of maternal oxygen in women with nonreassuring FHR tracings.4 Their data showed that maternal oxygen administration increased fetal oxygen as measured by fetal pulse oximetry. The lower the initial fetal oxygen levels prior to oxygen administration, the greater the increase.
Despite these findings, evidence for improved neonatal outcomes is lacking.5 While heart rate tracings and fetal oxygen saturation may be improved with maternal oxygen supplementation, neonatal morbidity appears to remain unchanged (FIGURE). In fact, newer research suggests potential harm. Although an improved FHR tracing may be comforting to the clinician, the end result may be less so. Given these findings on maternal oxygen supplementation, it is time to break this practice habit.

Maternal cardiovascular effects
Most of the literature on maternal hyperoxygenation focuses on fetal response. Before examining the effects on the fetus, however, we must consider the effect on the mother. Cardiovascular changes occur during and after maternal oxygen administration that should be taken into account.
McHugh and colleagues measured the hemodynamic changes in 46 pregnant and 20 nonpregnant women before, immediately, and 10 minutes after a 30-minute period of high-flow oxygen administration.6 While there were no changes in the nonpregnant women’s parameters, in the pregnant women heart rate and stroke volume were decreased after oxygen administration. Additionally, systemic vascular resistance increased and did not return to baseline by 10 minutes postadministration.
Since the purpose of the maternal oxygen administration is to increase oxygen to the fetus, this decrease in cardiac output and increase in systemic vascular resistance is concerning. These results may negate the intended effect of increased oxygen delivery to the fetus.
Continue to: Maternal and fetal oxidative stress...
Maternal and fetal oxidative stress
Assuming that the abnormal FHR tracing in our case patient is actually due to fetal hypoxia, it would seem prudent to increase fetal oxygenation. However, fetal hyperoxygenation may lead to free radical damage that could worsen neonatal outcomes. Oxidative stress, which can be caused by both hypoxia and hyperoxia, can lead to endothelial and cell receptor damage. This is known to contribute to the cerebral damage of hypoxic-ischemic encephalopathy.
In a randomized trial, Khaw and colleagues measured lipid peroxidases as a “free radical footprint” in women undergoing elective cesarean delivery who were administered oxygen or room air.7 Maternal and fetal oxygen levels were higher in the oxygen-supplementation group, but lipid peroxidases also were elevated. This finding suggests that the excess oxygen results in free radical formation and potentially negative effects on the neonate.
Although maternal oxygen supplementation frequently is viewed as harmless, this research shows that free radical damage may occur in the mother as well.
Additional research shows that longer durations of oxygen administration are correlated with worsening neonatal outcomes. In a study of liberal versus indicated oxygen use, the average time was approximately 90 minutes.8 Use for longer than 176 minutes was associated with lower oxygen levels in fetal blood. A proposed mechanism for this response is placental vasoconstriction thought to protect the fetus from free radical damage.
Again, if the goal is to increase oxygenation, prolonged maternal oxygen supplementation appears to produce the opposite effect.
Fetal acidemia and neonatal morbidity
If a fetus with an abnormal FHR tracing is thought to be hypoxic or acidemic, adding the potentially harmful effects of free radicals could worsen this condition. This is exactly what Raghuraman and colleagues demonstrated in a large prospective cohort analysis.9 While there was no difference in neonatal morbidity between those receiving oxygen and those on room air, there was a significant difference among infants with acidemia and hyperoxia. Composite morbidity (mechanical ventilation, hypothermic therapy, meconium aspiration, and death) was significantly increased in neonates with both hyperoxia and acidemia compared with nonacidemic hyperoxic infants.9 This is further supported by reports of an increased need for neonatal resuscitation and a fourfold increase in umbilical cord pH of less than 7.2.10
While intrauterine and extrauterine life certainly differ, these findings align with the pediatric literature that supports neonatal resuscitation with room air rather than 100% oxygen.11 Additionally, the intrauterine environment is relatively hypoxic, which may make free radical damage more severe.
Continue to: Oxygen use during the COVID-19 pandemic...
