User login
More than skin-deep
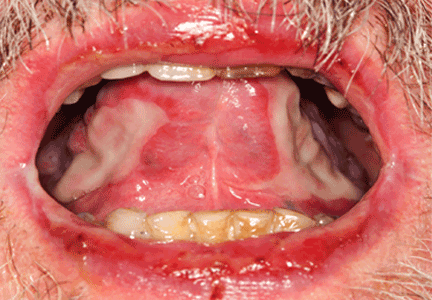
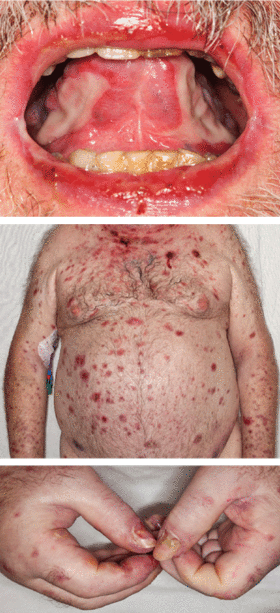
A 68-year-old man presented for evaluation of a diffuse rash with mucosal involvement. During the past 9 months, he had had oral ulcers, a truncal rash, and blistering lesions on his hands with fingernail erosion, and all of these had been getting worse (Figure 1). He also reported cycles of fever and a weight loss of 50 lb.
Computed tomography revealed diffuse lymphadenopathy, including a bulky 18-cm mediastinal mass. Lymph node biopsy confirmed follicular non-Hodgkin lymphoma.
On serum enzyme-linked immunosorbent assay (ELISA), the desmoglein 1 antibody titer was 62.4 U (< 14 is negative, > 20 is positive) and the desmoglein 3 antibody titer was 36.0 U (< 9 is negative, > 20 is positive). Indirect immunofluorescence testing on monkey esophagus substrate was reactive.
ELISA detected no immunoglobulin G (IgG) reactivity against the bullous pemphigoid autoantigens BP180 and BP230. Direct immunofluorescence testing revealed strong IgG and C3 deposition on epithelial cell surfaces, and indirect immunofluorescence on rat bladder substrate was positive, suggesting the diagnosis of paraneoplastic pemphigus.
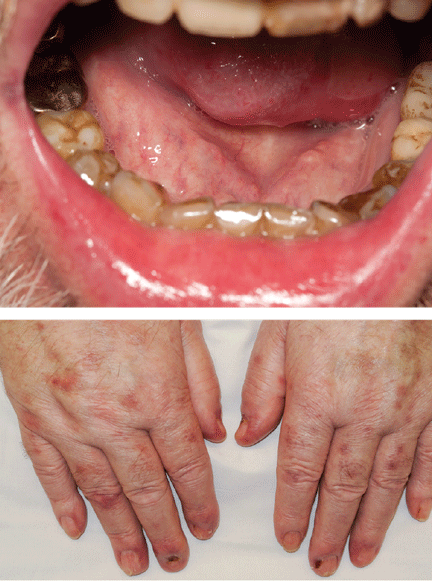
The patient was treated with dexamethasone and bendamustine-rituximab. Five months later, desmoglein 1 and 3 antibody titers were measured again and were within normal limits. His lesions had improved significantly (Figure 2), but he had endured multiple medical setbacks, including Pseudomonas peritonitis, fistulizing cytomegalovirus perianal ulceration, septic shock secondary to fulminant Clostridium difficile colitis, and toxic megacolon necessitating total colectomy with end-ileostomy.
PARANEOPLASTIC PEMPHIGUS
Paraneoplastic pemphigus usually occurs in the presence of underlying lymphoproliferative neoplasm, most often non-Hodgkin lymphoma, chronic lymphocytic leukemia, or Castleman disease. It may also occur with epithelial carcinoma, mesenchymal sarcoma, and, rarely, malignant melanoma.1 The association between malignancy and autoimmune mucocutaneous disease was first described in 1990.2 Autoantibodies against periplakin, envoplakin, and desmoglein 3 are often present and may play a role in pathogenesis.
