User login
Trends in Utilization of Total Hip Arthroplasty for Femoral Neck Fractures in the United States
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
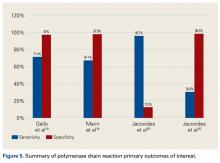
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.
Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
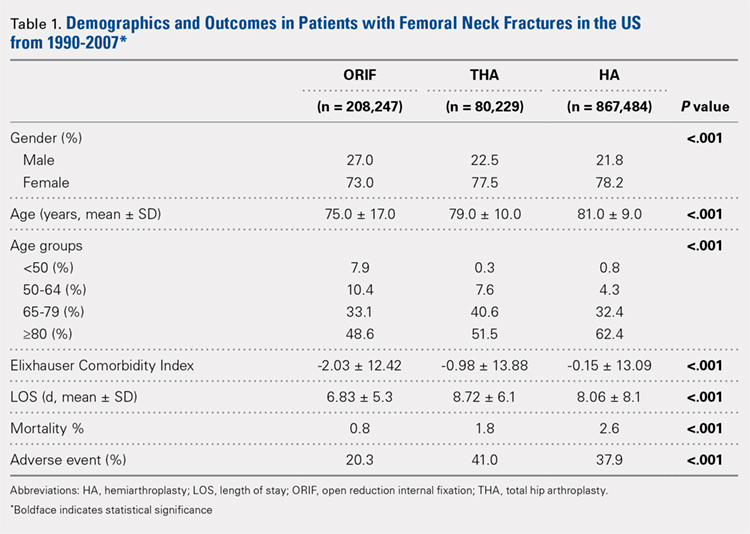
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.
Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
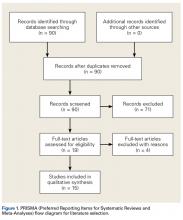
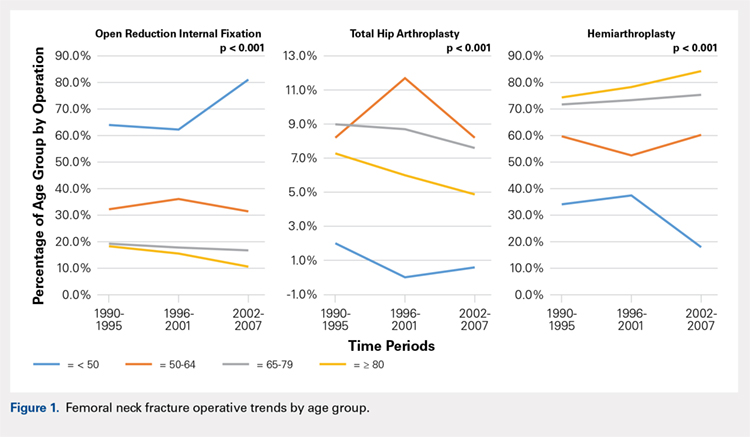
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.
Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).
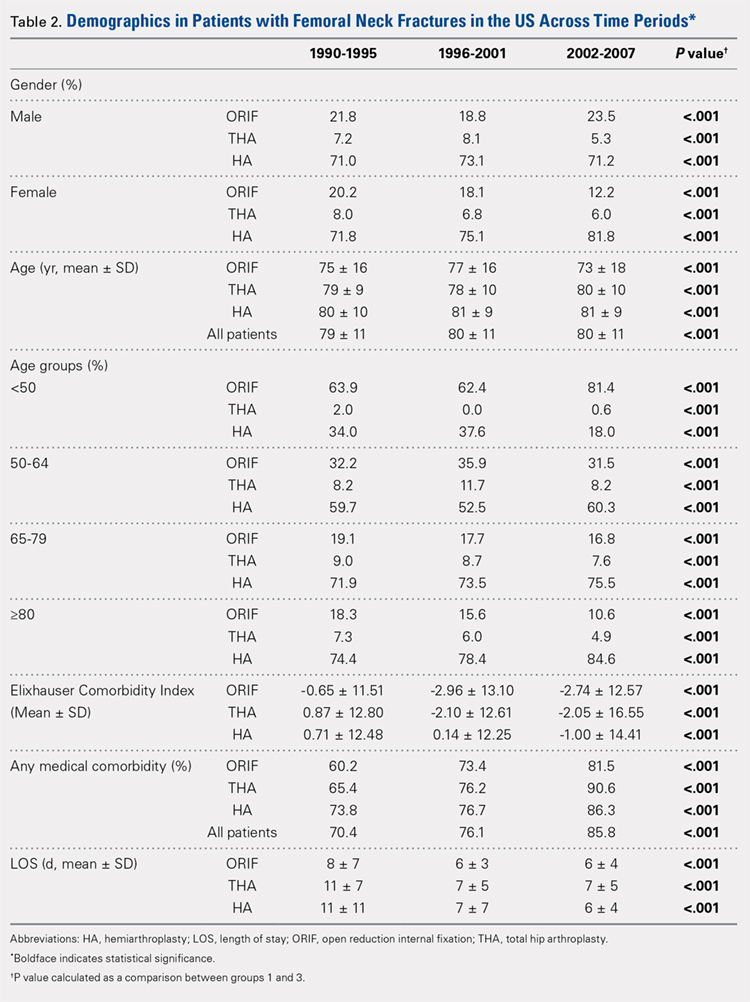
Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.
