User login
Anorectal Evaluations: Diagnosing & Treating Benign Conditions
CE/CME No: CR-1711
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand basic anorectal anatomy and how to perform a thorough anorectal exam.
• Describe the physical exam findings of common benign anorectal conditions.
• Discuss the different treatment options for benign anorectal conditions.
• Differentiate between common benign anorectal symptoms and red flags that should prompt referral to a colorectal specialist.
FACULTY
Priscilla Marsicovetere is an Assistant Professor of Medical Education and of Surgery at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire; Program Director for the Franklin Pierce University PA Program in Lebanon, New Hampshire; and practices with Emergency Services of New England at Springfield Hospital in Vermont. Srinivas Joga Ivatury is an Assistant Professor of Surgery at the Geisel School of Medicine at Dartmouth and practices in the Department of Surgery at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through October 31, 2018.
Article begins on next page >>
Anorectal pain and discomfort can stem from several possible etiologies, most of which are benign. As such, many patients with anorectal complaints who present in the primary care setting can be adequately evaluated, diagnosed, and treated without referral to a colorectal specialist. However, the clinician must be able to differentiate between common benign anorectal symptoms and red flags that should prompt referral.
Anorectal disorders are common in the United States and result in numerous primary care visits each year. Presentations range from pain and itching to bleeding and lesions. Common anorectal conditions include hemorrhoids, perianal skin tags, fissures, pruritus ani, perianal abscess, and condyloma. Most are benign and can be managed in the primary care setting.
Before a provider can competently diagnose and treat anorectal conditions, however, a comprehensive history and physical examination must be conducted. Grucela and colleagues documented that physicians’ diagnostic accuracy with anorectal conditions is about 50%—highlighting the need for providers to become more familiar with the history and clinical elements associated with anorectal complaints.1
This article reviews the assessment of the anorectum, diagnosis of common disorders and their recommended treatments, and red flags for referral to a colorectal specialist.
ANORECTAL ANATOMY
The beginning of the anal canal is demarcated by its moist, hairless appearance. Just inside the anal opening are two palpable circular muscles, the internal and external anal sphincters, separated by an intersphincteric groove. The sphincters are firmly closed in the resting state, which helps maintain continence.
The anal canal is generally 3 to 4 cm long and ends at the dentate line, a series of crypts in the anal mucosa.2 The crypts are openings into the anal glands, which are mucus-secreting structures in the anus. The dentate line is easily identified on anoscopy as a discrete change in the appearance of the mucosa. The dentate line is an important landmark because it delineates the boundary between somatic and visceral nerve supplies.3 Tissue proximal to the dentate line is innervated by visceral nerves and is insensate, and thus usually not a cause of pain; tissue distal to the dentate line, however, is highly innervated by somatic nerves and can be intensely painful.2
The anorectal canal is lined by three fibrovascular cushions, located in the left lateral, right posterior, and right anterior positions.4 Inside each cushion is a venous structure, called a hemorrhoid, which allows the cushion to enlarge and help maintain continence.5
Proximal to the anus is the rectum, the 12- to 15-cm long terminus of the colon. Anorectal examination in the primary care setting will typically not progress beyond the last 2 to 3 cm of the rectum.
TAKING THE HISTORY
A thorough history will provide clues about potential underlying anorectal pathology. Patients may not be forthcoming about symptoms due to embarrassment, fear of a cancer diagnosis, or cultural customs or habits. A thorough history should elicit information about all of the patient’s symptoms (see Table 1), including bleeding, change in bowel habits, and unintended weight loss.

