User login
Catatonia: How to identify and treat it
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14
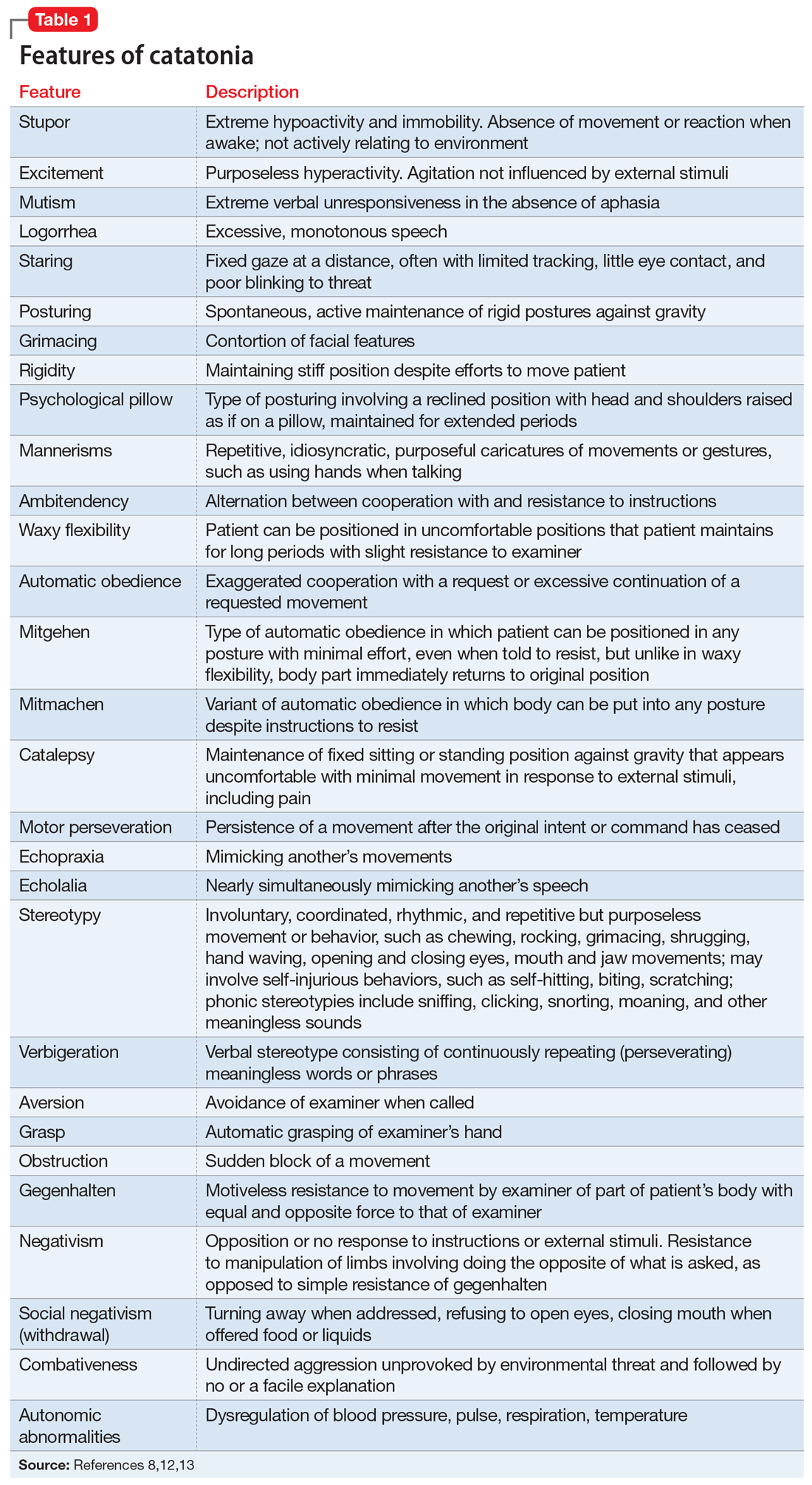
Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
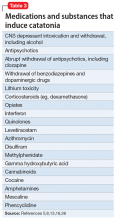
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
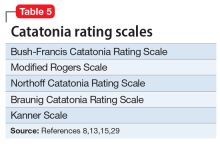
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
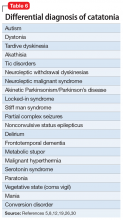
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.
