User login
Hepatitis B: Screening, Awareness, and the Need to Treat
Globally, chronic hepatitis B virus (HBV) infection is the leading cause of liver-related morbidity and mortality. Worldwide, more than 2 billion people have been exposed to HBV, and about 250 million are chronically infected.1
The prevalence of hepatitis B surface antigen (HBsAg), the serologic marker of chronicity, varies significantly worldwide. The highest rates of HBsAg are reported in Asia, Sub-Saharan Africa, and the Amazon basin. The overall prevalence of chronic HBV infection in the U.S. is low, 800,000 to 1.4 million persons. The disease is predominantly seen in immigrants, with > 90% of cases in persons from countries of intermediate or high HBV prevalence, such as East Asia, Africa, Pacific Islands, parts of Africa, and Eastern Europe.2
The prevalence of chronic HBV infection in the U.S. may be underestimated and closer to 2.2 million persons, because many foreign-born persons are generally excluded from national prevalence surveys.3 More worrisome, studies suggest that a majority of individuals with chronic HBV infection are unaware of their diagnosis, and consequently, many patients who might benefit from therapy do not receive appropriate care or treatment.4 This review will discuss screening recommendations for HBV in the U.S., identify knowledge gaps regarding the disease, and present a cogent argument for why treatment-eligible patients should be entered into management programs and evaluated for therapy.
Hepatitis B Screening
Chronic hepatitis B meets the criteria established by the World Health Organization as a disease for which screening would be beneficial to public health. Chronic HBV infection is an important health problem that can result in serious sequelae, such as cirrhosis, hepatocellular carcinoma, and liver-related mortality. Moreover, persons unaware of their diagnosis may unwittingly transmit the virus to unprotected individuals.
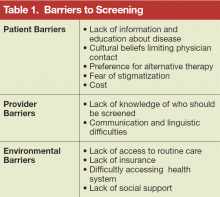
A simple, relatively inexpensive test is widely available to identify chronic HBV infection. The test allows physicians to confirm a diagnosis before symptoms develop and offer a safe and effective therapy. Modeling studies suggest that screening populations with a prevalence of chronic HBV infection ≥ 2% also would be cost-effective in reducing the burden of HBV-associated liver cancer and chronic liver disease in high-risk populations.5,6 However, a number of barriers exist that limit screening (Table 1).
Who to Screen?
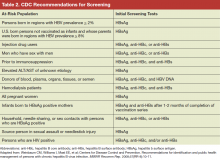
All guidelines recommend that persons at high risk for HBV infection should be screened. Broadly, these include persons from geographic areas with a high prevalence of chronic infection, persons at high risk for acquiring HBV infection, persons with increased risk of transmitting HBV, and persons at risk for reactivation of HBV. In addition to previous recommendations, the Centers for Disease Control and Prevention (CDC) updated 2008 guidelines now recommend testing all persons born in geographic areas with a HBsAg prevalence of ≥ 2%, U.S.-born persons not vaccinated as infants whose parents were born in regions with HBsAg prevalence ≥ 8%, persons who inject drugs, men who have sex with men, persons with elevated alanine transaminase and aspartate transaminase of unknown etiology, and persons with selected medical conditions that require immunosuppressive therapy (Table 2).7 In 2014, the U.S. Preventive Services Task Force provided updated guidance on screening of nonpregnant adolescents and adults that aligned with the CDC guidelines and also recommended screening individuals at high risk for HBV infection.8 The American Association for the Study of Liver Diseases and other professional liver organizations support these recommendations.9
Which Test to Use
Serologic testing for HBsAg is the recommended method to identify persons with chronic HBV infection. Testing for HBV infection in high-risk groups should be performed with a FDA-licensed or FDA-approved serologic assay for HBsAg (sensitivity and specificity of > 98%) according to the manufacturer’s recommendations. Initially, reactive specimens should be confirmed with a licensed confirmatory test.
A positive HBsAg result indicates active infection, either acute or chronic. Other serological markers of HBV infection, such as presence of hepatitis B core IgM antibody, and the clinical context are used to differentiate between acute, chronic, or resolving infection. For identification of individuals who are at risk for chronic infection, the screening strategy should be with HBsAg only. For identifying susceptible persons who should be offered HBV vaccination, or patients that are at risk of reactivation or transmission of HBV, screening should include
testing for HBsAg, hepatitis B core antibody (anti-HBc) and hepatitis B surface antibody (anti-HBs).
The interpretation of HBV screening serology is shown in Table 3. Persons with chronic HBV infection are treated, if needed, per practice guidelines based on the initial test results and interpretation of the stage of the disease and counseled regarding transmission of infection. 9,10 Vaccination is recommended for uninfected persons.
