User login
Head off complications in late preterm infants
• Delay discharge of late preterm infants to a minimum of 48 hours to prevent readmission. B
• Perform transcutaneous or total serum bilirubin testing before discharging late preterm infants. C
• Perform a formal feeding assessment of breastfed infants prior to discharge. C
• Ensure that a follow-up appointment is made for 24 to 48 hours after discharge. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Laura M delivers her third baby at 35 weeks 4 days after an uneventful spontaneous labor. Julia, 5 lb 1 oz, has Apgar scores of 8 and 9. You see them on the mother-baby unit Friday afternoon, 36 hours after the delivery.
Ms. M has successfully breastfed her other 2 children and is planning to breastfeed Julia as well. She says the infant is nursing well today but seemed sleepy yesterday. The nursing notes say that Julia went to the nursery overnight because her mother was tired, and the infant spit up after receiving a formula bottle. Ms. M is asking you to discharge her because tomorrow is her son’s birthday. After considering this and her experience with breastfeeding, you decide to discharge her with follow-up on Monday instead of checking her out to your partner for the weekend.
Ms. M returns to your office on Monday with Julia, whose weight is down 10% and is “glowing yellow.” The baby is not latching well at the breast and is spitting up when Ms. M tries to supplement with formula. After examining Julia and checking lab work (bilirubin, 20 mg/dL), you decide to readmit her for feeding difficulties and hyperbilirubinemia.
Complications are common with late preterm infants, which refers to babies born between 34 weeks 0 days and 36 weeks 6 days of pregnancy. Between 1990 and 2006, there was a dramatic (25%) increase in the rate of late preterm infants in the United States, although more recently this number has leveled off.1
Several factors have been linked to increased late preterm births: maternal obesity, increased maternal age, and the increasing rate of multiple gestation pregnancies that have resulted from the expanded use of reproductive technology.2 Similarly, treatment of severe preeclampsia and premature rupture of membranes often includes delivery after 34 weeks of gestation,3 further contributing to the problem. Finally, higher rates of antenatal screening have contributed to more inductions and cesarean sections at earlier gestational ages. One study even found a correlation between higher malpractice premiums and more frequent late preterm inductions.4
Don’t let their appearance fool you
At first appearance, late preterm infants are similar to term infants in terms of Apgar scores,5 size, and weight.2 However, the care of these infants can be complex. They are often placed in well-infant nurseries under the same protocols as term infants and discharged before an adequate observation period. These infants have both increased short- and long-term morbidity and mortality and use a significant amount of health care resources.5 Morbidity in these infants decreases with each week of gestation from 34 weeks to a nadir at 39 weeks and can be unrelated to maternal and pregnancy complications.6
The following are some important issues to keep in mind when caring for these infants.
Hypothermia and hypoglycemia
Late preterm infants experience increased cold stress because of their limited fat stores, reduced brown fat, an immature epidermal barrier, and increased surface area to body mass ratio.2 Hypothermia increases the metabolic demands on the neonate and can worsen hypoglycemia as well as respiratory distress.7
Ideally, clinicians should dry these infants with warm blankets, place them skin-to-skin with their mother, and cover them with a warm blanket and cap to avoid excess energy expenditure.8 If conditions necessitate, neonates can be placed in a radiant warmer. Infants’ temperature needs to be monitored within the first 30 minutes of life and frequently reassessed during the first 12 hours of life—the “transition period.”8 The infant’s axillary temperature should be maintained between 36.5°C and 37.4°C (97.7-99.3°F); the temperature should remain stable in an open crib for the 12 hours before discharge.2
Decreased glycogen stores, increased glucose utilization, and immature hepatic enzymes help to explain the fact that hypoglycemia is 3 times more common in late preterm infants compared with full-term neonates.7 In all newborns, glucose levels decrease to their nadir between 30 and 90 minutes of life and normally trigger the breakdown of glycogen if the infant does not eat.7 Hypoglycemia can manifest as a change in level of consciousness, apnea, cyanosis, tachypnea, hypothermia, and seizures.9 The evidence is limited and there is controversy as to what level of hypoglycemia and over what duration of time is harmful.9
Most experts agree that breastfeeding should be started in the delivery room, and plasma glucose checked at 60 minutes of life and any time an infant is symptomatic. A level less than 45 mg/dL during the transition period should prompt feeding.9 Glucose levels should be rechecked within an hour of feeding and every 3 hours thereafter. If the level remains low or the infant is not interested in feeding, give a bolus of D10W and consider an infusion.7,10 TABLE 110 highlights one proposed method of glucose management.
TABLE 1
How best to manage glucose in a late preterm newborn10
| Asymptomatic late preterm infants | Glucose level (mg/dL) | Response |
|---|---|---|
| Check plasma glucose at 1 hour of life | <45 | Feed, recheck 1 h |
| Subsequent glucose monitoring | <35 | IV infusion* |
| >35 | Rescreen q3h for 36 h | |
| Symptomatic late preterm infants | ||
| Infusion for goal >55 mg/dL | <45 | Minibolus;† IV infusion* |
| *IV infusion=D10W at 6-8 mg/kg/min. †Minibolus=200 mg/kg D10W (2 mL/kg). Adapted with permission from: Macmillan Publishers Ltd. Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29:S12-S17. Copyright 2009. | ||
Respiratory distress
At least 30% of late preterm infants will have some evidence of respiratory distress,5 which is defined as the need for oxygen supplementation due to tachypnea, grunting, nasal flaring, retractions, or cyanosis. Those at highest risk are white males born via cesarean section.11 Transient tachypnea of the newborn (TTN) appears to be the cause of almost half the cases of respiratory distress in these young patients.12 As this is a diagnosis by exclusion, consider other common causes: respiratory distress syndrome, neonatal pneumonia, meconium aspiration syndrome, and persistent pulmonary hypertension.12 The need for ventilator support is a significant concern in this group and increases exponentially with decreasing gestational age.6
Hyperbilirubinemia
More than half of all late preterm infants will present with clinically significant jaundice.5 Late preterm infants have an increased bilirubin load, decreased uptake of bilirubin, and delayed conjugation. They are less able to bind bilirubin to albumin, which increases their predisposition to bilirubin-induced neurological dysfunction and kernicterus.13 They also have more difficulty with breastfeeding, which exacerbates their hyperbilirubinemia.14
Infants born at 36 weeks have an 8-fold increased risk of developing severe hyperbilirubinemia (total serum bilirubin >20 mg/dL) than those born at 41 weeks,13 which explains why they are disproportionately represented in the US Pilot Kernicterus Registry.14 These infants can develop neurological sequelae at lower levels of bilirubin than their term counterparts and have less chance of complete recovery once intensive therapy has been implemented.14
The 2 main risk factors for severe hyperbilirubinemia have to do with discharge time and breastfeeding. Discharge at less than 48 hours is a risk factor for both term and late preterm infants.14,15 This is likely due to the fact that bilirubin peaks in late preterm infants between Days 5 and 7, and in term infants between Days 3 and 5.16 Difficulties with breastfeeding, the second risk factor,17 can be due to a combination of delayed maternal lactogenesis and ineffective milk removal on the part of the infant.18 When infants ingest smaller volumes of milk, bowel movements are infrequent and the bilirubin in the gut gets reabsorbed instead of excreted.
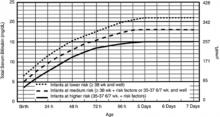
American Academy of Pediatrics (AAP) guidelines suggest measuring total serum bilirubin or transcutaneous bilirubin and plotting the value on an hour-specific, gestational-age-specific, risk-specific nomogram (FIGURE).19 Alternately, BiliTool (http://www.bilitool.org) can be used to individualize an infant’s risk.
FIGURE
Bilirubin levels and risk of significant hyperbilirubinemia19
Bilirubin levels should be plotted according to the infant’s hours of life and assessed according to risk factors on the appropriate risk line. If infants are 34 weeks 0 days to 34 weeks 6 days, they should be assessed on the high risk line.
- Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
- Risk factors include isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, or albumin <3.0 g/dL (if measured).
- For well infants 35-37 6/7 weeks, you can adjust the total serum bilirubin (TSB) levels for intervention around the medium risk line. It is an option to intervene at lower TSB levels for infants closer to 35 weeks and at higher TSB levels for those closer to 37 6/7 weeks.
- It is an option to provide conventional phototherapy in the hospital or at home at TSB levels of 2-3 mg/dL (35-50 mmol/L) below those shown, but home phototherapy should not be used in any infant with risk factors.
Reprinted with permission from: American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. Copyright © 2004 by the American Academy of Pediatrics.
Feeding difficulties
The advantages of breast milk feeding for these babies are great. Unfortunately, it is breastfed infants who are more likely to have feeding difficulties and are at higher risk for readmission.17 Early feeding skills are complex and challenging. Because of their immaturity, late preterm infants have less effective suck and swallow coordination. They can be sleepier and have less stamina.10 Infants require coordinated oral motor movements, breathing, and swallowing to avoid desaturation and aspiration.20 It is also important to note that almost every reported case of kernicterus in the past 20 years has been in breastfed infants whose feeding was not well established.13 First-born infants are especially at risk, as it takes longer to establish an adequate supply of milk.
For late preterm infants, it is imperative to establish successful breastfeeding to avoid dehydration and jaundice. Lactation consultation, education, and close follow-up are essential to successful breastfeeding in this group.18 The AAP Committee on Fetus and Newborn recommends a formal breastfeeding evaluation by trained caregivers at least twice daily prior to discharge.2
Brain injury
Late gestation is a critical period of brain growth. The 34-week-old brain weighs 65% of the term brain and increases linearly with each week. Fifty percent of the increase in cortical volume happens after 34 weeks.21 The risk of intraventricular hemorrhage and periventricular leukomalacia, while common in earlier preemies, is rare in infants born after 34 weeks. However, there is evidence to suggest other complications. Late preterm infants are more likely to be diagnosed with developmental delay and require special resources in preschool and less likely to be ready for school.22
Sepsis
While late preterm neonates do have a small but significant increase in culture-proven sepsis and pneumonia compared with term babies,6 the work-up for possible sepsis is 3 times more likely. When these infants have poor feeding, mild respiratory distress, or TTN, physicians become concerned about sepsis and initiate a work-up.5 Currently there are no management guidelines for sepsis evaluation in this subset of preterm infants.23
Readmission is a distinct possibility
One study of healthy late preterm infants showed a readmission rate of 4.8%. The most common reasons for readmission were jaundice and infection.17 Risk factors for readmission were breastfeeding, primiparity, labor and delivery complications, public payer source at delivery, and a mother of Asian/Pacific Islander ethnicity.17 Another study showed that discharge at less than 48 hours significantly increased the likelihood of readmission, even more so if the infant was breastfeeding.15
Several recent studies have highlighted a relationship between decreasing gestational age and a wide range of long-term adverse outcomes. In the early years of childhood, there is an increased risk for developmental delay and decreased kindergarten readiness.22 There is also a significant risk for disability, including cerebral palsy, mental retardation, and behavioral disorders.
Late preterm infants are at greater risk for several complications and the mortality rate is high in this group, when compared with term infants. By initial appearance and even weight, they rival their term counterparts. However, while they may look much like term babies and not weigh much less, they need more intense monitoring and should meet stringent discharge criteria (TABLE 22).
TABLE 2
Minimum discharge criteria for late preterm infants2
All criteria should be met prior to discharge.
|
| Adapted with permission from: Engle WA, Tomashek KM, Wallman C, Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401. Copyright © 2007 by the American Academy of Pediatrics. |
Clearly, more studies need to be done to address the unique needs and specific treatment guidelines for these infants. In fact, hospitals may need to consider introducing neonatal observation nurseries with protocols specifically tailored for late preterm infants.
CASE Ms. M and Julia likely would have been better served with an extra day in the hospital to address feeding issues and monitor for hyperbilirubinemia. Julia received phototherapy, IV fluids, and intensive lactation support, which included pumped breast milk given through a supplemental nursing system.