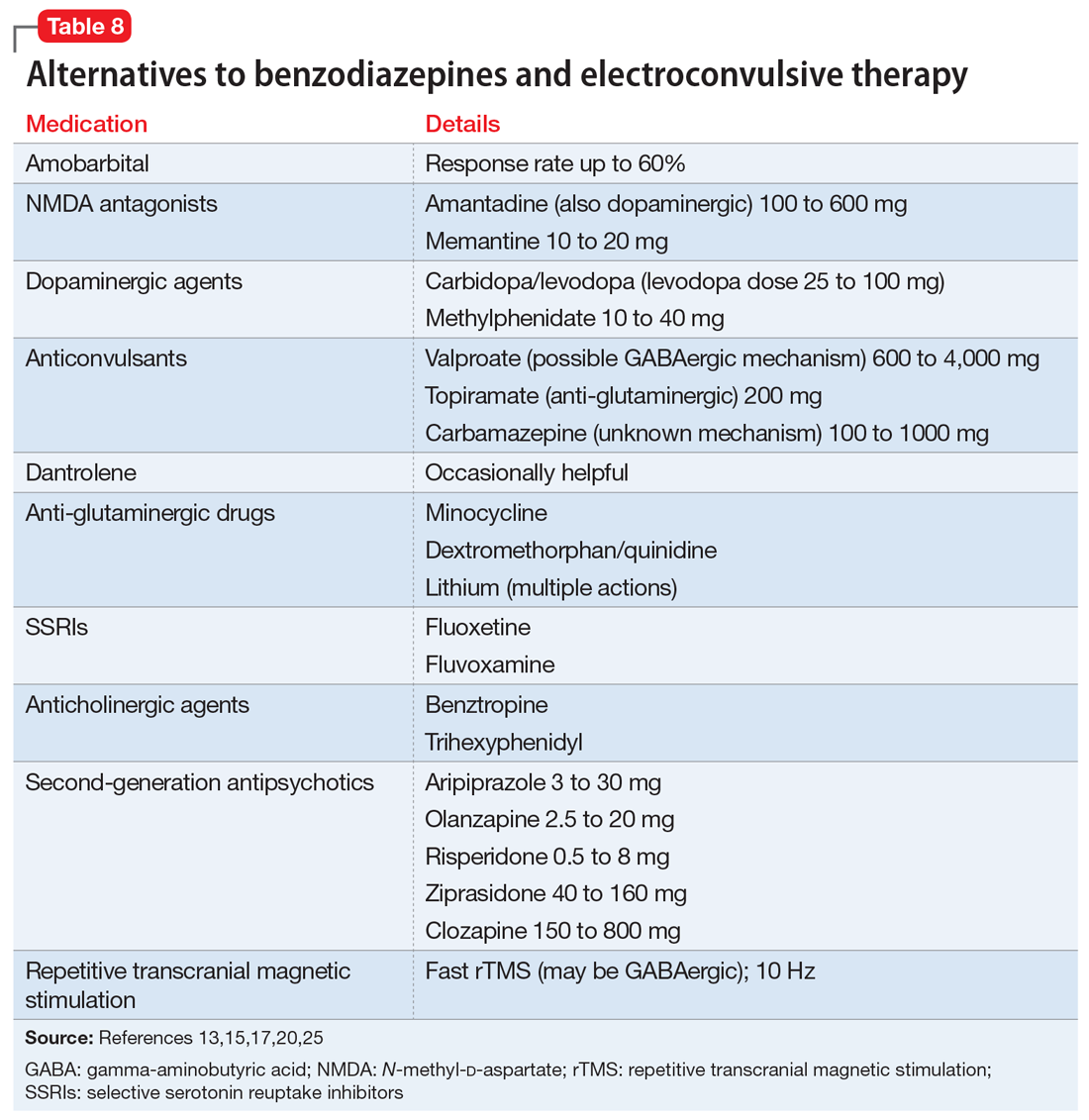
Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14

Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.

Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14

Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.

Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
When bipolar treatment fails: What’s your next step?
All phases of bipolar disorder can be difficult to treat, and patients remain symptomatic on average about half the time.1 Not all bipolar patients who experience continued illness and disability are treatment-resistant (Box 1), but when symptoms persist you may ask yourself: Was treatment suboptimal or simply ineffective?
Patients with severe symptoms may be satisfied with a substantial decrease in symptoms, but any residual symptoms cause ongoing distress and lower the threshold for recurrences.2 Finding the right combination of therapies for your patient is key to achieving an enduring response.
Future studies may tell us which treatments to combine and in what sequence for complex bipolar disorder, but—since most published studies exclude complex and comorbid cases—for now we must rely on limited controlled data and clinical experience. Using those resources, we offer comprehensive, practical recommendations for trouble-shooting (Box 2)3-6 and getting better results when bipolar disorder does not respond to standard treatment.
Some studies define treatment resistance as failure to respond to lithium, and in other settings it is viewed as failure to respond to ≥2 treatment courses. Because euthymia and normal functioning are important for long-term prognosis, we define treatment-resistance as failure to achieve both symptomatic and functional remission following an adequate course of therapy.
Effective strategies for treating bipolar disorder depend on:
- illness phase (later episodes are more difficult to treat than earlier ones)
- symptom complexity (mixed symptoms probably reflect more complex pathophysiology and are more likely to require combination therapies)
- predominant presentations (mania, depression, rapid and ultradian cycling)
- whether symptoms are acute or chronic.
Unfortunately, the findings of and strategies used in clinical trials of refractory bipolar disorder are difficult to extrapolate to everyday practice. Most studies exclude patients with a history of treatment resistance, severe symptoms, and important comorbidities such as substance abuse. In addition, the usual primary endpoint is response (≥50% reduction of symptoms) rather than remission (minimal symptoms and no longer meeting criteria for the disorder). Very few studies address functional remission, which is necessary to reduce the risk of symptomatic recurrence.
In clinical practice, when initial treatment for bipolar disorder fails to produce remission, systematically addressing 5 questions (Box 2) can help direct your next step.
Mania
When a patient with mania does not respond as expected, the next step depends on which antimanic agent you prescribed:
Lithium can take a month to become fully effective for mania, which is why a benzodiazepine or antipsychotic is often added acutely to reduce agitation. Do not mistake neurotoxic interactions between lithium and antipsychotics for increased mania.
Although data vary on lithium’s optimal serum level, adjust to approximately 0.8 to 1 mEq/L, if tolerated, when lower levels are not effective. Children and young adolescents may need higher serum levels (such as 1.5 mEq/L) because the difference between serum and brain lithium levels is greater in younger patients than in adults.
Consider the dosing schedule. Because lithium’s elimination half-life with repeated dosing is 24 hours, most adults can take any formulation once daily—which improves adherence and reduces adverse effects. Children eliminate lithium more rapidly and need more frequent dosing.
High loading doses result in more rapid control of agitation, probably as a result of sedation. In our experience, however, rapidly sedating patients may interfere with long-term adherence.