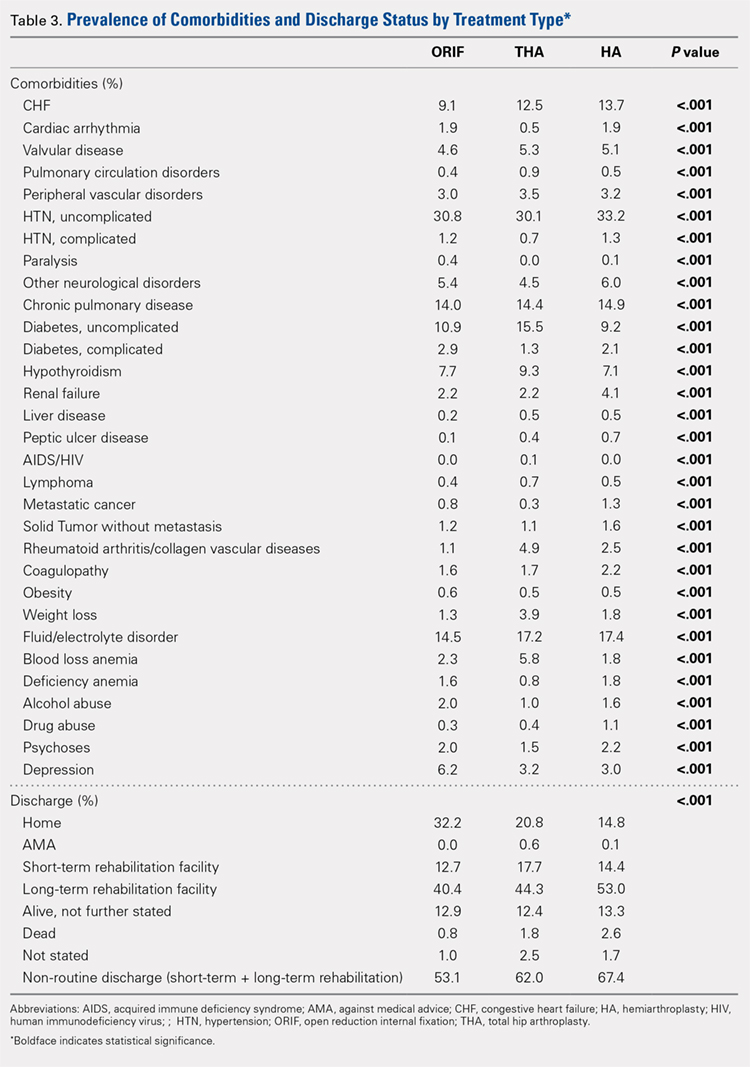
DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.
