User login
Highlights from the 2019 Society of Gynecologic Surgeons Scientific Meeting

- Rising to the challenges in gynecologic surgical care
- Anterior, apical, posterior: Vaginal anatomy for the gynecologic surgeon
- Beyond enhanced recovery after surgery
B. Star Hampton, MD
Professor
Department of Obstetrics and Gynecology
Division of Urogynecology and Reconstructive
Pelvic Surgery
The Warren Albert Medical School
of Brown University
Women & Infants Hospital
Providence, Rhode Island
Peter C. Jeppson, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of New Mexico School of Medicine
Albuquerque, New Mexico
Audra Jolyn Hill, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of Utah School of Medicine
Salt Lake City, Utah
Sunil Balgobin, MD
Assistant Professor
Department of Obstetrics and Gynecology
Division of Female Pelvic Medicine
and Reconstructive Surgery
University of Texas Southwestern Medical Center
Dallas, Texas
Sean C. Dowdy, MD
Professor and Chair
Division of Gynecologic Oncology
Mayo Clinic
Rochester, Minnesota

- Rising to the challenges in gynecologic surgical care
- Anterior, apical, posterior: Vaginal anatomy for the gynecologic surgeon
- Beyond enhanced recovery after surgery
B. Star Hampton, MD
Professor
Department of Obstetrics and Gynecology
Division of Urogynecology and Reconstructive
Pelvic Surgery
The Warren Albert Medical School
of Brown University
Women & Infants Hospital
Providence, Rhode Island
Peter C. Jeppson, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of New Mexico School of Medicine
Albuquerque, New Mexico
Audra Jolyn Hill, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of Utah School of Medicine
Salt Lake City, Utah
Sunil Balgobin, MD
Assistant Professor
Department of Obstetrics and Gynecology
Division of Female Pelvic Medicine
and Reconstructive Surgery
University of Texas Southwestern Medical Center
Dallas, Texas
Sean C. Dowdy, MD
Professor and Chair
Division of Gynecologic Oncology
Mayo Clinic
Rochester, Minnesota

- Rising to the challenges in gynecologic surgical care
- Anterior, apical, posterior: Vaginal anatomy for the gynecologic surgeon
- Beyond enhanced recovery after surgery
B. Star Hampton, MD
Professor
Department of Obstetrics and Gynecology
Division of Urogynecology and Reconstructive
Pelvic Surgery
The Warren Albert Medical School
of Brown University
Women & Infants Hospital
Providence, Rhode Island
Peter C. Jeppson, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of New Mexico School of Medicine
Albuquerque, New Mexico
Audra Jolyn Hill, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of Utah School of Medicine
Salt Lake City, Utah
Sunil Balgobin, MD
Assistant Professor
Department of Obstetrics and Gynecology
Division of Female Pelvic Medicine
and Reconstructive Surgery
University of Texas Southwestern Medical Center
Dallas, Texas
Sean C. Dowdy, MD
Professor and Chair
Division of Gynecologic Oncology
Mayo Clinic
Rochester, Minnesota
Beyond enhanced recovery after surgery
Our specialty is focusing now more intently on perioperative optimization, commonly referred to as enhanced recovery after surgery (ERAS), a concept championed first and most visibly by colorectal surgeons in the 1990s.1 Both academic and nonacademic practices are challenging long-held beliefs about perioperative management.
The 3 tenets of ERAS
In multiple surgical specialties, proper implementation of 3 tenets—early feeding, perioperative euvolemia, and multimodal pain control—reduces the length of hospital stay, improves patient satisfaction, reduces complications, lowers health care costs, and most importantly hastens patient recovery.
1 Early feeding
Just as athletes hydrate and carbohydrate load prior to a competition, patients benefit if fluids and calories are not withheld in anticipation of a physiologically stressful surgical procedure. Similarly, modest benefit is associated with carbohydrate loading as a liquid supplement 2 hours before surgery.2 The American Society of Anesthesiologists guidelines state that while solid foods should not be consumed after midnight before surgery, clear liquids safely may be withheld for only 2 hours prior to anesthesia induction, and systematic reviews have failed to show harm.3,4 All patients, including those undergoing colonic resections, are allowed to eat a general diet as tolerated the evening before surgery, supplemented with caloric-dense nutritional supplements.
2 Multimodal pain control
Postsurgical pain is a top patient concern. Pain control is critical for rapid recovery; it helps avoid upregulation of the sympathetic axis and permits ambulation and resumption of normal activities. Although opioids relieve pain, they should not be considered a primary pain control approach.
Responding to the opioid epidemic, in 2015 the Centers for Disease Control and Prevention identified opioid overdose prevention as one of the top 5 public health challenges; notably, approximately 6% of patients will experience new, persistent opioid use following surgery.5 Optimal pain management therefore should provide effective pain relief while minimizing opioid use.
Preemptive oral acetaminophen, gabapentin, and celecoxib should be used routinely prior to incision; nonsteroidal anti-inflammatory drugs should be scheduled postoperatively. Even after a complex cytoreductive laparotomy, pain may be controlled with oral rather than intravenous (IV) medications in most patients, with opioid requirements averaging just 2 to 4 tablets of oxycodone in the first 48 hours after surgery, in our experience. The most critical need for pain medications occurs in the first 48 hours after surgery, which highlights the importance of local or regional analgesia. In one investigation, implementation of multimodal pain management that included incisional injection of liposomal bupivacaine reduced patient-controlled analgesia use to less than 5% after laparotomy.6 The need for opioids more than a week postoperatively is uncommon even after a laparotomy.
3 Perioperative euvolemia
Maintaining euvolemia is a central and underrecognized tenet of enhanced recovery pathways, and it facilitates the other 2 tenets of early feeding and optimal pain control. Overhydrated patients have more pain and prolonged recovery of bowel function. Unfortunately, euvolemia is the most difficult ERAS component to implement, requiring seamless communication between all members of the surgical team.
Continue to: Fluid therapy...
Fluid therapy should be respected as a pharmacologic agent with both benefits and risks. Recognizing that a single liter of lactated Ringer’s solution contains the sodium load of more than 30 bags of potato chips (and normal saline contains far more), one can imagine the impact of 10 L of solution on peripheral and bowel edema and on overall recovery. Importantly, euvolemia must be initiated during surgery. A meta-analysis of nearly 1,000 randomly assigned patients showed that benefits were limited when euvolemia was initiated in the postoperative period.7
When it comes to maintaining euvolemia, particular care must be taken to avoid erring toward hyperadherence. No difference in hospital length of stay, complications, or ileus was observed when patients were randomly assigned to goal-directed fluid therapy or standard practice.8 However, differences in the volume of fluid administered were relatively small, and while there was evidence of underhydration (likely responsible for acute kidney injury), there was no evidence of overhydration. For example, 4 L of fluid is likely superior to 15 L, but it may not be clinically different from 4.5 L. A threshold of fluid restriction is likely to be reached; that is, additional benefit is not achieved and, instead, detrimental effects may occur.