Hepatitis B Education
There is relatively poor awareness of HBV among highrisk individuals and health care professionals (HCPs). A study examining the prevalence of chronic hepatitis B in an Asian and Pacific Islander population reported that about 15% of participants had not been previously tested for hepatitis B.11 Another study that surveyed 3,163 Asian American adults in the San Francisco Bay Area found that of those screened and identified with chronic HBV infection (8.9%), two-thirds were unaware that they were infected.12
Primary care providers in San Francisco correctly identified that Chinese immigrants have a higher prevalence of chronic hepatitis B than that of non-Hispanic white or U.S.-born Chinese people, but the providers incorrectly identified persons with HIV infection, men who have sex with men, and persons who inject drugs as having higher prevalence than that of Chinese immigrants in a survey.13 Lack of awareness probably contributes to poor outcomes from the infection among at-risk persons as well as continued transmission to susceptible individuals. Furthermore, lack of knowledge is a barrier to testing, prevention, and care. Increased awareness to identify the at-risk population and early treatment is an important step to prevent morbidity and mortality from chronic hepatitis B.
Public Awareness
Many at-risk populations are unaware of HBV, its possible routes of transmission, or that a safe and effective vaccine exists for HBV prevention. Moreover, many foreign-born persons with chronic HBV infection feel stigmatized by others or have cultural barriers against Western medicine and prefer alternative therapies. As a result, the Institute of Medicine (IOM) has suggested that innovative approaches need to be developed to promote a better understanding of transmission, prevent and treat HBV, increase HBV vaccination rates among children and at-risk adults, educate women about vertical transmission risk, reduce stigmatization, and provide culturally sensitive and understandable educational material.14
Awareness Among Health Care Professionals
Studies have identified knowledge gaps among HCPs regarding the prevalence of HBV in the general U.S. population, outcome of infection, who should be screened and vaccinated against HBV, appropriate methods for screening and interpretation of serologic tests for HBV, and proper treatment of persons with chronic infection. As a consequence, the IOM recommended educational programs for HCPs on the prevalence of HBV infection in the general U.S. population and at-risk populations, particularly foreign-born persons. In addition, these educational programs should target all levels of HCPs, including undergraduates and postgraduates, and include information on screening and prevention, testing, and interpretation of tests.14
Need to Treat
The global HBV disease burden remains high despite the existence of an effective vaccine. Worldwide, there are an estimated 4.5 million new infections and 780,000 HBVrelated deaths each year.15 In the U.S., the highest rates of mortality are seen in Asians and Pacific Islanders and among persons aged 55 to 64 years. Asians and Pacific Islanders also have the highest rate of liver cancer deaths.16,17
The natural history of chronic hepatitis B is highly variable and dependent on a complex interplay between the virus and the host immune response. It is estimated that between 25% and 40% of persons with chronic hepatitis B will be at risk for progression to cirrhosis.7,18,19 In a study among persons with cirrhosis, the 5-year cumulative risk of developing hepatocellular carcinoma was 17% in Asian patients and 10% in white Americans and Europeans. The 5-year liver-related death rate was 14% among East Asians and 15% among white Europeans.18
A significant proportion of individuals infected with HBV are unaware of their diagnosis, not enrolled in care, or not receiving therapy.7,12,20-24 Data from several prospective and retrospective cohort studies have demonstrated that prolonged viral suppression achieved with therapy is associated with regression of fibrosis and reversal of cirrhosis in a substantial proportion of individuals.25,26 Treatment has also been associated with a reduction in rates of liver decompensation, HCC, liverrelated, and all-cause mortality among patients with liver cirrhosis.27
Given the risk of serious complications and the availability of safe and effective therapy, it is imperative that persons identified as having chronic hepatitis B be referred for evaluation to determine whether therapy is warranted. However, it is also important to recognize that a cure for HBV infection is currently not available, and most patients who initiate therapy will require longterm treatment. In addition, persons who are not currently treatment candidates may become candidates due to changes in disease activity. This underscores the point that patients with chronic hepatitis B require lifelong monitoring regardless of whether they are receiving treatment.
Conclusions
The primary reasons to screen for HBV are to reduce morbidity and mortality related to liver disease and to prevent transmission. Significant barriers remain to screening and referral for care for HBV in the U.S. Educational programs to increase knowledge and awareness among HCPs and the public together with improved access to care are critical to improve disease outcomes and prevent transmission. Despite the availability of an effective vaccine for 3 decades, the global prevalence of HBV has not substantially declined. Further research is needed to explore strategies to overcome screening barriers, improve vaccination rates, and to develop new models of health care delivery to reduce the burden of disease-related to HBV.