Carbamazepine, other anticonvulsants. Because they less sedating, carbamazepine and other anticonvulsants might not appear to be rapidly effective for bipolar mania. If you wait up to a month, however, any antimanic effect will be obvious.
Antipsychotics are rapidly effective for mania. Higher doses work faster but produce more side effects. After an acute response, some patients can be maintained on a second-generation antipsychotic (SGA), but others do better on a standard mood stabilizer such as lithium or valproate.
Calcium channel blockers. Verapamil has been effective mostly for lithium-responsive mania in 27 of 30 studies. Nimodipine has been useful for more complex bipolar syndromes in a few studies using patients as their own controls.
To be effective for bipolar disorder, however, calcium channel blockers require frequent, high dosing (such as verapamil, 120 mg 4 times daily, or nimodipine, 60 to 120 mg 6 times daily), which makes adherence difficult.
1 Is the patient taking anything that is making symptoms worse?
Antidepressants can induce mania, hypomania, and cycle acceleration in bipolar disorder, even when mood stabilizers are co-prescribed.3 Stimulants also may destabilize bipolar mood disorders; consider this possibility when patients taking stimulants for apparent attention-deficit/hyperactivity disorder at first appear to improve and then deteriorate.
Alcohol and cocaine can induce mania and depression. Cocaine is a potent kindling stimulus that could contribute to enduring mood instability.
2 Is the patient taking the medication?
Treatment adherence by bipolar patients may be as low as 35%.4 Ask outpatients what kinds of problems they have encountered taking medications, not whether they have such problems. Talk with the patient about adherence after each dosage increase, and be readily available. Prescribe extended-release pills for patients who have trouble keeping track of medications.
3 Is treatment adequate?
Adjust mood-stabilizer dosing until the patient responds or cannot tolerate the medication; complex cases often require combination treatment. Give the medication sufficient time to work; most mood stabilizers take ≥1 month to become fully effective.
4 Is another condition interfering with treatment?
Up to 70% of patients with refractory mood disorders have subclinical hypothyroidism. Look for:
- elevated thyroid stimulating hormone (TSH) with or without decreased thyroxine (T4)
- elevated TSH response to thyrotrop-inreleasing hormone (TRH).5
Also consider hypercalcemia from chronic lithium therapy,6 anemia, sleep apnea, posttraumatic stress disorder, substance use disorders, and personality disorders.
5 Am I ignoring psychotherapy?
Address psychosocial issues that influence the course of illness. Attend to patients’ important relationships, loss, negative thinking, and biological and social rhythms.
- consider augmentation first when a patient has had a partial response to a given medication
- switch when a patient cannot tolerate or shows no response to a therapeutic dose of a given medication.
Combinations of lithium and carbamazepine or valproate can be more effective than either drug alone, but therapeutic doses of each usually are needed. Carbamazepine has been used successfully to augment a partial response to nimodipine.9 In a small open-label trial, adding oxcarbazepine to lithium, valproate or antidepressants improved response in some patients with mild refractory mania.10
Switching among anticonvulsants can be useful because their actions and side effects differ. Clozapine in a wide range of doses can be very effective for refractory mania,11 but its use is difficult to monitor in highly agitated manic patients.
Other options. Electroconvulsive therapy (ECT) is the most effective treatment for mania, producing higher response rates than any antimanic drug.12 In a study of repetitive transcranial magnetic stimulation (rTMS), 8 of 9 patients with mania refractory to mood stabilizers had a sustained response after 1 month of right-sided rTMS treatment.13 Conversely, left-sided rTMS can aggravate mania.
Bipolar depression
Continuing controversy about the best way to treat bipolar depression makes it difficult to know if treatment has been suboptimal or a patient is treatment-resistant.
Antidepressants. No antidepressant is approved (or recommended) as monotherapy for bipolar depression, and most experts recommend against prescribing antidepressants without concomitant mood stabilizers. Even so:
- Clinicians prescribing monotherapy for bipolar disorder choose antidepressants twice as often as mood stabilizers.
- Antidepressants are prescribed more frequently in combination with mood stabilizers than as monotherapy, although empiric trials have shown most antidepressants are not effective for bipolar depression.14
In many cases, an antidepressant seems to help at first and then induces a recurrence of depression, often mixed with dysphoric hypomanic symptoms. The recurrent episode improves when the clinician increases the antidepressant dose or changes to another antidepressant, only to be followed by another recurrence that may be interpreted as an incomplete antidepressant response.
Antipsychotics. Quetiapine16 and a combination of olanzapine and fluoxetine17 are approved for treating bipolar depression. The studies supporting this indication lasted only 8 weeks, however, and excluded patients with the kinds of complicated and comorbid mood disorders commonly seen in clinical practice.
Many patients dropped out before the studies were completed, and “screen fails” (patients with the diagnosis who were not enrolled in the study) were not reported. In addition, “remitted” patients remained symptomatic.
Therefore, FDA approval of this indication does not guarantee these medications’ long-term efficacy or safety for bipolar depression or that they are useful in patients with complex forms of bipolar depression.
Recommended approach. Treatment resistance of bipolar depression to multiple mood stabilizers—with or without an antidepressant—or to an antipsychotic may manifest as lack of response, partial response, or initial good response followed by relapse or recurrence. Sometimes depression improves but irritability or mood lability worsen.
No reliable controlled studies have addressed complex refractory bipolar depression, but clinical experience suggests 1 approach for all of these responses:
Reconsider possible hypothyroidism. A low-normal T4—especially if decreased over time—and a mid-range or high-normal TSH—especially if increased—may indicate that subclinical hypothyroidism is inhibiting a response to mood stabilizers and antidepressants.18
Stop the antidepressant. If your patient is taking an antidepressant, it may be ineffective, creating mixed dysphoric hypomania, and/or driving another recurrence of depression. This is especially likely if the patient shows an initial prompt antidepressant response, but depression returns with irritability, insomnia, restlessness, or other subtle symptoms of dysphoric hypomania.