Rather than a specific directive, a more clinically relevant goal may be to replace insensible fluid losses and to maintain perfusion and blood pressure with the lowest volume possible. Note that estimation of fluid requirements is vastly simplified by omitting mechanical bowel preparation. Postoperatively, permissive oliguria (20 mL/h) is allowed since reduced urine output is a normal response to surgery (as a result of inappropriate secretion of antidiuretic hormone) and does not necessitate administration of a fluid bolus. Above all, anesthesiologists should acknowledge that fluid administration’s effects on a patient extend past the postanesthesia care unit, and the entire surgical team should be invested in the patient’s long-term recovery.
Our experience with ERAS
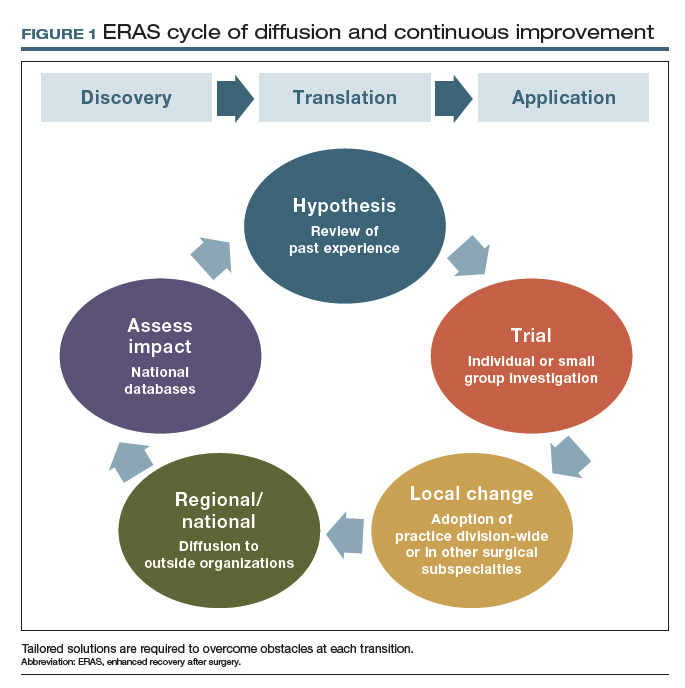
In 2011, Mayo Clinic was the first institution to implement enhanced recovery on a large scale in gynecologic surgery. We have subsequently made multiple pathway modifications in the spirit of continuous improvement (FIGURE 1).
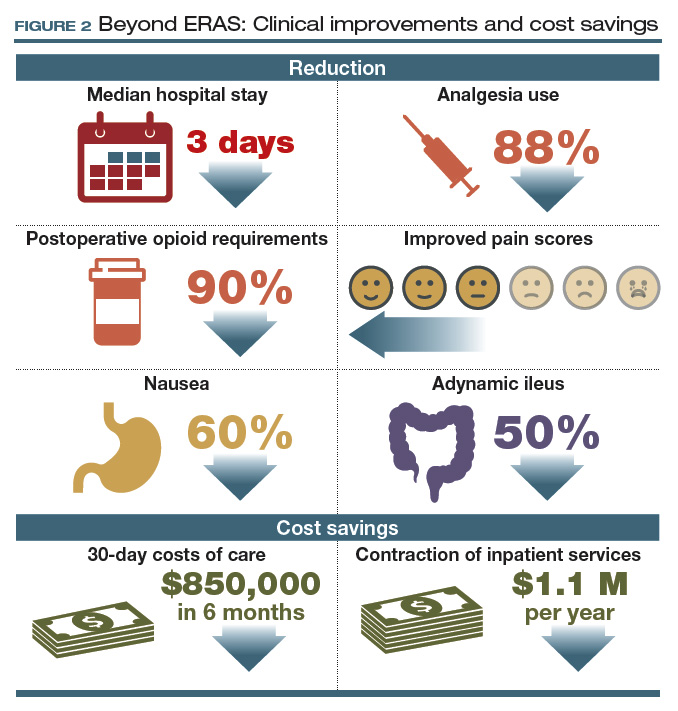
For patients with ovarian cancer requiring extended procedures for cytoreduction via laparotomy (such as colon resection, splenectomy, diaphragm resection), enhanced recovery reduced the median hospital stay by 3 days, patient-controlled IV analgesia use by 88%, and postoperative opioid requirements by 90%.9,10
At 48 hours after surgery, 40% of our patients require no opioids or tramadol, and epidurals are not utilized because of their effects on ambulation and the potential for hypotension. These reductions were met with stable to improved pain scores, a 60% decrease in nausea, and a 50% reduction in adynamic ileus.9,10
Our initial efforts reduced 30-day costs of care by more than $850,000 in just 6 months, with savings of more than $7,600 for each patient undergoing a complex cytoreduction. Furthermore, these improvements allowed consolidation of our inpatient unit with those of other surgical specialties, serving higher volumes of patients within a smaller inpatient footprint. This contraction of inpatient services has accounted for an additional $1.1 million in savings every year since implementation (FIGURE 2).9,10
Our group is not alone in realizing these benefits, and interest has intensified as demonstrated by the fact that the ERAS Society guidelines are among the all-time most downloaded articles in Gynecologic Oncology.11,12 Although our research to demonstrate safety has focused on women undergoing complex oncologic operations, ERAS nevertheless hastens recovery, improves patient satisfaction, and adds value for all patients undergoing gynecologic surgery.
Continue to: Collateral improvements to practice...
Collateral improvements to practice
Clinical optimization using evidence-based practices such as enhanced recovery pathways can result in immediate patient benefit. Affecting such profound clinical improvements is energizing and creates a unique opportunity to transform the culture of the entire health care team. Irrespective of our provider roles (surgeon, anesthesiologist, nurse) or areas of interest (practice, research, education, leadership), we are united by a common purpose: to improve the human condition.13 Reaffirming this common purpose, through the collective effort involved in establishing a standardized enhanced recovery pathway, has allowed our practice and those of others to move beyond enhanced recovery and improve other areas of practice.
Other positive effects. The long-term collateral impact of this culture change at our institution is arguably more important than enhanced recovery itself. Examples of downstream impact include14,15:
- 80% reduction in surgical site infection
- 50% reduction in anastomotic leaks
- 60% reduction in blood utilization for patients undergoing surgery for ovarian cancer.
Team-based pragmatic strategies. Additionally, our willingness to make decisions as a division rather than as individuals facilitated universal implementation of sentinel lymph node biopsy for patients with endometrial cancer and standardized imaging, testing, and surgical decision making for patients with ovarian and endometrial cancer.
The interventions associated with these improvements were not tested in a randomized fashion; however, rather than await perfect data, we made informed decisions based on imperfect data together with a commitment to continuous data review. We find this to be an effective strategy if our goal is to ensure that tomorrow’s outcomes will be better than yesterday’s. In this way, pragmatic trials can be extremely effective in rural settings and tertiary centers.
Barriers to innovation
The widely reported benefits of enhanced recovery beg the question, Why has enhanced recovery not been adopted universally as standard of care? The answer is multifaceted and highlights long-standing shortcomings in our health care system.