PHYSICAL EXAM
Positioning the patient
Undergoing an anorectal examination can be embarrassing, whether it be from exposure of sensitive body parts or the less-than-desirable prone jackknife positioning. Patients often have preconceived notions that the exam will be humiliating and/or painful. Care should be taken to minimize any embarrassment and discomfort.
Positioning of the patient is a matter of provider preference. Options include the left lateral decubitus, prone jackknife, or lithotomy positions.
Positioning should always be done with draping. Regardless of position, ensure the draping exposes only the perineum. This can be achieved by encircling the patient’s bare bottom with a plain white sheet that exposes only the anus and surrounding skin, keeping the lower back, lateral buttocks, and thighs covered.
Interestingly, data on patient preference for positioning during anorectal exams are limited. In a 2009 study of 178 patients undergoing anorectal exam, more than half of patients (up to 71.4%) expecting to or having already had a proctologic exam reported that no specific type of positioning (eg, Sims, lithotomy with lifted legs, knee-chest, knee-chest with patient’s body bent forward) was most embarrassing to them.6 The report revealed that while most patients would favor the Sims position if they had a choice, they deferred to their examiner to choose the position that seemed most suitable to get a reliable diagnosis.6
Inspection of the perineum
Once the patient is properly positioned and draped, inspection of the perineum can occur. Begin by gently spreading the buttocks. Describe any abnormality seen (eg, ulcer, lesion, dermatitis, prolapsing tissue, or blood), including size, color, and location.
A common pitfall is to describe the location of abnormalities using a clock face, such as “at 4 o’clock.” This is misleading and should be avoided, because depending on patient position, the clock face can point to different locations (eg, if the patient is in the lithotomy versus prone jackknife position).
A better approach is to divide the perianal area into four anatomic quadrants: right anterior, right posterior, left anterior, left posterior. Using this schematic, the patient's position is irrelevant, and accurate documentation of lesion location is assured.
Digital rectal exam
After visual inspection of the perianal skin, a digital rectal exam (DRE) should be performed. Slowly insert a gloved, lubricated index finger into the anus and lower rectum. Note the tone of the anus at rest (eg, excessively tight vs lax). Palpate the circumference of the anus, sweeping side to side while assessing for any tenderness, mass, or induration—if present, note the anatomic quadrant. If a mass is felt, note whether it is firm or soft, fixed or mobile, and broad-based or pedunculated. When the lubricated finger is removed from the anus, note whether blood is present.
Anoscopy
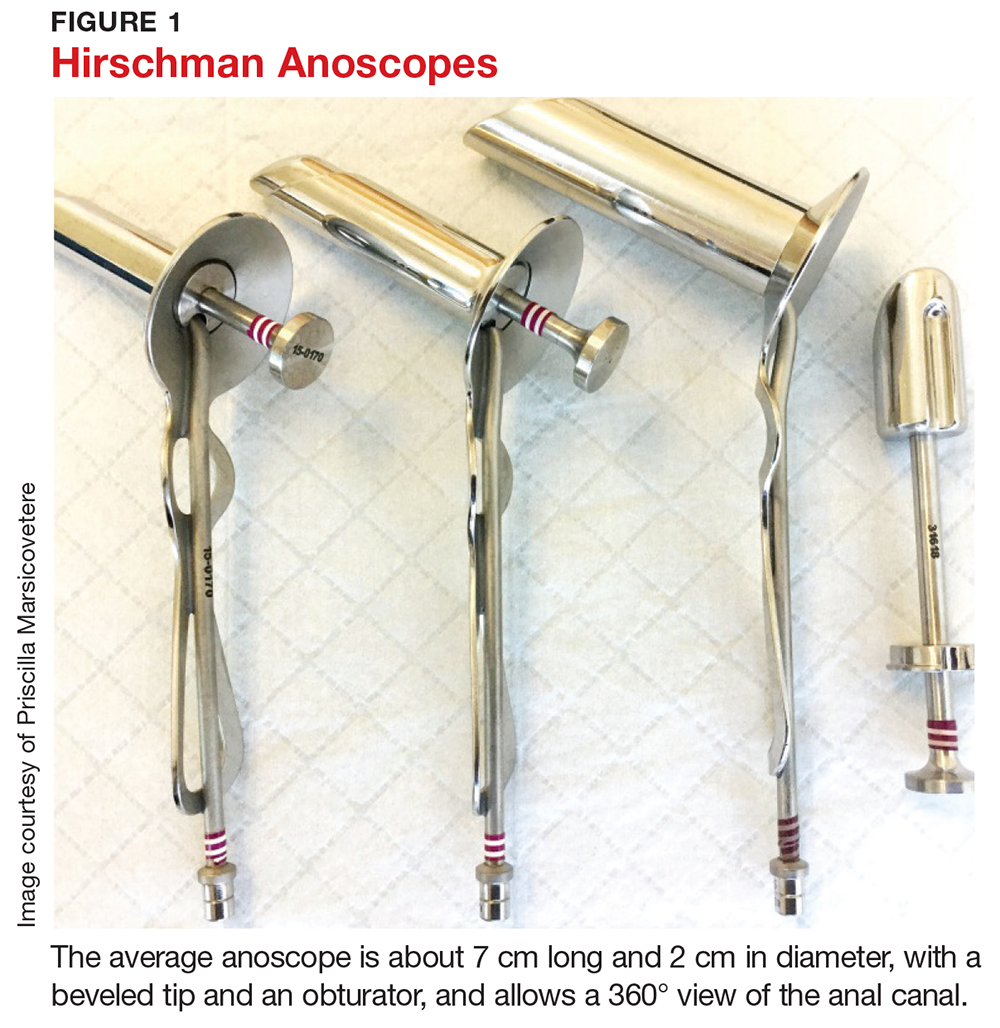
After DRE, visually inspect the anorectum. The instrument used varies from anoscope to rigid proctoscope to flexible sigmoidoscope. In a primary care setting, the most likely available instrument is an anoscope. The average anoscope is about 7 cm long and 2 cm in diameter, with a beveled tip and an obturator (see Figure 1), and allows a 360° view of the anal canal.7
Examination of the anal canal is accomplished by dividing the canal into the four anatomic quadrants described earlier and inserting the lubricated anoscope for inspection of each of the four quadrants. Observe the rectal mucosa and the anus as the scope is slowly withdrawn. If abnormalities are seen, note the location, size, shape, and any other descriptive features.
It is not necessary to perform a Hemoccult test after examination of the anorectum, as the presence of minor blood may be the direct result of the exam itself and thus provides no useful information to the examiner.
COMMON PATHOLOGIES
Once the history and physical exam are complete, a differential diagnosis can be formulated. Most anorectal disorders are benign conditions that pose no immediate health threat and can be managed in the primary care setting. Others, however, can be more serious and should prompt referral to a colorectal specialist for further evaluation. Knowing the difference can spare a patient unnecessary anxiety and referral; it can also lead to prompt, lifesaving interventions if red flags are recognized.
Hemorrhoids
Hemorrhoids are a common anorectal complaint.8,9 It is estimated that up to 75% of the population will experience symptoms of hemorrhoids during their lifetime.5,8 Whether internal or external, in their normal, nonpathologic, quiescent state, hemorrhoids are asymptomatic. Hemorrhoids become symptomatic when the supporting structures of hemorrhoidal tissue (ie, the anal cushions) deteriorate, resulting in venous dilation, inflammation, and thrombosis, which in turn lead to swelling, bright red bleeding, and/or prolapse.2,10 The most common causes of hemorrhoidal disease are chronic constipation and prolonged straining with bowel movements, though chronic diarrhea and pregnancy have also been identified as risk factors.2,8,11
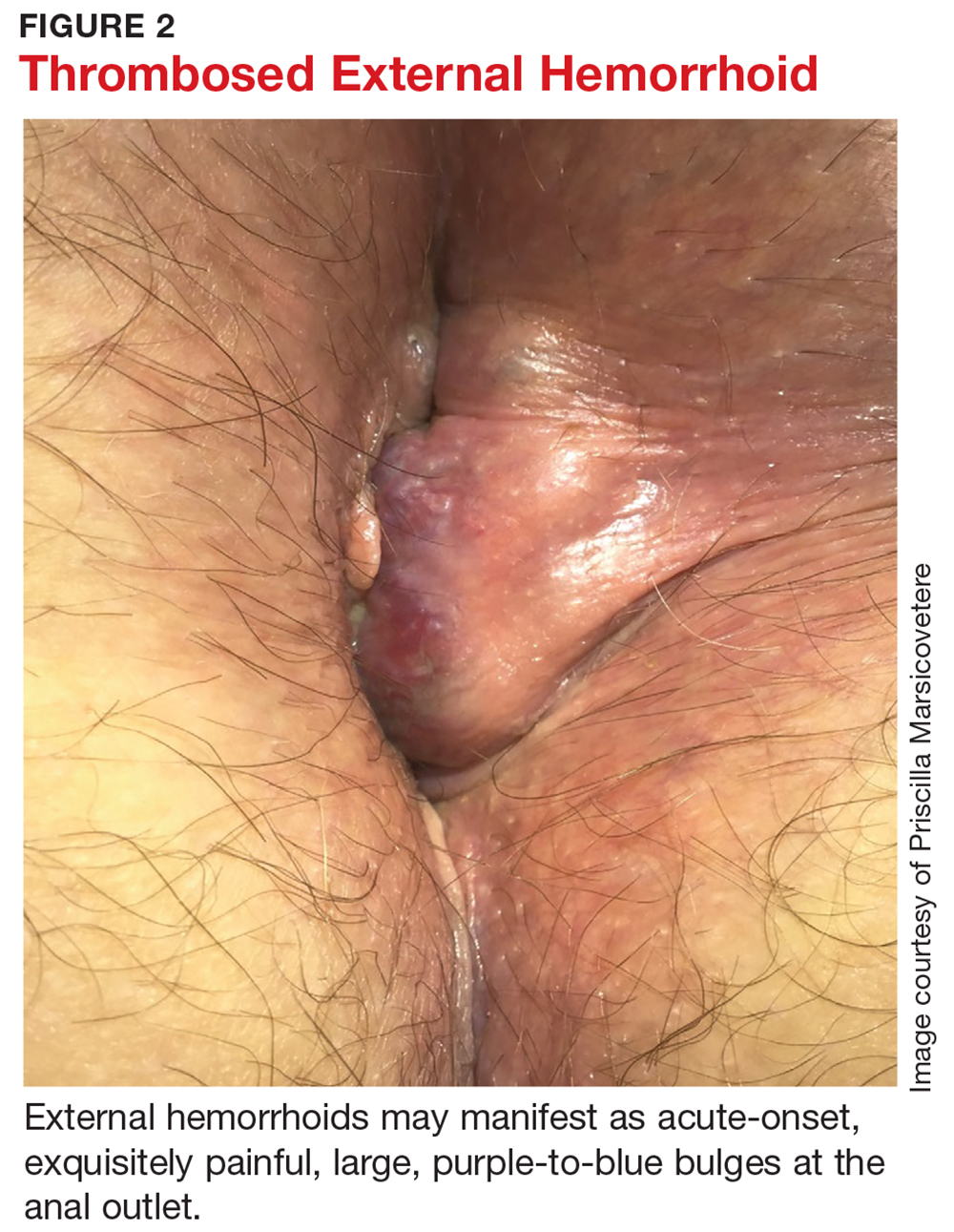
External hemorrhoids, which are located distal to the dentate line, are typically only visible when they become thrombosed or swollen. In this state, they may manifest as acute-onset, exquisitely painful, large, purple-to-blue bulges at the anal outlet (see Figure 2). The number and size of the lesions can vary. The patient may report pain when sitting or wiping, as well as bleeding from the lesion.12,13 The pain is typically severe in the first couple of days, then slowly starts to subside.2,12
For internal hemorrhoids, which are located proximal to the dentate line, the main symptom is usually painless bright red blood per rectum.8,11,12 Patients may also report a sensation of rectal fullness or experience prolapse of the hemorrhoid through the anus. Prolapse typically occurs with defecation; in more severe cases, it can also occur between bowel movements, usually with any activity that increases intra-abdominal pressure (eg, coughing, heavy lifting, pregnancy, portal hypertension). The prolapse may reduce spontaneously, or may have to be manually reduced. If it cannot be reduced, there is a risk for incarceration or strangulation, potentially leading to gangrene.
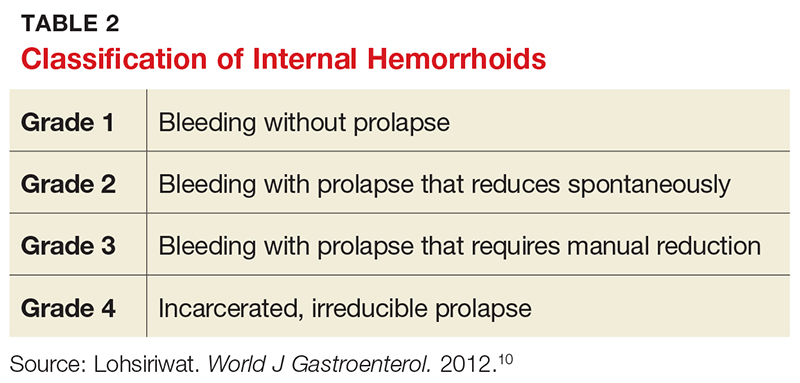
The presence of bleeding and/or prolapse determines the classification of internal hemorrhoids (see Table 2). Dietary and lifestyle modification are used in the management of all grades of hemorrhoids. In addition, for grade 1 and 2 lesions, topical medication (eg, anti-inflammatory cream) can be used, whereas grade 3 (and selected grade 2) lesions respond well to rubber band ligation. Given the severity of grade 4 lesions, surgical intervention (eg, hemorrhoidectomy) is usually indicated.10
About a third of patients with symptomatic hemorrhoids seek clinical treatment.14 Most are hemodynamically stable and require no imaging and usually no labs (unless anemia is suspected).2 Management depends on the location and degree of symptoms (eg, internal vs external, prolapse, or thrombosis). In the event of an acutely thrombosed external hemorrhoid, clot excision for pain relief is appropriate if symptoms have been present for less than 48 to 72 hours; after that amount of time, the pain from the procedure will likely exceed the degree of relief provided, and conservative management should instead be recommended.2,8,11
Firstline treatment consists of lifestyle modification with a high-fiber diet and daily fiber supplement to ensure stool is soft and easy to pass.2,8 A meta-analysis of seven clinical trials with a total of 378 patients with hemorrhoids showed that fiber supplementation resulted in a 50% decrease in bleeding risk from internal hemorrhoids.15
Adequate hydration, preferably with noncaffeinated liquids, is also recommended. This will prevent constipation and the need to strain or spend excessive time on the toilet. Sitz baths can help alleviate pain and discomfort.
Several OTC topical medications are marketed for hemorrhoid relief. Many of these preparations contain steroids for their anti-inflammatory effects or astringents to address skin irritation that can result from anal leakage if prolapsing hemorrhoids prevent the anal outlet from closing. Steroid use should be limited to five to seven days, due to atrophic effects on the skin. While OTC preparations may temporarily alleviate discomfort, they will not address the underlying cause of symptoms.
Indications for referral to a colorectal specialist for symptomatic hemorrhoids include failure to improve with conservative management, persistent patient discomfort, and prolapse, as these indicate potential need for more invasive treatment.
Perianal skin tags
Perianal skin tags, while a nuisance, are not pathologic in most instances and pose no threat to health. They are an outgrowth of normal skin, appearing as loose, flesh-colored perianal tissues (see Figure 3). Tags range in size from a few millimeters to a centimeter long and can occur alone or in multiples.
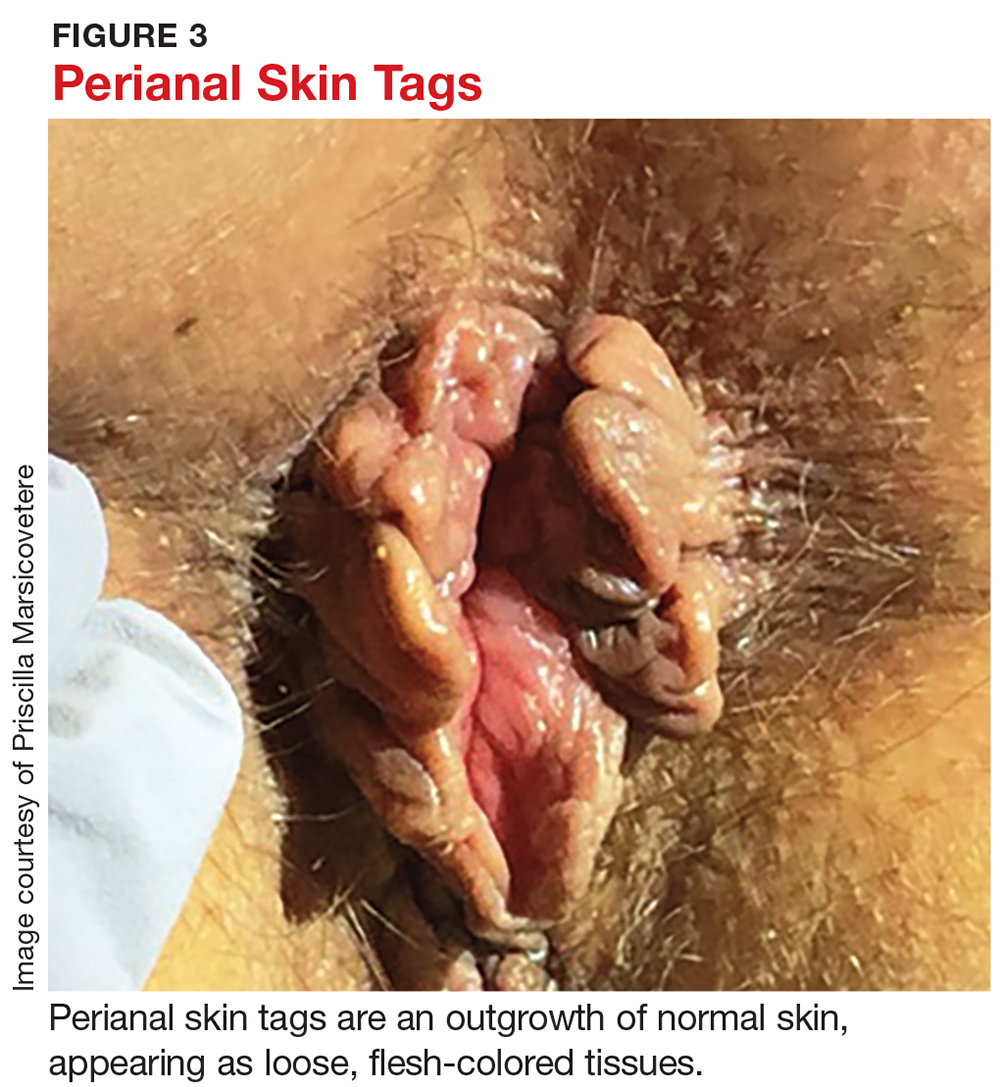
Perianal skin tags are diagnosed clinically and require no labwork or imaging. Visual inspection is typically sufficient to distinguish tags from pathologic lesions such as condyloma or abscess. If there is uncertainty, however, biopsy or referral to a specialist is warranted.
Certain medical conditions can predispose a patient to development of perianal skin tags. They can be sequelae of thrombosed external hemorrhoids.8,11 They are also common in patients with Crohn disease.11 Perianal skin tags are not, however, the result of anal intercourse or sexually transmitted infections.
Treatment is usually not indicated for perianal skin tags. If the tags interfere with hygiene or cause perianal discomfort or significantly decreased quality of life, however, patients may seek removal. These patients should be referred to a colorectal specialist for evaluation for excision.
Anal fissures (fissure in ano)
The most common cause of severe anorectal pain is fissure.4 A fissure is an elliptical tear, or split, in the lining of the anal canal that causes spasm of the anal sphincters. The tear is distal to the dentate line and thus intensely painful.2,5 Common cited causes of fissures are trauma from passage of large, hard stools, straining, or diarrhea.16
Fissures can usually be visualized by spreading the posterior anus apart (see Figure 4). They are most commonly located in the posterior or anterior midline, though they can occur anywhere around the anus.2,4 Often, a sentinel tag—appearing as a taut, flesh-colored skin tag—is present at the external pole of the fissure.5,11 DRE and anoscopy should be avoided, as they will trigger intense pain and spasm of the sphincters.
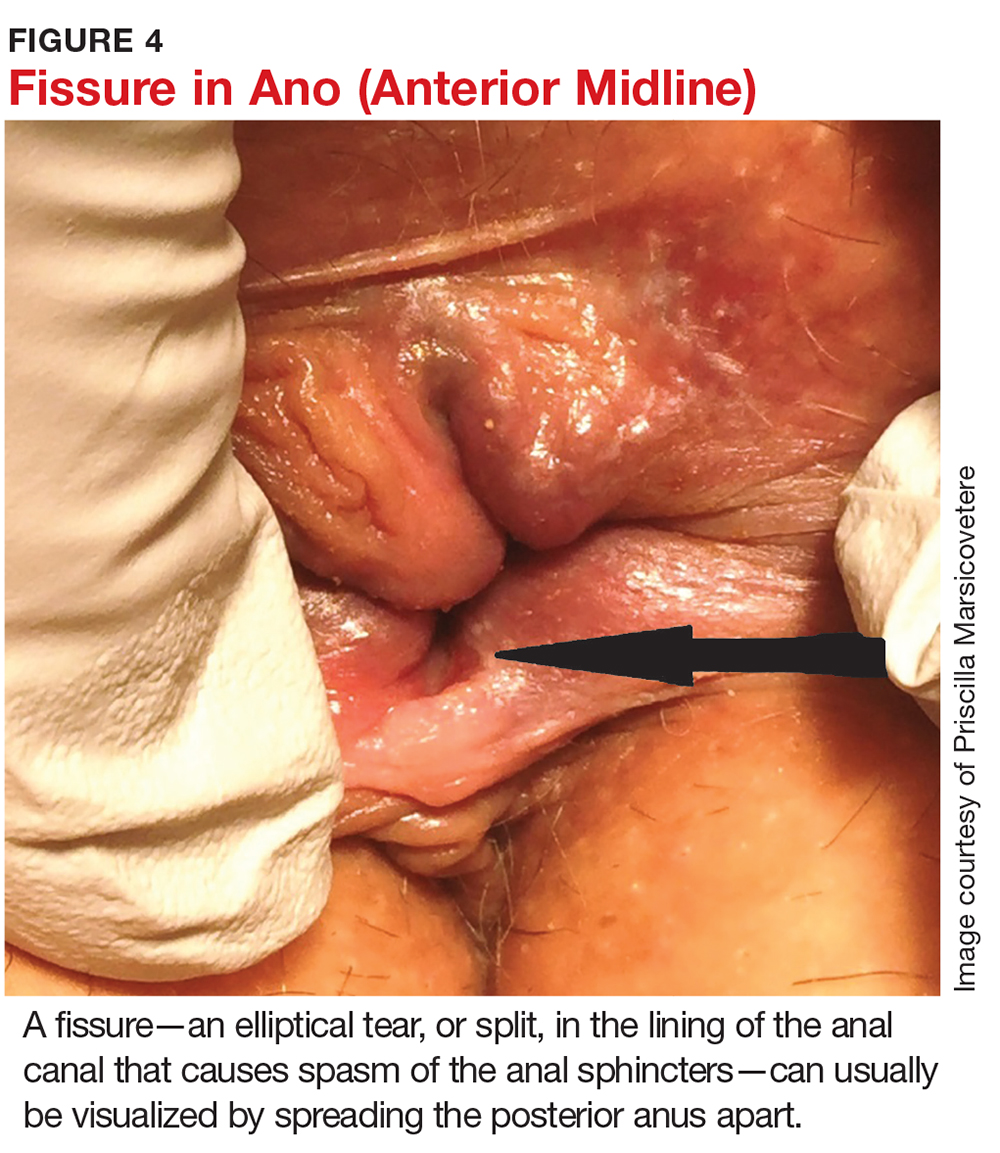
Fissures are characterized as acute (present ≤ 3 months) or chronic (> 3 months).9,11 Visually, acute fissures typically have clean edges, with the appearance of a paper cut to the mucosa, while chronic fissures have indurated, heaped-up edges, often with exposure of the underlying sphincter muscle.17
They tend to be exquisitely painful, as the mucosa distal to the dentate line is highly innervated. Patients report pain akin to “passing shards of broken glass” with bowel movements, which is often accompanied by a fear of defecation and bright red blood on the toilet paper or dripping into the water.11 The pain, caused by spasm of the sphincters, typically starts during a bowel movement and lasts minutes to hours afterward.
Initial treatment is aimed at relaxing the sphincters, as well as softening stool to prevent further trauma and allow the fissure to heal. Patients should be educated about the importance of adequate fiber intake to prevent constipation and straining. A daily bulk fiber supplement, in addition to a high-fiber diet (20-25 g/d), has been shown to result in healing of 87% of acute fissures.16 Sitz baths in plain warm water, three to four times a day, can encourage relaxation of the sphincters and increase local blood flow, both of which help with fissure healing.16 Topical medications can also be prescribed. These include compounded nitroglycerin 0.2% or nifedipine 2.0%, which act to reduce the spasm by relaxing smooth muscle, as well as increase blood flow to the lesion.12,14
Most acute fissures will heal with the regimen of high fiber intake, sitz baths, and topical medication. For refractory or chronic fissures, referral to a colorectal specialist for more invasive treatment is appropriate. Additionally, fissures that are not located in the typical posterior or anterior midline might indicate an atypical etiology, such as Crohn disease, tuberculosis, leukemia, or HIV, and thus patients who present with fissures in these locations should also be referred to a colorectal specialist.2,4,11,18
Pruritus ani
Pruritus ani, known as perianal dermatitis, is a benign condition that presents with intense perianal itching and burning. It is the second most common anorectal condition after hemorrhoids and affects up to 5% of the US population.2,9,11,19
Pruritus ani often develops secondary to local irritation of the skin (eg, from prolonged exposure to moisture), leading to an inflammatory response within the superficial skin layers. The irritation causes patients to scratch the skin, resulting in trauma, excoriation, and ulcer formation and leading to a cycle of further inflammation, exacerbation of symptoms, and persistent scratching.
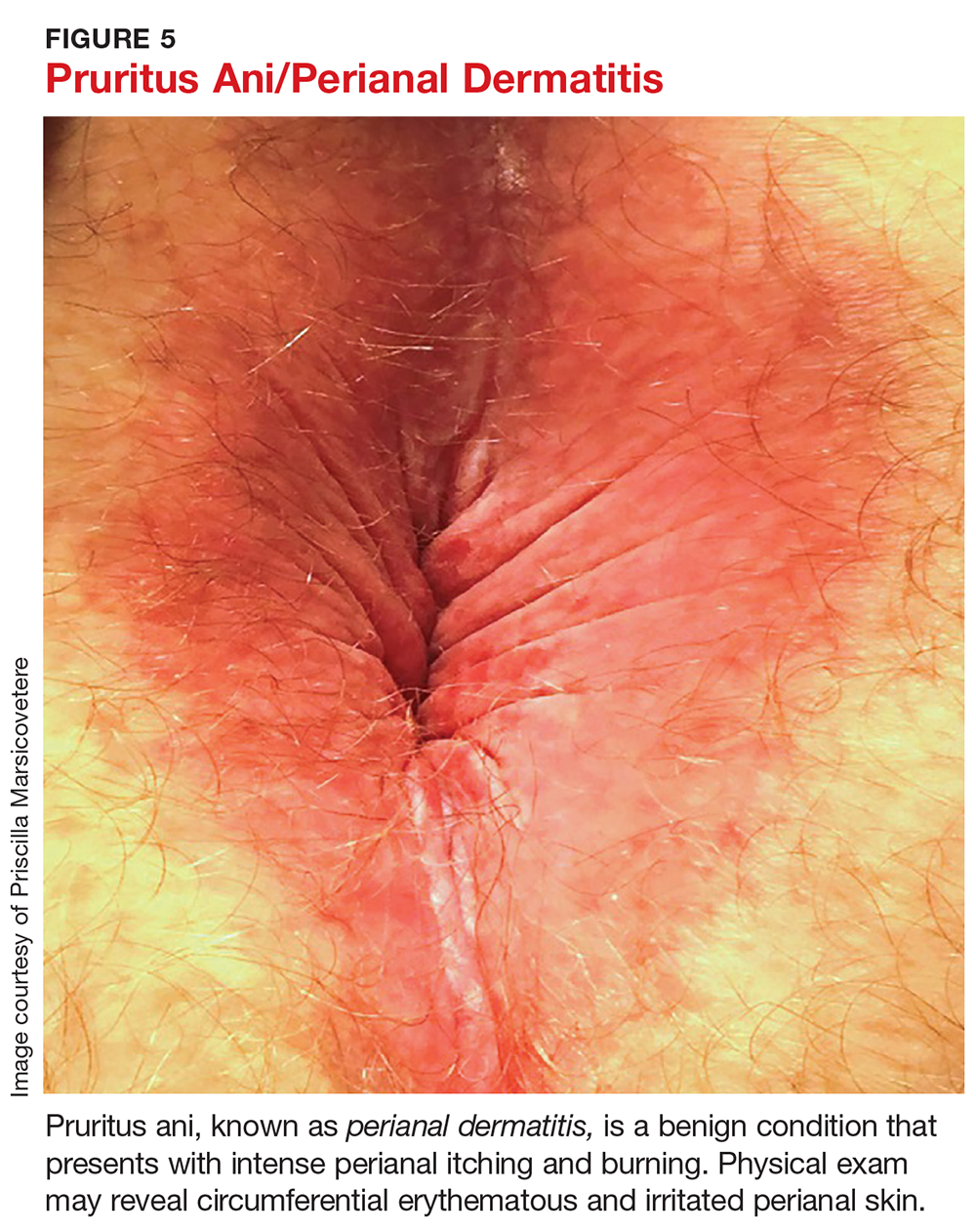
Physical exam may reveal circumferential erythematous and irritated perianal skin (see Figure 5). Linear or deep, punched-out excoriations may be present. Chronically, patients may develop lichenification with thick, whitened patches of skin.11 In the absence of red flags such as unintentional weight loss, anemia, rectal bleeding, or a family history of colon cancer, no additional evaluation is required during the initial visit, though anoscopy can be used to rule out associated anorectal pathology.
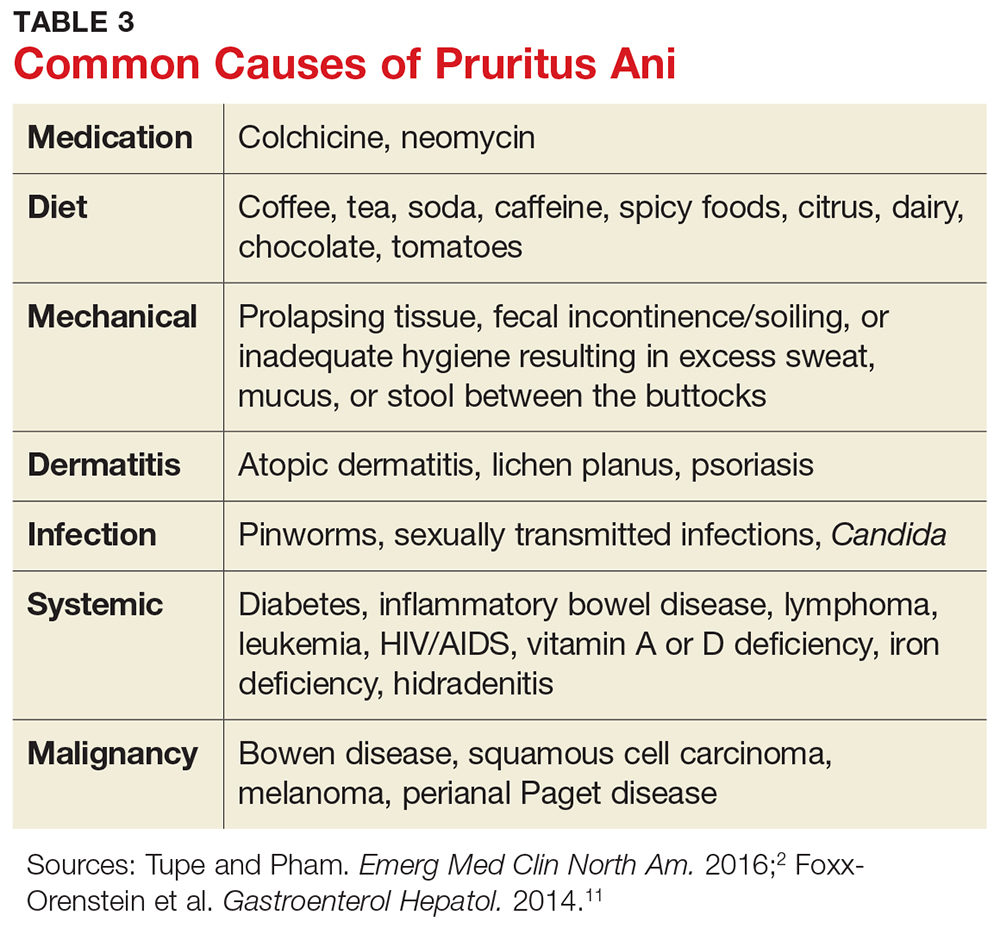
Many different causes of pruritus ani have been reported (see Table 3). In the case of an identifiable cause, symptoms tend to resolve once the offending agent is eliminated. Up to a quarter of cases, however, are idiopathic, with no identifiable trigger.20
Symptom management is thus key. Patients should be educated about lifestyle modification and informed that scratching will irritate the skin further and aggravate symptoms.5,11 If incontinence or diarrhea is thought to cause symptoms, dietary modifications, a fiber supplement, dysmotility agents, and Kegel exercises to strengthen the sphincters and decrease anal leakage can be recommended.
The affected skin should be kept clean and dry at all times. Aggressive wiping and overzealous hygiene should be avoided. Sitz baths can help with hygiene. A topical astringent such as witch hazel can help remove excess moisture from the skin. A layer of protective skin barrier cream with zinc oxide, when applied over dry skin, can help protect the skin from leakage throughout the day.
A sedating antihistamine can reduce scratching during sleep. Topical hydrocortisone 1% cream is effective for itch relief; this should be limited to five to seven days of consecutive use, however, as it can lead to pathologic thinning of the perianal skin. Topical capsaicin 0.006% cream has been shown to help alleviate intractable pruritus.2,21
The goal of these measures is to break the cycle of irritation and inflammation and give the skin an opportunity to heal. If the patient fails to improve, referral to a colorectal specialist or, alternatively, a dermatologist, is warranted for perianal skin biopsy and more invasive treatment options.
Perianal abscess
A perianal abscess is an infected cavity filled with pus under pressure, located near the anus or rectum. It most often results from an acute infection of the anorectal glands located at the dentate line that tracks outward to the perianal skin.12 Abscesses can also result from another disease process, such as Crohn disease, diabetes, or rectal trauma.2,11
Localized pain, swelling, and drainage are common presenting symptoms of an abscess.2,11 Systemic symptoms such as fever and chills may present later in the course but are rare.2
Treatment of the acute process is incision and drainage (I&D).11,12 This can be accomplished in the office with injection of local anesthesia, followed by excision of an ellipse of skin overlying the cavity large enough to allow full drainage of the abscess. Use of drains and packing of the wound are usually not necessary. There is also no role for antibiotics unless the patient is diabetic or immunosuppressed or cellulitis is present.11 In patients with systemic signs of illness, imaging such as CT or MRI of the pelvis can be used to assess for a deeper infection.2,12
For most patients, I&D resolves the process. However, up to half of perianal abscesses progress to form a fistula, a tunnel connecting the infected anal gland to the external skin.2,11,22 Indeed, abscesses and fistulae are part of the same infectious process, with the abscess representing the acute phase of infection and the fistula representing the chronic phase. On physical exam, in addition to the abscess site on the skin, a fistula may manifest as a palpable cord beneath the skin between the anus and the abscess opening.9 Additionally, the patient may report the abscess has been recurrent in nature, cyclically increasing in size and pain, then spontaneously draining.
A fistula requires surgical intervention for definitive treatment and should therefore prompt referral to a colorectal specialist. In the absence of fistula, however, a simple perianal abscess can be treated with I&D in the primary care setting.
Condyloma acuminatum
Condyloma acuminatum, also known as genital warts, is the most common sexually transmitted infection in the United States and a frequent anorectal complaint.23-25 More than 6 million new infections occur annually.25
Condyloma is caused by the human papillomavirus (HPV).24 More than 100 HPV subtypes have been identified.25 Types 6 and 11 are associated with typical condyloma acuminatum, while types 16 and 18 are found more commonly with dysplasia and malignant transformation.3,26
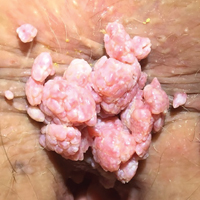
The lesions are spread by direct skin-to-skin or mucosa-to-mucosa contact, including anal intercourse. They appear as tiny finger-like projections on the perianal or genital skin, often with a cauliflower-like appearance, in clusters or as single entities, and ranging in size from a few millimeters to several centimeters (see Figure 6).
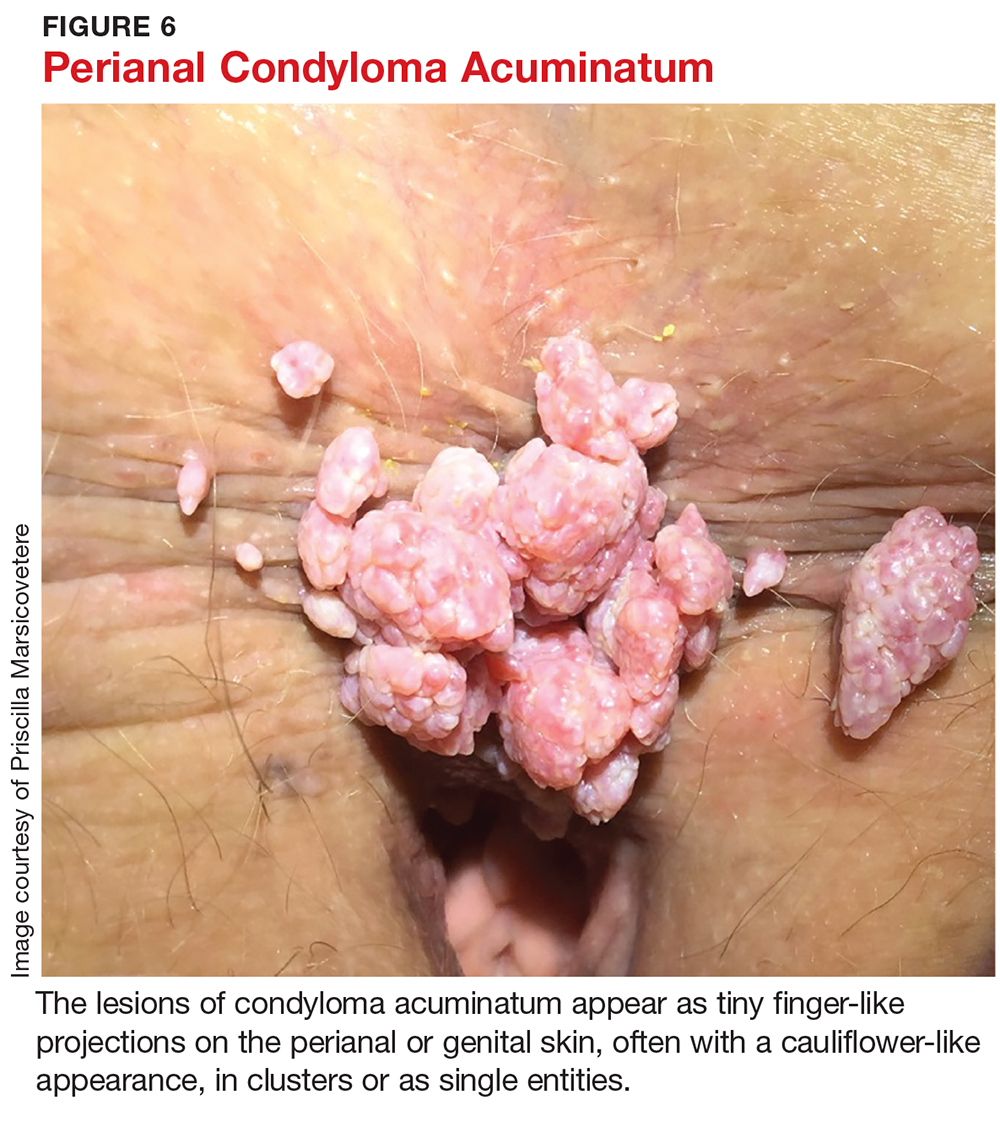
The patient may be asymptomatic or may complain of bleeding with defecation or leakage from the rectum between bowel movements. In up to 90% of perianal condyloma, concomitant anorectal lesions are present.3 Anoscopy is therefore indicated whenever perianal lesions are seen.
Treatment of genital warts varies depending on the number and location of lesions. The goal is lesion removal, but none of the available treatments are curative.24 If only a few small (diameter < 2 mm) warts are seen in the pe
Trichloroacetic acid (TCA), which is considered a form of chemical cautery, has been deemed more effective for tiny warts (diameter 1-2 mm) and better tolerated than podophyllin.24 Unlike podophyllin, however, TCA must be applied in clinic by a specialist, not at home by the patient. It works by chemically burning, cauterizing, and eroding skin and mucosa, thereby destroying old infected cells and allowing for growth of new, healthy cells.24 Using a solution with a higher concentration of TCA (80%-90%) has been shown to be more effective than lower concentration solutions (30%).24 The recurrence rate is high, generally about 36%.27 Due to its low risk for systemic absorption, TCA is safe for use during pregnancy.24
Local application of an immunomodulator, such as imiquimod 2%, 3.75%, or 5% cream, can also be used. Imiquimod works by upregulating tumor necrosis factor, leading to decreased viral replication and subsequent regression of warts.24 The most common side effects of imiquimod are erythema, erosions, burning, and pruritus.24 The higher concentration is associated with increased clearance rates as well as increased adverse effects.27 Therefore, the 3.75% cream is the preferred concentration of most providers.27 Recurrence rates range from 13% to 19%.28
In 2006, the FDA approved polyphenon E, a botanical ointment, to treat anogenital warts. The formulation is composed of eight different sinecatechins extracted from green tea leaves.29 Green tea sinecatechins have been shown to have antioxidant, antiproliferative, and antiviral properties, though the specific mechanisms of action in inhibiting anogenital wart growth have not been well studied to date. In a multicenter, randomized, double-blind, vehicle-controlled, three-arm parallel group phase 3 trial of 495 patients with anogenital warts, sinecatechins ointment 10% or 15% applied three times daily for 16 weeks resulted in complete clearance of warts in 57.2% and 56.3% of patients, respectively, compared to 33.7% clearance in the vehicle patients.30 Partial clearance (≥ 50%) of warts was observed in 78.4% of patients who used the 15% ointment, 74.0% of those who used the 10% ointment, and 51.5% of the vehicle patients. Rates of recurrence were 6.5% and 8.3% with use of the 10% and 15% sinecatechins ointment, respectively, which are much lower than recurrence rates observed with other topical applications (eg, imiquimod and podophyllotoxin).30
For larger warts or when there are anal lesions present, surgical excision and fulguration is required and referral to a colorectal specialist is warranted.
Warts recur from 4.6% to more than 70% of the time, most commonly due to activation of latent virus.23 Therefore, whether initially treated in an office or operating room, condyloma patients require follow-up on a regular basis, generally every six to 12 months, to assess for recurrent anal lesions so that treatment can be promptly initiated.
In the absence of a cure for anogenital warts, prevention through an HPV vaccine is important. The quadrivalent HPV vaccine (Gardasil) was approved by the FDA in 2006 for prophylactic vaccination of females ages 9 to 26. The vaccine triggers host formation of antibodies against four common subtypes of HPV: 6, 11, 16, and 18.31 In 2009, the FDA expanded the use of Gardasil to include males ages 9 to 26; subsequently, Gardasil 9 (which protects against nine types of HPV) was approved for males ages 9 to 15.32 In a group of 4,065 healthy males between the ages of 16 and 26, Gardasil reduced the incidence of external anogenital warts by 90.4%.33
HPV vaccine is now recommended for females through age 26 and males through age 21. The CDC also recommends HPV vaccine for the following individuals through age 26 if they did not get vaccinated when they were younger:
- Young men who have sex with men
- Young men who identify as gay or bisexual or who intend to have sex with men
- Young adults who are transgender
- Young adults with immunocompromising conditions (including HIV).34
CONCLUSION
Anorectal conditions are a common presentation in the primary care setting. A thorough history and physical exam will usually determine the etiology, and most benign pathologies can be successfully treated in the primary care clinic. Knowing how to perform an anorectal exam and having a thorough understanding of the most common anorectal pathologies can help alleviate examiner discomfort, while ensuring that patients receive prompt and adequate care. At the same time, recognizing the red flags can expedite referral for colorectal specialty evaluation when appropriate. Red flags that should prompt immediate referral to a colorectal specialist include older age, unintentional weight loss, iron deficiency anemia, family history of inflammatory bowel disease or colorectal cancer, and persistent anorectal bleeding or persistent symptoms despite adequate treatment of a suspected benign condition.
1. Grucela A, Salinas H, Khaitov S, et al. Prospective analysis of clinical accuracy in the diagnosis of benign anal pathology, comparison across specialties and years of experience. Dis Colon Rectum. 2010;53(1):47-52.
2. Tupe CL, Pham TV. Anorectal complaints in the emergency department. Emerg Med Clin North Am. 2016;34(2):251-270.
3. Billingham RP, Isler JT, Kimmins MH, et al. The diagnosis and management of common anorectal disorders. Curr Probl Surg. 2004;41(7):586-645.
4. Schubert MC, Sridhar S, Schade RR, Wexner SD. What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol. 2009;15(26):3201-3209.
5. Henderson PK, Cash BD. Common anorectal conditions: evaluation and treatment. Curr Gastroenterol Rep. 2014; 16(10):408.
6. Gebbensleben O, Hilger Y, Rohde H. Patients’ view of medical positioning for proctologic examination. Clin Exp Gastroenterol. 2009;2:133-138.
7. Gopal DV. Diseases of the rectum and anus: a clinical approach to common disorders. Clin Cornerstone. 2002;4(4): 34-48.
8. Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol. 2015;21(31):9245-9252.
9. Fargo MV, Latimer K. Evaluation and management of common anorectal conditions. Am Fam Physician. 2012;85(6):624-630.
10. Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17): 2009-2017.
11. Foxx-Orenstein AE, Umar SB, Crowell MD. Common anorectal disorders. Gastroenterol Hepatol. 2014;10(5):294-301.
12. Lohsiriwat V. Anorectal emergencies. World J Gastroenterol. 2016;22(26):5867-5878.
13. Greenspon J, Williams SB, Young HA, Orkin BA. Thrombosed external hemorrhoids: outcome after conservative treatment or surgical management. Dis Colon Rectum. 2004(9);47:1493-1498.
14. Summers A. Assessment and treatment of three common anorectal conditions. Emerg Nurse. 2013;21(2):28-33.
15. Alonso-Coello P, Mills E, Heels-Ansdell D, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101(1):181-188.
16. Medhi B, Rao RS, Prakash A, et al. Recent advances in the pharmacotherapy of chronic anal fissure: an update. Asian J Surg. 2008;31(3):154-163.
17. Nelson RL. Anal fissure (chronic). BMJ Clin Evid. 2014;2014. pii: 0407.
18. deRosa M, Cestaro G, Vitiello C, et al. Conservative versus surgical treatment for chronic idiopathic anal fissure: a prospective randomized trial. Updates Surg. 2013;65(3):197-200.
19. Lacy B, Weiser K. Common anorectal disorders: diagnosis and treatment. Curr Gastroenterol Rep. 2009;11(5):413-419.
20. Siddiqi S, Vijay V, Ward M, et al. Pruritus ani. Ann R Coll Surg Engl. 2008;90:457-463.
21. Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomized, placebo controlled, crossover study. Gut. 2003;52(9):1323-1326.
22. Burnstein M. Managing anorectal emergencies. Can Fam Physician. 1993;39:1782-1785.
23. Sasaki A, Nakajima T, Egashira H, et al. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol. 2016;22(8):2636-2641.
24. Kollipara R, Ekhlassi E, Downing C, et al. Advancements in pharmacotherapy for noncancerous manifestations of HPV. J Clin Med. 2015;4(5):832-846.
25. CDC. Manual for the Surveillance of Vaccine-preventable Diseases. Chapter 5: Human papillomavirus (HPV). www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Accessed October 18, 2017.
26. Leszczyszyn J, Lebski I, Lysenko L, et al. Anal warts (condyloma acuminatum)—current issues and treatment modalities. Adv Clin Exp Med. 2014;23(2):307-311.
27. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105.
28. Yan J, Chen SL, Wang HN, et al. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminate. Dermatology. 2006;213(3):218-223.
29. Hoy SM. Polyphenon E 10% ointment: in immunocompetent adults with external genital and perianal warts. Am J Clin Dermatol. 2012;13(4):275-281.
30. Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts. Obstet Gynecol. 2008;111(6):1371-1379.
31. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed October 18, 2017.
32. National Institutes of Health, National Cancer Institute. Human papillomavirus (HPV) vaccine. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet#q5. Accessed October 18, 2017.
33. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
34. CDC. HPV vaccines: vaccinating your preteen or teen. www.cdc.gov/hpv/parents/vaccine.html. Accessed October 18, 2017.
CE/CME No: CR-1711
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand basic anorectal anatomy and how to perform a thorough anorectal exam.
• Describe the physical exam findings of common benign anorectal conditions.
• Discuss the different treatment options for benign anorectal conditions.
• Differentiate between common benign anorectal symptoms and red flags that should prompt referral to a colorectal specialist.
FACULTY
Priscilla Marsicovetere is an Assistant Professor of Medical Education and of Surgery at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire; Program Director for the Franklin Pierce University PA Program in Lebanon, New Hampshire; and practices with Emergency Services of New England at Springfield Hospital in Vermont. Srinivas Joga Ivatury is an Assistant Professor of Surgery at the Geisel School of Medicine at Dartmouth and practices in the Department of Surgery at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through October 31, 2018.
Article begins on next page >>
Anorectal pain and discomfort can stem from several possible etiologies, most of which are benign. As such, many patients with anorectal complaints who present in the primary care setting can be adequately evaluated, diagnosed, and treated without referral to a colorectal specialist. However, the clinician must be able to differentiate between common benign anorectal symptoms and red flags that should prompt referral.
Anorectal disorders are common in the United States and result in numerous primary care visits each year. Presentations range from pain and itching to bleeding and lesions. Common anorectal conditions include hemorrhoids, perianal skin tags, fissures, pruritus ani, perianal abscess, and condyloma. Most are benign and can be managed in the primary care setting.
Before a provider can competently diagnose and treat anorectal conditions, however, a comprehensive history and physical examination must be conducted. Grucela and colleagues documented that physicians’ diagnostic accuracy with anorectal conditions is about 50%—highlighting the need for providers to become more familiar with the history and clinical elements associated with anorectal complaints.1
This article reviews the assessment of the anorectum, diagnosis of common disorders and their recommended treatments, and red flags for referral to a colorectal specialist.
ANORECTAL ANATOMY
The beginning of the anal canal is demarcated by its moist, hairless appearance. Just inside the anal opening are two palpable circular muscles, the internal and external anal sphincters, separated by an intersphincteric groove. The sphincters are firmly closed in the resting state, which helps maintain continence.
The anal canal is generally 3 to 4 cm long and ends at the dentate line, a series of crypts in the anal mucosa.2 The crypts are openings into the anal glands, which are mucus-secreting structures in the anus. The dentate line is easily identified on anoscopy as a discrete change in the appearance of the mucosa. The dentate line is an important landmark because it delineates the boundary between somatic and visceral nerve supplies.3 Tissue proximal to the dentate line is innervated by visceral nerves and is insensate, and thus usually not a cause of pain; tissue distal to the dentate line, however, is highly innervated by somatic nerves and can be intensely painful.2
The anorectal canal is lined by three fibrovascular cushions, located in the left lateral, right posterior, and right anterior positions.4 Inside each cushion is a venous structure, called a hemorrhoid, which allows the cushion to enlarge and help maintain continence.5
Proximal to the anus is the rectum, the 12- to 15-cm long terminus of the colon. Anorectal examination in the primary care setting will typically not progress beyond the last 2 to 3 cm of the rectum.
TAKING THE HISTORY
A thorough history will provide clues about potential underlying anorectal pathology. Patients may not be forthcoming about symptoms due to embarrassment, fear of a cancer diagnosis, or cultural customs or habits. A thorough history should elicit information about all of the patient’s symptoms (see Table 1), including bleeding, change in bowel habits, and unintended weight loss.