Withdraw the antidepressant gradually; for example, you might reduce the dose by 10% every few weeks so that the agent is discontinued across several months. Discontinuing an antidepressant too rapidly—even if it does not seem to be having any effect—can cause rebound depression that creates the mistaken impression that the antidepressant is needed.
Combine mood stabilizers. If a single mood stabilizer does not at least eliminate mood lability and other symptoms of activation, add a second agent. The combination of lithium and carbamazepine helps some depressed patients.20 Patients with considerable mood instability or psychotic symptoms may benefit from an adjunct antipsychotic.
Introduce mood stabilizers gradually. These medications may work more rapidly against mixed manic symptoms than they do against depression, especially when the dose is raised too quickly. The result is rapid control of irritability, hyperactivity, agitation, and related symptoms but an apparent increase in depression as mixed elements of elevated mood and energy are filtered out.
Add an antidepressant? If gradual adjustment of mood stabilizers eliminates mixed symptoms and mood fluctuations but the patient is still depressed, cautiously add an antidepressant. Antidepressants may be less likely to destabilize mood after all mixed elements have been treated completely.
Approximately 20% of bipolar patients are thought to experience rapid cycling, defined as ≥4 affective episodes/year separated by at least 2 weeks of euthymia between poles or with an immediate switch from one pole to the other.32 The prevalence of ultradian cycling—in which multiple brief affective episodes (usually subsyndromal or mixed) occur each day—is unclear.
Both cycling types probably represent stages in the evolution of bipolar mood disorders rather than distinct diagnoses. In many cases, mood cycling abates after months to years, but morbidity can be high and the wrong treatment may perpetuate mood cycling.
Complex mood cycling rarely responds to a single treatment, probably because its pathophysiology is complex. The need for polypharmacy may create the impression of treatment failure, but no one would expect a single medication to be sufficient for other complex illnesses such as cancer or AIDS.
21 Other medications have shown antidepressant effects in bipolar depression (Table).22-31 Although clinicians often use serotonin reuptake inhibitors, this practice has no empiric support in refractory bipolar depression—and our experience has not been particularly positive. Fluoxetine’s long half-life can perpetuate adverse effects long after the medication is withdrawn, and rebound depression is not uncommon when paroxetine or venlafaxine are withdrawn.
Some experts recommend discontinuing the antidepressant after depression remits to avoid driving more recurrences,3 but others do not think continuing antidepressants is risky. Apparently some patients do well with continued antidepressants, and others do not. In our experience, patients who have had mixed symptoms or mood lability are most likely to deteriorate with continued antidepressant treatment. Whenever depression returns after an initial and especially rapid response to an antidepressant, consider withdrawing the antidepressant and maximizing mood stabilizers first rather than changing or augmenting the antidepressant.
Treat seasonal symptoms. Many bipolar patients are most likely to be depressed in winter, and seasonal affective disorder is common in patients with a bipolar mood disorder. Their depression may respond to artificial bright light, usually given in the morning. Light therapy can help normalize the sleep-wake cycle, although it also can induce hypomania.
Other options. ECT is the most reliably effective treatment for bipolar depression. Because it treats both poles of the mood disorder, ECT also can be a useful maintenance treatment. A comparison of rTMS and placebo in 23 bipolar depressed patients failed to find any benefit of active treatment.32
Table
What now? Treatment options for refractory bipolar depression
| Treatment | Comment |
|---|---|
| Psychotherapy | Combine with somatic therapies for most patients with refractory mood disorders; adjunctive CBT, interpersonal and social rhythms therapy, or family-focused therapy speeded bipolar depression recovery in STEP-BD22 |
| Bupropion | Generally accepted as first-line antidepressant; the relatively low doses used may explain this agent’s lower risk of inducing mania compared with other antidepressants |
| MAO inhibitors | Can be combined with carbamazepine;23 tranylcypromine is best-studied antidepressant in bipolar depression and is especially useful for anergic states;24 selegiline also can be useful |
| Stimulants | Stimulants—such as methylphenidate, 15 to 30 mg/d—can be rapidly effective for lethargic, anergic depression (although evidence is limited); benefit wears off rapidly if mood is adversely affected |
| Pramipexole | Activating dopaminergic agent with rapid onset; investigational; has produced an antidepressant effect in patients with bipolar II depression when added to mood stabilizers25 |
| Modafinil | May be useful for residual fatigue in major depression and medication-induced sedation;26 improved depressive symptoms when used as an adjunct27 |
| Anticonvulsants | Anticonvulsants other than lamotrigine and carbamazepine-lithium combinations are considered later choices for bipolar depression; adjunctive zonisamide has been helpful in case series;28 gabapentin, pregabalin, and topiramate also can be useful adjuncts (although not supported by controlled studies in depression); adding levetiracetam may improve response29 |
| NMDA antagonist | Investigational; memantine30 was effective in a small controlled study, and riluzole (indicated for amyotrophic lateral sclerosis) was helpful in a small open study31 |
| CBT: cognitive-behavioral therapy; MAO: monoamine oxidase; NMDA: N-methyl-D-aspartate; STEP-BD: Systematic Treatment Enhancement Program for Bipolar Disorder | |
Rapid and ultradian cycling
No controlled studies have compared single-drug or combination therapies for rapid and ultradian cycling (Box 3).33 Thus, our recommendations for treating patients with cycling who have not responded to initial interventions are based on case series and clinical experience.
Reassess thyroid function. As many as 70% of patients with rapid cycling have subclinical hypothyroidism that contributes to mood instability.34 Thyroid replacement is indicated for any degree of hypothyroidism—even if medically unimportant—in patients with refractory mood disorders.