Most importantly, our health care system lacks a robust interface to link discovery of new techniques, treatments, and workflows to clinical practice. Perhaps the best example of this is the adoption of minimally invasive surgery (MIS) for endometrial cancer. Ten years have passed since randomized trials showed MIS has equivalent oncologic outcomes and superior recovery compared to laparotomy, yet in the United States less than 50% of women with endometrial cancer benefit.16,17
However, even surgeons who are knowledgeable about recent innovations and genuinely wish to promote improvements may face near-insurmountable skepticism. Blind faith in our abilities and outcomes, overprotection of autonomy, close-mindedness, and satisfaction with the status quo are common responses to innovation and are the enemies of change. Resistance often comes from good intentions, but our desire to avoid complications may result in actions that could just as accurately be labeled superstitious as conservative. These observations suggest that developing methods to incorporate evidence-based practice into routine clinical use is the rate-limiting step in improving surgical quality.
The national Improving Surgical Care and Recovery program is available to specifically aid with ERAS implementation. A collaboration between the Agency for Healthcare Research and Quality (AHRQ) and the American College of Surgeons, the program aims to diffuse enhanced recovery to 750 service lines in 4 surgical subspecialties, including gynecologic surgery, over the next 5 years. (Note: The author is the content expert for the gynecology portion of this program.) The program’s larger aim is to measurably improve patient outcomes, reduce health care utilization, and improve patient experience through the use of an adaptation to AHRQ’s Comprehensive Unit-based Safety Program (CUSP). The backbone for this program is the recent systematic review to establish best practices for gynecologic surgery.1 Free to all participants, the program includes resources such as webinars and coaching calls to assist with the inevitable barriers to ERAS implementation. For more information and to enroll, visit https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/enhanced-recovery /index.html. An important aspect of the program is a registry for tracking outcomes and identifying areas for improvement. For members who currently participate in the National Surgical Quality Improvement Program, clinical data are automatically uploaded into the database. Programs such as Improving Surgical Care and Recovery may be the most reliable way to facilitate diffusion of best practices and take collective responsibility for not only “my outcomes” but also for “our outcomes” as a national community of gynecologic surgeons.
Reference
1. Kalogera E, Nelson G, Liu J, et al. Surgical technical evidence review for gynecologic surgery conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery. Am J Obstet Gynecol. 2018;219:563.e1-563.e19.
Principles essential to change
Various methodologies have been described to manage change and facilitate implementation of new workflows and practices. Irrespective of the method used, including the more formal discipline of implementation science, at least 4 principles must be followed:
1. Teamwork. Mutual trust, mutual respect, and a sense of common purpose are minimum requirements for any successful initiative. Standardization is difficult or impossible without these elements. Thus, establishing a healthy team is the first step in implementing change.
2. Stakeholder analysis. Feedback from surgeons, nurses, residents, fellows, anesthesiologists, pharmacists, nurse anesthetists, and administrators is necessary to obtain diverse perspectives, facilitate engagement, and promote collaborative management. Negativity and resistance are common reactions to change, and it is particularly important to include those who are most skeptical in the stakeholder analysis to mitigate sabotage.
3. Concrete metrics. Success is possible only if defined a priori by specific and achievable goals. Counterbalances also are important to ensure that interventions do not have unintended consequences. Once a goal is met (for example, reduced hospital length of stay or costs), relevant metrics should be monitored after project completion for a minimum of 3 years to avoid regression to the pre-project state.
4. Leadership. The project champion responsible for the initiative must objectively facilitate all of the above and ensure excellent communication between stakeholders to nurture long-term engagement. Despite best efforts, if a minority of the group rejects compromise, this creates an opportunity to compare outcomes between those who do and do not accept the proposed change. Progress realized by early adopters may convince resistors to conform at a later time. Alternatively, the project champion also must have the insight to recognize when a proposed change is impossible at that point in time with that particular group. For example, our own initial attempts to implement enhanced recovery stalled in 2008, but they were successful 3 years later in a different environment.
Continue to: Although a discussion of leadership styles...
Although a discussion of leadership styles is beyond the scope of this article, in our experience, the most successful model is one of servant leadership that is team oriented rather than star dominated. Rather than being led by a single surgeon, each of the 4 quality improvement projects reviewed above (ERAS, and reductions in anastomotic leak, surgical site infection, and blood transfusion) that grew from enhanced recovery included trainees and was led by a different champion, encouraging teamwork and promoting career development. Such a model also supports the Accreditation Council for Graduate Medical Education’s emphasis on quality improvement education.
- Nygren J, Thacker J, Carli F, et al; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37:285-305.
- American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114: 495-511.
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423.
- Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17:65-71.
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504.
- Kalogera, E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Rahbari NN, Zimmermann JB, Schmidt T, et al. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96:331-341.
- Myles PS, Bellomo R, Corcoran T, et al; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263-2274.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part I. Gynecol Oncol. 2016;140:313-322.
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140:323-332.
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481.
- Johnson MP, Kim SJ, Langstraat CL, et al. Using bundled interventions to reduce surgical site infection after major gynecologic cancer surgery. Obstet Gynecol. 2016;127:1135-1144.
- Kalogera E, Nitschmann CC, Dowdy SC, et al. A prospective algorithm to reduce anastomotic leaks after rectosigmoid resection for gynecologic malignancies. Gynecol Oncol. 2017;144:343-347.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol. 2016;127:91-100.
Our specialty is focusing now more intently on perioperative optimization, commonly referred to as enhanced recovery after surgery (ERAS), a concept championed first and most visibly by colorectal surgeons in the 1990s.1 Both academic and nonacademic practices are challenging long-held beliefs about perioperative management.
The 3 tenets of ERAS
In multiple surgical specialties, proper implementation of 3 tenets—early feeding, perioperative euvolemia, and multimodal pain control—reduces the length of hospital stay, improves patient satisfaction, reduces complications, lowers health care costs, and most importantly hastens patient recovery.
1 Early feeding
Just as athletes hydrate and carbohydrate load prior to a competition, patients benefit if fluids and calories are not withheld in anticipation of a physiologically stressful surgical procedure. Similarly, modest benefit is associated with carbohydrate loading as a liquid supplement 2 hours before surgery.2 The American Society of Anesthesiologists guidelines state that while solid foods should not be consumed after midnight before surgery, clear liquids safely may be withheld for only 2 hours prior to anesthesia induction, and systematic reviews have failed to show harm.3,4 All patients, including those undergoing colonic resections, are allowed to eat a general diet as tolerated the evening before surgery, supplemented with caloric-dense nutritional supplements.
2 Multimodal pain control
Postsurgical pain is a top patient concern. Pain control is critical for rapid recovery; it helps avoid upregulation of the sympathetic axis and permits ambulation and resumption of normal activities. Although opioids relieve pain, they should not be considered a primary pain control approach.
Responding to the opioid epidemic, in 2015 the Centers for Disease Control and Prevention identified opioid overdose prevention as one of the top 5 public health challenges; notably, approximately 6% of patients will experience new, persistent opioid use following surgery.5 Optimal pain management therefore should provide effective pain relief while minimizing opioid use.