Click here to read the digital edition.
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-1555.
2. Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B--United States, 1974-2008. PLoS One. 2011;6(12):e27717.
3. Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56(2):422-433.
4. Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18(6):377-383.
5. Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147(7):460-469.
6. Eckman MH, Kaiser TE, Sherman KE. The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis. 2011;52(11):1294-1306.
7. Weinbaum CM, Williams I, Mast EE, et al; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1-20.
8. LeFevre ML; U.S. Preventive Services Task Force. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(1):58-66.
9. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
10. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283.
11. Centers for Disease Control and Prevention (CDC). Screening for chronic hepatitis B among Asian/Pacific Islander populations--New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(18):505-509.
12. Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46(4):1034-1040.
13. Lai CJ, Nguyen TT, Hwang J, Stewart SL, Kwan A, McPhee SJ. Provider knowledge and practice regarding hepatitis B screening in Chinese-speaking patients. J Cancer Educ. 2007;22(1):37-41.
14. Colvin HM, Mitchell AE, eds; Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: National Academies Press; 2010:xix, 232.
15. Hepatitis B Fact Sheet No. 204. World Health Organization Website. http://www.who.int/entity/mediacentre/factsheets/fs204/en/. Updated July 2015. Accessed March 17, 2016.
16. Perz JF, Openo K, Ahmed F, Bell BP. P.382 Trends in mortality from liver cancer in the USA, 1993-2002. J Clin Virol. 2006;36(suppl 2):S178.
17. Chang ET, Keegan TH, Gomez SL, et al. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109(10):2100-2108.
18. Fattovich G1, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335-352.
19. Chou R, Dana T, Bougatsos C, Blazina I, Khangura J, Zakher B. Screening for hepatitis B virus infection in adolescents and adults: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;161(1):31-45.
20. Wan KJ, Miyoshi T, Fryer G, et al. Screening for hepatitis B virus infection by primary care physicians in New York City: are screening recommendations for persons born inendemic countries being followed?[abstract 1454]. Hepatology. 2007;46(suppl):889A-890A.
21. Thompson MJ, Taylor VM, Jackson JC, et al. Hepatitis B knowledge and practices among Chinese American women in Seattle, Washington. J Cancer Educ. 2002;17(4):222-226.
22. Ma GX, Fang CY, Shive SE, Toubbeh J, Tan Y, Siu P. Risk perceptions and barriers to hepatitis B screening and vaccination among Vietnamese immigrants. J Immigr Minor Health. 2007;9(3):213-220.
23. Taylor VM, Choe JH, Yasui Y, Li Lin, Burke N, Jackson JC. Hepatitis B awareness, testing, and knowledge among Vietnamese American men and women. J Community Health. 2005;30(6):477-490.
24. Weinbaum CM, Lyerla R, Mackellar DA, et al; Young Men’s Survey Study Group. The young men’s survey phase II: hepatitis B immunization and infection among young men who have sex with men. Am J Public Health. 2008;98(5):839-845.
25. Marcellin P, Gane E, Buti M. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468-475.
26. Chang TT, Liaw YF, Wu SS. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886-893.
27. Wong GL, Chan HL, Mak CW. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology.2013;58(5):1537-1547.
Globally, chronic hepatitis B virus (HBV) infection is the leading cause of liver-related morbidity and mortality. Worldwide, more than 2 billion people have been exposed to HBV, and about 250 million are chronically infected.1
The prevalence of hepatitis B surface antigen (HBsAg), the serologic marker of chronicity, varies significantly worldwide. The highest rates of HBsAg are reported in Asia, Sub-Saharan Africa, and the Amazon basin. The overall prevalence of chronic HBV infection in the U.S. is low, 800,000 to 1.4 million persons. The disease is predominantly seen in immigrants, with > 90% of cases in persons from countries of intermediate or high HBV prevalence, such as East Asia, Africa, Pacific Islands, parts of Africa, and Eastern Europe.2
The prevalence of chronic HBV infection in the U.S. may be underestimated and closer to 2.2 million persons, because many foreign-born persons are generally excluded from national prevalence surveys.3 More worrisome, studies suggest that a majority of individuals with chronic HBV infection are unaware of their diagnosis, and consequently, many patients who might benefit from therapy do not receive appropriate care or treatment.4 This review will discuss screening recommendations for HBV in the U.S., identify knowledge gaps regarding the disease, and present a cogent argument for why treatment-eligible patients should be entered into management programs and evaluated for therapy.