Paraneoplastic pemphigus associated with malignancy portends a poor prognosis. Although historically reported to have an overall death rate ranging from 75% to 90%, with a mean survival of less than 1 year, a recent retrospective cohort has shown slightly improved outcomes, with 49% of patients remaining alive at 1 year and 38% alive at 5 years.3 The most common causes of death are sepsis, respiratory failure, and the underlying malignancy. Bronchiolitis obliterans may occur late in the disease course and is an ominous prognostic factor, with a death rate of 41% after a median interval of 13 months.4
Immunosuppressive drugs are often used to control the disease. First-line treatment is with high-dose steroids. Immunosuppressives such as azathioprine and cyclosporin may be used in conjunction to reduce the steroid dose that is required and to limit the adverse effects of steroid therapy. Treating the underlying malignancy may decrease autoantibody production and lead to clinical improvement.
- Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol 2004; 40:553–562.
- Anhalt GJ, Kim SC, Stanley JR, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990; 323:1729–1735.
- Leger S, Picard D, Ingen-Housz-Oro S, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol 2012; 148:1165–1172.
- Maldonado F, Pittelkow MR, Ryu JH. Constrictive bronchiolitis associated with pareaneoplastic autoimmune multi-organ syndrome. Respirology 2009; 14:129–133.
A 68-year-old man presented for evaluation of a diffuse rash with mucosal involvement. During the past 9 months, he had had oral ulcers, a truncal rash, and blistering lesions on his hands with fingernail erosion, and all of these had been getting worse (Figure 1). He also reported cycles of fever and a weight loss of 50 lb.

Computed tomography revealed diffuse lymphadenopathy, including a bulky 18-cm mediastinal mass. Lymph node biopsy confirmed follicular non-Hodgkin lymphoma.
On serum enzyme-linked immunosorbent assay (ELISA), the desmoglein 1 antibody titer was 62.4 U (< 14 is negative, > 20 is positive) and the desmoglein 3 antibody titer was 36.0 U (< 9 is negative, > 20 is positive). Indirect immunofluorescence testing on monkey esophagus substrate was reactive.
ELISA detected no immunoglobulin G (IgG) reactivity against the bullous pemphigoid autoantigens BP180 and BP230. Direct immunofluorescence testing revealed strong IgG and C3 deposition on epithelial cell surfaces, and indirect immunofluorescence on rat bladder substrate was positive, suggesting the diagnosis of paraneoplastic pemphigus.
The patient was treated with dexamethasone and bendamustine-rituximab. Five months later, desmoglein 1 and 3 antibody titers were measured again and were within normal limits. His lesions had improved significantly (Figure 2), but he had endured multiple medical setbacks, including Pseudomonas peritonitis, fistulizing cytomegalovirus perianal ulceration, septic shock secondary to fulminant Clostridium difficile colitis, and toxic megacolon necessitating total colectomy with end-ileostomy.

PARANEOPLASTIC PEMPHIGUS
Paraneoplastic pemphigus usually occurs in the presence of underlying lymphoproliferative neoplasm, most often non-Hodgkin lymphoma, chronic lymphocytic leukemia, or Castleman disease. It may also occur with epithelial carcinoma, mesenchymal sarcoma, and, rarely, malignant melanoma.1 The association between malignancy and autoimmune mucocutaneous disease was first described in 1990.2 Autoantibodies against periplakin, envoplakin, and desmoglein 3 are often present and may play a role in pathogenesis.
Paraneoplastic pemphigus associated with malignancy portends a poor prognosis. Although historically reported to have an overall death rate ranging from 75% to 90%, with a mean survival of less than 1 year, a recent retrospective cohort has shown slightly improved outcomes, with 49% of patients remaining alive at 1 year and 38% alive at 5 years.3 The most common causes of death are sepsis, respiratory failure, and the underlying malignancy. Bronchiolitis obliterans may occur late in the disease course and is an ominous prognostic factor, with a death rate of 41% after a median interval of 13 months.4
Immunosuppressive drugs are often used to control the disease. First-line treatment is with high-dose steroids. Immunosuppressives such as azathioprine and cyclosporin may be used in conjunction to reduce the steroid dose that is required and to limit the adverse effects of steroid therapy. Treating the underlying malignancy may decrease autoantibody production and lead to clinical improvement.