Over the course of 48 hours, her bilirubin decreased to 14 mg/dL and she was able to feed for longer periods of time without tiring. While both Ms. M and Julia’s initial outcomes were good, an extra day of feeding support may have prevented a readmission, thousands of dollars in care, and unnecessary stress on both mother and baby.
CORRESPONDENCE
Kimberly Stuckey-Schrock, MD, IU Health Goshen, Lincoln Avenue Family Medicine, 400 W Lincoln Avenue, Goshen, IN 46526; [email protected]
1. Martin JA, Hamilton BE, Sutton PD, et al. Birth: final data for 2008. Natl Vital Stat Rep. 2010;59:1, 3-71.
2. Engle WA, Tomashek KM, Wallman C. Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401.
3. Gabbe SG, Niebyl JR, Simpson JL. eds. Obstetrics Normal and Problem Pregnancies. Philadelphia, Pa: Churchill Livingstone; 2007.
4. Murthy K, Grobman WA, Lee TA, et al. Obstetricians’ rising liability insurance premiums and inductions at late preterm gestations. Med Care. 2009;47:425-430.
5. Wang ML, Dorer DJ, Fleming MP, et al. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372-376.
6. Melamed N, Klinger G, Tenenbaum-Gavish K, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114:253-260.
7. Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853-870.
8. Laptook A, Jackson G. Cold stress and hypoglycemia in the late preterm (“near-term”) infant: impact on nursery of admission. Semin Perinatol. 2006;30:24-27.
9. Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141-1145.
10. Adamkin D. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl):S12-S17.
11. Clark R. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251-257.
12. Kalyoncu O, Aygun C, Cetinoglu E, et al. Neonatal morbidity and mortality of late-preterm babies. J Matern Fetal Neonatal Med. 2010;23:607-612.
13. Watchko J. Hyperbilirubinemia and bilirubin toxicity in the late preterm infant. Clin Perinatol. 2006;33:839-852.
14. Bhutani V. Kernicterus in late preterm infants cared for as term healthy infants. Semin Perinatol. 2006;30:89-97.
15. Tomashek KM, Shapiro-Mendoza CK, Weiss J, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol. 2006;30:61-68.
16. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
17. Shapiro-Mendoza CK, Tomashek K, Kotelchuck M, et al. Risk factors for neonatal morbidity and mortality among “healthy” late preterm newborns. Semin Perinatol. 2006;30:54-60.
18. Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: evidence and management strategies to protect breastfeeding. J Midwifery Women’s Health. 2007;52:579-587.
19. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
20. Thoyre S, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw. 2005;24:7-16.
21. Adams-Chapman I. Neurodevelopmental outcomes of the late preterm infant. Clin Perinatol. 2006;33:947-964.
22. Morse SB, Zheng H, Tang Y, et al. Early school age outcomes of late preterm infants. Pediatrics. 2009;123:622-629.
23. Cohen-Wolkowiez M, Moran C, Benjamin D, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052-1056.
• Delay discharge of late preterm infants to a minimum of 48 hours to prevent readmission. B
• Perform transcutaneous or total serum bilirubin testing before discharging late preterm infants. C
• Perform a formal feeding assessment of breastfed infants prior to discharge. C
• Ensure that a follow-up appointment is made for 24 to 48 hours after discharge. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Laura M delivers her third baby at 35 weeks 4 days after an uneventful spontaneous labor. Julia, 5 lb 1 oz, has Apgar scores of 8 and 9. You see them on the mother-baby unit Friday afternoon, 36 hours after the delivery.
Ms. M has successfully breastfed her other 2 children and is planning to breastfeed Julia as well. She says the infant is nursing well today but seemed sleepy yesterday. The nursing notes say that Julia went to the nursery overnight because her mother was tired, and the infant spit up after receiving a formula bottle. Ms. M is asking you to discharge her because tomorrow is her son’s birthday. After considering this and her experience with breastfeeding, you decide to discharge her with follow-up on Monday instead of checking her out to your partner for the weekend.
Ms. M returns to your office on Monday with Julia, whose weight is down 10% and is “glowing yellow.” The baby is not latching well at the breast and is spitting up when Ms. M tries to supplement with formula. After examining Julia and checking lab work (bilirubin, 20 mg/dL), you decide to readmit her for feeding difficulties and hyperbilirubinemia.
Complications are common with late preterm infants, which refers to babies born between 34 weeks 0 days and 36 weeks 6 days of pregnancy. Between 1990 and 2006, there was a dramatic (25%) increase in the rate of late preterm infants in the United States, although more recently this number has leveled off.1
Several factors have been linked to increased late preterm births: maternal obesity, increased maternal age, and the increasing rate of multiple gestation pregnancies that have resulted from the expanded use of reproductive technology.2 Similarly, treatment of severe preeclampsia and premature rupture of membranes often includes delivery after 34 weeks of gestation,3 further contributing to the problem. Finally, higher rates of antenatal screening have contributed to more inductions and cesarean sections at earlier gestational ages. One study even found a correlation between higher malpractice premiums and more frequent late preterm inductions.4
Don’t let their appearance fool you
At first appearance, late preterm infants are similar to term infants in terms of Apgar scores,5 size, and weight.2 However, the care of these infants can be complex. They are often placed in well-infant nurseries under the same protocols as term infants and discharged before an adequate observation period. These infants have both increased short- and long-term morbidity and mortality and use a significant amount of health care resources.5 Morbidity in these infants decreases with each week of gestation from 34 weeks to a nadir at 39 weeks and can be unrelated to maternal and pregnancy complications.6
The following are some important issues to keep in mind when caring for these infants.
Hypothermia and hypoglycemia
Late preterm infants experience increased cold stress because of their limited fat stores, reduced brown fat, an immature epidermal barrier, and increased surface area to body mass ratio.2 Hypothermia increases the metabolic demands on the neonate and can worsen hypoglycemia as well as respiratory distress.7
Ideally, clinicians should dry these infants with warm blankets, place them skin-to-skin with their mother, and cover them with a warm blanket and cap to avoid excess energy expenditure.8 If conditions necessitate, neonates can be placed in a radiant warmer. Infants’ temperature needs to be monitored within the first 30 minutes of life and frequently reassessed during the first 12 hours of life—the “transition period.”8 The infant’s axillary temperature should be maintained between 36.5°C and 37.4°C (97.7-99.3°F); the temperature should remain stable in an open crib for the 12 hours before discharge.2
Decreased glycogen stores, increased glucose utilization, and immature hepatic enzymes help to explain the fact that hypoglycemia is 3 times more common in late preterm infants compared with full-term neonates.7 In all newborns, glucose levels decrease to their nadir between 30 and 90 minutes of life and normally trigger the breakdown of glycogen if the infant does not eat.7 Hypoglycemia can manifest as a change in level of consciousness, apnea, cyanosis, tachypnea, hypothermia, and seizures.9 The evidence is limited and there is controversy as to what level of hypoglycemia and over what duration of time is harmful.9
Most experts agree that breastfeeding should be started in the delivery room, and plasma glucose checked at 60 minutes of life and any time an infant is symptomatic. A level less than 45 mg/dL during the transition period should prompt feeding.9 Glucose levels should be rechecked within an hour of feeding and every 3 hours thereafter. If the level remains low or the infant is not interested in feeding, give a bolus of D10W and consider an infusion.7,10 TABLE 110 highlights one proposed method of glucose management.
TABLE 1
How best to manage glucose in a late preterm newborn10
| Asymptomatic late preterm infants | Glucose level (mg/dL) | Response |
|---|---|---|
| Check plasma glucose at 1 hour of life | <45 | Feed, recheck 1 h |
| Subsequent glucose monitoring | <35 | IV infusion* |
| >35 | Rescreen q3h for 36 h | |
| Symptomatic late preterm infants | ||
| Infusion for goal >55 mg/dL | <45 | Minibolus;† IV infusion* |
| *IV infusion=D10W at 6-8 mg/kg/min. †Minibolus=200 mg/kg D10W (2 mL/kg). Adapted with permission from: Macmillan Publishers Ltd. Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29:S12-S17. Copyright 2009. | ||
Respiratory distress
At least 30% of late preterm infants will have some evidence of respiratory distress,5 which is defined as the need for oxygen supplementation due to tachypnea, grunting, nasal flaring, retractions, or cyanosis. Those at highest risk are white males born via cesarean section.11 Transient tachypnea of the newborn (TTN) appears to be the cause of almost half the cases of respiratory distress in these young patients.12 As this is a diagnosis by exclusion, consider other common causes: respiratory distress syndrome, neonatal pneumonia, meconium aspiration syndrome, and persistent pulmonary hypertension.12 The need for ventilator support is a significant concern in this group and increases exponentially with decreasing gestational age.6
Hyperbilirubinemia
More than half of all late preterm infants will present with clinically significant jaundice.5 Late preterm infants have an increased bilirubin load, decreased uptake of bilirubin, and delayed conjugation. They are less able to bind bilirubin to albumin, which increases their predisposition to bilirubin-induced neurological dysfunction and kernicterus.13 They also have more difficulty with breastfeeding, which exacerbates their hyperbilirubinemia.14
Infants born at 36 weeks have an 8-fold increased risk of developing severe hyperbilirubinemia (total serum bilirubin >20 mg/dL) than those born at 41 weeks,13 which explains why they are disproportionately represented in the US Pilot Kernicterus Registry.14 These infants can develop neurological sequelae at lower levels of bilirubin than their term counterparts and have less chance of complete recovery once intensive therapy has been implemented.14
The 2 main risk factors for severe hyperbilirubinemia have to do with discharge time and breastfeeding. Discharge at less than 48 hours is a risk factor for both term and late preterm infants.14,15 This is likely due to the fact that bilirubin peaks in late preterm infants between Days 5 and 7, and in term infants between Days 3 and 5.16 Difficulties with breastfeeding, the second risk factor,17 can be due to a combination of delayed maternal lactogenesis and ineffective milk removal on the part of the infant.18 When infants ingest smaller volumes of milk, bowel movements are infrequent and the bilirubin in the gut gets reabsorbed instead of excreted.
American Academy of Pediatrics (AAP) guidelines suggest measuring total serum bilirubin or transcutaneous bilirubin and plotting the value on an hour-specific, gestational-age-specific, risk-specific nomogram (FIGURE).19 Alternately, BiliTool (http://www.bilitool.org) can be used to individualize an infant’s risk.
FIGURE
Bilirubin levels and risk of significant hyperbilirubinemia19
Bilirubin levels should be plotted according to the infant’s hours of life and assessed according to risk factors on the appropriate risk line. If infants are 34 weeks 0 days to 34 weeks 6 days, they should be assessed on the high risk line.
- Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
- Risk factors include isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, or albumin <3.0 g/dL (if measured).
- For well infants 35-37 6/7 weeks, you can adjust the total serum bilirubin (TSB) levels for intervention around the medium risk line. It is an option to intervene at lower TSB levels for infants closer to 35 weeks and at higher TSB levels for those closer to 37 6/7 weeks.
- It is an option to provide conventional phototherapy in the hospital or at home at TSB levels of 2-3 mg/dL (35-50 mmol/L) below those shown, but home phototherapy should not be used in any infant with risk factors.
Reprinted with permission from: American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. Copyright © 2004 by the American Academy of Pediatrics.
Feeding difficulties
The advantages of breast milk feeding for these babies are great. Unfortunately, it is breastfed infants who are more likely to have feeding difficulties and are at higher risk for readmission.17 Early feeding skills are complex and challenging. Because of their immaturity, late preterm infants have less effective suck and swallow coordination. They can be sleepier and have less stamina.10 Infants require coordinated oral motor movements, breathing, and swallowing to avoid desaturation and aspiration.20 It is also important to note that almost every reported case of kernicterus in the past 20 years has been in breastfed infants whose feeding was not well established.13 First-born infants are especially at risk, as it takes longer to establish an adequate supply of milk.