PHYSICAL EXAM
Positioning the patient
Undergoing an anorectal examination can be embarrassing, whether it be from exposure of sensitive body parts or the less-than-desirable prone jackknife positioning. Patients often have preconceived notions that the exam will be humiliating and/or painful. Care should be taken to minimize any embarrassment and discomfort.
Positioning of the patient is a matter of provider preference. Options include the left lateral decubitus, prone jackknife, or lithotomy positions.
Positioning should always be done with draping. Regardless of position, ensure the draping exposes only the perineum. This can be achieved by encircling the patient’s bare bottom with a plain white sheet that exposes only the anus and surrounding skin, keeping the lower back, lateral buttocks, and thighs covered.
Interestingly, data on patient preference for positioning during anorectal exams are limited. In a 2009 study of 178 patients undergoing anorectal exam, more than half of patients (up to 71.4%) expecting to or having already had a proctologic exam reported that no specific type of positioning (eg, Sims, lithotomy with lifted legs, knee-chest, knee-chest with patient’s body bent forward) was most embarrassing to them.6 The report revealed that while most patients would favor the Sims position if they had a choice, they deferred to their examiner to choose the position that seemed most suitable to get a reliable diagnosis.6
Inspection of the perineum
Once the patient is properly positioned and draped, inspection of the perineum can occur. Begin by gently spreading the buttocks. Describe any abnormality seen (eg, ulcer, lesion, dermatitis, prolapsing tissue, or blood), including size, color, and location.
A common pitfall is to describe the location of abnormalities using a clock face, such as “at 4 o’clock.” This is misleading and should be avoided, because depending on patient position, the clock face can point to different locations (eg, if the patient is in the lithotomy versus prone jackknife position).
A better approach is to divide the perianal area into four anatomic quadrants: right anterior, right posterior, left anterior, left posterior. Using this schematic, the patient's position is irrelevant, and accurate documentation of lesion location is assured.
Digital rectal exam
After visual inspection of the perianal skin, a digital rectal exam (DRE) should be performed. Slowly insert a gloved, lubricated index finger into the anus and lower rectum. Note the tone of the anus at rest (eg, excessively tight vs lax). Palpate the circumference of the anus, sweeping side to side while assessing for any tenderness, mass, or induration—if present, note the anatomic quadrant. If a mass is felt, note whether it is firm or soft, fixed or mobile, and broad-based or pedunculated. When the lubricated finger is removed from the anus, note whether blood is present.
Anoscopy
After DRE, visually inspect the anorectum. The instrument used varies from anoscope to rigid proctoscope to flexible sigmoidoscope. In a primary care setting, the most likely available instrument is an anoscope. The average anoscope is about 7 cm long and 2 cm in diameter, with a beveled tip and an obturator (see Figure 1), and allows a 360° view of the anal canal.7