Hepatitis B Screening
Chronic hepatitis B meets the criteria established by the World Health Organization as a disease for which screening would be beneficial to public health. Chronic HBV infection is an important health problem that can result in serious sequelae, such as cirrhosis, hepatocellular carcinoma, and liver-related mortality. Moreover, persons unaware of their diagnosis may unwittingly transmit the virus to unprotected individuals.
A simple, relatively inexpensive test is widely available to identify chronic HBV infection. The test allows physicians to confirm a diagnosis before symptoms develop and offer a safe and effective therapy. Modeling studies suggest that screening populations with a prevalence of chronic HBV infection ≥ 2% also would be cost-effective in reducing the burden of HBV-associated liver cancer and chronic liver disease in high-risk populations.5,6 However, a number of barriers exist that limit screening (Table 1).
Who to Screen?
All guidelines recommend that persons at high risk for HBV infection should be screened. Broadly, these include persons from geographic areas with a high prevalence of chronic infection, persons at high risk for acquiring HBV infection, persons with increased risk of transmitting HBV, and persons at risk for reactivation of HBV. In addition to previous recommendations, the Centers for Disease Control and Prevention (CDC) updated 2008 guidelines now recommend testing all persons born in geographic areas with a HBsAg prevalence of ≥ 2%, U.S.-born persons not vaccinated as infants whose parents were born in regions with HBsAg prevalence ≥ 8%, persons who inject drugs, men who have sex with men, persons with elevated alanine transaminase and aspartate transaminase of unknown etiology, and persons with selected medical conditions that require immunosuppressive therapy (Table 2).7 In 2014, the U.S. Preventive Services Task Force provided updated guidance on screening of nonpregnant adolescents and adults that aligned with the CDC guidelines and also recommended screening individuals at high risk for HBV infection.8 The American Association for the Study of Liver Diseases and other professional liver organizations support these recommendations.9
Which Test to Use
Serologic testing for HBsAg is the recommended method to identify persons with chronic HBV infection. Testing for HBV infection in high-risk groups should be performed with a FDA-licensed or FDA-approved serologic assay for HBsAg (sensitivity and specificity of > 98%) according to the manufacturer’s recommendations. Initially, reactive specimens should be confirmed with a licensed confirmatory test.
A positive HBsAg result indicates active infection, either acute or chronic. Other serological markers of HBV infection, such as presence of hepatitis B core IgM antibody, and the clinical context are used to differentiate between acute, chronic, or resolving infection. For identification of individuals who are at risk for chronic infection, the screening strategy should be with HBsAg only. For identifying susceptible persons who should be offered HBV vaccination, or patients that are at risk of reactivation or transmission of HBV, screening should include
testing for HBsAg, hepatitis B core antibody (anti-HBc) and hepatitis B surface antibody (anti-HBs).
The interpretation of HBV screening serology is shown in Table 3. Persons with chronic HBV infection are treated, if needed, per practice guidelines based on the initial test results and interpretation of the stage of the disease and counseled regarding transmission of infection. 9,10 Vaccination is recommended for uninfected persons.
Hepatitis B Education
There is relatively poor awareness of HBV among highrisk individuals and health care professionals (HCPs). A study examining the prevalence of chronic hepatitis B in an Asian and Pacific Islander population reported that about 15% of participants had not been previously tested for hepatitis B.11 Another study that surveyed 3,163 Asian American adults in the San Francisco Bay Area found that of those screened and identified with chronic HBV infection (8.9%), two-thirds were unaware that they were infected.12
Primary care providers in San Francisco correctly identified that Chinese immigrants have a higher prevalence of chronic hepatitis B than that of non-Hispanic white or U.S.-born Chinese people, but the providers incorrectly identified persons with HIV infection, men who have sex with men, and persons who inject drugs as having higher prevalence than that of Chinese immigrants in a survey.13 Lack of awareness probably contributes to poor outcomes from the infection among at-risk persons as well as continued transmission to susceptible individuals. Furthermore, lack of knowledge is a barrier to testing, prevention, and care. Increased awareness to identify the at-risk population and early treatment is an important step to prevent morbidity and mortality from chronic hepatitis B.