Slowly withdraw antidepressants. Most patients with rapid cycling are taking antidepressants. If your patient is experiencing depressive symptoms while taking an antidepressant, this means the antidepressant is not working and there is little point in continuing it. For patients being withdrawn from multiple antidepressants, rotate dose decrements to help you monitor the effect of each reduction.
Combine mood stabilizers. After optimizing the dose of a single mood stabilizer, add a second one from a different class. In an open trial, adding oxcarbazepine, up to 2,400 mg/d, helped approximately one-third of 20 patients with refractory mood cycling.10 Lithium is generally considered less effective than anticonvulsants in rapid cycling, but at least one study showed it was equivalent to carbamazepine for this problem.35 Lithium combined with other mood stabilizers may be more effective than lithium monotherapy in refractory bipolar states.
Other options to consider in combination with mood stabilizers:
- an antipsychotic, especially in the presence of psychotic symptoms, when mixed symptoms are present
- clozapine, which can be a highly effective adjunct for refractory mood cycling and mixed states36 (but is a later adjunct because of required monitoring, common adverse effects, and risk of interactions with carbamazepine and benzodiazepines)
- nimodipine, which has empiric support for complex mood cycling37 and is well-tolerated with fewer interactions than other mood stabilizers (but cost and need for frequent dosing make it a second-line adjunct)
- supraphysiologic doses of thyroxine (≤0.4 mg/d, with T4 levels in the hyperthyroid range), which can improve response to mood-stabilizing regimens34 (but risks of inducing hyperthyroidism make this intervention third-line).
Related resources
- Dubovsky SL. Clinical guide to psychotropic medications. New York: WW Norton; 2005.
- Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin North Am 2005;28:349-70.
- Phillip Long, MD. Internet Mental Health. Online psychiatric diagnosis for the two-thirds of individuals with mental illness who do not seek treatment. www.mentalhealth.com/dis/p20-md02.html.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium • Lithobid, others
- Lorazepam • Ativan
- Memantine • Namenda
- Methylphenidate • Concerta, Ritalin, others
- Modafinil • Provigil
- Nimodipine • Nimotop
- Olanzapine/fluoxetine • Symbyax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Pregabalin • Lyrica
- Quetiapine • Seroquel
- Riluzole • Rilutek
- Selegiline • Eldepryl
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
- Verapamil • Calan, Isoptin, others
- Zonisamide • Zonegran
Dr. Mostert reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Dubovsky receives research/grant support from Eli Lilly and Company, Organon, Pfizer, UCB Pharma, anhd Forest Laboratories. He is a consultant to Oganon and Biovail Pharmaceuticals.
1. Judd JL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003;61:261-9.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEPBD). Am J Psychiatry 2006;163:217-24.
3. Altschuler LL, Post RM, Leverich GS. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152:1130-8.
4. Osterberg L, Blaschke T. Drug therapy: adherence to medication. N Engl J Med 2005;353:487-97.
5. Kusalic M. Grade II and grade III hypothyroidism in rapid cycling bipolar patients. Biol Psychiatry 1992;25:177-81.
6. Franks RD, Dubovsky SL, Lifshitz M, et al. Long-term lithium carbonate therapy causes hyperparathyroidism. Arch Gen Psychiatry 1982;39:1074-7.
7. Allen MH, Hirschfeld RMA, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry 2006;163:272-5.
8. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially responsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
9. Pazzaglia P, Post RM, Ketter TA, et al. Nimodipine monotherapy and carbamazepine augmentation in patients with refractory recurrent affective illness. J Clin Psychopharmacol 1998;18:404-13.
10. Conway CR, Chibnall JT, Nelson LA, et al. An open-label trial of adjunctive oxcarbazepine for bipolar disorder. J Clin Psychopharmacol 2006;26:95-7.
11. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153:759-64.
12. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episodes: a review of 50 years’ experience. Am J Psychiatry 1994;151:169-76.
13. Michael N, Erfurth A. Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Disord 2004;78:253-7.
14. Baldessarini RJ, Leahy L, Arcona S, et al. Patterns of psychotropic drug prescription for U.S. patients with diagnoses of bipolar disorders. Psychiatr Serv 2007;58:85-91.
15. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007;356:1711-22.
16. Cookson J, Keck PE, Jr, Ketter TA, Macfadden W. Number needed to treat and time to response/remission for quetiapine monotherapy efficacy in acute bipolar depression: evidence from a large, randomized, placebo-controlled study. Int Clin Psychopharmacol 2007;22(2):93-100.
17. Tohen M, Vieta E, Calabrese JR, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
18. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry 2002;159:116-21.
19. Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet 2007;369:935-45.
20. Kishimoto A. The treatment of affective disorder with carbamazepine: prophylactic synergism of lithium and carbamazepine combination. Prog Neuropsychopharmacol Biol Psychiatry 1992;16:483-93.
21. McElroy SL, Zarate CA, Cookson J, et al. A 52-week, open-label continuation study of lamotrigine in the treatment of bipolar depression. J Clin Psychiatry 2004;65:204-10.
22. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry 2007;64:419-26.
23. Ketter TA, Post RM, Parekh PI, Worthington K. Addition of monoamine oxidase inhibitors to carbamazepine: preliminary evidence of safety and antidepressant efficacy in treatment-resistant depression. J Clin Psychiatry 1995;56:471-5.
24. Himmelhoch JM, Thase ME, Mallinger AG, Houck PR. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991;148:910-6.
25. Zarate CAJ, Payne JL, Singh J, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 2004;56:54-60.
26. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother 2007;41:1005-12.
27. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164:1242-9.