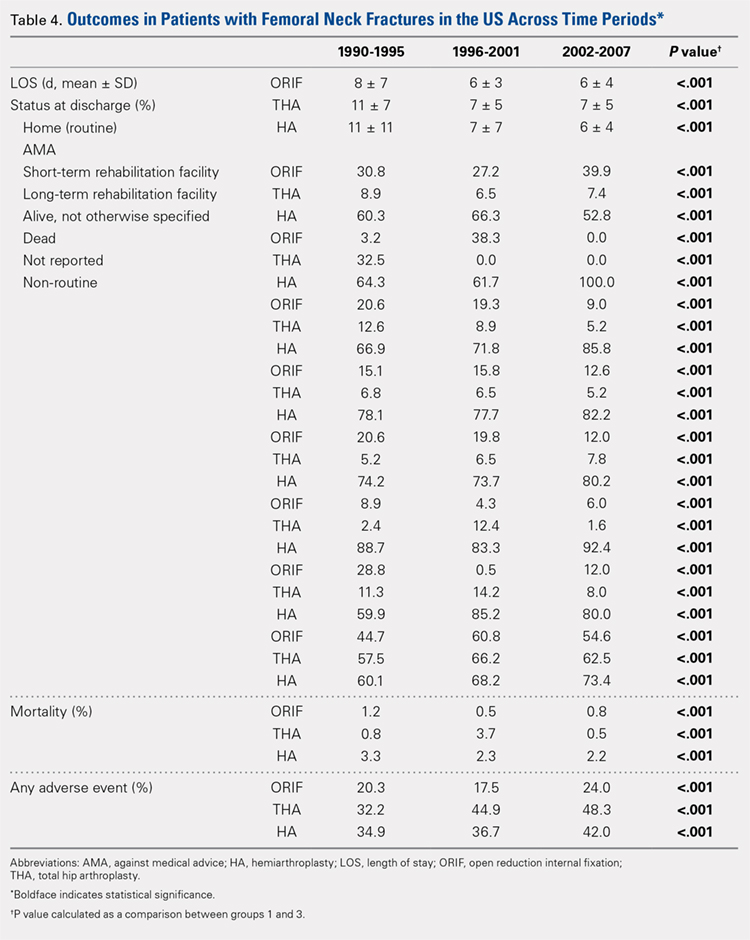
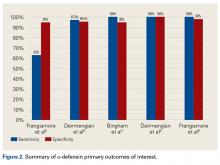
Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).
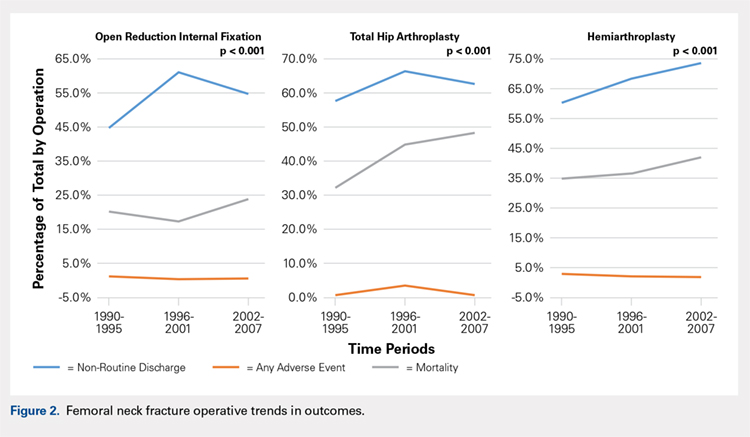
GENERAL ADVERSE EVENTS
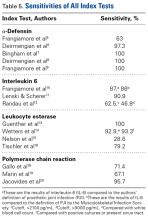
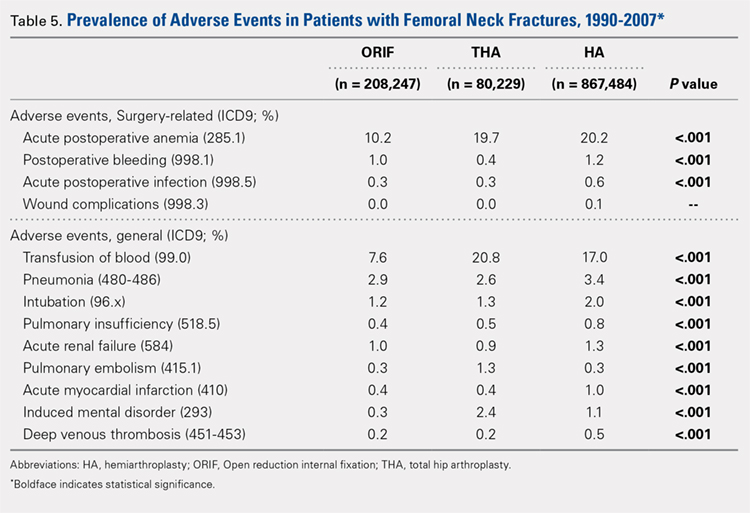
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.
Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).
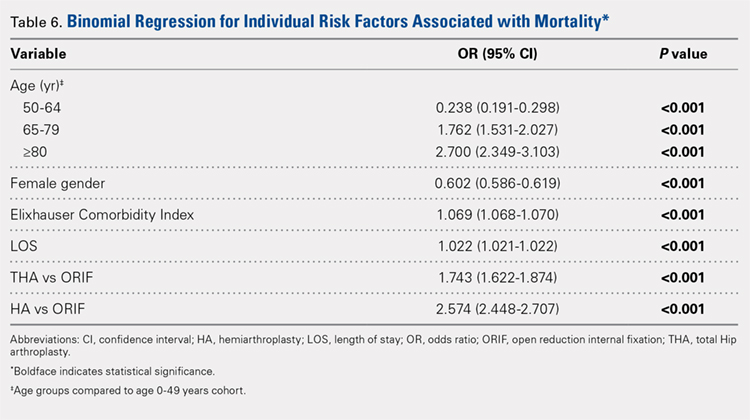
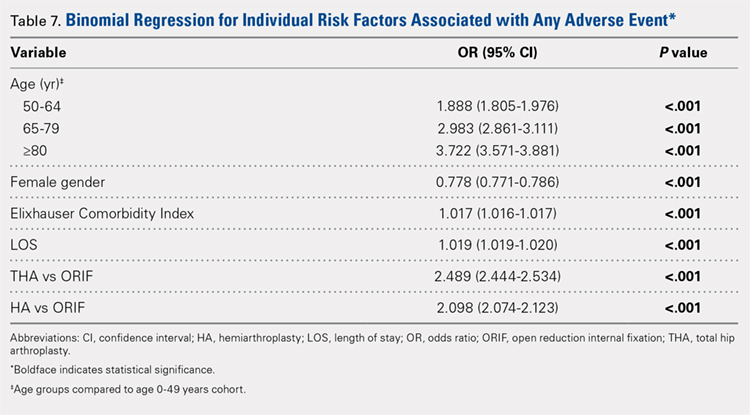
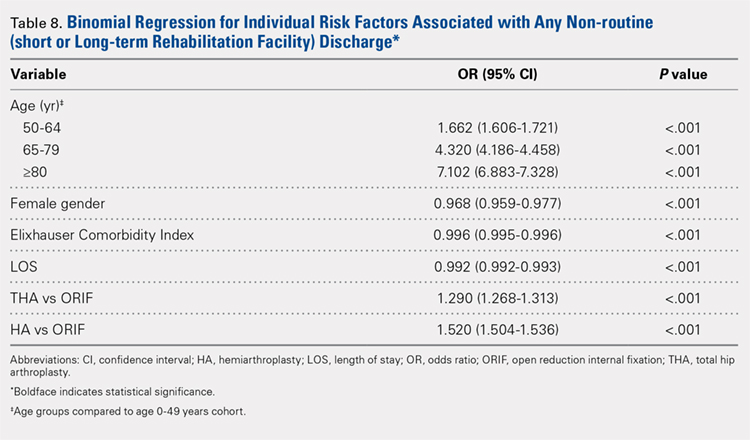
Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).
DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.
Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
TAKE-HOME POINTS
- The femoral neck patient population is older and has more medical comorbidities.
- Hemiarthroplasty (HA) is being performed more commonly in patients > 50 years old for femoral neck fractures.
- Open reduction and internal fixation is being performed more commonly in patients > 80 years old for femoral neck fractures.
- The rate of adverse events following femoral neck fracture is higher in the total hip arthroplasty (THA) group than in the HA group.
- THA is an independent risk factor for adverse events following femoral neck fracture.
Systematic Review of Novel Synovial Fluid Markers and Polymerase Chain Reaction in the Diagnosis of Prosthetic Joint Infection
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.