Public Awareness
Many at-risk populations are unaware of HBV, its possible routes of transmission, or that a safe and effective vaccine exists for HBV prevention. Moreover, many foreign-born persons with chronic HBV infection feel stigmatized by others or have cultural barriers against Western medicine and prefer alternative therapies. As a result, the Institute of Medicine (IOM) has suggested that innovative approaches need to be developed to promote a better understanding of transmission, prevent and treat HBV, increase HBV vaccination rates among children and at-risk adults, educate women about vertical transmission risk, reduce stigmatization, and provide culturally sensitive and understandable educational material.14
Awareness Among Health Care Professionals
Studies have identified knowledge gaps among HCPs regarding the prevalence of HBV in the general U.S. population, outcome of infection, who should be screened and vaccinated against HBV, appropriate methods for screening and interpretation of serologic tests for HBV, and proper treatment of persons with chronic infection. As a consequence, the IOM recommended educational programs for HCPs on the prevalence of HBV infection in the general U.S. population and at-risk populations, particularly foreign-born persons. In addition, these educational programs should target all levels of HCPs, including undergraduates and postgraduates, and include information on screening and prevention, testing, and interpretation of tests.14
Need to Treat
The global HBV disease burden remains high despite the existence of an effective vaccine. Worldwide, there are an estimated 4.5 million new infections and 780,000 HBVrelated deaths each year.15 In the U.S., the highest rates of mortality are seen in Asians and Pacific Islanders and among persons aged 55 to 64 years. Asians and Pacific Islanders also have the highest rate of liver cancer deaths.16,17
The natural history of chronic hepatitis B is highly variable and dependent on a complex interplay between the virus and the host immune response. It is estimated that between 25% and 40% of persons with chronic hepatitis B will be at risk for progression to cirrhosis.7,18,19 In a study among persons with cirrhosis, the 5-year cumulative risk of developing hepatocellular carcinoma was 17% in Asian patients and 10% in white Americans and Europeans. The 5-year liver-related death rate was 14% among East Asians and 15% among white Europeans.18
A significant proportion of individuals infected with HBV are unaware of their diagnosis, not enrolled in care, or not receiving therapy.7,12,20-24 Data from several prospective and retrospective cohort studies have demonstrated that prolonged viral suppression achieved with therapy is associated with regression of fibrosis and reversal of cirrhosis in a substantial proportion of individuals.25,26 Treatment has also been associated with a reduction in rates of liver decompensation, HCC, liverrelated, and all-cause mortality among patients with liver cirrhosis.27
Given the risk of serious complications and the availability of safe and effective therapy, it is imperative that persons identified as having chronic hepatitis B be referred for evaluation to determine whether therapy is warranted. However, it is also important to recognize that a cure for HBV infection is currently not available, and most patients who initiate therapy will require longterm treatment. In addition, persons who are not currently treatment candidates may become candidates due to changes in disease activity. This underscores the point that patients with chronic hepatitis B require lifelong monitoring regardless of whether they are receiving treatment.
Conclusions
The primary reasons to screen for HBV are to reduce morbidity and mortality related to liver disease and to prevent transmission. Significant barriers remain to screening and referral for care for HBV in the U.S. Educational programs to increase knowledge and awareness among HCPs and the public together with improved access to care are critical to improve disease outcomes and prevent transmission. Despite the availability of an effective vaccine for 3 decades, the global prevalence of HBV has not substantially declined. Further research is needed to explore strategies to overcome screening barriers, improve vaccination rates, and to develop new models of health care delivery to reduce the burden of disease-related to HBV.
Click here to read the digital edition.
Globally, chronic hepatitis B virus (HBV) infection is the leading cause of liver-related morbidity and mortality. Worldwide, more than 2 billion people have been exposed to HBV, and about 250 million are chronically infected.1
The prevalence of hepatitis B surface antigen (HBsAg), the serologic marker of chronicity, varies significantly worldwide. The highest rates of HBsAg are reported in Asia, Sub-Saharan Africa, and the Amazon basin. The overall prevalence of chronic HBV infection in the U.S. is low, 800,000 to 1.4 million persons. The disease is predominantly seen in immigrants, with > 90% of cases in persons from countries of intermediate or high HBV prevalence, such as East Asia, Africa, Pacific Islands, parts of Africa, and Eastern Europe.2
The prevalence of chronic HBV infection in the U.S. may be underestimated and closer to 2.2 million persons, because many foreign-born persons are generally excluded from national prevalence surveys.3 More worrisome, studies suggest that a majority of individuals with chronic HBV infection are unaware of their diagnosis, and consequently, many patients who might benefit from therapy do not receive appropriate care or treatment.4 This review will discuss screening recommendations for HBV in the U.S., identify knowledge gaps regarding the disease, and present a cogent argument for why treatment-eligible patients should be entered into management programs and evaluated for therapy.
Hepatitis B Screening
Chronic hepatitis B meets the criteria established by the World Health Organization as a disease for which screening would be beneficial to public health. Chronic HBV infection is an important health problem that can result in serious sequelae, such as cirrhosis, hepatocellular carcinoma, and liver-related mortality. Moreover, persons unaware of their diagnosis may unwittingly transmit the virus to unprotected individuals.