28. Anand A, Bukhari L, Jennings SA, et al. A preliminary open-label study of zonisamide treatment for bipolar depression in 10 patients. J Clin Psychiatry 2005;66:195-8.
29. Post RM, Altshuler LL, Frye MA, et al. Preliminary observations on the effectiveness of levetiracetam in the open adjunctive treatment of refractory bipolar disorder. J Clin Psychiatry 2005;66:370-4.
30. Zarate CAJ, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006;63:856-64.
31. Zarate CAJ, Quiroz JA, Singh JB, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 2005;57:430-2.
32. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord 2003;5:40-7.
33. Schneck CD. Treatment of rapid-cycling bipolar disorder. J Clin Psychiatry 2006;67(suppl 11):22-7.
34. Bauer MS, Whybrow PC, Winokur A. Rapid cycling bipolar affective disorder, I: Association with grade I hypothyroidism. Arch Gen Psychiatry 1990;47:427-32.
35. Okuma T, Yamashita I, Takahashi R, et al. Comparison of the antimanic efficacy of carbamazepine and lithium carbonate by double-blind controlled study. Pharmacopsychiatry 1990;23:143-50.
36. Calabrese JR, Meltzer HY, Markovitz PJ. Clozapine prophylaxis in rapid cycling bipolar disorder. J Clin Psychopharmacol 1991;11:396-7.
37. Goodnick PJ. Nimodipine treatment of rapid cycling bipolar disorder. J Clin Psychiatry 1995;56:330.-
All phases of bipolar disorder can be difficult to treat, and patients remain symptomatic on average about half the time.1 Not all bipolar patients who experience continued illness and disability are treatment-resistant (Box 1), but when symptoms persist you may ask yourself: Was treatment suboptimal or simply ineffective?
Patients with severe symptoms may be satisfied with a substantial decrease in symptoms, but any residual symptoms cause ongoing distress and lower the threshold for recurrences.2 Finding the right combination of therapies for your patient is key to achieving an enduring response.
Future studies may tell us which treatments to combine and in what sequence for complex bipolar disorder, but—since most published studies exclude complex and comorbid cases—for now we must rely on limited controlled data and clinical experience. Using those resources, we offer comprehensive, practical recommendations for trouble-shooting (Box 2)3-6 and getting better results when bipolar disorder does not respond to standard treatment.
Some studies define treatment resistance as failure to respond to lithium, and in other settings it is viewed as failure to respond to ≥2 treatment courses. Because euthymia and normal functioning are important for long-term prognosis, we define treatment-resistance as failure to achieve both symptomatic and functional remission following an adequate course of therapy.
Effective strategies for treating bipolar disorder depend on:
- illness phase (later episodes are more difficult to treat than earlier ones)
- symptom complexity (mixed symptoms probably reflect more complex pathophysiology and are more likely to require combination therapies)
- predominant presentations (mania, depression, rapid and ultradian cycling)
- whether symptoms are acute or chronic.
Unfortunately, the findings of and strategies used in clinical trials of refractory bipolar disorder are difficult to extrapolate to everyday practice. Most studies exclude patients with a history of treatment resistance, severe symptoms, and important comorbidities such as substance abuse. In addition, the usual primary endpoint is response (≥50% reduction of symptoms) rather than remission (minimal symptoms and no longer meeting criteria for the disorder). Very few studies address functional remission, which is necessary to reduce the risk of symptomatic recurrence.
In clinical practice, when initial treatment for bipolar disorder fails to produce remission, systematically addressing 5 questions (Box 2) can help direct your next step.
Mania
When a patient with mania does not respond as expected, the next step depends on which antimanic agent you prescribed:
Lithium can take a month to become fully effective for mania, which is why a benzodiazepine or antipsychotic is often added acutely to reduce agitation. Do not mistake neurotoxic interactions between lithium and antipsychotics for increased mania.
Although data vary on lithium’s optimal serum level, adjust to approximately 0.8 to 1 mEq/L, if tolerated, when lower levels are not effective. Children and young adolescents may need higher serum levels (such as 1.5 mEq/L) because the difference between serum and brain lithium levels is greater in younger patients than in adults.
Consider the dosing schedule. Because lithium’s elimination half-life with repeated dosing is 24 hours, most adults can take any formulation once daily—which improves adherence and reduces adverse effects. Children eliminate lithium more rapidly and need more frequent dosing.
High loading doses result in more rapid control of agitation, probably as a result of sedation. In our experience, however, rapidly sedating patients may interfere with long-term adherence.
Carbamazepine, other anticonvulsants. Because they less sedating, carbamazepine and other anticonvulsants might not appear to be rapidly effective for bipolar mania. If you wait up to a month, however, any antimanic effect will be obvious.
Antipsychotics are rapidly effective for mania. Higher doses work faster but produce more side effects. After an acute response, some patients can be maintained on a second-generation antipsychotic (SGA), but others do better on a standard mood stabilizer such as lithium or valproate.
Calcium channel blockers. Verapamil has been effective mostly for lithium-responsive mania in 27 of 30 studies. Nimodipine has been useful for more complex bipolar syndromes in a few studies using patients as their own controls.
To be effective for bipolar disorder, however, calcium channel blockers require frequent, high dosing (such as verapamil, 120 mg 4 times daily, or nimodipine, 60 to 120 mg 6 times daily), which makes adherence difficult.
1 Is the patient taking anything that is making symptoms worse?
Antidepressants can induce mania, hypomania, and cycle acceleration in bipolar disorder, even when mood stabilizers are co-prescribed.3 Stimulants also may destabilize bipolar mood disorders; consider this possibility when patients taking stimulants for apparent attention-deficit/hyperactivity disorder at first appear to improve and then deteriorate.
Alcohol and cocaine can induce mania and depression. Cocaine is a potent kindling stimulus that could contribute to enduring mood instability.