Preemptive oral acetaminophen, gabapentin, and celecoxib should be used routinely prior to incision; nonsteroidal anti-inflammatory drugs should be scheduled postoperatively. Even after a complex cytoreductive laparotomy, pain may be controlled with oral rather than intravenous (IV) medications in most patients, with opioid requirements averaging just 2 to 4 tablets of oxycodone in the first 48 hours after surgery, in our experience. The most critical need for pain medications occurs in the first 48 hours after surgery, which highlights the importance of local or regional analgesia. In one investigation, implementation of multimodal pain management that included incisional injection of liposomal bupivacaine reduced patient-controlled analgesia use to less than 5% after laparotomy.6 The need for opioids more than a week postoperatively is uncommon even after a laparotomy.
3 Perioperative euvolemia
Maintaining euvolemia is a central and underrecognized tenet of enhanced recovery pathways, and it facilitates the other 2 tenets of early feeding and optimal pain control. Overhydrated patients have more pain and prolonged recovery of bowel function. Unfortunately, euvolemia is the most difficult ERAS component to implement, requiring seamless communication between all members of the surgical team.
Continue to: Fluid therapy...
Fluid therapy should be respected as a pharmacologic agent with both benefits and risks. Recognizing that a single liter of lactated Ringer’s solution contains the sodium load of more than 30 bags of potato chips (and normal saline contains far more), one can imagine the impact of 10 L of solution on peripheral and bowel edema and on overall recovery. Importantly, euvolemia must be initiated during surgery. A meta-analysis of nearly 1,000 randomly assigned patients showed that benefits were limited when euvolemia was initiated in the postoperative period.7
When it comes to maintaining euvolemia, particular care must be taken to avoid erring toward hyperadherence. No difference in hospital length of stay, complications, or ileus was observed when patients were randomly assigned to goal-directed fluid therapy or standard practice.8 However, differences in the volume of fluid administered were relatively small, and while there was evidence of underhydration (likely responsible for acute kidney injury), there was no evidence of overhydration. For example, 4 L of fluid is likely superior to 15 L, but it may not be clinically different from 4.5 L. A threshold of fluid restriction is likely to be reached; that is, additional benefit is not achieved and, instead, detrimental effects may occur.
Rather than a specific directive, a more clinically relevant goal may be to replace insensible fluid losses and to maintain perfusion and blood pressure with the lowest volume possible. Note that estimation of fluid requirements is vastly simplified by omitting mechanical bowel preparation. Postoperatively, permissive oliguria (20 mL/h) is allowed since reduced urine output is a normal response to surgery (as a result of inappropriate secretion of antidiuretic hormone) and does not necessitate administration of a fluid bolus. Above all, anesthesiologists should acknowledge that fluid administration’s effects on a patient extend past the postanesthesia care unit, and the entire surgical team should be invested in the patient’s long-term recovery.
Our experience with ERAS
In 2011, Mayo Clinic was the first institution to implement enhanced recovery on a large scale in gynecologic surgery. We have subsequently made multiple pathway modifications in the spirit of continuous improvement (FIGURE 1).

For patients with ovarian cancer requiring extended procedures for cytoreduction via laparotomy (such as colon resection, splenectomy, diaphragm resection), enhanced recovery reduced the median hospital stay by 3 days, patient-controlled IV analgesia use by 88%, and postoperative opioid requirements by 90%.9,10
At 48 hours after surgery, 40% of our patients require no opioids or tramadol, and epidurals are not utilized because of their effects on ambulation and the potential for hypotension. These reductions were met with stable to improved pain scores, a 60% decrease in nausea, and a 50% reduction in adynamic ileus.9,10
Our initial efforts reduced 30-day costs of care by more than $850,000 in just 6 months, with savings of more than $7,600 for each patient undergoing a complex cytoreduction. Furthermore, these improvements allowed consolidation of our inpatient unit with those of other surgical specialties, serving higher volumes of patients within a smaller inpatient footprint. This contraction of inpatient services has accounted for an additional $1.1 million in savings every year since implementation (FIGURE 2).9,10
Our group is not alone in realizing these benefits, and interest has intensified as demonstrated by the fact that the ERAS Society guidelines are among the all-time most downloaded articles in Gynecologic Oncology.11,12 Although our research to demonstrate safety has focused on women undergoing complex oncologic operations, ERAS nevertheless hastens recovery, improves patient satisfaction, and adds value for all patients undergoing gynecologic surgery.

Continue to: Collateral improvements to practice...
Collateral improvements to practice
Clinical optimization using evidence-based practices such as enhanced recovery pathways can result in immediate patient benefit. Affecting such profound clinical improvements is energizing and creates a unique opportunity to transform the culture of the entire health care team. Irrespective of our provider roles (surgeon, anesthesiologist, nurse) or areas of interest (practice, research, education, leadership), we are united by a common purpose: to improve the human condition.13 Reaffirming this common purpose, through the collective effort involved in establishing a standardized enhanced recovery pathway, has allowed our practice and those of others to move beyond enhanced recovery and improve other areas of practice.
Other positive effects. The long-term collateral impact of this culture change at our institution is arguably more important than enhanced recovery itself. Examples of downstream impact include14,15:
- 80% reduction in surgical site infection
- 50% reduction in anastomotic leaks
- 60% reduction in blood utilization for patients undergoing surgery for ovarian cancer.
Team-based pragmatic strategies. Additionally, our willingness to make decisions as a division rather than as individuals facilitated universal implementation of sentinel lymph node biopsy for patients with endometrial cancer and standardized imaging, testing, and surgical decision making for patients with ovarian and endometrial cancer.
The interventions associated with these improvements were not tested in a randomized fashion; however, rather than await perfect data, we made informed decisions based on imperfect data together with a commitment to continuous data review. We find this to be an effective strategy if our goal is to ensure that tomorrow’s outcomes will be better than yesterday’s. In this way, pragmatic trials can be extremely effective in rural settings and tertiary centers.
Barriers to innovation
The widely reported benefits of enhanced recovery beg the question, Why has enhanced recovery not been adopted universally as standard of care? The answer is multifaceted and highlights long-standing shortcomings in our health care system.
Most importantly, our health care system lacks a robust interface to link discovery of new techniques, treatments, and workflows to clinical practice. Perhaps the best example of this is the adoption of minimally invasive surgery (MIS) for endometrial cancer. Ten years have passed since randomized trials showed MIS has equivalent oncologic outcomes and superior recovery compared to laparotomy, yet in the United States less than 50% of women with endometrial cancer benefit.16,17
However, even surgeons who are knowledgeable about recent innovations and genuinely wish to promote improvements may face near-insurmountable skepticism. Blind faith in our abilities and outcomes, overprotection of autonomy, close-mindedness, and satisfaction with the status quo are common responses to innovation and are the enemies of change. Resistance often comes from good intentions, but our desire to avoid complications may result in actions that could just as accurately be labeled superstitious as conservative. These observations suggest that developing methods to incorporate evidence-based practice into routine clinical use is the rate-limiting step in improving surgical quality.