A simple, relatively inexpensive test is widely available to identify chronic HBV infection. The test allows physicians to confirm a diagnosis before symptoms develop and offer a safe and effective therapy. Modeling studies suggest that screening populations with a prevalence of chronic HBV infection ≥ 2% also would be cost-effective in reducing the burden of HBV-associated liver cancer and chronic liver disease in high-risk populations.5,6 However, a number of barriers exist that limit screening (Table 1).
Who to Screen?
All guidelines recommend that persons at high risk for HBV infection should be screened. Broadly, these include persons from geographic areas with a high prevalence of chronic infection, persons at high risk for acquiring HBV infection, persons with increased risk of transmitting HBV, and persons at risk for reactivation of HBV. In addition to previous recommendations, the Centers for Disease Control and Prevention (CDC) updated 2008 guidelines now recommend testing all persons born in geographic areas with a HBsAg prevalence of ≥ 2%, U.S.-born persons not vaccinated as infants whose parents were born in regions with HBsAg prevalence ≥ 8%, persons who inject drugs, men who have sex with men, persons with elevated alanine transaminase and aspartate transaminase of unknown etiology, and persons with selected medical conditions that require immunosuppressive therapy (Table 2).7 In 2014, the U.S. Preventive Services Task Force provided updated guidance on screening of nonpregnant adolescents and adults that aligned with the CDC guidelines and also recommended screening individuals at high risk for HBV infection.8 The American Association for the Study of Liver Diseases and other professional liver organizations support these recommendations.9
Which Test to Use
Serologic testing for HBsAg is the recommended method to identify persons with chronic HBV infection. Testing for HBV infection in high-risk groups should be performed with a FDA-licensed or FDA-approved serologic assay for HBsAg (sensitivity and specificity of > 98%) according to the manufacturer’s recommendations. Initially, reactive specimens should be confirmed with a licensed confirmatory test.
A positive HBsAg result indicates active infection, either acute or chronic. Other serological markers of HBV infection, such as presence of hepatitis B core IgM antibody, and the clinical context are used to differentiate between acute, chronic, or resolving infection. For identification of individuals who are at risk for chronic infection, the screening strategy should be with HBsAg only. For identifying susceptible persons who should be offered HBV vaccination, or patients that are at risk of reactivation or transmission of HBV, screening should include
testing for HBsAg, hepatitis B core antibody (anti-HBc) and hepatitis B surface antibody (anti-HBs).
The interpretation of HBV screening serology is shown in Table 3. Persons with chronic HBV infection are treated, if needed, per practice guidelines based on the initial test results and interpretation of the stage of the disease and counseled regarding transmission of infection. 9,10 Vaccination is recommended for uninfected persons.
Hepatitis B Education
There is relatively poor awareness of HBV among highrisk individuals and health care professionals (HCPs). A study examining the prevalence of chronic hepatitis B in an Asian and Pacific Islander population reported that about 15% of participants had not been previously tested for hepatitis B.11 Another study that surveyed 3,163 Asian American adults in the San Francisco Bay Area found that of those screened and identified with chronic HBV infection (8.9%), two-thirds were unaware that they were infected.12
Primary care providers in San Francisco correctly identified that Chinese immigrants have a higher prevalence of chronic hepatitis B than that of non-Hispanic white or U.S.-born Chinese people, but the providers incorrectly identified persons with HIV infection, men who have sex with men, and persons who inject drugs as having higher prevalence than that of Chinese immigrants in a survey.13 Lack of awareness probably contributes to poor outcomes from the infection among at-risk persons as well as continued transmission to susceptible individuals. Furthermore, lack of knowledge is a barrier to testing, prevention, and care. Increased awareness to identify the at-risk population and early treatment is an important step to prevent morbidity and mortality from chronic hepatitis B.