Examination of the anal canal is accomplished by dividing the canal into the four anatomic quadrants described earlier and inserting the lubricated anoscope for inspection of each of the four quadrants. Observe the rectal mucosa and the anus as the scope is slowly withdrawn. If abnormalities are seen, note the location, size, shape, and any other descriptive features.
It is not necessary to perform a Hemoccult test after examination of the anorectum, as the presence of minor blood may be the direct result of the exam itself and thus provides no useful information to the examiner.
COMMON PATHOLOGIES
Once the history and physical exam are complete, a differential diagnosis can be formulated. Most anorectal disorders are benign conditions that pose no immediate health threat and can be managed in the primary care setting. Others, however, can be more serious and should prompt referral to a colorectal specialist for further evaluation. Knowing the difference can spare a patient unnecessary anxiety and referral; it can also lead to prompt, lifesaving interventions if red flags are recognized.
Hemorrhoids
Hemorrhoids are a common anorectal complaint.8,9 It is estimated that up to 75% of the population will experience symptoms of hemorrhoids during their lifetime.5,8 Whether internal or external, in their normal, nonpathologic, quiescent state, hemorrhoids are asymptomatic. Hemorrhoids become symptomatic when the supporting structures of hemorrhoidal tissue (ie, the anal cushions) deteriorate, resulting in venous dilation, inflammation, and thrombosis, which in turn lead to swelling, bright red bleeding, and/or prolapse.2,10 The most common causes of hemorrhoidal disease are chronic constipation and prolonged straining with bowel movements, though chronic diarrhea and pregnancy have also been identified as risk factors.2,8,11
External hemorrhoids, which are located distal to the dentate line, are typically only visible when they become thrombosed or swollen. In this state, they may manifest as acute-onset, exquisitely painful, large, purple-to-blue bulges at the anal outlet (see Figure 2). The number and size of the lesions can vary. The patient may report pain when sitting or wiping, as well as bleeding from the lesion.12,13 The pain is typically severe in the first couple of days, then slowly starts to subside.2,12