The national Improving Surgical Care and Recovery program is available to specifically aid with ERAS implementation. A collaboration between the Agency for Healthcare Research and Quality (AHRQ) and the American College of Surgeons, the program aims to diffuse enhanced recovery to 750 service lines in 4 surgical subspecialties, including gynecologic surgery, over the next 5 years. (Note: The author is the content expert for the gynecology portion of this program.) The program’s larger aim is to measurably improve patient outcomes, reduce health care utilization, and improve patient experience through the use of an adaptation to AHRQ’s Comprehensive Unit-based Safety Program (CUSP). The backbone for this program is the recent systematic review to establish best practices for gynecologic surgery.1 Free to all participants, the program includes resources such as webinars and coaching calls to assist with the inevitable barriers to ERAS implementation. For more information and to enroll, visit https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/enhanced-recovery /index.html. An important aspect of the program is a registry for tracking outcomes and identifying areas for improvement. For members who currently participate in the National Surgical Quality Improvement Program, clinical data are automatically uploaded into the database. Programs such as Improving Surgical Care and Recovery may be the most reliable way to facilitate diffusion of best practices and take collective responsibility for not only “my outcomes” but also for “our outcomes” as a national community of gynecologic surgeons.
Reference
1. Kalogera E, Nelson G, Liu J, et al. Surgical technical evidence review for gynecologic surgery conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery. Am J Obstet Gynecol. 2018;219:563.e1-563.e19.
Principles essential to change
Various methodologies have been described to manage change and facilitate implementation of new workflows and practices. Irrespective of the method used, including the more formal discipline of implementation science, at least 4 principles must be followed:
1. Teamwork. Mutual trust, mutual respect, and a sense of common purpose are minimum requirements for any successful initiative. Standardization is difficult or impossible without these elements. Thus, establishing a healthy team is the first step in implementing change.
2. Stakeholder analysis. Feedback from surgeons, nurses, residents, fellows, anesthesiologists, pharmacists, nurse anesthetists, and administrators is necessary to obtain diverse perspectives, facilitate engagement, and promote collaborative management. Negativity and resistance are common reactions to change, and it is particularly important to include those who are most skeptical in the stakeholder analysis to mitigate sabotage.
3. Concrete metrics. Success is possible only if defined a priori by specific and achievable goals. Counterbalances also are important to ensure that interventions do not have unintended consequences. Once a goal is met (for example, reduced hospital length of stay or costs), relevant metrics should be monitored after project completion for a minimum of 3 years to avoid regression to the pre-project state.
4. Leadership. The project champion responsible for the initiative must objectively facilitate all of the above and ensure excellent communication between stakeholders to nurture long-term engagement. Despite best efforts, if a minority of the group rejects compromise, this creates an opportunity to compare outcomes between those who do and do not accept the proposed change. Progress realized by early adopters may convince resistors to conform at a later time. Alternatively, the project champion also must have the insight to recognize when a proposed change is impossible at that point in time with that particular group. For example, our own initial attempts to implement enhanced recovery stalled in 2008, but they were successful 3 years later in a different environment.
Continue to: Although a discussion of leadership styles...
Although a discussion of leadership styles is beyond the scope of this article, in our experience, the most successful model is one of servant leadership that is team oriented rather than star dominated. Rather than being led by a single surgeon, each of the 4 quality improvement projects reviewed above (ERAS, and reductions in anastomotic leak, surgical site infection, and blood transfusion) that grew from enhanced recovery included trainees and was led by a different champion, encouraging teamwork and promoting career development. Such a model also supports the Accreditation Council for Graduate Medical Education’s emphasis on quality improvement education.
Our specialty is focusing now more intently on perioperative optimization, commonly referred to as enhanced recovery after surgery (ERAS), a concept championed first and most visibly by colorectal surgeons in the 1990s.1 Both academic and nonacademic practices are challenging long-held beliefs about perioperative management.
The 3 tenets of ERAS
In multiple surgical specialties, proper implementation of 3 tenets—early feeding, perioperative euvolemia, and multimodal pain control—reduces the length of hospital stay, improves patient satisfaction, reduces complications, lowers health care costs, and most importantly hastens patient recovery.
1 Early feeding
Just as athletes hydrate and carbohydrate load prior to a competition, patients benefit if fluids and calories are not withheld in anticipation of a physiologically stressful surgical procedure. Similarly, modest benefit is associated with carbohydrate loading as a liquid supplement 2 hours before surgery.2 The American Society of Anesthesiologists guidelines state that while solid foods should not be consumed after midnight before surgery, clear liquids safely may be withheld for only 2 hours prior to anesthesia induction, and systematic reviews have failed to show harm.3,4 All patients, including those undergoing colonic resections, are allowed to eat a general diet as tolerated the evening before surgery, supplemented with caloric-dense nutritional supplements.
2 Multimodal pain control
Postsurgical pain is a top patient concern. Pain control is critical for rapid recovery; it helps avoid upregulation of the sympathetic axis and permits ambulation and resumption of normal activities. Although opioids relieve pain, they should not be considered a primary pain control approach.
Responding to the opioid epidemic, in 2015 the Centers for Disease Control and Prevention identified opioid overdose prevention as one of the top 5 public health challenges; notably, approximately 6% of patients will experience new, persistent opioid use following surgery.5 Optimal pain management therefore should provide effective pain relief while minimizing opioid use.
Preemptive oral acetaminophen, gabapentin, and celecoxib should be used routinely prior to incision; nonsteroidal anti-inflammatory drugs should be scheduled postoperatively. Even after a complex cytoreductive laparotomy, pain may be controlled with oral rather than intravenous (IV) medications in most patients, with opioid requirements averaging just 2 to 4 tablets of oxycodone in the first 48 hours after surgery, in our experience. The most critical need for pain medications occurs in the first 48 hours after surgery, which highlights the importance of local or regional analgesia. In one investigation, implementation of multimodal pain management that included incisional injection of liposomal bupivacaine reduced patient-controlled analgesia use to less than 5% after laparotomy.6 The need for opioids more than a week postoperatively is uncommon even after a laparotomy.
3 Perioperative euvolemia
Maintaining euvolemia is a central and underrecognized tenet of enhanced recovery pathways, and it facilitates the other 2 tenets of early feeding and optimal pain control. Overhydrated patients have more pain and prolonged recovery of bowel function. Unfortunately, euvolemia is the most difficult ERAS component to implement, requiring seamless communication between all members of the surgical team.
Continue to: Fluid therapy...
Fluid therapy should be respected as a pharmacologic agent with both benefits and risks. Recognizing that a single liter of lactated Ringer’s solution contains the sodium load of more than 30 bags of potato chips (and normal saline contains far more), one can imagine the impact of 10 L of solution on peripheral and bowel edema and on overall recovery. Importantly, euvolemia must be initiated during surgery. A meta-analysis of nearly 1,000 randomly assigned patients showed that benefits were limited when euvolemia was initiated in the postoperative period.7
When it comes to maintaining euvolemia, particular care must be taken to avoid erring toward hyperadherence. No difference in hospital length of stay, complications, or ileus was observed when patients were randomly assigned to goal-directed fluid therapy or standard practice.8 However, differences in the volume of fluid administered were relatively small, and while there was evidence of underhydration (likely responsible for acute kidney injury), there was no evidence of overhydration. For example, 4 L of fluid is likely superior to 15 L, but it may not be clinically different from 4.5 L. A threshold of fluid restriction is likely to be reached; that is, additional benefit is not achieved and, instead, detrimental effects may occur.
