User login
Diagnosis of Severe Acute Lower Gastrointestinal Bleeding with CTA
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
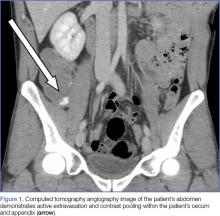
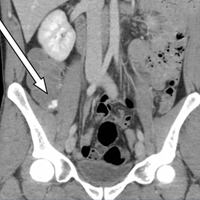
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
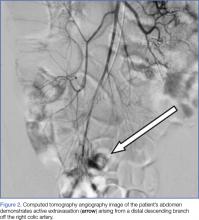
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.