Public Awareness
Many at-risk populations are unaware of HBV, its possible routes of transmission, or that a safe and effective vaccine exists for HBV prevention. Moreover, many foreign-born persons with chronic HBV infection feel stigmatized by others or have cultural barriers against Western medicine and prefer alternative therapies. As a result, the Institute of Medicine (IOM) has suggested that innovative approaches need to be developed to promote a better understanding of transmission, prevent and treat HBV, increase HBV vaccination rates among children and at-risk adults, educate women about vertical transmission risk, reduce stigmatization, and provide culturally sensitive and understandable educational material.14
Awareness Among Health Care Professionals
Studies have identified knowledge gaps among HCPs regarding the prevalence of HBV in the general U.S. population, outcome of infection, who should be screened and vaccinated against HBV, appropriate methods for screening and interpretation of serologic tests for HBV, and proper treatment of persons with chronic infection. As a consequence, the IOM recommended educational programs for HCPs on the prevalence of HBV infection in the general U.S. population and at-risk populations, particularly foreign-born persons. In addition, these educational programs should target all levels of HCPs, including undergraduates and postgraduates, and include information on screening and prevention, testing, and interpretation of tests.14
Need to Treat
The global HBV disease burden remains high despite the existence of an effective vaccine. Worldwide, there are an estimated 4.5 million new infections and 780,000 HBVrelated deaths each year.15 In the U.S., the highest rates of mortality are seen in Asians and Pacific Islanders and among persons aged 55 to 64 years. Asians and Pacific Islanders also have the highest rate of liver cancer deaths.16,17
The natural history of chronic hepatitis B is highly variable and dependent on a complex interplay between the virus and the host immune response. It is estimated that between 25% and 40% of persons with chronic hepatitis B will be at risk for progression to cirrhosis.7,18,19 In a study among persons with cirrhosis, the 5-year cumulative risk of developing hepatocellular carcinoma was 17% in Asian patients and 10% in white Americans and Europeans. The 5-year liver-related death rate was 14% among East Asians and 15% among white Europeans.18
A significant proportion of individuals infected with HBV are unaware of their diagnosis, not enrolled in care, or not receiving therapy.7,12,20-24 Data from several prospective and retrospective cohort studies have demonstrated that prolonged viral suppression achieved with therapy is associated with regression of fibrosis and reversal of cirrhosis in a substantial proportion of individuals.25,26 Treatment has also been associated with a reduction in rates of liver decompensation, HCC, liverrelated, and all-cause mortality among patients with liver cirrhosis.27
Given the risk of serious complications and the availability of safe and effective therapy, it is imperative that persons identified as having chronic hepatitis B be referred for evaluation to determine whether therapy is warranted. However, it is also important to recognize that a cure for HBV infection is currently not available, and most patients who initiate therapy will require longterm treatment. In addition, persons who are not currently treatment candidates may become candidates due to changes in disease activity. This underscores the point that patients with chronic hepatitis B require lifelong monitoring regardless of whether they are receiving treatment.
Conclusions
The primary reasons to screen for HBV are to reduce morbidity and mortality related to liver disease and to prevent transmission. Significant barriers remain to screening and referral for care for HBV in the U.S. Educational programs to increase knowledge and awareness among HCPs and the public together with improved access to care are critical to improve disease outcomes and prevent transmission. Despite the availability of an effective vaccine for 3 decades, the global prevalence of HBV has not substantially declined. Further research is needed to explore strategies to overcome screening barriers, improve vaccination rates, and to develop new models of health care delivery to reduce the burden of disease-related to HBV.
Click here to read the digital edition.
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-1555.
2. Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B--United States, 1974-2008. PLoS One. 2011;6(12):e27717.
3. Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56(2):422-433.
4. Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18(6):377-383.
5. Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147(7):460-469.
6. Eckman MH, Kaiser TE, Sherman KE. The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis. 2011;52(11):1294-1306.
7. Weinbaum CM, Williams I, Mast EE, et al; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1-20.
8. LeFevre ML; U.S. Preventive Services Task Force. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(1):58-66.
9. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
10. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283.
11. Centers for Disease Control and Prevention (CDC). Screening for chronic hepatitis B among Asian/Pacific Islander populations--New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(18):505-509.
12. Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46(4):1034-1040.
13. Lai CJ, Nguyen TT, Hwang J, Stewart SL, Kwan A, McPhee SJ. Provider knowledge and practice regarding hepatitis B screening in Chinese-speaking patients. J Cancer Educ. 2007;22(1):37-41.
14. Colvin HM, Mitchell AE, eds; Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: National Academies Press; 2010:xix, 232.
15. Hepatitis B Fact Sheet No. 204. World Health Organization Website. http://www.who.int/entity/mediacentre/factsheets/fs204/en/. Updated July 2015. Accessed March 17, 2016.
16. Perz JF, Openo K, Ahmed F, Bell BP. P.382 Trends in mortality from liver cancer in the USA, 1993-2002. J Clin Virol. 2006;36(suppl 2):S178.
17. Chang ET, Keegan TH, Gomez SL, et al. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109(10):2100-2108.
18. Fattovich G1, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335-352.
19. Chou R, Dana T, Bougatsos C, Blazina I, Khangura J, Zakher B. Screening for hepatitis B virus infection in adolescents and adults: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;161(1):31-45.