Rather than a specific directive, a more clinically relevant goal may be to replace insensible fluid losses and to maintain perfusion and blood pressure with the lowest volume possible. Note that estimation of fluid requirements is vastly simplified by omitting mechanical bowel preparation. Postoperatively, permissive oliguria (20 mL/h) is allowed since reduced urine output is a normal response to surgery (as a result of inappropriate secretion of antidiuretic hormone) and does not necessitate administration of a fluid bolus. Above all, anesthesiologists should acknowledge that fluid administration’s effects on a patient extend past the postanesthesia care unit, and the entire surgical team should be invested in the patient’s long-term recovery.
Our experience with ERAS
In 2011, Mayo Clinic was the first institution to implement enhanced recovery on a large scale in gynecologic surgery. We have subsequently made multiple pathway modifications in the spirit of continuous improvement (FIGURE 1).

For patients with ovarian cancer requiring extended procedures for cytoreduction via laparotomy (such as colon resection, splenectomy, diaphragm resection), enhanced recovery reduced the median hospital stay by 3 days, patient-controlled IV analgesia use by 88%, and postoperative opioid requirements by 90%.9,10
At 48 hours after surgery, 40% of our patients require no opioids or tramadol, and epidurals are not utilized because of their effects on ambulation and the potential for hypotension. These reductions were met with stable to improved pain scores, a 60% decrease in nausea, and a 50% reduction in adynamic ileus.9,10
Our initial efforts reduced 30-day costs of care by more than $850,000 in just 6 months, with savings of more than $7,600 for each patient undergoing a complex cytoreduction. Furthermore, these improvements allowed consolidation of our inpatient unit with those of other surgical specialties, serving higher volumes of patients within a smaller inpatient footprint. This contraction of inpatient services has accounted for an additional $1.1 million in savings every year since implementation (FIGURE 2).9,10
Our group is not alone in realizing these benefits, and interest has intensified as demonstrated by the fact that the ERAS Society guidelines are among the all-time most downloaded articles in Gynecologic Oncology.11,12 Although our research to demonstrate safety has focused on women undergoing complex oncologic operations, ERAS nevertheless hastens recovery, improves patient satisfaction, and adds value for all patients undergoing gynecologic surgery.

Continue to: Collateral improvements to practice...
Collateral improvements to practice
Clinical optimization using evidence-based practices such as enhanced recovery pathways can result in immediate patient benefit. Affecting such profound clinical improvements is energizing and creates a unique opportunity to transform the culture of the entire health care team. Irrespective of our provider roles (surgeon, anesthesiologist, nurse) or areas of interest (practice, research, education, leadership), we are united by a common purpose: to improve the human condition.13 Reaffirming this common purpose, through the collective effort involved in establishing a standardized enhanced recovery pathway, has allowed our practice and those of others to move beyond enhanced recovery and improve other areas of practice.
Other positive effects. The long-term collateral impact of this culture change at our institution is arguably more important than enhanced recovery itself. Examples of downstream impact include14,15:
- 80% reduction in surgical site infection
- 50% reduction in anastomotic leaks
- 60% reduction in blood utilization for patients undergoing surgery for ovarian cancer.
Team-based pragmatic strategies. Additionally, our willingness to make decisions as a division rather than as individuals facilitated universal implementation of sentinel lymph node biopsy for patients with endometrial cancer and standardized imaging, testing, and surgical decision making for patients with ovarian and endometrial cancer.
The interventions associated with these improvements were not tested in a randomized fashion; however, rather than await perfect data, we made informed decisions based on imperfect data together with a commitment to continuous data review. We find this to be an effective strategy if our goal is to ensure that tomorrow’s outcomes will be better than yesterday’s. In this way, pragmatic trials can be extremely effective in rural settings and tertiary centers.
Barriers to innovation
The widely reported benefits of enhanced recovery beg the question, Why has enhanced recovery not been adopted universally as standard of care? The answer is multifaceted and highlights long-standing shortcomings in our health care system.
Most importantly, our health care system lacks a robust interface to link discovery of new techniques, treatments, and workflows to clinical practice. Perhaps the best example of this is the adoption of minimally invasive surgery (MIS) for endometrial cancer. Ten years have passed since randomized trials showed MIS has equivalent oncologic outcomes and superior recovery compared to laparotomy, yet in the United States less than 50% of women with endometrial cancer benefit.16,17
However, even surgeons who are knowledgeable about recent innovations and genuinely wish to promote improvements may face near-insurmountable skepticism. Blind faith in our abilities and outcomes, overprotection of autonomy, close-mindedness, and satisfaction with the status quo are common responses to innovation and are the enemies of change. Resistance often comes from good intentions, but our desire to avoid complications may result in actions that could just as accurately be labeled superstitious as conservative. These observations suggest that developing methods to incorporate evidence-based practice into routine clinical use is the rate-limiting step in improving surgical quality.
The national Improving Surgical Care and Recovery program is available to specifically aid with ERAS implementation. A collaboration between the Agency for Healthcare Research and Quality (AHRQ) and the American College of Surgeons, the program aims to diffuse enhanced recovery to 750 service lines in 4 surgical subspecialties, including gynecologic surgery, over the next 5 years. (Note: The author is the content expert for the gynecology portion of this program.) The program’s larger aim is to measurably improve patient outcomes, reduce health care utilization, and improve patient experience through the use of an adaptation to AHRQ’s Comprehensive Unit-based Safety Program (CUSP). The backbone for this program is the recent systematic review to establish best practices for gynecologic surgery.1 Free to all participants, the program includes resources such as webinars and coaching calls to assist with the inevitable barriers to ERAS implementation. For more information and to enroll, visit https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/enhanced-recovery /index.html. An important aspect of the program is a registry for tracking outcomes and identifying areas for improvement. For members who currently participate in the National Surgical Quality Improvement Program, clinical data are automatically uploaded into the database. Programs such as Improving Surgical Care and Recovery may be the most reliable way to facilitate diffusion of best practices and take collective responsibility for not only “my outcomes” but also for “our outcomes” as a national community of gynecologic surgeons.
Reference
1. Kalogera E, Nelson G, Liu J, et al. Surgical technical evidence review for gynecologic surgery conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery. Am J Obstet Gynecol. 2018;219:563.e1-563.e19.
Principles essential to change
Various methodologies have been described to manage change and facilitate implementation of new workflows and practices. Irrespective of the method used, including the more formal discipline of implementation science, at least 4 principles must be followed:
1. Teamwork. Mutual trust, mutual respect, and a sense of common purpose are minimum requirements for any successful initiative. Standardization is difficult or impossible without these elements. Thus, establishing a healthy team is the first step in implementing change.