For late preterm infants, it is imperative to establish successful breastfeeding to avoid dehydration and jaundice. Lactation consultation, education, and close follow-up are essential to successful breastfeeding in this group.18 The AAP Committee on Fetus and Newborn recommends a formal breastfeeding evaluation by trained caregivers at least twice daily prior to discharge.2
Brain injury
Late gestation is a critical period of brain growth. The 34-week-old brain weighs 65% of the term brain and increases linearly with each week. Fifty percent of the increase in cortical volume happens after 34 weeks.21 The risk of intraventricular hemorrhage and periventricular leukomalacia, while common in earlier preemies, is rare in infants born after 34 weeks. However, there is evidence to suggest other complications. Late preterm infants are more likely to be diagnosed with developmental delay and require special resources in preschool and less likely to be ready for school.22
Sepsis
While late preterm neonates do have a small but significant increase in culture-proven sepsis and pneumonia compared with term babies,6 the work-up for possible sepsis is 3 times more likely. When these infants have poor feeding, mild respiratory distress, or TTN, physicians become concerned about sepsis and initiate a work-up.5 Currently there are no management guidelines for sepsis evaluation in this subset of preterm infants.23
Readmission is a distinct possibility
One study of healthy late preterm infants showed a readmission rate of 4.8%. The most common reasons for readmission were jaundice and infection.17 Risk factors for readmission were breastfeeding, primiparity, labor and delivery complications, public payer source at delivery, and a mother of Asian/Pacific Islander ethnicity.17 Another study showed that discharge at less than 48 hours significantly increased the likelihood of readmission, even more so if the infant was breastfeeding.15
Several recent studies have highlighted a relationship between decreasing gestational age and a wide range of long-term adverse outcomes. In the early years of childhood, there is an increased risk for developmental delay and decreased kindergarten readiness.22 There is also a significant risk for disability, including cerebral palsy, mental retardation, and behavioral disorders.
Late preterm infants are at greater risk for several complications and the mortality rate is high in this group, when compared with term infants. By initial appearance and even weight, they rival their term counterparts. However, while they may look much like term babies and not weigh much less, they need more intense monitoring and should meet stringent discharge criteria (TABLE 22).
TABLE 2
Minimum discharge criteria for late preterm infants2
All criteria should be met prior to discharge.
|
| Adapted with permission from: Engle WA, Tomashek KM, Wallman C, Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401. Copyright © 2007 by the American Academy of Pediatrics. |
Clearly, more studies need to be done to address the unique needs and specific treatment guidelines for these infants. In fact, hospitals may need to consider introducing neonatal observation nurseries with protocols specifically tailored for late preterm infants.
CASE Ms. M and Julia likely would have been better served with an extra day in the hospital to address feeding issues and monitor for hyperbilirubinemia. Julia received phototherapy, IV fluids, and intensive lactation support, which included pumped breast milk given through a supplemental nursing system.
Over the course of 48 hours, her bilirubin decreased to 14 mg/dL and she was able to feed for longer periods of time without tiring. While both Ms. M and Julia’s initial outcomes were good, an extra day of feeding support may have prevented a readmission, thousands of dollars in care, and unnecessary stress on both mother and baby.
CORRESPONDENCE
Kimberly Stuckey-Schrock, MD, IU Health Goshen, Lincoln Avenue Family Medicine, 400 W Lincoln Avenue, Goshen, IN 46526; [email protected]
• Delay discharge of late preterm infants to a minimum of 48 hours to prevent readmission. B
• Perform transcutaneous or total serum bilirubin testing before discharging late preterm infants. C
• Perform a formal feeding assessment of breastfed infants prior to discharge. C
• Ensure that a follow-up appointment is made for 24 to 48 hours after discharge. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Laura M delivers her third baby at 35 weeks 4 days after an uneventful spontaneous labor. Julia, 5 lb 1 oz, has Apgar scores of 8 and 9. You see them on the mother-baby unit Friday afternoon, 36 hours after the delivery.
Ms. M has successfully breastfed her other 2 children and is planning to breastfeed Julia as well. She says the infant is nursing well today but seemed sleepy yesterday. The nursing notes say that Julia went to the nursery overnight because her mother was tired, and the infant spit up after receiving a formula bottle. Ms. M is asking you to discharge her because tomorrow is her son’s birthday. After considering this and her experience with breastfeeding, you decide to discharge her with follow-up on Monday instead of checking her out to your partner for the weekend.
Ms. M returns to your office on Monday with Julia, whose weight is down 10% and is “glowing yellow.” The baby is not latching well at the breast and is spitting up when Ms. M tries to supplement with formula. After examining Julia and checking lab work (bilirubin, 20 mg/dL), you decide to readmit her for feeding difficulties and hyperbilirubinemia.
Complications are common with late preterm infants, which refers to babies born between 34 weeks 0 days and 36 weeks 6 days of pregnancy. Between 1990 and 2006, there was a dramatic (25%) increase in the rate of late preterm infants in the United States, although more recently this number has leveled off.1
Several factors have been linked to increased late preterm births: maternal obesity, increased maternal age, and the increasing rate of multiple gestation pregnancies that have resulted from the expanded use of reproductive technology.2 Similarly, treatment of severe preeclampsia and premature rupture of membranes often includes delivery after 34 weeks of gestation,3 further contributing to the problem. Finally, higher rates of antenatal screening have contributed to more inductions and cesarean sections at earlier gestational ages. One study even found a correlation between higher malpractice premiums and more frequent late preterm inductions.4
Don’t let their appearance fool you
At first appearance, late preterm infants are similar to term infants in terms of Apgar scores,5 size, and weight.2 However, the care of these infants can be complex. They are often placed in well-infant nurseries under the same protocols as term infants and discharged before an adequate observation period. These infants have both increased short- and long-term morbidity and mortality and use a significant amount of health care resources.5 Morbidity in these infants decreases with each week of gestation from 34 weeks to a nadir at 39 weeks and can be unrelated to maternal and pregnancy complications.6
The following are some important issues to keep in mind when caring for these infants.
Hypothermia and hypoglycemia
Late preterm infants experience increased cold stress because of their limited fat stores, reduced brown fat, an immature epidermal barrier, and increased surface area to body mass ratio.2 Hypothermia increases the metabolic demands on the neonate and can worsen hypoglycemia as well as respiratory distress.7
Ideally, clinicians should dry these infants with warm blankets, place them skin-to-skin with their mother, and cover them with a warm blanket and cap to avoid excess energy expenditure.8 If conditions necessitate, neonates can be placed in a radiant warmer. Infants’ temperature needs to be monitored within the first 30 minutes of life and frequently reassessed during the first 12 hours of life—the “transition period.”8 The infant’s axillary temperature should be maintained between 36.5°C and 37.4°C (97.7-99.3°F); the temperature should remain stable in an open crib for the 12 hours before discharge.2
Decreased glycogen stores, increased glucose utilization, and immature hepatic enzymes help to explain the fact that hypoglycemia is 3 times more common in late preterm infants compared with full-term neonates.7 In all newborns, glucose levels decrease to their nadir between 30 and 90 minutes of life and normally trigger the breakdown of glycogen if the infant does not eat.7 Hypoglycemia can manifest as a change in level of consciousness, apnea, cyanosis, tachypnea, hypothermia, and seizures.9 The evidence is limited and there is controversy as to what level of hypoglycemia and over what duration of time is harmful.9
Most experts agree that breastfeeding should be started in the delivery room, and plasma glucose checked at 60 minutes of life and any time an infant is symptomatic. A level less than 45 mg/dL during the transition period should prompt feeding.9 Glucose levels should be rechecked within an hour of feeding and every 3 hours thereafter. If the level remains low or the infant is not interested in feeding, give a bolus of D10W and consider an infusion.7,10 TABLE 110 highlights one proposed method of glucose management.
TABLE 1
How best to manage glucose in a late preterm newborn10
| Asymptomatic late preterm infants | Glucose level (mg/dL) | Response |
|---|---|---|
| Check plasma glucose at 1 hour of life | <45 | Feed, recheck 1 h |
| Subsequent glucose monitoring | <35 | IV infusion* |
| >35 | Rescreen q3h for 36 h | |
| Symptomatic late preterm infants | ||
| Infusion for goal >55 mg/dL | <45 | Minibolus;† IV infusion* |
| *IV infusion=D10W at 6-8 mg/kg/min. †Minibolus=200 mg/kg D10W (2 mL/kg). Adapted with permission from: Macmillan Publishers Ltd. Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29:S12-S17. Copyright 2009. | ||
Respiratory distress
At least 30% of late preterm infants will have some evidence of respiratory distress,5 which is defined as the need for oxygen supplementation due to tachypnea, grunting, nasal flaring, retractions, or cyanosis. Those at highest risk are white males born via cesarean section.11 Transient tachypnea of the newborn (TTN) appears to be the cause of almost half the cases of respiratory distress in these young patients.12 As this is a diagnosis by exclusion, consider other common causes: respiratory distress syndrome, neonatal pneumonia, meconium aspiration syndrome, and persistent pulmonary hypertension.12 The need for ventilator support is a significant concern in this group and increases exponentially with decreasing gestational age.6
Hyperbilirubinemia
More than half of all late preterm infants will present with clinically significant jaundice.5 Late preterm infants have an increased bilirubin load, decreased uptake of bilirubin, and delayed conjugation. They are less able to bind bilirubin to albumin, which increases their predisposition to bilirubin-induced neurological dysfunction and kernicterus.13 They also have more difficulty with breastfeeding, which exacerbates their hyperbilirubinemia.14
Infants born at 36 weeks have an 8-fold increased risk of developing severe hyperbilirubinemia (total serum bilirubin >20 mg/dL) than those born at 41 weeks,13 which explains why they are disproportionately represented in the US Pilot Kernicterus Registry.14 These infants can develop neurological sequelae at lower levels of bilirubin than their term counterparts and have less chance of complete recovery once intensive therapy has been implemented.14
The 2 main risk factors for severe hyperbilirubinemia have to do with discharge time and breastfeeding. Discharge at less than 48 hours is a risk factor for both term and late preterm infants.14,15 This is likely due to the fact that bilirubin peaks in late preterm infants between Days 5 and 7, and in term infants between Days 3 and 5.16 Difficulties with breastfeeding, the second risk factor,17 can be due to a combination of delayed maternal lactogenesis and ineffective milk removal on the part of the infant.18 When infants ingest smaller volumes of milk, bowel movements are infrequent and the bilirubin in the gut gets reabsorbed instead of excreted.
American Academy of Pediatrics (AAP) guidelines suggest measuring total serum bilirubin or transcutaneous bilirubin and plotting the value on an hour-specific, gestational-age-specific, risk-specific nomogram (FIGURE).19 Alternately, BiliTool (http://www.bilitool.org) can be used to individualize an infant’s risk.
FIGURE
Bilirubin levels and risk of significant hyperbilirubinemia19
Bilirubin levels should be plotted according to the infant’s hours of life and assessed according to risk factors on the appropriate risk line. If infants are 34 weeks 0 days to 34 weeks 6 days, they should be assessed on the high risk line.
- Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
- Risk factors include isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, or albumin <3.0 g/dL (if measured).
- For well infants 35-37 6/7 weeks, you can adjust the total serum bilirubin (TSB) levels for intervention around the medium risk line. It is an option to intervene at lower TSB levels for infants closer to 35 weeks and at higher TSB levels for those closer to 37 6/7 weeks.
- It is an option to provide conventional phototherapy in the hospital or at home at TSB levels of 2-3 mg/dL (35-50 mmol/L) below those shown, but home phototherapy should not be used in any infant with risk factors.
Reprinted with permission from: American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. Copyright © 2004 by the American Academy of Pediatrics.
Feeding difficulties
The advantages of breast milk feeding for these babies are great. Unfortunately, it is breastfed infants who are more likely to have feeding difficulties and are at higher risk for readmission.17 Early feeding skills are complex and challenging. Because of their immaturity, late preterm infants have less effective suck and swallow coordination. They can be sleepier and have less stamina.10 Infants require coordinated oral motor movements, breathing, and swallowing to avoid desaturation and aspiration.20 It is also important to note that almost every reported case of kernicterus in the past 20 years has been in breastfed infants whose feeding was not well established.13 First-born infants are especially at risk, as it takes longer to establish an adequate supply of milk.