For internal hemorrhoids, which are located proximal to the dentate line, the main symptom is usually painless bright red blood per rectum.8,11,12 Patients may also report a sensation of rectal fullness or experience prolapse of the hemorrhoid through the anus. Prolapse typically occurs with defecation; in more severe cases, it can also occur between bowel movements, usually with any activity that increases intra-abdominal pressure (eg, coughing, heavy lifting, pregnancy, portal hypertension). The prolapse may reduce spontaneously, or may have to be manually reduced. If it cannot be reduced, there is a risk for incarceration or strangulation, potentially leading to gangrene.
The presence of bleeding and/or prolapse determines the classification of internal hemorrhoids (see Table 2). Dietary and lifestyle modification are used in the management of all grades of hemorrhoids. In addition, for grade 1 and 2 lesions, topical medication (eg, anti-inflammatory cream) can be used, whereas grade 3 (and selected grade 2) lesions respond well to rubber band ligation. Given the severity of grade 4 lesions, surgical intervention (eg, hemorrhoidectomy) is usually indicated.10

About a third of patients with symptomatic hemorrhoids seek clinical treatment.14 Most are hemodynamically stable and require no imaging and usually no labs (unless anemia is suspected).2 Management depends on the location and degree of symptoms (eg, internal vs external, prolapse, or thrombosis). In the event of an acutely thrombosed external hemorrhoid, clot excision for pain relief is appropriate if symptoms have been present for less than 48 to 72 hours; after that amount of time, the pain from the procedure will likely exceed the degree of relief provided, and conservative management should instead be recommended.2,8,11
Firstline treatment consists of lifestyle modification with a high-fiber diet and daily fiber supplement to ensure stool is soft and easy to pass.2,8 A meta-analysis of seven clinical trials with a total of 378 patients with hemorrhoids showed that fiber supplementation resulted in a 50% decrease in bleeding risk from internal hemorrhoids.15
Adequate hydration, preferably with noncaffeinated liquids, is also recommended. This will prevent constipation and the need to strain or spend excessive time on the toilet. Sitz baths can help alleviate pain and discomfort.
Several OTC topical medications are marketed for hemorrhoid relief. Many of these preparations contain steroids for their anti-inflammatory effects or astringents to address skin irritation that can result from anal leakage if prolapsing hemorrhoids prevent the anal outlet from closing. Steroid use should be limited to five to seven days, due to atrophic effects on the skin. While OTC preparations may temporarily alleviate discomfort, they will not address the underlying cause of symptoms.
Indications for referral to a colorectal specialist for symptomatic hemorrhoids include failure to improve with conservative management, persistent patient discomfort, and prolapse, as these indicate potential need for more invasive treatment.
Perianal skin tags
Perianal skin tags, while a nuisance, are not pathologic in most instances and pose no threat to health. They are an outgrowth of normal skin, appearing as loose, flesh-colored perianal tissues (see Figure 3). Tags range in size from a few millimeters to a centimeter long and can occur alone or in multiples.

Perianal skin tags are diagnosed clinically and require no labwork or imaging. Visual inspection is typically sufficient to distinguish tags from pathologic lesions such as condyloma or abscess. If there is uncertainty, however, biopsy or referral to a specialist is warranted.
Certain medical conditions can predispose a patient to development of perianal skin tags. They can be sequelae of thrombosed external hemorrhoids.8,11 They are also common in patients with Crohn disease.11 Perianal skin tags are not, however, the result of anal intercourse or sexually transmitted infections.
Treatment is usually not indicated for perianal skin tags. If the tags interfere with hygiene or cause perianal discomfort or significantly decreased quality of life, however, patients may seek removal. These patients should be referred to a colorectal specialist for evaluation for excision.
Anal fissures (fissure in ano)
The most common cause of severe anorectal pain is fissure.4 A fissure is an elliptical tear, or split, in the lining of the anal canal that causes spasm of the anal sphincters. The tear is distal to the dentate line and thus intensely painful.2,5 Common cited causes of fissures are trauma from passage of large, hard stools, straining, or diarrhea.16
Fissures can usually be visualized by spreading the posterior anus apart (see Figure 4). They are most commonly located in the posterior or anterior midline, though they can occur anywhere around the anus.2,4 Often, a sentinel tag—appearing as a taut, flesh-colored skin tag—is present at the external pole of the fissure.5,11 DRE and anoscopy should be avoided, as they will trigger intense pain and spasm of the sphincters.

Fissures are characterized as acute (present ≤ 3 months) or chronic (> 3 months).9,11 Visually, acute fissures typically have clean edges, with the appearance of a paper cut to the mucosa, while chronic fissures have indurated, heaped-up edges, often with exposure of the underlying sphincter muscle.17
They tend to be exquisitely painful, as the mucosa distal to the dentate line is highly innervated. Patients report pain akin to “passing shards of broken glass” with bowel movements, which is often accompanied by a fear of defecation and bright red blood on the toilet paper or dripping into the water.11 The pain, caused by spasm of the sphincters, typically starts during a bowel movement and lasts minutes to hours afterward.
Initial treatment is aimed at relaxing the sphincters, as well as softening stool to prevent further trauma and allow the fissure to heal. Patients should be educated about the importance of adequate fiber intake to prevent constipation and straining. A daily bulk fiber supplement, in addition to a high-fiber diet (20-25 g/d), has been shown to result in healing of 87% of acute fissures.16 Sitz baths in plain warm water, three to four times a day, can encourage relaxation of the sphincters and increase local blood flow, both of which help with fissure healing.16 Topical medications can also be prescribed. These include compounded nitroglycerin 0.2% or nifedipine 2.0%, which act to reduce the spasm by relaxing smooth muscle, as well as increase blood flow to the lesion.12,14
Most acute fissures will heal with the regimen of high fiber intake, sitz baths, and topical medication. For refractory or chronic fissures, referral to a colorectal specialist for more invasive treatment is appropriate. Additionally, fissures that are not located in the typical posterior or anterior midline might indicate an atypical etiology, such as Crohn disease, tuberculosis, leukemia, or HIV, and thus patients who present with fissures in these locations should also be referred to a colorectal specialist.2,4,11,18
Pruritus ani
Pruritus ani, known as perianal dermatitis, is a benign condition that presents with intense perianal itching and burning. It is the second most common anorectal condition after hemorrhoids and affects up to 5% of the US population.2,9,11,19
Pruritus ani often develops secondary to local irritation of the skin (eg, from prolonged exposure to moisture), leading to an inflammatory response within the superficial skin layers. The irritation causes patients to scratch the skin, resulting in trauma, excoriation, and ulcer formation and leading to a cycle of further inflammation, exacerbation of symptoms, and persistent scratching.
Physical exam may reveal circumferential erythematous and irritated perianal skin (see Figure 5). Linear or deep, punched-out excoriations may be present. Chronically, patients may develop lichenification with thick, whitened patches of skin.11 In the absence of red flags such as unintentional weight loss, anemia, rectal bleeding, or a family history of colon cancer, no additional evaluation is required during the initial visit, though anoscopy can be used to rule out associated anorectal pathology.