2. Stakeholder analysis. Feedback from surgeons, nurses, residents, fellows, anesthesiologists, pharmacists, nurse anesthetists, and administrators is necessary to obtain diverse perspectives, facilitate engagement, and promote collaborative management. Negativity and resistance are common reactions to change, and it is particularly important to include those who are most skeptical in the stakeholder analysis to mitigate sabotage.
3. Concrete metrics. Success is possible only if defined a priori by specific and achievable goals. Counterbalances also are important to ensure that interventions do not have unintended consequences. Once a goal is met (for example, reduced hospital length of stay or costs), relevant metrics should be monitored after project completion for a minimum of 3 years to avoid regression to the pre-project state.
4. Leadership. The project champion responsible for the initiative must objectively facilitate all of the above and ensure excellent communication between stakeholders to nurture long-term engagement. Despite best efforts, if a minority of the group rejects compromise, this creates an opportunity to compare outcomes between those who do and do not accept the proposed change. Progress realized by early adopters may convince resistors to conform at a later time. Alternatively, the project champion also must have the insight to recognize when a proposed change is impossible at that point in time with that particular group. For example, our own initial attempts to implement enhanced recovery stalled in 2008, but they were successful 3 years later in a different environment.
Continue to: Although a discussion of leadership styles...
Although a discussion of leadership styles is beyond the scope of this article, in our experience, the most successful model is one of servant leadership that is team oriented rather than star dominated. Rather than being led by a single surgeon, each of the 4 quality improvement projects reviewed above (ERAS, and reductions in anastomotic leak, surgical site infection, and blood transfusion) that grew from enhanced recovery included trainees and was led by a different champion, encouraging teamwork and promoting career development. Such a model also supports the Accreditation Council for Graduate Medical Education’s emphasis on quality improvement education.
- Nygren J, Thacker J, Carli F, et al; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37:285-305.
- American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114: 495-511.
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423.
- Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17:65-71.
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504.
- Kalogera, E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Rahbari NN, Zimmermann JB, Schmidt T, et al. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96:331-341.
- Myles PS, Bellomo R, Corcoran T, et al; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263-2274.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part I. Gynecol Oncol. 2016;140:313-322.
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140:323-332.
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481.
- Johnson MP, Kim SJ, Langstraat CL, et al. Using bundled interventions to reduce surgical site infection after major gynecologic cancer surgery. Obstet Gynecol. 2016;127:1135-1144.
- Kalogera E, Nitschmann CC, Dowdy SC, et al. A prospective algorithm to reduce anastomotic leaks after rectosigmoid resection for gynecologic malignancies. Gynecol Oncol. 2017;144:343-347.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol. 2016;127:91-100.
- Nygren J, Thacker J, Carli F, et al; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37:285-305.
- American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114: 495-511.
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423.
- Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17:65-71.
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504.
- Kalogera, E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Rahbari NN, Zimmermann JB, Schmidt T, et al. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96:331-341.
- Myles PS, Bellomo R, Corcoran T, et al; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263-2274.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part I. Gynecol Oncol. 2016;140:313-322.
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140:323-332.
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481.
- Johnson MP, Kim SJ, Langstraat CL, et al. Using bundled interventions to reduce surgical site infection after major gynecologic cancer surgery. Obstet Gynecol. 2016;127:1135-1144.
- Kalogera E, Nitschmann CC, Dowdy SC, et al. A prospective algorithm to reduce anastomotic leaks after rectosigmoid resection for gynecologic malignancies. Gynecol Oncol. 2017;144:343-347.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol. 2016;127:91-100.
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
EXPERT COMMENTARY
Postoperative ileus is a common complication following abdominal surgery, particularly for patients undergoing laparotomy. Ileus is frustrating for patients and providers alike, and its occurrence may prolong the length of hospital stay, increase the cost of care, worsen patient satisfaction, and potentially delay postoperative treatments, such as chemotherapy for patients with gynecologic malignancies. The etiology of ileus is multifactorial, but it is thought to be caused primarily by a local inflammatory response from mechanical handling and irritation of the bowel. Although various interventions, such as laxatives, peripheral mu antagonists, and chewing gum, have been shown to reduce the occurrence of ileus, the effectiveness of these interventions varies, and ileus remains a major source of morbidity.1
Details of the study
To investigate whether coffee consumption accelerates recovery of bowel function following surgery, Güngördük and colleagues conducted a randomized controlled trial of coffee consumption after laparotomy with hysterectomy and staging for patients with gynecologic malignancies. This intervention avoids costs associated with drugs such as oral mu antagonists.
The trial included 114 women; after surgery, 58 were assigned to consume coffee 3 times daily and 56 received routine postoperative care without coffee consumption. The primary outcome measure was the time to the first passage of flatus after surgery. Time to first bowel movement and time to tolerance of a solid diet were secondary outcomes.
The results of this trial are consistent with prior study findings in colorectal surgery.2 Güngördük and associates found that patients in the coffee-consumption group, compared with controls, had reduced the time to flatus by 12 hours (mean [SD] time to flatus, 30.2 [8.0] vs 40.2 [12.1] hours; P<.001), shortened time to full diet by 1.3 days (mean [SD] time to tolerate food, 3.4 [1.2] vs 4.7 [1.6] days; P<.001), reduced time to first bowel movement by 12 hours (43.1 [9.4] vs 58.5 [17.0] hours; P<.001), and shortened length of hospital stay by 1 day. Symptoms of ileus were reduced from 52% to 14% with coffee consumption.
Study limitation. An important weakness of this study is that although the authors defined the severity of ileus by time to resolution, they did not define what constituted a diagnosis of ileus in the first place.
Unanswered questions. Coffee is a known diarrhetic, so it is not unexpected that its use shortened time to flatus and first bowel movement. What is not known, however, is whether coffee consumption improves recovery. The significance of a 1-day reduction in hospital stay is unclear given the relatively prolonged hospitalization (6 to 7 days) seen in this investigation of patients with mixed gynecologic malignancies who underwent staging only. In contrast, another study showed that, for patients managed within an enhanced recovery pathway (a multimodal perioperative care enhancement protocol), median length of stay was 4 days for patients who underwent staging alone and 5 days for patients with ovarian cancer (40% underwent enteric resections).3 Thus, the effects of coffee consumption are unclear for patients managed with an optimized perioperative pathway.
The improvement in oral intake is also of questionable significance since these patients tolerated a solid diet 3 to 4 days after surgery, compared with the evening of surgery for most patients managed with enhanced recovery.
Incisional injection of liposomal bupivacaine has been associated with a reduction in the rate of ileus from 22% to 11% after complex cytoreduction for ovarian cancer when added to an existing enhanced recovery pathway; rates were only 5% for patients undergoing staging alone.4 These findings may be due to the significant reduction in opioid use that accompanied the use of liposomal bupivacaine.
--SEAN C. DOWDY, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nelson G, Altman AD, Nick A, et al. Guidelines for post- operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140(2):323-332.
- Müller SA, Rahbari NN, Schneider F, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg. 2012;99(11):1530-1538.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122 (2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128(5):1009-1017.