For late preterm infants, it is imperative to establish successful breastfeeding to avoid dehydration and jaundice. Lactation consultation, education, and close follow-up are essential to successful breastfeeding in this group.18 The AAP Committee on Fetus and Newborn recommends a formal breastfeeding evaluation by trained caregivers at least twice daily prior to discharge.2
Brain injury
Late gestation is a critical period of brain growth. The 34-week-old brain weighs 65% of the term brain and increases linearly with each week. Fifty percent of the increase in cortical volume happens after 34 weeks.21 The risk of intraventricular hemorrhage and periventricular leukomalacia, while common in earlier preemies, is rare in infants born after 34 weeks. However, there is evidence to suggest other complications. Late preterm infants are more likely to be diagnosed with developmental delay and require special resources in preschool and less likely to be ready for school.22
Sepsis
While late preterm neonates do have a small but significant increase in culture-proven sepsis and pneumonia compared with term babies,6 the work-up for possible sepsis is 3 times more likely. When these infants have poor feeding, mild respiratory distress, or TTN, physicians become concerned about sepsis and initiate a work-up.5 Currently there are no management guidelines for sepsis evaluation in this subset of preterm infants.23
Readmission is a distinct possibility
One study of healthy late preterm infants showed a readmission rate of 4.8%. The most common reasons for readmission were jaundice and infection.17 Risk factors for readmission were breastfeeding, primiparity, labor and delivery complications, public payer source at delivery, and a mother of Asian/Pacific Islander ethnicity.17 Another study showed that discharge at less than 48 hours significantly increased the likelihood of readmission, even more so if the infant was breastfeeding.15
Several recent studies have highlighted a relationship between decreasing gestational age and a wide range of long-term adverse outcomes. In the early years of childhood, there is an increased risk for developmental delay and decreased kindergarten readiness.22 There is also a significant risk for disability, including cerebral palsy, mental retardation, and behavioral disorders.
Late preterm infants are at greater risk for several complications and the mortality rate is high in this group, when compared with term infants. By initial appearance and even weight, they rival their term counterparts. However, while they may look much like term babies and not weigh much less, they need more intense monitoring and should meet stringent discharge criteria (TABLE 22).
TABLE 2
Minimum discharge criteria for late preterm infants2
All criteria should be met prior to discharge.
|
| Adapted with permission from: Engle WA, Tomashek KM, Wallman C, Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401. Copyright © 2007 by the American Academy of Pediatrics. |
Clearly, more studies need to be done to address the unique needs and specific treatment guidelines for these infants. In fact, hospitals may need to consider introducing neonatal observation nurseries with protocols specifically tailored for late preterm infants.
CASE Ms. M and Julia likely would have been better served with an extra day in the hospital to address feeding issues and monitor for hyperbilirubinemia. Julia received phototherapy, IV fluids, and intensive lactation support, which included pumped breast milk given through a supplemental nursing system.
Over the course of 48 hours, her bilirubin decreased to 14 mg/dL and she was able to feed for longer periods of time without tiring. While both Ms. M and Julia’s initial outcomes were good, an extra day of feeding support may have prevented a readmission, thousands of dollars in care, and unnecessary stress on both mother and baby.
CORRESPONDENCE
Kimberly Stuckey-Schrock, MD, IU Health Goshen, Lincoln Avenue Family Medicine, 400 W Lincoln Avenue, Goshen, IN 46526; [email protected]
1. Martin JA, Hamilton BE, Sutton PD, et al. Birth: final data for 2008. Natl Vital Stat Rep. 2010;59:1, 3-71.
2. Engle WA, Tomashek KM, Wallman C. Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401.
3. Gabbe SG, Niebyl JR, Simpson JL. eds. Obstetrics Normal and Problem Pregnancies. Philadelphia, Pa: Churchill Livingstone; 2007.
4. Murthy K, Grobman WA, Lee TA, et al. Obstetricians’ rising liability insurance premiums and inductions at late preterm gestations. Med Care. 2009;47:425-430.
5. Wang ML, Dorer DJ, Fleming MP, et al. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372-376.
6. Melamed N, Klinger G, Tenenbaum-Gavish K, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114:253-260.
7. Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853-870.
8. Laptook A, Jackson G. Cold stress and hypoglycemia in the late preterm (“near-term”) infant: impact on nursery of admission. Semin Perinatol. 2006;30:24-27.
9. Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141-1145.
10. Adamkin D. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl):S12-S17.
11. Clark R. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251-257.
12. Kalyoncu O, Aygun C, Cetinoglu E, et al. Neonatal morbidity and mortality of late-preterm babies. J Matern Fetal Neonatal Med. 2010;23:607-612.
13. Watchko J. Hyperbilirubinemia and bilirubin toxicity in the late preterm infant. Clin Perinatol. 2006;33:839-852.
14. Bhutani V. Kernicterus in late preterm infants cared for as term healthy infants. Semin Perinatol. 2006;30:89-97.
15. Tomashek KM, Shapiro-Mendoza CK, Weiss J, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol. 2006;30:61-68.
16. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
17. Shapiro-Mendoza CK, Tomashek K, Kotelchuck M, et al. Risk factors for neonatal morbidity and mortality among “healthy” late preterm newborns. Semin Perinatol. 2006;30:54-60.
18. Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: evidence and management strategies to protect breastfeeding. J Midwifery Women’s Health. 2007;52:579-587.
19. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
20. Thoyre S, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw. 2005;24:7-16.
21. Adams-Chapman I. Neurodevelopmental outcomes of the late preterm infant. Clin Perinatol. 2006;33:947-964.
22. Morse SB, Zheng H, Tang Y, et al. Early school age outcomes of late preterm infants. Pediatrics. 2009;123:622-629.
23. Cohen-Wolkowiez M, Moran C, Benjamin D, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052-1056.
1. Martin JA, Hamilton BE, Sutton PD, et al. Birth: final data for 2008. Natl Vital Stat Rep. 2010;59:1, 3-71.
2. Engle WA, Tomashek KM, Wallman C. Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401.
3. Gabbe SG, Niebyl JR, Simpson JL. eds. Obstetrics Normal and Problem Pregnancies. Philadelphia, Pa: Churchill Livingstone; 2007.
4. Murthy K, Grobman WA, Lee TA, et al. Obstetricians’ rising liability insurance premiums and inductions at late preterm gestations. Med Care. 2009;47:425-430.
5. Wang ML, Dorer DJ, Fleming MP, et al. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372-376.
6. Melamed N, Klinger G, Tenenbaum-Gavish K, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114:253-260.
7. Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853-870.
8. Laptook A, Jackson G. Cold stress and hypoglycemia in the late preterm (“near-term”) infant: impact on nursery of admission. Semin Perinatol. 2006;30:24-27.
9. Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141-1145.
10. Adamkin D. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl):S12-S17.
11. Clark R. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251-257.
12. Kalyoncu O, Aygun C, Cetinoglu E, et al. Neonatal morbidity and mortality of late-preterm babies. J Matern Fetal Neonatal Med. 2010;23:607-612.
13. Watchko J. Hyperbilirubinemia and bilirubin toxicity in the late preterm infant. Clin Perinatol. 2006;33:839-852.
14. Bhutani V. Kernicterus in late preterm infants cared for as term healthy infants. Semin Perinatol. 2006;30:89-97.
15. Tomashek KM, Shapiro-Mendoza CK, Weiss J, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol. 2006;30:61-68.
16. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
17. Shapiro-Mendoza CK, Tomashek K, Kotelchuck M, et al. Risk factors for neonatal morbidity and mortality among “healthy” late preterm newborns. Semin Perinatol. 2006;30:54-60.
18. Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: evidence and management strategies to protect breastfeeding. J Midwifery Women’s Health. 2007;52:579-587.
19. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
20. Thoyre S, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw. 2005;24:7-16.
21. Adams-Chapman I. Neurodevelopmental outcomes of the late preterm infant. Clin Perinatol. 2006;33:947-964.
22. Morse SB, Zheng H, Tang Y, et al. Early school age outcomes of late preterm infants. Pediatrics. 2009;123:622-629.
23. Cohen-Wolkowiez M, Moran C, Benjamin D, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052-1056.
Inhalation therapy: Help patients avoid these mistakes
• Stress the importance of exhaling gently for a few seconds before inhaling (deeply and slowly for a metered dose inhaler, and deeply and rapidly for most dry powder inhalers). C
• Observe the inhaler technique of every patient receiving inhalation therapy on more than one occasion. C
• Don’t rely on self-reports regarding inhaler technique; despite claims of proficiency, most patients make at least one mistake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For patients with asthma or chronic obstructive pulmonary disease (COPD), inhalation therapy is the foundation of treatment. Yet all too often, patients don’t get the full value of their inhaled medications because they use their inhaler incorrectly. When technique is markedly flawed, suboptimal outcomes typically result.
Given the number of Americans with asthma (at least 22 million)1 and COPD (more than 13 million adults),2 faulty inhaler technique is a major public health problem. In fact, the number of people suffering from COPD may be even larger: Close to 24 million US adults are believed to have impaired lung function.3,4 For patients with asthma or COPD—many of whom are treated by family physicians—comprehensive education with a focus on correct use of an inhaler is essential.
In this review, we present evidence of frequent inhaler errors (from clinical studies) and highlight some of the more common mistakes (based on our clinical experience [TABLE]5). Finally, we offer ‘‘time-efficient’’ solutions to inhaler problems—steps that physicians in busy primary care practices can take to ensure that patients with asthma or COPD get the maximum benefit from inhalation therapy.
TABLE
Caution patients about these device-specific mistakes*
| Metered dose inhaler |
|---|
|
| Metered dose inhaler plus spacer/VHC |
|
| Dry powder inhaler |
|
| *These are examples based on the experience of the authors; other errors are possible. †Timing is not as crucial as it is for an MDI without a spacer, but the drug is still lost if inhalation is delayed. ‡Correct use varies by type of product (see product literature for specifics). DPI, dry powder inhaler; MDI, metered dose inhaler; VHC, valved holding chamber. Source: Adapted with permission from Self TH, et al. Consultant. 2003.5 |
Inhaler error is well documented
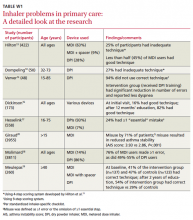
Since 1965, when it was first reported that many patients used metered dose inhalers (MDIs) incorrectly,6 evidence has accumulated supporting the magnitude of the problem.7-12 (Studies conducted in family practice settings are described in “Researchers look at inhaler problems in primary care” and in TABLE W1.13-20)
A number of studies of various sizes (from 41 to 3955 patients) have assessed inhaler technique in patients being treated by clinicians in primary care. The researchers used a variety of scoring methods, as well. Among them were a simple 4-step (0-4) rating system, a 9-step system, a standardized inhaler-specific checklist, and a system that tracked the number of omissions patients made.13-20 All found significant problems with inhaler technique. (You’ll find a detailed look at the studies in TABLE W1 at jfponline.com.)
In one study of 422 patients,13 including young children, adolescents, and adults, participants received one point for correctly performing each of the following steps:
- Adequate preparation (shaking well for those using a metered dose inhaler [MDI]; loading correctly for patients using a dry powder inhaler [DPI])
- Adequate expiration, correct head position
- Adequate inspiratory technique
- Holding breath afterwards.
The researchers found that 25% of the patients had inadequate technique (≤2 on a 0-4 point scale). In this study, as in others that included patients using various types of devices, use of an MDI was associated with a higher rate of incorrect technique.
Another much-smaller study14 used the same 4-step system to assess the technique of 50 patients, all of whom had the same type of DPI and had received extensive training in the correct use of the device. Despite the training, 27% of the patients received scores of ≤2 (inadequate technique). Sixty-eight percent received a score of 3 (adequate); only 5% received a score of 4 (good).