2 Is the patient taking the medication?
Treatment adherence by bipolar patients may be as low as 35%.4 Ask outpatients what kinds of problems they have encountered taking medications, not whether they have such problems. Talk with the patient about adherence after each dosage increase, and be readily available. Prescribe extended-release pills for patients who have trouble keeping track of medications.
3 Is treatment adequate?
Adjust mood-stabilizer dosing until the patient responds or cannot tolerate the medication; complex cases often require combination treatment. Give the medication sufficient time to work; most mood stabilizers take ≥1 month to become fully effective.
4 Is another condition interfering with treatment?
Up to 70% of patients with refractory mood disorders have subclinical hypothyroidism. Look for:
- elevated thyroid stimulating hormone (TSH) with or without decreased thyroxine (T4)
- elevated TSH response to thyrotrop-inreleasing hormone (TRH).5
Also consider hypercalcemia from chronic lithium therapy,6 anemia, sleep apnea, posttraumatic stress disorder, substance use disorders, and personality disorders.
5 Am I ignoring psychotherapy?
Address psychosocial issues that influence the course of illness. Attend to patients’ important relationships, loss, negative thinking, and biological and social rhythms.
- consider augmentation first when a patient has had a partial response to a given medication
- switch when a patient cannot tolerate or shows no response to a therapeutic dose of a given medication.
Combinations of lithium and carbamazepine or valproate can be more effective than either drug alone, but therapeutic doses of each usually are needed. Carbamazepine has been used successfully to augment a partial response to nimodipine.9 In a small open-label trial, adding oxcarbazepine to lithium, valproate or antidepressants improved response in some patients with mild refractory mania.10
Switching among anticonvulsants can be useful because their actions and side effects differ. Clozapine in a wide range of doses can be very effective for refractory mania,11 but its use is difficult to monitor in highly agitated manic patients.
Other options. Electroconvulsive therapy (ECT) is the most effective treatment for mania, producing higher response rates than any antimanic drug.12 In a study of repetitive transcranial magnetic stimulation (rTMS), 8 of 9 patients with mania refractory to mood stabilizers had a sustained response after 1 month of right-sided rTMS treatment.13 Conversely, left-sided rTMS can aggravate mania.
Bipolar depression
Continuing controversy about the best way to treat bipolar depression makes it difficult to know if treatment has been suboptimal or a patient is treatment-resistant.
Antidepressants. No antidepressant is approved (or recommended) as monotherapy for bipolar depression, and most experts recommend against prescribing antidepressants without concomitant mood stabilizers. Even so:
- Clinicians prescribing monotherapy for bipolar disorder choose antidepressants twice as often as mood stabilizers.
- Antidepressants are prescribed more frequently in combination with mood stabilizers than as monotherapy, although empiric trials have shown most antidepressants are not effective for bipolar depression.14
In many cases, an antidepressant seems to help at first and then induces a recurrence of depression, often mixed with dysphoric hypomanic symptoms. The recurrent episode improves when the clinician increases the antidepressant dose or changes to another antidepressant, only to be followed by another recurrence that may be interpreted as an incomplete antidepressant response.
Antipsychotics. Quetiapine16 and a combination of olanzapine and fluoxetine17 are approved for treating bipolar depression. The studies supporting this indication lasted only 8 weeks, however, and excluded patients with the kinds of complicated and comorbid mood disorders commonly seen in clinical practice.
Many patients dropped out before the studies were completed, and “screen fails” (patients with the diagnosis who were not enrolled in the study) were not reported. In addition, “remitted” patients remained symptomatic.
Therefore, FDA approval of this indication does not guarantee these medications’ long-term efficacy or safety for bipolar depression or that they are useful in patients with complex forms of bipolar depression.
Recommended approach. Treatment resistance of bipolar depression to multiple mood stabilizers—with or without an antidepressant—or to an antipsychotic may manifest as lack of response, partial response, or initial good response followed by relapse or recurrence. Sometimes depression improves but irritability or mood lability worsen.
No reliable controlled studies have addressed complex refractory bipolar depression, but clinical experience suggests 1 approach for all of these responses:
Reconsider possible hypothyroidism. A low-normal T4—especially if decreased over time—and a mid-range or high-normal TSH—especially if increased—may indicate that subclinical hypothyroidism is inhibiting a response to mood stabilizers and antidepressants.18
Stop the antidepressant. If your patient is taking an antidepressant, it may be ineffective, creating mixed dysphoric hypomania, and/or driving another recurrence of depression. This is especially likely if the patient shows an initial prompt antidepressant response, but depression returns with irritability, insomnia, restlessness, or other subtle symptoms of dysphoric hypomania.
Withdraw the antidepressant gradually; for example, you might reduce the dose by 10% every few weeks so that the agent is discontinued across several months. Discontinuing an antidepressant too rapidly—even if it does not seem to be having any effect—can cause rebound depression that creates the mistaken impression that the antidepressant is needed.
Combine mood stabilizers. If a single mood stabilizer does not at least eliminate mood lability and other symptoms of activation, add a second agent. The combination of lithium and carbamazepine helps some depressed patients.20 Patients with considerable mood instability or psychotic symptoms may benefit from an adjunct antipsychotic.
Introduce mood stabilizers gradually. These medications may work more rapidly against mixed manic symptoms than they do against depression, especially when the dose is raised too quickly. The result is rapid control of irritability, hyperactivity, agitation, and related symptoms but an apparent increase in depression as mixed elements of elevated mood and energy are filtered out.
Add an antidepressant? If gradual adjustment of mood stabilizers eliminates mixed symptoms and mood fluctuations but the patient is still depressed, cautiously add an antidepressant. Antidepressants may be less likely to destabilize mood after all mixed elements have been treated completely.