Oxygen use during the COVID-19 pandemic
While high-flow oxygen by mask is not considered an aerosol-generating procedure according to the Centers for Disease Control and Prevention, data are limited regarding the cleaning and filtering of oxygen. It is unknown if high-flow oxygen by mask increases the risk of infectious disease transmission to care providers. Therefore, in the midst of the COVID-19 pandemic, ACOG currently recommends against using supplemental oxygen for Category II and Category III tracings, since the benefits are not well established and the possibility of harm to providers may be increased.12 Oxygen supplementation still should be used in mothers with hypoxia.
Other intrauterine resuscitation options
Maternal oxygen administration does not appear beneficial for neonatal outcomes, but other methods can be used. An intravenous fluid bolus and lateral positioning of the mother, for example, are both associated with increased fetal oxygenation. Reducing uterine activity by discontinuing oxytocin or cervical ripening agents or by administering a tocolytic also can improve FHR abnormalities. Oxygen use should be reserved for patients with maternal hypoxia.
The bottom line
The liberal use of maternal oxygenation for the management of abnormal FHR tracings should be stopped. Clear evidence of its benefit is lacking, and the real possibility of fetal and maternal harm remains. This may be especially true during the COVID-19 pandemic. ●
- American College of Obstetricians and Gynecologists. Practice bulletin No. 116. Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116:1232-1240.
- Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. Am J Obstet Gynecol. 2014;211:124-127.
- Althabe O, Schwarcz RL, Pose SV, et al. Effects on fetal heart rate and fetal pO2 of oxygen administration to the mother. Am J Obstet Gynecol. 1967;98:858-870.
- Haydon ML, Gorenberg DM, Nageotte MP, et al. The effect of maternal oxygen administration on fetal pulse oximetry during labor in fetuses with nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2006;195:735-738.
- Fawole B, Hofmeyr GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev. 2012;12:CD0000136.
- McHugh A, El-Khuffash A, Bussmann N, et al. Hyperoxygenation in pregnancy exerts a more profound effect on cardiovascular hemodynamics than is observed in the nonpregnant state. Am J Obstet Gynecol. 2019;220:397.e1-397.e8.
- Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18-23.
- Watkins VY, Martin S, Macones GA, et al. The duration of intrapartum supplemental oxygen administration and umbilical cord oxygen content. Am J Obstet Gynecol. 2020;223:440.e1-440.e7.
- Raghuraman N, Temming LA, Stout MJ, et al. Intrauterine hyperoxemia and risk of neonatal morbidity. Obstet Gynecol. 2017;129:676-682.
- Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172(2 pt 1):465-474.
- Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353-363.
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed October 15, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 116. Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116:1232-1240.
- Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. Am J Obstet Gynecol. 2014;211:124-127.
- Althabe O, Schwarcz RL, Pose SV, et al. Effects on fetal heart rate and fetal pO2 of oxygen administration to the mother. Am J Obstet Gynecol. 1967;98:858-870.
- Haydon ML, Gorenberg DM, Nageotte MP, et al. The effect of maternal oxygen administration on fetal pulse oximetry during labor in fetuses with nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2006;195:735-738.
- Fawole B, Hofmeyr GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev. 2012;12:CD0000136.
- McHugh A, El-Khuffash A, Bussmann N, et al. Hyperoxygenation in pregnancy exerts a more profound effect on cardiovascular hemodynamics than is observed in the nonpregnant state. Am J Obstet Gynecol. 2019;220:397.e1-397.e8.
- Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18-23.
- Watkins VY, Martin S, Macones GA, et al. The duration of intrapartum supplemental oxygen administration and umbilical cord oxygen content. Am J Obstet Gynecol. 2020;223:440.e1-440.e7.
- Raghuraman N, Temming LA, Stout MJ, et al. Intrauterine hyperoxemia and risk of neonatal morbidity. Obstet Gynecol. 2017;129:676-682.
- Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172(2 pt 1):465-474.
- Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353-363.
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed October 15, 2020.
False reassurance?
False reassurance?