Many different causes of pruritus ani have been reported (see Table 3). In the case of an identifiable cause, symptoms tend to resolve once the offending agent is eliminated. Up to a quarter of cases, however, are idiopathic, with no identifiable trigger.20

Symptom management is thus key. Patients should be educated about lifestyle modification and informed that scratching will irritate the skin further and aggravate symptoms.5,11 If incontinence or diarrhea is thought to cause symptoms, dietary modifications, a fiber supplement, dysmotility agents, and Kegel exercises to strengthen the sphincters and decrease anal leakage can be recommended.
The affected skin should be kept clean and dry at all times. Aggressive wiping and overzealous hygiene should be avoided. Sitz baths can help with hygiene. A topical astringent such as witch hazel can help remove excess moisture from the skin. A layer of protective skin barrier cream with zinc oxide, when applied over dry skin, can help protect the skin from leakage throughout the day.
A sedating antihistamine can reduce scratching during sleep. Topical hydrocortisone 1% cream is effective for itch relief; this should be limited to five to seven days of consecutive use, however, as it can lead to pathologic thinning of the perianal skin. Topical capsaicin 0.006% cream has been shown to help alleviate intractable pruritus.2,21
The goal of these measures is to break the cycle of irritation and inflammation and give the skin an opportunity to heal. If the patient fails to improve, referral to a colorectal specialist or, alternatively, a dermatologist, is warranted for perianal skin biopsy and more invasive treatment options.
Perianal abscess
A perianal abscess is an infected cavity filled with pus under pressure, located near the anus or rectum. It most often results from an acute infection of the anorectal glands located at the dentate line that tracks outward to the perianal skin.12 Abscesses can also result from another disease process, such as Crohn disease, diabetes, or rectal trauma.2,11
Localized pain, swelling, and drainage are common presenting symptoms of an abscess.2,11 Systemic symptoms such as fever and chills may present later in the course but are rare.2
Treatment of the acute process is incision and drainage (I&D).11,12 This can be accomplished in the office with injection of local anesthesia, followed by excision of an ellipse of skin overlying the cavity large enough to allow full drainage of the abscess. Use of drains and packing of the wound are usually not necessary. There is also no role for antibiotics unless the patient is diabetic or immunosuppressed or cellulitis is present.11 In patients with systemic signs of illness, imaging such as CT or MRI of the pelvis can be used to assess for a deeper infection.2,12
For most patients, I&D resolves the process. However, up to half of perianal abscesses progress to form a fistula, a tunnel connecting the infected anal gland to the external skin.2,11,22 Indeed, abscesses and fistulae are part of the same infectious process, with the abscess representing the acute phase of infection and the fistula representing the chronic phase. On physical exam, in addition to the abscess site on the skin, a fistula may manifest as a palpable cord beneath the skin between the anus and the abscess opening.9 Additionally, the patient may report the abscess has been recurrent in nature, cyclically increasing in size and pain, then spontaneously draining.
A fistula requires surgical intervention for definitive treatment and should therefore prompt referral to a colorectal specialist. In the absence of fistula, however, a simple perianal abscess can be treated with I&D in the primary care setting.
Condyloma acuminatum
Condyloma acuminatum, also known as genital warts, is the most common sexually transmitted infection in the United States and a frequent anorectal complaint.23-25 More than 6 million new infections occur annually.25
Condyloma is caused by the human papillomavirus (HPV).24 More than 100 HPV subtypes have been identified.25 Types 6 and 11 are associated with typical condyloma acuminatum, while types 16 and 18 are found more commonly with dysplasia and malignant transformation.3,26
The lesions are spread by direct skin-to-skin or mucosa-to-mucosa contact, including anal intercourse. They appear as tiny finger-like projections on the perianal or genital skin, often with a cauliflower-like appearance, in clusters or as single entities, and ranging in size from a few millimeters to several centimeters (see Figure 6).

The patient may be asymptomatic or may complain of bleeding with defecation or leakage from the rectum between bowel movements. In up to 90% of perianal condyloma, concomitant anorectal lesions are present.3 Anoscopy is therefore indicated whenever perianal lesions are seen.
Treatment of genital warts varies depending on the number and location of lesions. The goal is lesion removal, but none of the available treatments are curative.24 If only a few small (diameter < 2 mm) warts are seen in the pe
Trichloroacetic acid (TCA), which is considered a form of chemical cautery, has been deemed more effective for tiny warts (diameter 1-2 mm) and better tolerated than podophyllin.24 Unlike podophyllin, however, TCA must be applied in clinic by a specialist, not at home by the patient. It works by chemically burning, cauterizing, and eroding skin and mucosa, thereby destroying old infected cells and allowing for growth of new, healthy cells.24 Using a solution with a higher concentration of TCA (80%-90%) has been shown to be more effective than lower concentration solutions (30%).24 The recurrence rate is high, generally about 36%.27 Due to its low risk for systemic absorption, TCA is safe for use during pregnancy.24
Local application of an immunomodulator, such as imiquimod 2%, 3.75%, or 5% cream, can also be used. Imiquimod works by upregulating tumor necrosis factor, leading to decreased viral replication and subsequent regression of warts.24 The most common side effects of imiquimod are erythema, erosions, burning, and pruritus.24 The higher concentration is associated with increased clearance rates as well as increased adverse effects.27 Therefore, the 3.75% cream is the preferred concentration of most providers.27 Recurrence rates range from 13% to 19%.28
In 2006, the FDA approved polyphenon E, a botanical ointment, to treat anogenital warts. The formulation is composed of eight different sinecatechins extracted from green tea leaves.29 Green tea sinecatechins have been shown to have antioxidant, antiproliferative, and antiviral properties, though the specific mechanisms of action in inhibiting anogenital wart growth have not been well studied to date. In a multicenter, randomized, double-blind, vehicle-controlled, three-arm parallel group phase 3 trial of 495 patients with anogenital warts, sinecatechins ointment 10% or 15% applied three times daily for 16 weeks resulted in complete clearance of warts in 57.2% and 56.3% of patients, respectively, compared to 33.7% clearance in the vehicle patients.30 Partial clearance (≥ 50%) of warts was observed in 78.4% of patients who used the 15% ointment, 74.0% of those who used the 10% ointment, and 51.5% of the vehicle patients. Rates of recurrence were 6.5% and 8.3% with use of the 10% and 15% sinecatechins ointment, respectively, which are much lower than recurrence rates observed with other topical applications (eg, imiquimod and podophyllotoxin).30
For larger warts or when there are anal lesions present, surgical excision and fulguration is required and referral to a colorectal specialist is warranted.
Warts recur from 4.6% to more than 70% of the time, most commonly due to activation of latent virus.23 Therefore, whether initially treated in an office or operating room, condyloma patients require follow-up on a regular basis, generally every six to 12 months, to assess for recurrent anal lesions so that treatment can be promptly initiated.
In the absence of a cure for anogenital warts, prevention through an HPV vaccine is important. The quadrivalent HPV vaccine (Gardasil) was approved by the FDA in 2006 for prophylactic vaccination of females ages 9 to 26. The vaccine triggers host formation of antibodies against four common subtypes of HPV: 6, 11, 16, and 18.31 In 2009, the FDA expanded the use of Gardasil to include males ages 9 to 26; subsequently, Gardasil 9 (which protects against nine types of HPV) was approved for males ages 9 to 15.32 In a group of 4,065 healthy males between the ages of 16 and 26, Gardasil reduced the incidence of external anogenital warts by 90.4%.33
HPV vaccine is now recommended for females through age 26 and males through age 21. The CDC also recommends HPV vaccine for the following individuals through age 26 if they did not get vaccinated when they were younger:
- Young men who have sex with men
- Young men who identify as gay or bisexual or who intend to have sex with men
- Young adults who are transgender
- Young adults with immunocompromising conditions (including HIV).34
CONCLUSION
Anorectal conditions are a common presentation in the primary care setting. A thorough history and physical exam will usually determine the etiology, and most benign pathologies can be successfully treated in the primary care clinic. Knowing how to perform an anorectal exam and having a thorough understanding of the most common anorectal pathologies can help alleviate examiner discomfort, while ensuring that patients receive prompt and adequate care. At the same time, recognizing the red flags can expedite referral for colorectal specialty evaluation when appropriate. Red flags that should prompt immediate referral to a colorectal specialist include older age, unintentional weight loss, iron deficiency anemia, family history of inflammatory bowel disease or colorectal cancer, and persistent anorectal bleeding or persistent symptoms despite adequate treatment of a suspected benign condition.
CE/CME No: CR-1711
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand basic anorectal anatomy and how to perform a thorough anorectal exam.
• Describe the physical exam findings of common benign anorectal conditions.
• Discuss the different treatment options for benign anorectal conditions.
• Differentiate between common benign anorectal symptoms and red flags that should prompt referral to a colorectal specialist.
FACULTY
Priscilla Marsicovetere is an Assistant Professor of Medical Education and of Surgery at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire; Program Director for the Franklin Pierce University PA Program in Lebanon, New Hampshire; and practices with Emergency Services of New England at Springfield Hospital in Vermont. Srinivas Joga Ivatury is an Assistant Professor of Surgery at the Geisel School of Medicine at Dartmouth and practices in the Department of Surgery at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through October 31, 2018.
Article begins on next page >>
Anorectal pain and discomfort can stem from several possible etiologies, most of which are benign. As such, many patients with anorectal complaints who present in the primary care setting can be adequately evaluated, diagnosed, and treated without referral to a colorectal specialist. However, the clinician must be able to differentiate between common benign anorectal symptoms and red flags that should prompt referral.
Anorectal disorders are common in the United States and result in numerous primary care visits each year. Presentations range from pain and itching to bleeding and lesions. Common anorectal conditions include hemorrhoids, perianal skin tags, fissures, pruritus ani, perianal abscess, and condyloma. Most are benign and can be managed in the primary care setting.
Before a provider can competently diagnose and treat anorectal conditions, however, a comprehensive history and physical examination must be conducted. Grucela and colleagues documented that physicians’ diagnostic accuracy with anorectal conditions is about 50%—highlighting the need for providers to become more familiar with the history and clinical elements associated with anorectal complaints.1
This article reviews the assessment of the anorectum, diagnosis of common disorders and their recommended treatments, and red flags for referral to a colorectal specialist.
ANORECTAL ANATOMY
The beginning of the anal canal is demarcated by its moist, hairless appearance. Just inside the anal opening are two palpable circular muscles, the internal and external anal sphincters, separated by an intersphincteric groove. The sphincters are firmly closed in the resting state, which helps maintain continence.
The anal canal is generally 3 to 4 cm long and ends at the dentate line, a series of crypts in the anal mucosa.2 The crypts are openings into the anal glands, which are mucus-secreting structures in the anus. The dentate line is easily identified on anoscopy as a discrete change in the appearance of the mucosa. The dentate line is an important landmark because it delineates the boundary between somatic and visceral nerve supplies.3 Tissue proximal to the dentate line is innervated by visceral nerves and is insensate, and thus usually not a cause of pain; tissue distal to the dentate line, however, is highly innervated by somatic nerves and can be intensely painful.2
The anorectal canal is lined by three fibrovascular cushions, located in the left lateral, right posterior, and right anterior positions.4 Inside each cushion is a venous structure, called a hemorrhoid, which allows the cushion to enlarge and help maintain continence.5
Proximal to the anus is the rectum, the 12- to 15-cm long terminus of the colon. Anorectal examination in the primary care setting will typically not progress beyond the last 2 to 3 cm of the rectum.
TAKING THE HISTORY
A thorough history will provide clues about potential underlying anorectal pathology. Patients may not be forthcoming about symptoms due to embarrassment, fear of a cancer diagnosis, or cultural customs or habits. A thorough history should elicit information about all of the patient’s symptoms (see Table 1), including bleeding, change in bowel habits, and unintended weight loss.