EXPERT COMMENTARY
Postoperative ileus is a common complication following abdominal surgery, particularly for patients undergoing laparotomy. Ileus is frustrating for patients and providers alike, and its occurrence may prolong the length of hospital stay, increase the cost of care, worsen patient satisfaction, and potentially delay postoperative treatments, such as chemotherapy for patients with gynecologic malignancies. The etiology of ileus is multifactorial, but it is thought to be caused primarily by a local inflammatory response from mechanical handling and irritation of the bowel. Although various interventions, such as laxatives, peripheral mu antagonists, and chewing gum, have been shown to reduce the occurrence of ileus, the effectiveness of these interventions varies, and ileus remains a major source of morbidity.1
Details of the study
To investigate whether coffee consumption accelerates recovery of bowel function following surgery, Güngördük and colleagues conducted a randomized controlled trial of coffee consumption after laparotomy with hysterectomy and staging for patients with gynecologic malignancies. This intervention avoids costs associated with drugs such as oral mu antagonists.
The trial included 114 women; after surgery, 58 were assigned to consume coffee 3 times daily and 56 received routine postoperative care without coffee consumption. The primary outcome measure was the time to the first passage of flatus after surgery. Time to first bowel movement and time to tolerance of a solid diet were secondary outcomes.
The results of this trial are consistent with prior study findings in colorectal surgery.2 Güngördük and associates found that patients in the coffee-consumption group, compared with controls, had reduced the time to flatus by 12 hours (mean [SD] time to flatus, 30.2 [8.0] vs 40.2 [12.1] hours; P<.001), shortened time to full diet by 1.3 days (mean [SD] time to tolerate food, 3.4 [1.2] vs 4.7 [1.6] days; P<.001), reduced time to first bowel movement by 12 hours (43.1 [9.4] vs 58.5 [17.0] hours; P<.001), and shortened length of hospital stay by 1 day. Symptoms of ileus were reduced from 52% to 14% with coffee consumption.
Study limitation. An important weakness of this study is that although the authors defined the severity of ileus by time to resolution, they did not define what constituted a diagnosis of ileus in the first place.
Unanswered questions. Coffee is a known diarrhetic, so it is not unexpected that its use shortened time to flatus and first bowel movement. What is not known, however, is whether coffee consumption improves recovery. The significance of a 1-day reduction in hospital stay is unclear given the relatively prolonged hospitalization (6 to 7 days) seen in this investigation of patients with mixed gynecologic malignancies who underwent staging only. In contrast, another study showed that, for patients managed within an enhanced recovery pathway (a multimodal perioperative care enhancement protocol), median length of stay was 4 days for patients who underwent staging alone and 5 days for patients with ovarian cancer (40% underwent enteric resections).3 Thus, the effects of coffee consumption are unclear for patients managed with an optimized perioperative pathway.
The improvement in oral intake is also of questionable significance since these patients tolerated a solid diet 3 to 4 days after surgery, compared with the evening of surgery for most patients managed with enhanced recovery.
Incisional injection of liposomal bupivacaine has been associated with a reduction in the rate of ileus from 22% to 11% after complex cytoreduction for ovarian cancer when added to an existing enhanced recovery pathway; rates were only 5% for patients undergoing staging alone.4 These findings may be due to the significant reduction in opioid use that accompanied the use of liposomal bupivacaine.
--SEAN C. DOWDY, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Postoperative ileus is a common complication following abdominal surgery, particularly for patients undergoing laparotomy. Ileus is frustrating for patients and providers alike, and its occurrence may prolong the length of hospital stay, increase the cost of care, worsen patient satisfaction, and potentially delay postoperative treatments, such as chemotherapy for patients with gynecologic malignancies. The etiology of ileus is multifactorial, but it is thought to be caused primarily by a local inflammatory response from mechanical handling and irritation of the bowel. Although various interventions, such as laxatives, peripheral mu antagonists, and chewing gum, have been shown to reduce the occurrence of ileus, the effectiveness of these interventions varies, and ileus remains a major source of morbidity.1
Details of the study
To investigate whether coffee consumption accelerates recovery of bowel function following surgery, Güngördük and colleagues conducted a randomized controlled trial of coffee consumption after laparotomy with hysterectomy and staging for patients with gynecologic malignancies. This intervention avoids costs associated with drugs such as oral mu antagonists.
The trial included 114 women; after surgery, 58 were assigned to consume coffee 3 times daily and 56 received routine postoperative care without coffee consumption. The primary outcome measure was the time to the first passage of flatus after surgery. Time to first bowel movement and time to tolerance of a solid diet were secondary outcomes.
The results of this trial are consistent with prior study findings in colorectal surgery.2 Güngördük and associates found that patients in the coffee-consumption group, compared with controls, had reduced the time to flatus by 12 hours (mean [SD] time to flatus, 30.2 [8.0] vs 40.2 [12.1] hours; P<.001), shortened time to full diet by 1.3 days (mean [SD] time to tolerate food, 3.4 [1.2] vs 4.7 [1.6] days; P<.001), reduced time to first bowel movement by 12 hours (43.1 [9.4] vs 58.5 [17.0] hours; P<.001), and shortened length of hospital stay by 1 day. Symptoms of ileus were reduced from 52% to 14% with coffee consumption.
Study limitation. An important weakness of this study is that although the authors defined the severity of ileus by time to resolution, they did not define what constituted a diagnosis of ileus in the first place.
Unanswered questions. Coffee is a known diarrhetic, so it is not unexpected that its use shortened time to flatus and first bowel movement. What is not known, however, is whether coffee consumption improves recovery. The significance of a 1-day reduction in hospital stay is unclear given the relatively prolonged hospitalization (6 to 7 days) seen in this investigation of patients with mixed gynecologic malignancies who underwent staging only. In contrast, another study showed that, for patients managed within an enhanced recovery pathway (a multimodal perioperative care enhancement protocol), median length of stay was 4 days for patients who underwent staging alone and 5 days for patients with ovarian cancer (40% underwent enteric resections).3 Thus, the effects of coffee consumption are unclear for patients managed with an optimized perioperative pathway.
The improvement in oral intake is also of questionable significance since these patients tolerated a solid diet 3 to 4 days after surgery, compared with the evening of surgery for most patients managed with enhanced recovery.
Incisional injection of liposomal bupivacaine has been associated with a reduction in the rate of ileus from 22% to 11% after complex cytoreduction for ovarian cancer when added to an existing enhanced recovery pathway; rates were only 5% for patients undergoing staging alone.4 These findings may be due to the significant reduction in opioid use that accompanied the use of liposomal bupivacaine.
--SEAN C. DOWDY, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nelson G, Altman AD, Nick A, et al. Guidelines for post- operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140(2):323-332.
- Müller SA, Rahbari NN, Schneider F, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg. 2012;99(11):1530-1538.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122 (2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128(5):1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for post- operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140(2):323-332.
- Müller SA, Rahbari NN, Schneider F, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg. 2012;99(11):1530-1538.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122 (2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128(5):1009-1017.