The 2 largest studies—one including 3955 patients using MDIs20 and the other looking at 3811 patients using various kinds of devices18—found high levels of errors, as well. In the latter study, 76% of patients with MDIs made at least one error vs 49% to 55% of patients using DPIs.18 The results convinced a large majority of the physicians caring for these patients of the need to check inhaler technique more frequently. In the study of MDI users alone, 71% of the patients made at least one mistake.20 inhaler misuse was associated with higher asthma instability scores, this study showed.
More recently, a researcher assessed the effects of an integrated primary care model on the management of asthma and/or COPD in middle-aged and elderly patients, in a study of 260 patients in 44 family practices.19 The study included an evaluation of inhaler technique.
Participants were divided into an intervention group—137 patients who received education regarding inhaler use from a nurse—and a usual care group (123 patients). After 2 years, correct inhaler technique among those in the intervention group went from 41% at baseline to 54%. At the same time, the proportion of those in the usual care group with correct technique fell from 47% to 29%.19
Error rates vary widely from one clinical trial to another, depending on study criteria, type of device, and extent of patient education, among other factors. Nonetheless, several studies (spanning 3 decades) found the error rate to be close to, or greater than, 90%.7,10,21
The most recent of these, published in 2009,21 was based on observation of the inhaler technique used by patients with asthma or COPD directly following appointments in an outpatient clinic. The authors found that, although >98% of the study participants claimed to know how to use their inhalers, 94% committed at least one error. In this study and a number of others, user error was more likely in patients using MDIs.13,18,21,22
Adding a spacer (eg, a valved holding chamber such as the AeroChamber) can be helpful, as the spacer affords the patient more time to inhale the medication. But patients who use an MDI with a spacer often make mistakes, too, and patient education is essential.23-26
Breath-activated dry powder inhalers (DPIs)—such as the Flexhaler, HandiHaler, Aerolizer, and Diskus—also reduce the likelihood of error. DPIs eliminate a step that MDI users often struggle with: the need to simultaneously press down on the canister and begin a slow, deep inhalation.
What’s more, DPIs do not have to be shaken before use. Nonetheless, using a DPI still involves a series of actions. For the HandiHaler and Aerolizer, patients must load the dose, and some patients fail to read the directions and swallow the capsule instead of loading it into the device. Patients must remember to exhale away from the device (ie, not into the dry powder) before inhaling, then hold their breath for approximately 10 seconds. There is potential for error at each step.
Stress the need to exhale before using the inhaler
Forgetting to exhale before inhaling is a common, and significant, mistake regardless of the type of device. It is paramount to stress the need to exhale gently for a few seconds before inhaling (slowly and deeply for patients using an MDI, rapidly and deeply with most DPIs). For MDI users, poor timing, described earlier, is another common and serious mistake. Patients using an MDI with a valved holding chamber sometimes inhale for too long before pressing down on the inhaler, then are unable to continue inhaling although the aerosol is still in the chamber. A common error made by patients using multidose DPIs is simply to forget to load the dose.
Physicians need to brush up on their skills, too
It’s not just patients who lack proficiency in inhaler technique. Numerous studies have demonstrated poor skill among physicians and other health care professionals.27-34 Evidence also shows that targeted education results in substantial improvement.32,35
In one study undertaken to evaluate family medicine residents’ proficiency in using asthma inhalers, participants (an intervention group at one clinic and a control group at another) all were given a pretest. The intervention group then received educational materials and a tutorial, as well as the opportunity for hands-on practice, after which both groups were given a post-test. The residents who received the training had a 170% jump, on average, in proficiency score, vs a 55% increase for the control group (P<.001).35
Inhaled Medication Instructional Videos
Courtesy of: National Jewish Health
Go to http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos
Another study—this one involving first-year interns—looked at level of improvement based on the type of education provided. Initially, only 5% of the interns could use an MDI without error. After a lecture and demonstration, 13% had an error-free technique. But when each intern participated in an intensive one-on-one session, the error-free rate reached 73%. The researchers’ conclusion: Lectures are relatively ineffective in teaching interns inhaler technique compared with a one-on-one approach.32
The Chicago Breathe Project,36 a new program aimed at improving education in the use of asthma inhalers for physicians and minority patients, provides further evidence of the value of clinician education. After a series of workshops for residents at 5 academic institutions, the physicians’ knowledge of proper use of inhalers rose dramatically—from just 5% preprogram to 91% postprogram (P<.001). Six months after the educational activity, the residents (n=161) were more likely (44% vs 11% preprogram) to assess patients’ inhaler technique.36
Teaching patients when time is tight
National and international guidelines stress the need to teach patients correct use of asthma and COPD inhalers.1,37,38 Providing the requisite education includes observation of each patient’s inhaler technique with proper use demonstrated, as needed.
The problem, of course, is how to provide that level of patient education within the time constraints of a busy family practice. We recommend these time-efficient solutions:
Enlist the help of other clinicians. While it is important that someone in your office be well trained and able to instruct patients in the proper use of inhalers, that individual need not be you. The National Institutes of Health recommends that the “principal clinician” introduce key educational messages, which can be reinforced and expanded on by other members of the health care team.1
After you advise patients that it is crucial for them to be trained in and adhere to proper inhaler technique, another health care professional—often a clinic nurse or pharmacist who has had special training—can provide the hands-on education. Studies have shown that when pharmacists who are competent in asthma management, including inhaler technique, work with physicians to optimize the education and overall management of patients with asthma, better outcomes often result, including a reduction in both emergency department visits and hospitalizations.1,39,40
Use videos to demonstrate correct technique. Videos are an effective teaching tool,9 and many of them are device-specific. National Jewish Health, which is world renowned for its asthma care, has a set of instructional videos posted on You-Tube and accessible from its Web site (http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos). In addition to videos that demonstrate the use of an MDI alone and an MDI plus a valved holding chamber, the site has links to 6 DPI videos, each covering a different device.
Use intermittent observation. After the patient views the appropriate video, you or a member of your staff will still need to observe the patient’s inhaler technique to ensure correct use. Ideally, this should occur at every visit.1,37 When that’s not possible, use intermittent observation, starting with the first 2 or 3 visits after the introduction of inhalation therapy and then switching to periodic observation to ensure that the patient is maintaining good technique.
In determining how often observation is necessary, keep in mind that simply asking patients whether they are having inhaler problems is not sufficient.1 Patients tend to say they have little or no trouble when, in fact, most struggle, at times, with the devices. What’s more, good technique tends to decrease over time, and repetitive education is important.
To motivate patients, try this communication technique
Motivational interviewing, a technique that has been used to help patients battle obesity, quit smoking, and control hypertension,41-43 among other health problems, can help you identify inhaler problems that need to be addressed. It involves the use of open-ended questions (eg, “What worries you most about your asthma?”), affirmations (“You’ve done a great job testing your peak flow level every morning”), reflective listening (“You’re tired of taking medicine every day”), and summary statements (“You know you should take your medicine every day but you’re having trouble remembering it. Is that right?”).
A pilot study44 showed that when this technique was incorporated into an asthma education session, patient motivation increased. The ratio of perceived advantages vs disadvantages of taking asthma medication correctly improved, as well. Another study45 found that when motivational interviewing was used during home visits to inner-city African American adolescents for asthma care, the patients’ motivation, readiness to adhere to treatment, and asthma-related quality of life improved, although self-reported adherence to asthma medication did not. Further studies involving patients with asthma are under way (www.clinicaltrials.gov/ct2/results?term=asthma).
It is important to note that the use of motivational interviewing does not require a lengthy visit. One study found that on average, visits in which primary care physicians used this communication technique lasted less than 10 minutes.46
CORRESPONDENCE Timothy H. Self, PharmD, University of Tennessee Health Science Center, 881 Madison Avenue, Room 235, Memphis, TN 38163; [email protected]
1. National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007.
2. Centers for Disease Control and Prevention. National Center for Health Statistics: National health interview survey raw data, 2008. Analysis performed by American Lung Association Research and Program Services.
3. American Lung Association. COPD—Helping the missing millions. February 24, 2010. Available at: http://www.lungusa.org/about-us/our-impact/top-stories/copd-helping-the-missing.html. Accessed November 9, 2011.
4. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1-16.
5. Self TH, Kilgore KE, Shelton V. MDIs, spacers, and dry powder inhalers: what patients are likely to do wrong. Consultant. 2003;49:702-705.
6. Saunders KB. Misuse of inhaled bronchodilator agents. Br Med J. 1965;1:1037-1038.
7. Epstein SW, Manning CPR, Ashley MJ, et al. Survey of the clinical uses of pressurized aerosol inhalers. Can Med Assoc J. 1979;120:813-816.
8. Shim C, Williams MH. The adequacy of inhalation of aerosol from canister nebulizers. Am J Med. 1980;69:891-894.
9. Self TH, Brooks JB, Lieberman P, et al. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Can Med Assoc J. 1983;128:129-131.
10. Hartert TV, Windom HH, Peeples RS, et al. Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am J Med. 1996;100:386-394.
11. Goodman DE, Israel E, Rosenberg M, et al. The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med. 1994;150:1256-1261.
12. Newman SP, Pavia D, Clarke SW. How should a pressurized beta-adrenergic bronchodilator be inhaled? Eur J Respir Dis. 1981;62:3-21.
13. Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br J Gen Pract. 1990;40:505-506.
14. Dompeling E, Van Grunsven PM, Van Schayck GP, et al. Treatment with inhaled steroids in asthma and chronic bronchitis: long-term compliance and inhaler technique. Fam Pract. 1992;9:161-166.
15. Verver S, Poelman M, Bogels A, et al. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam Pract. 1996;13:35-40.
16. Dickinson J, Hutton S, Atkin A, et al. Reducing asthma morbidity in the community: the effect of a targeted nurse-run asthma clinic in an English general practice. Respir Med. 1997;91:634-640.
17. Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care. 2001;19:255-260.
18. Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249-254.
19. Meulepas MA, Jacobs JE, Smeenk FW, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scand J Prim Health Care. 2007;25:186-192.
20. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246-251.
21. Souza ML, Meneghini AC, Ferraz E, et al. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35:824-831.
22. Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323-328.
23. Rachelefsky GS, Rohr AS, Wo J, et al. Use of a tube spacer to improve the efficacy of a metered dose inhaler in asthmatic children. Am J Dis Child. 1986;140:1191-1193.
24. Demirkan K, Tolley E, Mastin T, et al. Salmeterol administration by metered-dose inhaler alone vs metered-dose inhaler plus valved holding chamber. Chest. 2000;117:1314-1318.
25. Pedersen S, Ostergaard PA. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983;38:191-194.
26. Dolovich MD, Ahrens RS, Hess DR, et al. Device selection an outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335-371.
27. Interiano B, Guntupalli KK. Metered-dose inhalers: do health care providers know what to teach? Arch Intern Med. 1993;153:81-85.
28. Hanania NA, Wittman R, Kesten S, et al. Medical personnel’s knowledge of and ability to use inhaling devices. Metered-dose inhalers, spacing chambers, and breath-actuated dry powder inhalers. Chest. 1994;105:111-116.
29. Amirav I, Goren A, Pawlowski NA. What do pediatricians in training know about the correct use of inhalers and spacer devices? J Allergy Clin Immunol. 1994;94:669-675.
30. Chopra N, Oprescu N, Fask A, et al. Does introduction of new “easy to use” inhalational devices improve medical personnel’s knowledge of their proper use? Ann Allergy Asthma Immunol. 2002;88:395-400.
31. Self TH, Arnold LB, Czosnowski LM, et al. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44:593-598.
32. Lee-Wong M, Mayo PH. Results of a programme to improve house staff use of metered dose inhalers and spacers. Postgrad Med J. 2003;79:221-225.
33. Muchao FP, Pern SL, Rodriques JC, et al. Evaluation of the knowledge of health professionals at a pediatric hospital regarding the use of metered dose inhalers. J Bras Pneumol. 2008;34:4-12.
34. Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, Diskus, and Turbuhaler): changes after a single teaching session. J Asthma. 2009;46:944-950.
35. Kelcher S, Brownoff R. Teaching residents to use asthma devices. Assessing family residents’ skill and a brief intervention. Can Fam Physician. 1994;40:2090-2095.
36. Press VG, Pincayage AT, Pappalardo AA, et al. The Chicago Breathe Project: a regional approach to improving education on asthma inhalers for resident physicians and minority patients. J Natl Med Assoc. 2010;102:548-555.
37. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2010. Available at: www.ginasthma.org. Accessed November 9, 2011.
38. Executive Summary: global strategy on the diagnosis and management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report, 2009. Available at: www.goldcopd.com. Accessed November 9, 2011.
39. Self TH, Chrisman CR, Mason DL, et al. Reducing emergency department visits and hospitalizations in African American and Hispanic patients: a 15-year review. J Asthma. 2005;42:807-812.
40. Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy asthma care program (PACP) improves outcomes for patients in the community. Thorax. 2007;62:496-502.
41. DiLillo V, Nicole J, West DS. Incorporating motivational interviewing into behavioral obesity treatment. Cogn Behav Pract. 2003;10:120-130.
42. Borrelli B, Novak S, Hecht J, et al. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking). Prev Med. 2005;41:815-821.
43. Woollard L, Beilin L, Lord T, et al. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466-468.
44. Schmaling K, Blume A, Afari N. A randomized controlled pilot study of motivational interviewing to change attitudes about adherence to medications for asthma. J Clin Psych Med Settings. 2001;8:167-172.
45. Riekert KA, Borrelli B, Bilderback A, et al. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
46. Butler C, Rollnick S, Cohen D, et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49:611-616.
• Stress the importance of exhaling gently for a few seconds before inhaling (deeply and slowly for a metered dose inhaler, and deeply and rapidly for most dry powder inhalers). C
• Observe the inhaler technique of every patient receiving inhalation therapy on more than one occasion. C
• Don’t rely on self-reports regarding inhaler technique; despite claims of proficiency, most patients make at least one mistake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For patients with asthma or chronic obstructive pulmonary disease (COPD), inhalation therapy is the foundation of treatment. Yet all too often, patients don’t get the full value of their inhaled medications because they use their inhaler incorrectly. When technique is markedly flawed, suboptimal outcomes typically result.
Given the number of Americans with asthma (at least 22 million)1 and COPD (more than 13 million adults),2 faulty inhaler technique is a major public health problem. In fact, the number of people suffering from COPD may be even larger: Close to 24 million US adults are believed to have impaired lung function.3,4 For patients with asthma or COPD—many of whom are treated by family physicians—comprehensive education with a focus on correct use of an inhaler is essential.
In this review, we present evidence of frequent inhaler errors (from clinical studies) and highlight some of the more common mistakes (based on our clinical experience [TABLE]5). Finally, we offer ‘‘time-efficient’’ solutions to inhaler problems—steps that physicians in busy primary care practices can take to ensure that patients with asthma or COPD get the maximum benefit from inhalation therapy.
TABLE
Caution patients about these device-specific mistakes*
| Metered dose inhaler |
|---|
|
| Metered dose inhaler plus spacer/VHC |
|
| Dry powder inhaler |
|
| *These are examples based on the experience of the authors; other errors are possible. †Timing is not as crucial as it is for an MDI without a spacer, but the drug is still lost if inhalation is delayed. ‡Correct use varies by type of product (see product literature for specifics). DPI, dry powder inhaler; MDI, metered dose inhaler; VHC, valved holding chamber. Source: Adapted with permission from Self TH, et al. Consultant. 2003.5 |
Inhaler error is well documented
Since 1965, when it was first reported that many patients used metered dose inhalers (MDIs) incorrectly,6 evidence has accumulated supporting the magnitude of the problem.7-12 (Studies conducted in family practice settings are described in “Researchers look at inhaler problems in primary care” and in TABLE W1.13-20)
A number of studies of various sizes (from 41 to 3955 patients) have assessed inhaler technique in patients being treated by clinicians in primary care. The researchers used a variety of scoring methods, as well. Among them were a simple 4-step (0-4) rating system, a 9-step system, a standardized inhaler-specific checklist, and a system that tracked the number of omissions patients made.13-20 All found significant problems with inhaler technique. (You’ll find a detailed look at the studies in TABLE W1 at jfponline.com.)
In one study of 422 patients,13 including young children, adolescents, and adults, participants received one point for correctly performing each of the following steps:
- Adequate preparation (shaking well for those using a metered dose inhaler [MDI]; loading correctly for patients using a dry powder inhaler [DPI])
- Adequate expiration, correct head position
- Adequate inspiratory technique
- Holding breath afterwards.
The researchers found that 25% of the patients had inadequate technique (≤2 on a 0-4 point scale). In this study, as in others that included patients using various types of devices, use of an MDI was associated with a higher rate of incorrect technique.
Another much-smaller study14 used the same 4-step system to assess the technique of 50 patients, all of whom had the same type of DPI and had received extensive training in the correct use of the device. Despite the training, 27% of the patients received scores of ≤2 (inadequate technique). Sixty-eight percent received a score of 3 (adequate); only 5% received a score of 4 (good).
The 2 largest studies—one including 3955 patients using MDIs20 and the other looking at 3811 patients using various kinds of devices18—found high levels of errors, as well. In the latter study, 76% of patients with MDIs made at least one error vs 49% to 55% of patients using DPIs.18 The results convinced a large majority of the physicians caring for these patients of the need to check inhaler technique more frequently. In the study of MDI users alone, 71% of the patients made at least one mistake.20 inhaler misuse was associated with higher asthma instability scores, this study showed.
More recently, a researcher assessed the effects of an integrated primary care model on the management of asthma and/or COPD in middle-aged and elderly patients, in a study of 260 patients in 44 family practices.19 The study included an evaluation of inhaler technique.
Participants were divided into an intervention group—137 patients who received education regarding inhaler use from a nurse—and a usual care group (123 patients). After 2 years, correct inhaler technique among those in the intervention group went from 41% at baseline to 54%. At the same time, the proportion of those in the usual care group with correct technique fell from 47% to 29%.19
Error rates vary widely from one clinical trial to another, depending on study criteria, type of device, and extent of patient education, among other factors. Nonetheless, several studies (spanning 3 decades) found the error rate to be close to, or greater than, 90%.7,10,21
The most recent of these, published in 2009,21 was based on observation of the inhaler technique used by patients with asthma or COPD directly following appointments in an outpatient clinic. The authors found that, although >98% of the study participants claimed to know how to use their inhalers, 94% committed at least one error. In this study and a number of others, user error was more likely in patients using MDIs.13,18,21,22
Adding a spacer (eg, a valved holding chamber such as the AeroChamber) can be helpful, as the spacer affords the patient more time to inhale the medication. But patients who use an MDI with a spacer often make mistakes, too, and patient education is essential.23-26
Breath-activated dry powder inhalers (DPIs)—such as the Flexhaler, HandiHaler, Aerolizer, and Diskus—also reduce the likelihood of error. DPIs eliminate a step that MDI users often struggle with: the need to simultaneously press down on the canister and begin a slow, deep inhalation.
What’s more, DPIs do not have to be shaken before use. Nonetheless, using a DPI still involves a series of actions. For the HandiHaler and Aerolizer, patients must load the dose, and some patients fail to read the directions and swallow the capsule instead of loading it into the device. Patients must remember to exhale away from the device (ie, not into the dry powder) before inhaling, then hold their breath for approximately 10 seconds. There is potential for error at each step.
Stress the need to exhale before using the inhaler
Forgetting to exhale before inhaling is a common, and significant, mistake regardless of the type of device. It is paramount to stress the need to exhale gently for a few seconds before inhaling (slowly and deeply for patients using an MDI, rapidly and deeply with most DPIs). For MDI users, poor timing, described earlier, is another common and serious mistake. Patients using an MDI with a valved holding chamber sometimes inhale for too long before pressing down on the inhaler, then are unable to continue inhaling although the aerosol is still in the chamber. A common error made by patients using multidose DPIs is simply to forget to load the dose.
Physicians need to brush up on their skills, too
It’s not just patients who lack proficiency in inhaler technique. Numerous studies have demonstrated poor skill among physicians and other health care professionals.27-34 Evidence also shows that targeted education results in substantial improvement.32,35
In one study undertaken to evaluate family medicine residents’ proficiency in using asthma inhalers, participants (an intervention group at one clinic and a control group at another) all were given a pretest. The intervention group then received educational materials and a tutorial, as well as the opportunity for hands-on practice, after which both groups were given a post-test. The residents who received the training had a 170% jump, on average, in proficiency score, vs a 55% increase for the control group (P<.001).35
Inhaled Medication Instructional Videos
Courtesy of: National Jewish Health
Go to http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos
Another study—this one involving first-year interns—looked at level of improvement based on the type of education provided. Initially, only 5% of the interns could use an MDI without error. After a lecture and demonstration, 13% had an error-free technique. But when each intern participated in an intensive one-on-one session, the error-free rate reached 73%. The researchers’ conclusion: Lectures are relatively ineffective in teaching interns inhaler technique compared with a one-on-one approach.32
The Chicago Breathe Project,36 a new program aimed at improving education in the use of asthma inhalers for physicians and minority patients, provides further evidence of the value of clinician education. After a series of workshops for residents at 5 academic institutions, the physicians’ knowledge of proper use of inhalers rose dramatically—from just 5% preprogram to 91% postprogram (P<.001). Six months after the educational activity, the residents (n=161) were more likely (44% vs 11% preprogram) to assess patients’ inhaler technique.36
Teaching patients when time is tight
National and international guidelines stress the need to teach patients correct use of asthma and COPD inhalers.1,37,38 Providing the requisite education includes observation of each patient’s inhaler technique with proper use demonstrated, as needed.
The problem, of course, is how to provide that level of patient education within the time constraints of a busy family practice. We recommend these time-efficient solutions:
Enlist the help of other clinicians. While it is important that someone in your office be well trained and able to instruct patients in the proper use of inhalers, that individual need not be you. The National Institutes of Health recommends that the “principal clinician” introduce key educational messages, which can be reinforced and expanded on by other members of the health care team.1
After you advise patients that it is crucial for them to be trained in and adhere to proper inhaler technique, another health care professional—often a clinic nurse or pharmacist who has had special training—can provide the hands-on education. Studies have shown that when pharmacists who are competent in asthma management, including inhaler technique, work with physicians to optimize the education and overall management of patients with asthma, better outcomes often result, including a reduction in both emergency department visits and hospitalizations.1,39,40
Use videos to demonstrate correct technique. Videos are an effective teaching tool,9 and many of them are device-specific. National Jewish Health, which is world renowned for its asthma care, has a set of instructional videos posted on You-Tube and accessible from its Web site (http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos). In addition to videos that demonstrate the use of an MDI alone and an MDI plus a valved holding chamber, the site has links to 6 DPI videos, each covering a different device.
Use intermittent observation. After the patient views the appropriate video, you or a member of your staff will still need to observe the patient’s inhaler technique to ensure correct use. Ideally, this should occur at every visit.1,37 When that’s not possible, use intermittent observation, starting with the first 2 or 3 visits after the introduction of inhalation therapy and then switching to periodic observation to ensure that the patient is maintaining good technique.
In determining how often observation is necessary, keep in mind that simply asking patients whether they are having inhaler problems is not sufficient.1 Patients tend to say they have little or no trouble when, in fact, most struggle, at times, with the devices. What’s more, good technique tends to decrease over time, and repetitive education is important.
To motivate patients, try this communication technique
Motivational interviewing, a technique that has been used to help patients battle obesity, quit smoking, and control hypertension,41-43 among other health problems, can help you identify inhaler problems that need to be addressed. It involves the use of open-ended questions (eg, “What worries you most about your asthma?”), affirmations (“You’ve done a great job testing your peak flow level every morning”), reflective listening (“You’re tired of taking medicine every day”), and summary statements (“You know you should take your medicine every day but you’re having trouble remembering it. Is that right?”).