20. Wan KJ, Miyoshi T, Fryer G, et al. Screening for hepatitis B virus infection by primary care physicians in New York City: are screening recommendations for persons born inendemic countries being followed?[abstract 1454]. Hepatology. 2007;46(suppl):889A-890A.
21. Thompson MJ, Taylor VM, Jackson JC, et al. Hepatitis B knowledge and practices among Chinese American women in Seattle, Washington. J Cancer Educ. 2002;17(4):222-226.
22. Ma GX, Fang CY, Shive SE, Toubbeh J, Tan Y, Siu P. Risk perceptions and barriers to hepatitis B screening and vaccination among Vietnamese immigrants. J Immigr Minor Health. 2007;9(3):213-220.
23. Taylor VM, Choe JH, Yasui Y, Li Lin, Burke N, Jackson JC. Hepatitis B awareness, testing, and knowledge among Vietnamese American men and women. J Community Health. 2005;30(6):477-490.
24. Weinbaum CM, Lyerla R, Mackellar DA, et al; Young Men’s Survey Study Group. The young men’s survey phase II: hepatitis B immunization and infection among young men who have sex with men. Am J Public Health. 2008;98(5):839-845.
25. Marcellin P, Gane E, Buti M. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468-475.
26. Chang TT, Liaw YF, Wu SS. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886-893.
27. Wong GL, Chan HL, Mak CW. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology.2013;58(5):1537-1547.
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-1555.
2. Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B--United States, 1974-2008. PLoS One. 2011;6(12):e27717.
3. Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56(2):422-433.
4. Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18(6):377-383.
5. Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147(7):460-469.
6. Eckman MH, Kaiser TE, Sherman KE. The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis. 2011;52(11):1294-1306.
7. Weinbaum CM, Williams I, Mast EE, et al; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1-20.
8. LeFevre ML; U.S. Preventive Services Task Force. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(1):58-66.
9. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
10. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283.
11. Centers for Disease Control and Prevention (CDC). Screening for chronic hepatitis B among Asian/Pacific Islander populations--New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(18):505-509.
12. Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46(4):1034-1040.
13. Lai CJ, Nguyen TT, Hwang J, Stewart SL, Kwan A, McPhee SJ. Provider knowledge and practice regarding hepatitis B screening in Chinese-speaking patients. J Cancer Educ. 2007;22(1):37-41.
14. Colvin HM, Mitchell AE, eds; Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: National Academies Press; 2010:xix, 232.
15. Hepatitis B Fact Sheet No. 204. World Health Organization Website. http://www.who.int/entity/mediacentre/factsheets/fs204/en/. Updated July 2015. Accessed March 17, 2016.
16. Perz JF, Openo K, Ahmed F, Bell BP. P.382 Trends in mortality from liver cancer in the USA, 1993-2002. J Clin Virol. 2006;36(suppl 2):S178.
17. Chang ET, Keegan TH, Gomez SL, et al. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109(10):2100-2108.
18. Fattovich G1, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335-352.
19. Chou R, Dana T, Bougatsos C, Blazina I, Khangura J, Zakher B. Screening for hepatitis B virus infection in adolescents and adults: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;161(1):31-45.
20. Wan KJ, Miyoshi T, Fryer G, et al. Screening for hepatitis B virus infection by primary care physicians in New York City: are screening recommendations for persons born inendemic countries being followed?[abstract 1454]. Hepatology. 2007;46(suppl):889A-890A.
21. Thompson MJ, Taylor VM, Jackson JC, et al. Hepatitis B knowledge and practices among Chinese American women in Seattle, Washington. J Cancer Educ. 2002;17(4):222-226.
22. Ma GX, Fang CY, Shive SE, Toubbeh J, Tan Y, Siu P. Risk perceptions and barriers to hepatitis B screening and vaccination among Vietnamese immigrants. J Immigr Minor Health. 2007;9(3):213-220.
23. Taylor VM, Choe JH, Yasui Y, Li Lin, Burke N, Jackson JC. Hepatitis B awareness, testing, and knowledge among Vietnamese American men and women. J Community Health. 2005;30(6):477-490.
24. Weinbaum CM, Lyerla R, Mackellar DA, et al; Young Men’s Survey Study Group. The young men’s survey phase II: hepatitis B immunization and infection among young men who have sex with men. Am J Public Health. 2008;98(5):839-845.
25. Marcellin P, Gane E, Buti M. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468-475.
26. Chang TT, Liaw YF, Wu SS. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886-893.
27. Wong GL, Chan HL, Mak CW. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology.2013;58(5):1537-1547.