PHYSICAL EXAM
Positioning the patient
Undergoing an anorectal examination can be embarrassing, whether it be from exposure of sensitive body parts or the less-than-desirable prone jackknife positioning. Patients often have preconceived notions that the exam will be humiliating and/or painful. Care should be taken to minimize any embarrassment and discomfort.
Positioning of the patient is a matter of provider preference. Options include the left lateral decubitus, prone jackknife, or lithotomy positions.
Positioning should always be done with draping. Regardless of position, ensure the draping exposes only the perineum. This can be achieved by encircling the patient’s bare bottom with a plain white sheet that exposes only the anus and surrounding skin, keeping the lower back, lateral buttocks, and thighs covered.
Interestingly, data on patient preference for positioning during anorectal exams are limited. In a 2009 study of 178 patients undergoing anorectal exam, more than half of patients (up to 71.4%) expecting to or having already had a proctologic exam reported that no specific type of positioning (eg, Sims, lithotomy with lifted legs, knee-chest, knee-chest with patient’s body bent forward) was most embarrassing to them.6 The report revealed that while most patients would favor the Sims position if they had a choice, they deferred to their examiner to choose the position that seemed most suitable to get a reliable diagnosis.6
Inspection of the perineum
Once the patient is properly positioned and draped, inspection of the perineum can occur. Begin by gently spreading the buttocks. Describe any abnormality seen (eg, ulcer, lesion, dermatitis, prolapsing tissue, or blood), including size, color, and location.
A common pitfall is to describe the location of abnormalities using a clock face, such as “at 4 o’clock.” This is misleading and should be avoided, because depending on patient position, the clock face can point to different locations (eg, if the patient is in the lithotomy versus prone jackknife position).
A better approach is to divide the perianal area into four anatomic quadrants: right anterior, right posterior, left anterior, left posterior. Using this schematic, the patient's position is irrelevant, and accurate documentation of lesion location is assured.
Digital rectal exam
After visual inspection of the perianal skin, a digital rectal exam (DRE) should be performed. Slowly insert a gloved, lubricated index finger into the anus and lower rectum. Note the tone of the anus at rest (eg, excessively tight vs lax). Palpate the circumference of the anus, sweeping side to side while assessing for any tenderness, mass, or induration—if present, note the anatomic quadrant. If a mass is felt, note whether it is firm or soft, fixed or mobile, and broad-based or pedunculated. When the lubricated finger is removed from the anus, note whether blood is present.
Anoscopy
After DRE, visually inspect the anorectum. The instrument used varies from anoscope to rigid proctoscope to flexible sigmoidoscope. In a primary care setting, the most likely available instrument is an anoscope. The average anoscope is about 7 cm long and 2 cm in diameter, with a beveled tip and an obturator (see Figure 1), and allows a 360° view of the anal canal.7

Examination of the anal canal is accomplished by dividing the canal into the four anatomic quadrants described earlier and inserting the lubricated anoscope for inspection of each of the four quadrants. Observe the rectal mucosa and the anus as the scope is slowly withdrawn. If abnormalities are seen, note the location, size, shape, and any other descriptive features.
It is not necessary to perform a Hemoccult test after examination of the anorectum, as the presence of minor blood may be the direct result of the exam itself and thus provides no useful information to the examiner.
COMMON PATHOLOGIES
Once the history and physical exam are complete, a differential diagnosis can be formulated. Most anorectal disorders are benign conditions that pose no immediate health threat and can be managed in the primary care setting. Others, however, can be more serious and should prompt referral to a colorectal specialist for further evaluation. Knowing the difference can spare a patient unnecessary anxiety and referral; it can also lead to prompt, lifesaving interventions if red flags are recognized.
Hemorrhoids
Hemorrhoids are a common anorectal complaint.8,9 It is estimated that up to 75% of the population will experience symptoms of hemorrhoids during their lifetime.5,8 Whether internal or external, in their normal, nonpathologic, quiescent state, hemorrhoids are asymptomatic. Hemorrhoids become symptomatic when the supporting structures of hemorrhoidal tissue (ie, the anal cushions) deteriorate, resulting in venous dilation, inflammation, and thrombosis, which in turn lead to swelling, bright red bleeding, and/or prolapse.2,10 The most common causes of hemorrhoidal disease are chronic constipation and prolonged straining with bowel movements, though chronic diarrhea and pregnancy have also been identified as risk factors.2,8,11
External hemorrhoids, which are located distal to the dentate line, are typically only visible when they become thrombosed or swollen. In this state, they may manifest as acute-onset, exquisitely painful, large, purple-to-blue bulges at the anal outlet (see Figure 2). The number and size of the lesions can vary. The patient may report pain when sitting or wiping, as well as bleeding from the lesion.12,13 The pain is typically severe in the first couple of days, then slowly starts to subside.2,12

For internal hemorrhoids, which are located proximal to the dentate line, the main symptom is usually painless bright red blood per rectum.8,11,12 Patients may also report a sensation of rectal fullness or experience prolapse of the hemorrhoid through the anus. Prolapse typically occurs with defecation; in more severe cases, it can also occur between bowel movements, usually with any activity that increases intra-abdominal pressure (eg, coughing, heavy lifting, pregnancy, portal hypertension). The prolapse may reduce spontaneously, or may have to be manually reduced. If it cannot be reduced, there is a risk for incarceration or strangulation, potentially leading to gangrene.
The presence of bleeding and/or prolapse determines the classification of internal hemorrhoids (see Table 2). Dietary and lifestyle modification are used in the management of all grades of hemorrhoids. In addition, for grade 1 and 2 lesions, topical medication (eg, anti-inflammatory cream) can be used, whereas grade 3 (and selected grade 2) lesions respond well to rubber band ligation. Given the severity of grade 4 lesions, surgical intervention (eg, hemorrhoidectomy) is usually indicated.10

About a third of patients with symptomatic hemorrhoids seek clinical treatment.14 Most are hemodynamically stable and require no imaging and usually no labs (unless anemia is suspected).2 Management depends on the location and degree of symptoms (eg, internal vs external, prolapse, or thrombosis). In the event of an acutely thrombosed external hemorrhoid, clot excision for pain relief is appropriate if symptoms have been present for less than 48 to 72 hours; after that amount of time, the pain from the procedure will likely exceed the degree of relief provided, and conservative management should instead be recommended.2,8,11
Firstline treatment consists of lifestyle modification with a high-fiber diet and daily fiber supplement to ensure stool is soft and easy to pass.2,8 A meta-analysis of seven clinical trials with a total of 378 patients with hemorrhoids showed that fiber supplementation resulted in a 50% decrease in bleeding risk from internal hemorrhoids.15
Adequate hydration, preferably with noncaffeinated liquids, is also recommended. This will prevent constipation and the need to strain or spend excessive time on the toilet. Sitz baths can help alleviate pain and discomfort.
Several OTC topical medications are marketed for hemorrhoid relief. Many of these preparations contain steroids for their anti-inflammatory effects or astringents to address skin irritation that can result from anal leakage if prolapsing hemorrhoids prevent the anal outlet from closing. Steroid use should be limited to five to seven days, due to atrophic effects on the skin. While OTC preparations may temporarily alleviate discomfort, they will not address the underlying cause of symptoms.
Indications for referral to a colorectal specialist for symptomatic hemorrhoids include failure to improve with conservative management, persistent patient discomfort, and prolapse, as these indicate potential need for more invasive treatment.
Perianal skin tags
Perianal skin tags, while a nuisance, are not pathologic in most instances and pose no threat to health. They are an outgrowth of normal skin, appearing as loose, flesh-colored perianal tissues (see Figure 3). Tags range in size from a few millimeters to a centimeter long and can occur alone or in multiples.

Perianal skin tags are diagnosed clinically and require no labwork or imaging. Visual inspection is typically sufficient to distinguish tags from pathologic lesions such as condyloma or abscess. If there is uncertainty, however, biopsy or referral to a specialist is warranted.
Certain medical conditions can predispose a patient to development of perianal skin tags. They can be sequelae of thrombosed external hemorrhoids.8,11 They are also common in patients with Crohn disease.11 Perianal skin tags are not, however, the result of anal intercourse or sexually transmitted infections.
Treatment is usually not indicated for perianal skin tags. If the tags interfere with hygiene or cause perianal discomfort or significantly decreased quality of life, however, patients may seek removal. These patients should be referred to a colorectal specialist for evaluation for excision.
Anal fissures (fissure in ano)
The most common cause of severe anorectal pain is fissure.4 A fissure is an elliptical tear, or split, in the lining of the anal canal that causes spasm of the anal sphincters. The tear is distal to the dentate line and thus intensely painful.2,5 Common cited causes of fissures are trauma from passage of large, hard stools, straining, or diarrhea.16
Fissures can usually be visualized by spreading the posterior anus apart (see Figure 4). They are most commonly located in the posterior or anterior midline, though they can occur anywhere around the anus.2,4 Often, a sentinel tag—appearing as a taut, flesh-colored skin tag—is present at the external pole of the fissure.5,11 DRE and anoscopy should be avoided, as they will trigger intense pain and spasm of the sphincters.

Fissures are characterized as acute (present ≤ 3 months) or chronic (> 3 months).9,11 Visually, acute fissures typically have clean edges, with the appearance of a paper cut to the mucosa, while chronic fissures have indurated, heaped-up edges, often with exposure of the underlying sphincter muscle.17
They tend to be exquisitely painful, as the mucosa distal to the dentate line is highly innervated. Patients report pain akin to “passing shards of broken glass” with bowel movements, which is often accompanied by a fear of defecation and bright red blood on the toilet paper or dripping into the water.11 The pain, caused by spasm of the sphincters, typically starts during a bowel movement and lasts minutes to hours afterward.
Initial treatment is aimed at relaxing the sphincters, as well as softening stool to prevent further trauma and allow the fissure to heal. Patients should be educated about the importance of adequate fiber intake to prevent constipation and straining. A daily bulk fiber supplement, in addition to a high-fiber diet (20-25 g/d), has been shown to result in healing of 87% of acute fissures.16 Sitz baths in plain warm water, three to four times a day, can encourage relaxation of the sphincters and increase local blood flow, both of which help with fissure healing.16 Topical medications can also be prescribed. These include compounded nitroglycerin 0.2% or nifedipine 2.0%, which act to reduce the spasm by relaxing smooth muscle, as well as increase blood flow to the lesion.12,14
Most acute fissures will heal with the regimen of high fiber intake, sitz baths, and topical medication. For refractory or chronic fissures, referral to a colorectal specialist for more invasive treatment is appropriate. Additionally, fissures that are not located in the typical posterior or anterior midline might indicate an atypical etiology, such as Crohn disease, tuberculosis, leukemia, or HIV, and thus patients who present with fissures in these locations should also be referred to a colorectal specialist.2,4,11,18
Pruritus ani
Pruritus ani, known as perianal dermatitis, is a benign condition that presents with intense perianal itching and burning. It is the second most common anorectal condition after hemorrhoids and affects up to 5% of the US population.2,9,11,19
Pruritus ani often develops secondary to local irritation of the skin (eg, from prolonged exposure to moisture), leading to an inflammatory response within the superficial skin layers. The irritation causes patients to scratch the skin, resulting in trauma, excoriation, and ulcer formation and leading to a cycle of further inflammation, exacerbation of symptoms, and persistent scratching.
Physical exam may reveal circumferential erythematous and irritated perianal skin (see Figure 5). Linear or deep, punched-out excoriations may be present. Chronically, patients may develop lichenification with thick, whitened patches of skin.11 In the absence of red flags such as unintentional weight loss, anemia, rectal bleeding, or a family history of colon cancer, no additional evaluation is required during the initial visit, though anoscopy can be used to rule out associated anorectal pathology.

Many different causes of pruritus ani have been reported (see Table 3). In the case of an identifiable cause, symptoms tend to resolve once the offending agent is eliminated. Up to a quarter of cases, however, are idiopathic, with no identifiable trigger.20

Symptom management is thus key. Patients should be educated about lifestyle modification and informed that scratching will irritate the skin further and aggravate symptoms.5,11 If incontinence or diarrhea is thought to cause symptoms, dietary modifications, a fiber supplement, dysmotility agents, and Kegel exercises to strengthen the sphincters and decrease anal leakage can be recommended.
The affected skin should be kept clean and dry at all times. Aggressive wiping and overzealous hygiene should be avoided. Sitz baths can help with hygiene. A topical astringent such as witch hazel can help remove excess moisture from the skin. A layer of protective skin barrier cream with zinc oxide, when applied over dry skin, can help protect the skin from leakage throughout the day.
A sedating antihistamine can reduce scratching during sleep. Topical hydrocortisone 1% cream is effective for itch relief; this should be limited to five to seven days of consecutive use, however, as it can lead to pathologic thinning of the perianal skin. Topical capsaicin 0.006% cream has been shown to help alleviate intractable pruritus.2,21
The goal of these measures is to break the cycle of irritation and inflammation and give the skin an opportunity to heal. If the patient fails to improve, referral to a colorectal specialist or, alternatively, a dermatologist, is warranted for perianal skin biopsy and more invasive treatment options.
Perianal abscess
A perianal abscess is an infected cavity filled with pus under pressure, located near the anus or rectum. It most often results from an acute infection of the anorectal glands located at the dentate line that tracks outward to the perianal skin.12 Abscesses can also result from another disease process, such as Crohn disease, diabetes, or rectal trauma.2,11
Localized pain, swelling, and drainage are common presenting symptoms of an abscess.2,11 Systemic symptoms such as fever and chills may present later in the course but are rare.2
Treatment of the acute process is incision and drainage (I&D).11,12 This can be accomplished in the office with injection of local anesthesia, followed by excision of an ellipse of skin overlying the cavity large enough to allow full drainage of the abscess. Use of drains and packing of the wound are usually not necessary. There is also no role for antibiotics unless the patient is diabetic or immunosuppressed or cellulitis is present.11 In patients with systemic signs of illness, imaging such as CT or MRI of the pelvis can be used to assess for a deeper infection.2,12
For most patients, I&D resolves the process. However, up to half of perianal abscesses progress to form a fistula, a tunnel connecting the infected anal gland to the external skin.2,11,22 Indeed, abscesses and fistulae are part of the same infectious process, with the abscess representing the acute phase of infection and the fistula representing the chronic phase. On physical exam, in addition to the abscess site on the skin, a fistula may manifest as a palpable cord beneath the skin between the anus and the abscess opening.9 Additionally, the patient may report the abscess has been recurrent in nature, cyclically increasing in size and pain, then spontaneously draining.
A fistula requires surgical intervention for definitive treatment and should therefore prompt referral to a colorectal specialist. In the absence of fistula, however, a simple perianal abscess can be treated with I&D in the primary care setting.
Condyloma acuminatum
Condyloma acuminatum, also known as genital warts, is the most common sexually transmitted infection in the United States and a frequent anorectal complaint.23-25 More than 6 million new infections occur annually.25
Condyloma is caused by the human papillomavirus (HPV).24 More than 100 HPV subtypes have been identified.25 Types 6 and 11 are associated with typical condyloma acuminatum, while types 16 and 18 are found more commonly with dysplasia and malignant transformation.3,26
The lesions are spread by direct skin-to-skin or mucosa-to-mucosa contact, including anal intercourse. They appear as tiny finger-like projections on the perianal or genital skin, often with a cauliflower-like appearance, in clusters or as single entities, and ranging in size from a few millimeters to several centimeters (see Figure 6).