A pilot study44 showed that when this technique was incorporated into an asthma education session, patient motivation increased. The ratio of perceived advantages vs disadvantages of taking asthma medication correctly improved, as well. Another study45 found that when motivational interviewing was used during home visits to inner-city African American adolescents for asthma care, the patients’ motivation, readiness to adhere to treatment, and asthma-related quality of life improved, although self-reported adherence to asthma medication did not. Further studies involving patients with asthma are under way (www.clinicaltrials.gov/ct2/results?term=asthma).
It is important to note that the use of motivational interviewing does not require a lengthy visit. One study found that on average, visits in which primary care physicians used this communication technique lasted less than 10 minutes.46
CORRESPONDENCE Timothy H. Self, PharmD, University of Tennessee Health Science Center, 881 Madison Avenue, Room 235, Memphis, TN 38163; [email protected]
• Stress the importance of exhaling gently for a few seconds before inhaling (deeply and slowly for a metered dose inhaler, and deeply and rapidly for most dry powder inhalers). C
• Observe the inhaler technique of every patient receiving inhalation therapy on more than one occasion. C
• Don’t rely on self-reports regarding inhaler technique; despite claims of proficiency, most patients make at least one mistake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For patients with asthma or chronic obstructive pulmonary disease (COPD), inhalation therapy is the foundation of treatment. Yet all too often, patients don’t get the full value of their inhaled medications because they use their inhaler incorrectly. When technique is markedly flawed, suboptimal outcomes typically result.
Given the number of Americans with asthma (at least 22 million)1 and COPD (more than 13 million adults),2 faulty inhaler technique is a major public health problem. In fact, the number of people suffering from COPD may be even larger: Close to 24 million US adults are believed to have impaired lung function.3,4 For patients with asthma or COPD—many of whom are treated by family physicians—comprehensive education with a focus on correct use of an inhaler is essential.
In this review, we present evidence of frequent inhaler errors (from clinical studies) and highlight some of the more common mistakes (based on our clinical experience [TABLE]5). Finally, we offer ‘‘time-efficient’’ solutions to inhaler problems—steps that physicians in busy primary care practices can take to ensure that patients with asthma or COPD get the maximum benefit from inhalation therapy.
TABLE
Caution patients about these device-specific mistakes*
| Metered dose inhaler |
|---|
|
| Metered dose inhaler plus spacer/VHC |
|
| Dry powder inhaler |
|
| *These are examples based on the experience of the authors; other errors are possible. †Timing is not as crucial as it is for an MDI without a spacer, but the drug is still lost if inhalation is delayed. ‡Correct use varies by type of product (see product literature for specifics). DPI, dry powder inhaler; MDI, metered dose inhaler; VHC, valved holding chamber. Source: Adapted with permission from Self TH, et al. Consultant. 2003.5 |
Inhaler error is well documented
Since 1965, when it was first reported that many patients used metered dose inhalers (MDIs) incorrectly,6 evidence has accumulated supporting the magnitude of the problem.7-12 (Studies conducted in family practice settings are described in “Researchers look at inhaler problems in primary care” and in TABLE W1.13-20)
A number of studies of various sizes (from 41 to 3955 patients) have assessed inhaler technique in patients being treated by clinicians in primary care. The researchers used a variety of scoring methods, as well. Among them were a simple 4-step (0-4) rating system, a 9-step system, a standardized inhaler-specific checklist, and a system that tracked the number of omissions patients made.13-20 All found significant problems with inhaler technique. (You’ll find a detailed look at the studies in TABLE W1 at jfponline.com.)
In one study of 422 patients,13 including young children, adolescents, and adults, participants received one point for correctly performing each of the following steps:
- Adequate preparation (shaking well for those using a metered dose inhaler [MDI]; loading correctly for patients using a dry powder inhaler [DPI])
- Adequate expiration, correct head position
- Adequate inspiratory technique
- Holding breath afterwards.
The researchers found that 25% of the patients had inadequate technique (≤2 on a 0-4 point scale). In this study, as in others that included patients using various types of devices, use of an MDI was associated with a higher rate of incorrect technique.
Another much-smaller study14 used the same 4-step system to assess the technique of 50 patients, all of whom had the same type of DPI and had received extensive training in the correct use of the device. Despite the training, 27% of the patients received scores of ≤2 (inadequate technique). Sixty-eight percent received a score of 3 (adequate); only 5% received a score of 4 (good).
The 2 largest studies—one including 3955 patients using MDIs20 and the other looking at 3811 patients using various kinds of devices18—found high levels of errors, as well. In the latter study, 76% of patients with MDIs made at least one error vs 49% to 55% of patients using DPIs.18 The results convinced a large majority of the physicians caring for these patients of the need to check inhaler technique more frequently. In the study of MDI users alone, 71% of the patients made at least one mistake.20 inhaler misuse was associated with higher asthma instability scores, this study showed.
More recently, a researcher assessed the effects of an integrated primary care model on the management of asthma and/or COPD in middle-aged and elderly patients, in a study of 260 patients in 44 family practices.19 The study included an evaluation of inhaler technique.
Participants were divided into an intervention group—137 patients who received education regarding inhaler use from a nurse—and a usual care group (123 patients). After 2 years, correct inhaler technique among those in the intervention group went from 41% at baseline to 54%. At the same time, the proportion of those in the usual care group with correct technique fell from 47% to 29%.19
Error rates vary widely from one clinical trial to another, depending on study criteria, type of device, and extent of patient education, among other factors. Nonetheless, several studies (spanning 3 decades) found the error rate to be close to, or greater than, 90%.7,10,21
The most recent of these, published in 2009,21 was based on observation of the inhaler technique used by patients with asthma or COPD directly following appointments in an outpatient clinic. The authors found that, although >98% of the study participants claimed to know how to use their inhalers, 94% committed at least one error. In this study and a number of others, user error was more likely in patients using MDIs.13,18,21,22
Adding a spacer (eg, a valved holding chamber such as the AeroChamber) can be helpful, as the spacer affords the patient more time to inhale the medication. But patients who use an MDI with a spacer often make mistakes, too, and patient education is essential.23-26
Breath-activated dry powder inhalers (DPIs)—such as the Flexhaler, HandiHaler, Aerolizer, and Diskus—also reduce the likelihood of error. DPIs eliminate a step that MDI users often struggle with: the need to simultaneously press down on the canister and begin a slow, deep inhalation.
What’s more, DPIs do not have to be shaken before use. Nonetheless, using a DPI still involves a series of actions. For the HandiHaler and Aerolizer, patients must load the dose, and some patients fail to read the directions and swallow the capsule instead of loading it into the device. Patients must remember to exhale away from the device (ie, not into the dry powder) before inhaling, then hold their breath for approximately 10 seconds. There is potential for error at each step.
Stress the need to exhale before using the inhaler
Forgetting to exhale before inhaling is a common, and significant, mistake regardless of the type of device. It is paramount to stress the need to exhale gently for a few seconds before inhaling (slowly and deeply for patients using an MDI, rapidly and deeply with most DPIs). For MDI users, poor timing, described earlier, is another common and serious mistake. Patients using an MDI with a valved holding chamber sometimes inhale for too long before pressing down on the inhaler, then are unable to continue inhaling although the aerosol is still in the chamber. A common error made by patients using multidose DPIs is simply to forget to load the dose.
Physicians need to brush up on their skills, too
It’s not just patients who lack proficiency in inhaler technique. Numerous studies have demonstrated poor skill among physicians and other health care professionals.27-34 Evidence also shows that targeted education results in substantial improvement.32,35
In one study undertaken to evaluate family medicine residents’ proficiency in using asthma inhalers, participants (an intervention group at one clinic and a control group at another) all were given a pretest. The intervention group then received educational materials and a tutorial, as well as the opportunity for hands-on practice, after which both groups were given a post-test. The residents who received the training had a 170% jump, on average, in proficiency score, vs a 55% increase for the control group (P<.001).35
Inhaled Medication Instructional Videos
Courtesy of: National Jewish Health
Go to http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos
Another study—this one involving first-year interns—looked at level of improvement based on the type of education provided. Initially, only 5% of the interns could use an MDI without error. After a lecture and demonstration, 13% had an error-free technique. But when each intern participated in an intensive one-on-one session, the error-free rate reached 73%. The researchers’ conclusion: Lectures are relatively ineffective in teaching interns inhaler technique compared with a one-on-one approach.32
The Chicago Breathe Project,36 a new program aimed at improving education in the use of asthma inhalers for physicians and minority patients, provides further evidence of the value of clinician education. After a series of workshops for residents at 5 academic institutions, the physicians’ knowledge of proper use of inhalers rose dramatically—from just 5% preprogram to 91% postprogram (P<.001). Six months after the educational activity, the residents (n=161) were more likely (44% vs 11% preprogram) to assess patients’ inhaler technique.36
Teaching patients when time is tight
National and international guidelines stress the need to teach patients correct use of asthma and COPD inhalers.1,37,38 Providing the requisite education includes observation of each patient’s inhaler technique with proper use demonstrated, as needed.
The problem, of course, is how to provide that level of patient education within the time constraints of a busy family practice. We recommend these time-efficient solutions:
Enlist the help of other clinicians. While it is important that someone in your office be well trained and able to instruct patients in the proper use of inhalers, that individual need not be you. The National Institutes of Health recommends that the “principal clinician” introduce key educational messages, which can be reinforced and expanded on by other members of the health care team.1
After you advise patients that it is crucial for them to be trained in and adhere to proper inhaler technique, another health care professional—often a clinic nurse or pharmacist who has had special training—can provide the hands-on education. Studies have shown that when pharmacists who are competent in asthma management, including inhaler technique, work with physicians to optimize the education and overall management of patients with asthma, better outcomes often result, including a reduction in both emergency department visits and hospitalizations.1,39,40
Use videos to demonstrate correct technique. Videos are an effective teaching tool,9 and many of them are device-specific. National Jewish Health, which is world renowned for its asthma care, has a set of instructional videos posted on You-Tube and accessible from its Web site (http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos). In addition to videos that demonstrate the use of an MDI alone and an MDI plus a valved holding chamber, the site has links to 6 DPI videos, each covering a different device.
Use intermittent observation. After the patient views the appropriate video, you or a member of your staff will still need to observe the patient’s inhaler technique to ensure correct use. Ideally, this should occur at every visit.1,37 When that’s not possible, use intermittent observation, starting with the first 2 or 3 visits after the introduction of inhalation therapy and then switching to periodic observation to ensure that the patient is maintaining good technique.
In determining how often observation is necessary, keep in mind that simply asking patients whether they are having inhaler problems is not sufficient.1 Patients tend to say they have little or no trouble when, in fact, most struggle, at times, with the devices. What’s more, good technique tends to decrease over time, and repetitive education is important.
To motivate patients, try this communication technique
Motivational interviewing, a technique that has been used to help patients battle obesity, quit smoking, and control hypertension,41-43 among other health problems, can help you identify inhaler problems that need to be addressed. It involves the use of open-ended questions (eg, “What worries you most about your asthma?”), affirmations (“You’ve done a great job testing your peak flow level every morning”), reflective listening (“You’re tired of taking medicine every day”), and summary statements (“You know you should take your medicine every day but you’re having trouble remembering it. Is that right?”).
A pilot study44 showed that when this technique was incorporated into an asthma education session, patient motivation increased. The ratio of perceived advantages vs disadvantages of taking asthma medication correctly improved, as well. Another study45 found that when motivational interviewing was used during home visits to inner-city African American adolescents for asthma care, the patients’ motivation, readiness to adhere to treatment, and asthma-related quality of life improved, although self-reported adherence to asthma medication did not. Further studies involving patients with asthma are under way (www.clinicaltrials.gov/ct2/results?term=asthma).