A 68-year-old man presented for evaluation of a diffuse rash with mucosal involvement. During the past 9 months, he had had oral ulcers, a truncal rash, and blistering lesions on his hands with fingernail erosion, and all of these had been getting worse (Figure 1). He also reported cycles of fever and a weight loss of 50 lb.

Computed tomography revealed diffuse lymphadenopathy, including a bulky 18-cm mediastinal mass. Lymph node biopsy confirmed follicular non-Hodgkin lymphoma.
On serum enzyme-linked immunosorbent assay (ELISA), the desmoglein 1 antibody titer was 62.4 U (< 14 is negative, > 20 is positive) and the desmoglein 3 antibody titer was 36.0 U (< 9 is negative, > 20 is positive). Indirect immunofluorescence testing on monkey esophagus substrate was reactive.
ELISA detected no immunoglobulin G (IgG) reactivity against the bullous pemphigoid autoantigens BP180 and BP230. Direct immunofluorescence testing revealed strong IgG and C3 deposition on epithelial cell surfaces, and indirect immunofluorescence on rat bladder substrate was positive, suggesting the diagnosis of paraneoplastic pemphigus.
The patient was treated with dexamethasone and bendamustine-rituximab. Five months later, desmoglein 1 and 3 antibody titers were measured again and were within normal limits. His lesions had improved significantly (Figure 2), but he had endured multiple medical setbacks, including Pseudomonas peritonitis, fistulizing cytomegalovirus perianal ulceration, septic shock secondary to fulminant Clostridium difficile colitis, and toxic megacolon necessitating total colectomy with end-ileostomy.

PARANEOPLASTIC PEMPHIGUS
Paraneoplastic pemphigus usually occurs in the presence of underlying lymphoproliferative neoplasm, most often non-Hodgkin lymphoma, chronic lymphocytic leukemia, or Castleman disease. It may also occur with epithelial carcinoma, mesenchymal sarcoma, and, rarely, malignant melanoma.1 The association between malignancy and autoimmune mucocutaneous disease was first described in 1990.2 Autoantibodies against periplakin, envoplakin, and desmoglein 3 are often present and may play a role in pathogenesis.
Paraneoplastic pemphigus associated with malignancy portends a poor prognosis. Although historically reported to have an overall death rate ranging from 75% to 90%, with a mean survival of less than 1 year, a recent retrospective cohort has shown slightly improved outcomes, with 49% of patients remaining alive at 1 year and 38% alive at 5 years.3 The most common causes of death are sepsis, respiratory failure, and the underlying malignancy. Bronchiolitis obliterans may occur late in the disease course and is an ominous prognostic factor, with a death rate of 41% after a median interval of 13 months.4
Immunosuppressive drugs are often used to control the disease. First-line treatment is with high-dose steroids. Immunosuppressives such as azathioprine and cyclosporin may be used in conjunction to reduce the steroid dose that is required and to limit the adverse effects of steroid therapy. Treating the underlying malignancy may decrease autoantibody production and lead to clinical improvement.
- Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol 2004; 40:553–562.
- Anhalt GJ, Kim SC, Stanley JR, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990; 323:1729–1735.
- Leger S, Picard D, Ingen-Housz-Oro S, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol 2012; 148:1165–1172.
- Maldonado F, Pittelkow MR, Ryu JH. Constrictive bronchiolitis associated with pareaneoplastic autoimmune multi-organ syndrome. Respirology 2009; 14:129–133.
- Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol 2004; 40:553–562.
- Anhalt GJ, Kim SC, Stanley JR, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990; 323:1729–1735.
- Leger S, Picard D, Ingen-Housz-Oro S, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol 2012; 148:1165–1172.
- Maldonado F, Pittelkow MR, Ryu JH. Constrictive bronchiolitis associated with pareaneoplastic autoimmune multi-organ syndrome. Respirology 2009; 14:129–133.