The patient may be asymptomatic or may complain of bleeding with defecation or leakage from the rectum between bowel movements. In up to 90% of perianal condyloma, concomitant anorectal lesions are present.3 Anoscopy is therefore indicated whenever perianal lesions are seen.
Treatment of genital warts varies depending on the number and location of lesions. The goal is lesion removal, but none of the available treatments are curative.24 If only a few small (diameter < 2 mm) warts are seen in the pe
Trichloroacetic acid (TCA), which is considered a form of chemical cautery, has been deemed more effective for tiny warts (diameter 1-2 mm) and better tolerated than podophyllin.24 Unlike podophyllin, however, TCA must be applied in clinic by a specialist, not at home by the patient. It works by chemically burning, cauterizing, and eroding skin and mucosa, thereby destroying old infected cells and allowing for growth of new, healthy cells.24 Using a solution with a higher concentration of TCA (80%-90%) has been shown to be more effective than lower concentration solutions (30%).24 The recurrence rate is high, generally about 36%.27 Due to its low risk for systemic absorption, TCA is safe for use during pregnancy.24
Local application of an immunomodulator, such as imiquimod 2%, 3.75%, or 5% cream, can also be used. Imiquimod works by upregulating tumor necrosis factor, leading to decreased viral replication and subsequent regression of warts.24 The most common side effects of imiquimod are erythema, erosions, burning, and pruritus.24 The higher concentration is associated with increased clearance rates as well as increased adverse effects.27 Therefore, the 3.75% cream is the preferred concentration of most providers.27 Recurrence rates range from 13% to 19%.28
In 2006, the FDA approved polyphenon E, a botanical ointment, to treat anogenital warts. The formulation is composed of eight different sinecatechins extracted from green tea leaves.29 Green tea sinecatechins have been shown to have antioxidant, antiproliferative, and antiviral properties, though the specific mechanisms of action in inhibiting anogenital wart growth have not been well studied to date. In a multicenter, randomized, double-blind, vehicle-controlled, three-arm parallel group phase 3 trial of 495 patients with anogenital warts, sinecatechins ointment 10% or 15% applied three times daily for 16 weeks resulted in complete clearance of warts in 57.2% and 56.3% of patients, respectively, compared to 33.7% clearance in the vehicle patients.30 Partial clearance (≥ 50%) of warts was observed in 78.4% of patients who used the 15% ointment, 74.0% of those who used the 10% ointment, and 51.5% of the vehicle patients. Rates of recurrence were 6.5% and 8.3% with use of the 10% and 15% sinecatechins ointment, respectively, which are much lower than recurrence rates observed with other topical applications (eg, imiquimod and podophyllotoxin).30
For larger warts or when there are anal lesions present, surgical excision and fulguration is required and referral to a colorectal specialist is warranted.
Warts recur from 4.6% to more than 70% of the time, most commonly due to activation of latent virus.23 Therefore, whether initially treated in an office or operating room, condyloma patients require follow-up on a regular basis, generally every six to 12 months, to assess for recurrent anal lesions so that treatment can be promptly initiated.
In the absence of a cure for anogenital warts, prevention through an HPV vaccine is important. The quadrivalent HPV vaccine (Gardasil) was approved by the FDA in 2006 for prophylactic vaccination of females ages 9 to 26. The vaccine triggers host formation of antibodies against four common subtypes of HPV: 6, 11, 16, and 18.31 In 2009, the FDA expanded the use of Gardasil to include males ages 9 to 26; subsequently, Gardasil 9 (which protects against nine types of HPV) was approved for males ages 9 to 15.32 In a group of 4,065 healthy males between the ages of 16 and 26, Gardasil reduced the incidence of external anogenital warts by 90.4%.33
HPV vaccine is now recommended for females through age 26 and males through age 21. The CDC also recommends HPV vaccine for the following individuals through age 26 if they did not get vaccinated when they were younger:
- Young men who have sex with men
- Young men who identify as gay or bisexual or who intend to have sex with men
- Young adults who are transgender
- Young adults with immunocompromising conditions (including HIV).34
CONCLUSION
Anorectal conditions are a common presentation in the primary care setting. A thorough history and physical exam will usually determine the etiology, and most benign pathologies can be successfully treated in the primary care clinic. Knowing how to perform an anorectal exam and having a thorough understanding of the most common anorectal pathologies can help alleviate examiner discomfort, while ensuring that patients receive prompt and adequate care. At the same time, recognizing the red flags can expedite referral for colorectal specialty evaluation when appropriate. Red flags that should prompt immediate referral to a colorectal specialist include older age, unintentional weight loss, iron deficiency anemia, family history of inflammatory bowel disease or colorectal cancer, and persistent anorectal bleeding or persistent symptoms despite adequate treatment of a suspected benign condition.
1. Grucela A, Salinas H, Khaitov S, et al. Prospective analysis of clinical accuracy in the diagnosis of benign anal pathology, comparison across specialties and years of experience. Dis Colon Rectum. 2010;53(1):47-52.
2. Tupe CL, Pham TV. Anorectal complaints in the emergency department. Emerg Med Clin North Am. 2016;34(2):251-270.
3. Billingham RP, Isler JT, Kimmins MH, et al. The diagnosis and management of common anorectal disorders. Curr Probl Surg. 2004;41(7):586-645.
4. Schubert MC, Sridhar S, Schade RR, Wexner SD. What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol. 2009;15(26):3201-3209.
5. Henderson PK, Cash BD. Common anorectal conditions: evaluation and treatment. Curr Gastroenterol Rep. 2014; 16(10):408.
6. Gebbensleben O, Hilger Y, Rohde H. Patients’ view of medical positioning for proctologic examination. Clin Exp Gastroenterol. 2009;2:133-138.
7. Gopal DV. Diseases of the rectum and anus: a clinical approach to common disorders. Clin Cornerstone. 2002;4(4): 34-48.
8. Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol. 2015;21(31):9245-9252.
9. Fargo MV, Latimer K. Evaluation and management of common anorectal conditions. Am Fam Physician. 2012;85(6):624-630.
10. Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17): 2009-2017.
11. Foxx-Orenstein AE, Umar SB, Crowell MD. Common anorectal disorders. Gastroenterol Hepatol. 2014;10(5):294-301.
12. Lohsiriwat V. Anorectal emergencies. World J Gastroenterol. 2016;22(26):5867-5878.
13. Greenspon J, Williams SB, Young HA, Orkin BA. Thrombosed external hemorrhoids: outcome after conservative treatment or surgical management. Dis Colon Rectum. 2004(9);47:1493-1498.
14. Summers A. Assessment and treatment of three common anorectal conditions. Emerg Nurse. 2013;21(2):28-33.
15. Alonso-Coello P, Mills E, Heels-Ansdell D, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101(1):181-188.
16. Medhi B, Rao RS, Prakash A, et al. Recent advances in the pharmacotherapy of chronic anal fissure: an update. Asian J Surg. 2008;31(3):154-163.
17. Nelson RL. Anal fissure (chronic). BMJ Clin Evid. 2014;2014. pii: 0407.
18. deRosa M, Cestaro G, Vitiello C, et al. Conservative versus surgical treatment for chronic idiopathic anal fissure: a prospective randomized trial. Updates Surg. 2013;65(3):197-200.
19. Lacy B, Weiser K. Common anorectal disorders: diagnosis and treatment. Curr Gastroenterol Rep. 2009;11(5):413-419.
20. Siddiqi S, Vijay V, Ward M, et al. Pruritus ani. Ann R Coll Surg Engl. 2008;90:457-463.
21. Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomized, placebo controlled, crossover study. Gut. 2003;52(9):1323-1326.
22. Burnstein M. Managing anorectal emergencies. Can Fam Physician. 1993;39:1782-1785.
23. Sasaki A, Nakajima T, Egashira H, et al. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol. 2016;22(8):2636-2641.
24. Kollipara R, Ekhlassi E, Downing C, et al. Advancements in pharmacotherapy for noncancerous manifestations of HPV. J Clin Med. 2015;4(5):832-846.
25. CDC. Manual for the Surveillance of Vaccine-preventable Diseases. Chapter 5: Human papillomavirus (HPV). www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Accessed October 18, 2017.
26. Leszczyszyn J, Lebski I, Lysenko L, et al. Anal warts (condyloma acuminatum)—current issues and treatment modalities. Adv Clin Exp Med. 2014;23(2):307-311.
27. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105.
28. Yan J, Chen SL, Wang HN, et al. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminate. Dermatology. 2006;213(3):218-223.
29. Hoy SM. Polyphenon E 10% ointment: in immunocompetent adults with external genital and perianal warts. Am J Clin Dermatol. 2012;13(4):275-281.
30. Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts. Obstet Gynecol. 2008;111(6):1371-1379.
31. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed October 18, 2017.
32. National Institutes of Health, National Cancer Institute. Human papillomavirus (HPV) vaccine. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet#q5. Accessed October 18, 2017.
33. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
34. CDC. HPV vaccines: vaccinating your preteen or teen. www.cdc.gov/hpv/parents/vaccine.html. Accessed October 18, 2017.
1. Grucela A, Salinas H, Khaitov S, et al. Prospective analysis of clinical accuracy in the diagnosis of benign anal pathology, comparison across specialties and years of experience. Dis Colon Rectum. 2010;53(1):47-52.
2. Tupe CL, Pham TV. Anorectal complaints in the emergency department. Emerg Med Clin North Am. 2016;34(2):251-270.
3. Billingham RP, Isler JT, Kimmins MH, et al. The diagnosis and management of common anorectal disorders. Curr Probl Surg. 2004;41(7):586-645.
4. Schubert MC, Sridhar S, Schade RR, Wexner SD. What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol. 2009;15(26):3201-3209.
5. Henderson PK, Cash BD. Common anorectal conditions: evaluation and treatment. Curr Gastroenterol Rep. 2014; 16(10):408.
6. Gebbensleben O, Hilger Y, Rohde H. Patients’ view of medical positioning for proctologic examination. Clin Exp Gastroenterol. 2009;2:133-138.
7. Gopal DV. Diseases of the rectum and anus: a clinical approach to common disorders. Clin Cornerstone. 2002;4(4): 34-48.
8. Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol. 2015;21(31):9245-9252.
9. Fargo MV, Latimer K. Evaluation and management of common anorectal conditions. Am Fam Physician. 2012;85(6):624-630.
10. Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17): 2009-2017.
11. Foxx-Orenstein AE, Umar SB, Crowell MD. Common anorectal disorders. Gastroenterol Hepatol. 2014;10(5):294-301.
12. Lohsiriwat V. Anorectal emergencies. World J Gastroenterol. 2016;22(26):5867-5878.
13. Greenspon J, Williams SB, Young HA, Orkin BA. Thrombosed external hemorrhoids: outcome after conservative treatment or surgical management. Dis Colon Rectum. 2004(9);47:1493-1498.
14. Summers A. Assessment and treatment of three common anorectal conditions. Emerg Nurse. 2013;21(2):28-33.
15. Alonso-Coello P, Mills E, Heels-Ansdell D, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101(1):181-188.
16. Medhi B, Rao RS, Prakash A, et al. Recent advances in the pharmacotherapy of chronic anal fissure: an update. Asian J Surg. 2008;31(3):154-163.
17. Nelson RL. Anal fissure (chronic). BMJ Clin Evid. 2014;2014. pii: 0407.
18. deRosa M, Cestaro G, Vitiello C, et al. Conservative versus surgical treatment for chronic idiopathic anal fissure: a prospective randomized trial. Updates Surg. 2013;65(3):197-200.
19. Lacy B, Weiser K. Common anorectal disorders: diagnosis and treatment. Curr Gastroenterol Rep. 2009;11(5):413-419.
20. Siddiqi S, Vijay V, Ward M, et al. Pruritus ani. Ann R Coll Surg Engl. 2008;90:457-463.
21. Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomized, placebo controlled, crossover study. Gut. 2003;52(9):1323-1326.
22. Burnstein M. Managing anorectal emergencies. Can Fam Physician. 1993;39:1782-1785.
23. Sasaki A, Nakajima T, Egashira H, et al. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol. 2016;22(8):2636-2641.
24. Kollipara R, Ekhlassi E, Downing C, et al. Advancements in pharmacotherapy for noncancerous manifestations of HPV. J Clin Med. 2015;4(5):832-846.
25. CDC. Manual for the Surveillance of Vaccine-preventable Diseases. Chapter 5: Human papillomavirus (HPV). www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Accessed October 18, 2017.
26. Leszczyszyn J, Lebski I, Lysenko L, et al. Anal warts (condyloma acuminatum)—current issues and treatment modalities. Adv Clin Exp Med. 2014;23(2):307-311.
27. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105.
28. Yan J, Chen SL, Wang HN, et al. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminate. Dermatology. 2006;213(3):218-223.
29. Hoy SM. Polyphenon E 10% ointment: in immunocompetent adults with external genital and perianal warts. Am J Clin Dermatol. 2012;13(4):275-281.
30. Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts. Obstet Gynecol. 2008;111(6):1371-1379.
31. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed October 18, 2017.
32. National Institutes of Health, National Cancer Institute. Human papillomavirus (HPV) vaccine. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet#q5. Accessed October 18, 2017.
33. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
34. CDC. HPV vaccines: vaccinating your preteen or teen. www.cdc.gov/hpv/parents/vaccine.html. Accessed October 18, 2017.