It is important to note that the use of motivational interviewing does not require a lengthy visit. One study found that on average, visits in which primary care physicians used this communication technique lasted less than 10 minutes.46
CORRESPONDENCE Timothy H. Self, PharmD, University of Tennessee Health Science Center, 881 Madison Avenue, Room 235, Memphis, TN 38163; [email protected]
1. National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007.
2. Centers for Disease Control and Prevention. National Center for Health Statistics: National health interview survey raw data, 2008. Analysis performed by American Lung Association Research and Program Services.
3. American Lung Association. COPD—Helping the missing millions. February 24, 2010. Available at: http://www.lungusa.org/about-us/our-impact/top-stories/copd-helping-the-missing.html. Accessed November 9, 2011.
4. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1-16.
5. Self TH, Kilgore KE, Shelton V. MDIs, spacers, and dry powder inhalers: what patients are likely to do wrong. Consultant. 2003;49:702-705.
6. Saunders KB. Misuse of inhaled bronchodilator agents. Br Med J. 1965;1:1037-1038.
7. Epstein SW, Manning CPR, Ashley MJ, et al. Survey of the clinical uses of pressurized aerosol inhalers. Can Med Assoc J. 1979;120:813-816.
8. Shim C, Williams MH. The adequacy of inhalation of aerosol from canister nebulizers. Am J Med. 1980;69:891-894.
9. Self TH, Brooks JB, Lieberman P, et al. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Can Med Assoc J. 1983;128:129-131.
10. Hartert TV, Windom HH, Peeples RS, et al. Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am J Med. 1996;100:386-394.
11. Goodman DE, Israel E, Rosenberg M, et al. The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med. 1994;150:1256-1261.
12. Newman SP, Pavia D, Clarke SW. How should a pressurized beta-adrenergic bronchodilator be inhaled? Eur J Respir Dis. 1981;62:3-21.
13. Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br J Gen Pract. 1990;40:505-506.
14. Dompeling E, Van Grunsven PM, Van Schayck GP, et al. Treatment with inhaled steroids in asthma and chronic bronchitis: long-term compliance and inhaler technique. Fam Pract. 1992;9:161-166.
15. Verver S, Poelman M, Bogels A, et al. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam Pract. 1996;13:35-40.
16. Dickinson J, Hutton S, Atkin A, et al. Reducing asthma morbidity in the community: the effect of a targeted nurse-run asthma clinic in an English general practice. Respir Med. 1997;91:634-640.
17. Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care. 2001;19:255-260.
18. Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249-254.
19. Meulepas MA, Jacobs JE, Smeenk FW, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scand J Prim Health Care. 2007;25:186-192.
20. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246-251.
21. Souza ML, Meneghini AC, Ferraz E, et al. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35:824-831.
22. Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323-328.
23. Rachelefsky GS, Rohr AS, Wo J, et al. Use of a tube spacer to improve the efficacy of a metered dose inhaler in asthmatic children. Am J Dis Child. 1986;140:1191-1193.
24. Demirkan K, Tolley E, Mastin T, et al. Salmeterol administration by metered-dose inhaler alone vs metered-dose inhaler plus valved holding chamber. Chest. 2000;117:1314-1318.
25. Pedersen S, Ostergaard PA. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983;38:191-194.
26. Dolovich MD, Ahrens RS, Hess DR, et al. Device selection an outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335-371.
27. Interiano B, Guntupalli KK. Metered-dose inhalers: do health care providers know what to teach? Arch Intern Med. 1993;153:81-85.
28. Hanania NA, Wittman R, Kesten S, et al. Medical personnel’s knowledge of and ability to use inhaling devices. Metered-dose inhalers, spacing chambers, and breath-actuated dry powder inhalers. Chest. 1994;105:111-116.
29. Amirav I, Goren A, Pawlowski NA. What do pediatricians in training know about the correct use of inhalers and spacer devices? J Allergy Clin Immunol. 1994;94:669-675.
30. Chopra N, Oprescu N, Fask A, et al. Does introduction of new “easy to use” inhalational devices improve medical personnel’s knowledge of their proper use? Ann Allergy Asthma Immunol. 2002;88:395-400.
31. Self TH, Arnold LB, Czosnowski LM, et al. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44:593-598.
32. Lee-Wong M, Mayo PH. Results of a programme to improve house staff use of metered dose inhalers and spacers. Postgrad Med J. 2003;79:221-225.
33. Muchao FP, Pern SL, Rodriques JC, et al. Evaluation of the knowledge of health professionals at a pediatric hospital regarding the use of metered dose inhalers. J Bras Pneumol. 2008;34:4-12.
34. Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, Diskus, and Turbuhaler): changes after a single teaching session. J Asthma. 2009;46:944-950.
35. Kelcher S, Brownoff R. Teaching residents to use asthma devices. Assessing family residents’ skill and a brief intervention. Can Fam Physician. 1994;40:2090-2095.
36. Press VG, Pincayage AT, Pappalardo AA, et al. The Chicago Breathe Project: a regional approach to improving education on asthma inhalers for resident physicians and minority patients. J Natl Med Assoc. 2010;102:548-555.
37. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2010. Available at: www.ginasthma.org. Accessed November 9, 2011.
38. Executive Summary: global strategy on the diagnosis and management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report, 2009. Available at: www.goldcopd.com. Accessed November 9, 2011.
39. Self TH, Chrisman CR, Mason DL, et al. Reducing emergency department visits and hospitalizations in African American and Hispanic patients: a 15-year review. J Asthma. 2005;42:807-812.
40. Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy asthma care program (PACP) improves outcomes for patients in the community. Thorax. 2007;62:496-502.
41. DiLillo V, Nicole J, West DS. Incorporating motivational interviewing into behavioral obesity treatment. Cogn Behav Pract. 2003;10:120-130.
42. Borrelli B, Novak S, Hecht J, et al. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking). Prev Med. 2005;41:815-821.
43. Woollard L, Beilin L, Lord T, et al. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466-468.
44. Schmaling K, Blume A, Afari N. A randomized controlled pilot study of motivational interviewing to change attitudes about adherence to medications for asthma. J Clin Psych Med Settings. 2001;8:167-172.
45. Riekert KA, Borrelli B, Bilderback A, et al. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
46. Butler C, Rollnick S, Cohen D, et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49:611-616.
1. National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007.
2. Centers for Disease Control and Prevention. National Center for Health Statistics: National health interview survey raw data, 2008. Analysis performed by American Lung Association Research and Program Services.
3. American Lung Association. COPD—Helping the missing millions. February 24, 2010. Available at: http://www.lungusa.org/about-us/our-impact/top-stories/copd-helping-the-missing.html. Accessed November 9, 2011.
4. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1-16.
5. Self TH, Kilgore KE, Shelton V. MDIs, spacers, and dry powder inhalers: what patients are likely to do wrong. Consultant. 2003;49:702-705.
6. Saunders KB. Misuse of inhaled bronchodilator agents. Br Med J. 1965;1:1037-1038.
7. Epstein SW, Manning CPR, Ashley MJ, et al. Survey of the clinical uses of pressurized aerosol inhalers. Can Med Assoc J. 1979;120:813-816.
8. Shim C, Williams MH. The adequacy of inhalation of aerosol from canister nebulizers. Am J Med. 1980;69:891-894.
9. Self TH, Brooks JB, Lieberman P, et al. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Can Med Assoc J. 1983;128:129-131.
10. Hartert TV, Windom HH, Peeples RS, et al. Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am J Med. 1996;100:386-394.
11. Goodman DE, Israel E, Rosenberg M, et al. The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med. 1994;150:1256-1261.
12. Newman SP, Pavia D, Clarke SW. How should a pressurized beta-adrenergic bronchodilator be inhaled? Eur J Respir Dis. 1981;62:3-21.
13. Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br J Gen Pract. 1990;40:505-506.
14. Dompeling E, Van Grunsven PM, Van Schayck GP, et al. Treatment with inhaled steroids in asthma and chronic bronchitis: long-term compliance and inhaler technique. Fam Pract. 1992;9:161-166.
15. Verver S, Poelman M, Bogels A, et al. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam Pract. 1996;13:35-40.
16. Dickinson J, Hutton S, Atkin A, et al. Reducing asthma morbidity in the community: the effect of a targeted nurse-run asthma clinic in an English general practice. Respir Med. 1997;91:634-640.
17. Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care. 2001;19:255-260.
18. Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249-254.
19. Meulepas MA, Jacobs JE, Smeenk FW, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scand J Prim Health Care. 2007;25:186-192.
20. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246-251.
21. Souza ML, Meneghini AC, Ferraz E, et al. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35:824-831.
22. Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323-328.
23. Rachelefsky GS, Rohr AS, Wo J, et al. Use of a tube spacer to improve the efficacy of a metered dose inhaler in asthmatic children. Am J Dis Child. 1986;140:1191-1193.
24. Demirkan K, Tolley E, Mastin T, et al. Salmeterol administration by metered-dose inhaler alone vs metered-dose inhaler plus valved holding chamber. Chest. 2000;117:1314-1318.
25. Pedersen S, Ostergaard PA. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983;38:191-194.
26. Dolovich MD, Ahrens RS, Hess DR, et al. Device selection an outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335-371.
27. Interiano B, Guntupalli KK. Metered-dose inhalers: do health care providers know what to teach? Arch Intern Med. 1993;153:81-85.
28. Hanania NA, Wittman R, Kesten S, et al. Medical personnel’s knowledge of and ability to use inhaling devices. Metered-dose inhalers, spacing chambers, and breath-actuated dry powder inhalers. Chest. 1994;105:111-116.
29. Amirav I, Goren A, Pawlowski NA. What do pediatricians in training know about the correct use of inhalers and spacer devices? J Allergy Clin Immunol. 1994;94:669-675.
30. Chopra N, Oprescu N, Fask A, et al. Does introduction of new “easy to use” inhalational devices improve medical personnel’s knowledge of their proper use? Ann Allergy Asthma Immunol. 2002;88:395-400.
31. Self TH, Arnold LB, Czosnowski LM, et al. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44:593-598.
32. Lee-Wong M, Mayo PH. Results of a programme to improve house staff use of metered dose inhalers and spacers. Postgrad Med J. 2003;79:221-225.
33. Muchao FP, Pern SL, Rodriques JC, et al. Evaluation of the knowledge of health professionals at a pediatric hospital regarding the use of metered dose inhalers. J Bras Pneumol. 2008;34:4-12.
34. Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, Diskus, and Turbuhaler): changes after a single teaching session. J Asthma. 2009;46:944-950.
35. Kelcher S, Brownoff R. Teaching residents to use asthma devices. Assessing family residents’ skill and a brief intervention. Can Fam Physician. 1994;40:2090-2095.
36. Press VG, Pincayage AT, Pappalardo AA, et al. The Chicago Breathe Project: a regional approach to improving education on asthma inhalers for resident physicians and minority patients. J Natl Med Assoc. 2010;102:548-555.
37. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2010. Available at: www.ginasthma.org. Accessed November 9, 2011.
38. Executive Summary: global strategy on the diagnosis and management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report, 2009. Available at: www.goldcopd.com. Accessed November 9, 2011.
39. Self TH, Chrisman CR, Mason DL, et al. Reducing emergency department visits and hospitalizations in African American and Hispanic patients: a 15-year review. J Asthma. 2005;42:807-812.
40. Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy asthma care program (PACP) improves outcomes for patients in the community. Thorax. 2007;62:496-502.
41. DiLillo V, Nicole J, West DS. Incorporating motivational interviewing into behavioral obesity treatment. Cogn Behav Pract. 2003;10:120-130.
42. Borrelli B, Novak S, Hecht J, et al. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking). Prev Med. 2005;41:815-821.
43. Woollard L, Beilin L, Lord T, et al. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466-468.
44. Schmaling K, Blume A, Afari N. A randomized controlled pilot study of motivational interviewing to change attitudes about adherence to medications for asthma. J Clin Psych Med Settings. 2001;8:167-172.
45. Riekert KA, Borrelli B, Bilderback A, et al. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
46. Butler C, Rollnick S, Cohen D, et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49:611-616.