User login
Recurrent UTIs in Women: How to Refine Your Care

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9
FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
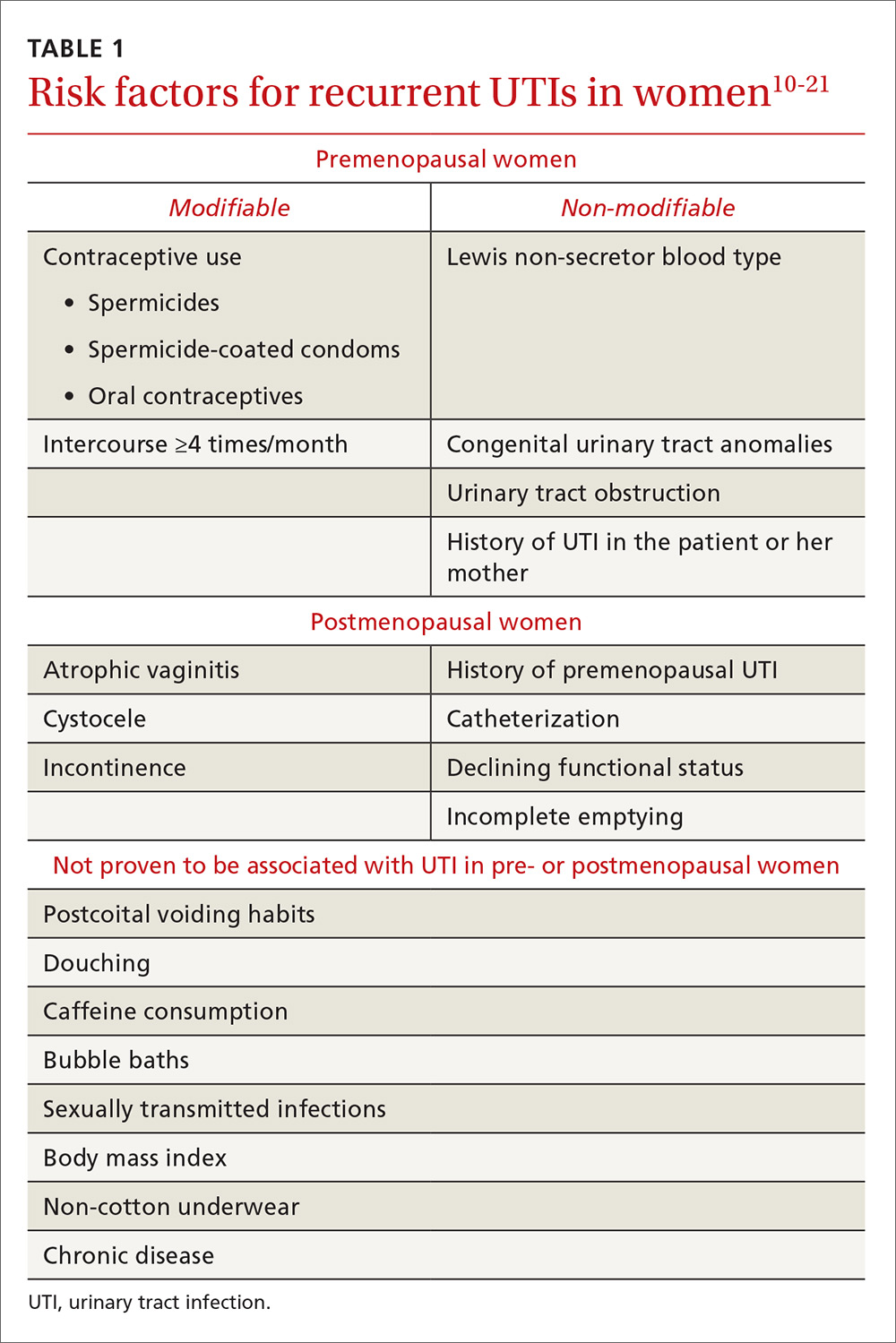
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
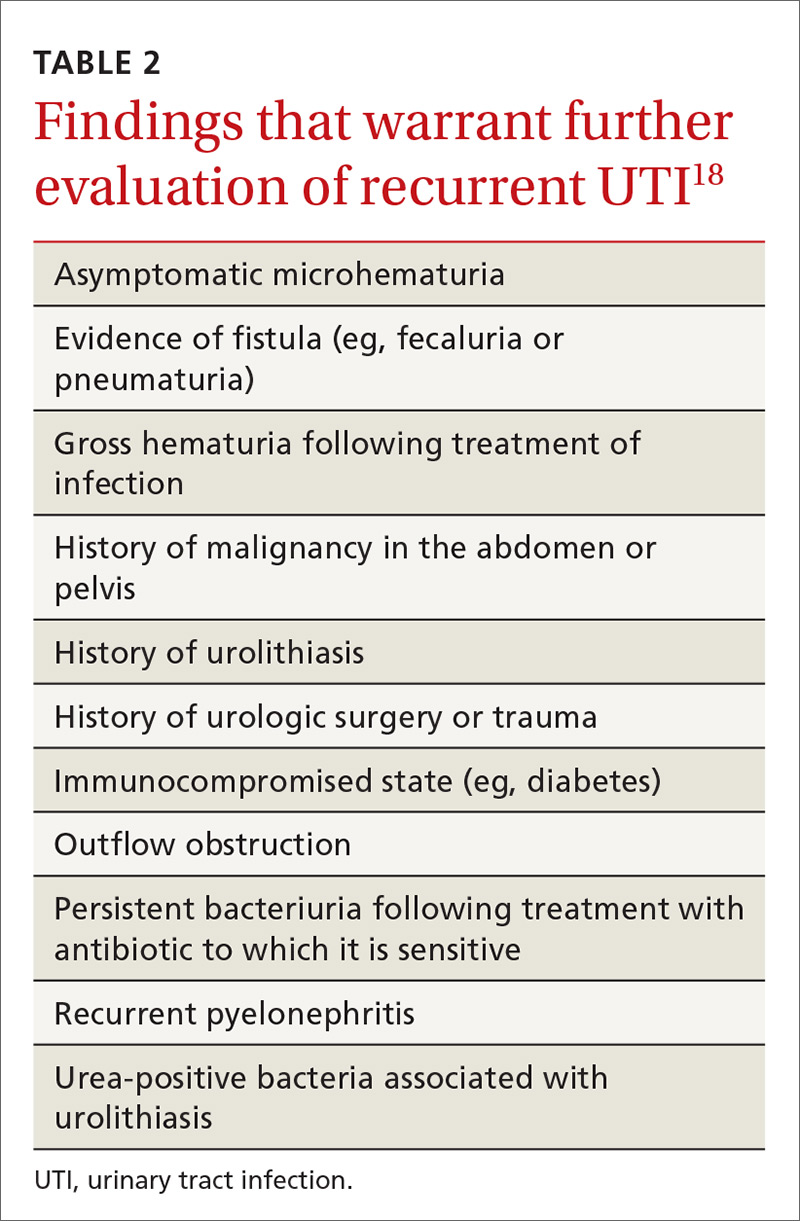
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
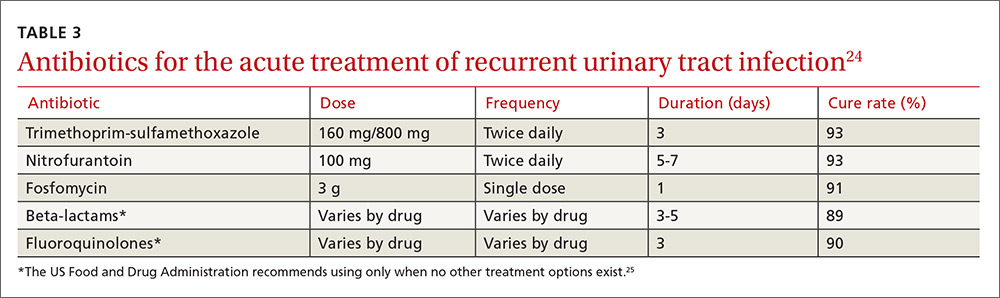
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24
Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33
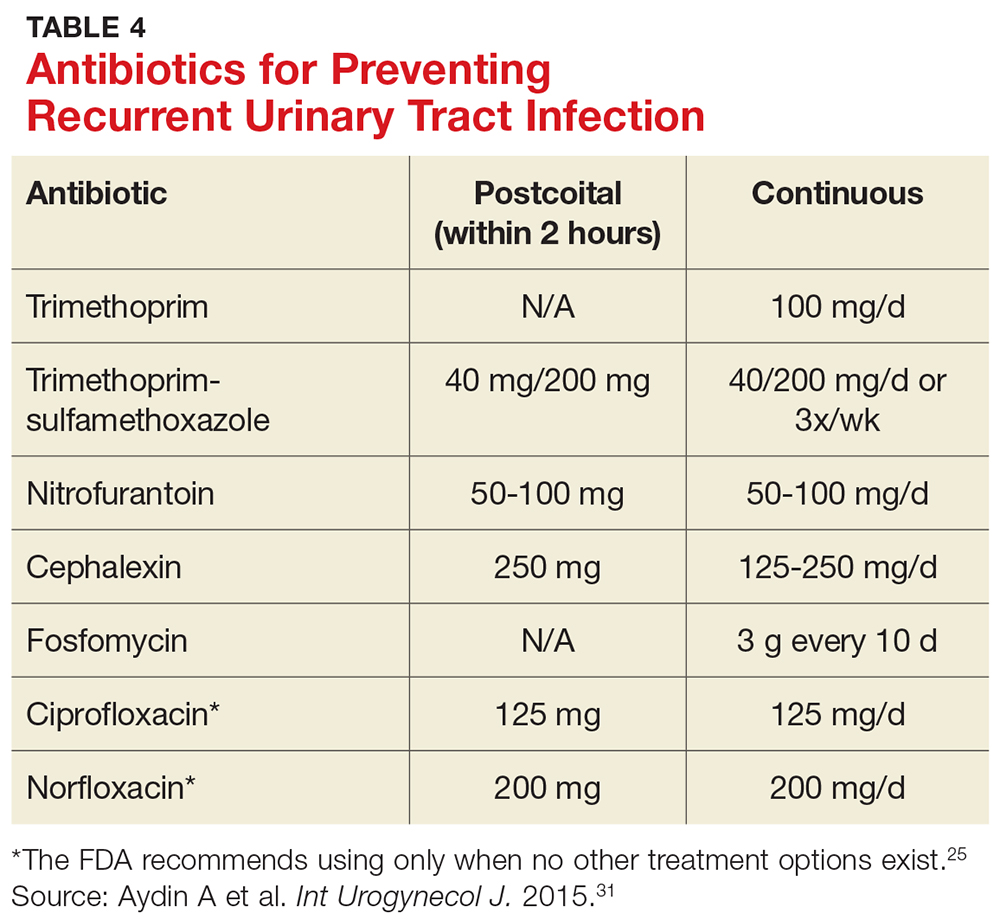
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9

FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13

Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24

Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33

A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9

FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13

Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24

Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33

A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.
Recurrent UTIs in women: How you can refine your care
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21
Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18
Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.
Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18 Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21

Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.

Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18 Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21

Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.

Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18 Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
PRACTICE RECOMMENDATIONS
› Avoid routine use of cystoscopy and imaging when evaluating women with recurrent urinary tract infection (UTI).
› Keep in mind that 3- to 5-day courses of antibiotics (nitrofurantoin, trimethoprim- sulfamethoxazole, fosfomycin, or beta-lactams) for UTIs are as effective as longer courses, and are associated with better compliance and fewer adverse effects.
› Assure patients considering prophylaxis for recurrent UTI that either continuous or postcoital antibiotics are effective.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series