Approximately 20% of bipolar patients are thought to experience rapid cycling, defined as ≥4 affective episodes/year separated by at least 2 weeks of euthymia between poles or with an immediate switch from one pole to the other.32 The prevalence of ultradian cycling—in which multiple brief affective episodes (usually subsyndromal or mixed) occur each day—is unclear.
Both cycling types probably represent stages in the evolution of bipolar mood disorders rather than distinct diagnoses. In many cases, mood cycling abates after months to years, but morbidity can be high and the wrong treatment may perpetuate mood cycling.
Complex mood cycling rarely responds to a single treatment, probably because its pathophysiology is complex. The need for polypharmacy may create the impression of treatment failure, but no one would expect a single medication to be sufficient for other complex illnesses such as cancer or AIDS.
21 Other medications have shown antidepressant effects in bipolar depression (Table).22-31 Although clinicians often use serotonin reuptake inhibitors, this practice has no empiric support in refractory bipolar depression—and our experience has not been particularly positive. Fluoxetine’s long half-life can perpetuate adverse effects long after the medication is withdrawn, and rebound depression is not uncommon when paroxetine or venlafaxine are withdrawn.
Some experts recommend discontinuing the antidepressant after depression remits to avoid driving more recurrences,3 but others do not think continuing antidepressants is risky. Apparently some patients do well with continued antidepressants, and others do not. In our experience, patients who have had mixed symptoms or mood lability are most likely to deteriorate with continued antidepressant treatment. Whenever depression returns after an initial and especially rapid response to an antidepressant, consider withdrawing the antidepressant and maximizing mood stabilizers first rather than changing or augmenting the antidepressant.
Treat seasonal symptoms. Many bipolar patients are most likely to be depressed in winter, and seasonal affective disorder is common in patients with a bipolar mood disorder. Their depression may respond to artificial bright light, usually given in the morning. Light therapy can help normalize the sleep-wake cycle, although it also can induce hypomania.
Other options. ECT is the most reliably effective treatment for bipolar depression. Because it treats both poles of the mood disorder, ECT also can be a useful maintenance treatment. A comparison of rTMS and placebo in 23 bipolar depressed patients failed to find any benefit of active treatment.32
Table
What now? Treatment options for refractory bipolar depression
| Treatment | Comment |
|---|---|
| Psychotherapy | Combine with somatic therapies for most patients with refractory mood disorders; adjunctive CBT, interpersonal and social rhythms therapy, or family-focused therapy speeded bipolar depression recovery in STEP-BD22 |
| Bupropion | Generally accepted as first-line antidepressant; the relatively low doses used may explain this agent’s lower risk of inducing mania compared with other antidepressants |
| MAO inhibitors | Can be combined with carbamazepine;23 tranylcypromine is best-studied antidepressant in bipolar depression and is especially useful for anergic states;24 selegiline also can be useful |
| Stimulants | Stimulants—such as methylphenidate, 15 to 30 mg/d—can be rapidly effective for lethargic, anergic depression (although evidence is limited); benefit wears off rapidly if mood is adversely affected |
| Pramipexole | Activating dopaminergic agent with rapid onset; investigational; has produced an antidepressant effect in patients with bipolar II depression when added to mood stabilizers25 |
| Modafinil | May be useful for residual fatigue in major depression and medication-induced sedation;26 improved depressive symptoms when used as an adjunct27 |
| Anticonvulsants | Anticonvulsants other than lamotrigine and carbamazepine-lithium combinations are considered later choices for bipolar depression; adjunctive zonisamide has been helpful in case series;28 gabapentin, pregabalin, and topiramate also can be useful adjuncts (although not supported by controlled studies in depression); adding levetiracetam may improve response29 |
| NMDA antagonist | Investigational; memantine30 was effective in a small controlled study, and riluzole (indicated for amyotrophic lateral sclerosis) was helpful in a small open study31 |
| CBT: cognitive-behavioral therapy; MAO: monoamine oxidase; NMDA: N-methyl-D-aspartate; STEP-BD: Systematic Treatment Enhancement Program for Bipolar Disorder | |
Rapid and ultradian cycling
No controlled studies have compared single-drug or combination therapies for rapid and ultradian cycling (Box 3).33 Thus, our recommendations for treating patients with cycling who have not responded to initial interventions are based on case series and clinical experience.
Reassess thyroid function. As many as 70% of patients with rapid cycling have subclinical hypothyroidism that contributes to mood instability.34 Thyroid replacement is indicated for any degree of hypothyroidism—even if medically unimportant—in patients with refractory mood disorders.
Slowly withdraw antidepressants. Most patients with rapid cycling are taking antidepressants. If your patient is experiencing depressive symptoms while taking an antidepressant, this means the antidepressant is not working and there is little point in continuing it. For patients being withdrawn from multiple antidepressants, rotate dose decrements to help you monitor the effect of each reduction.
Combine mood stabilizers. After optimizing the dose of a single mood stabilizer, add a second one from a different class. In an open trial, adding oxcarbazepine, up to 2,400 mg/d, helped approximately one-third of 20 patients with refractory mood cycling.10 Lithium is generally considered less effective than anticonvulsants in rapid cycling, but at least one study showed it was equivalent to carbamazepine for this problem.35 Lithium combined with other mood stabilizers may be more effective than lithium monotherapy in refractory bipolar states.
Other options to consider in combination with mood stabilizers:
- an antipsychotic, especially in the presence of psychotic symptoms, when mixed symptoms are present
- clozapine, which can be a highly effective adjunct for refractory mood cycling and mixed states36 (but is a later adjunct because of required monitoring, common adverse effects, and risk of interactions with carbamazepine and benzodiazepines)
- nimodipine, which has empiric support for complex mood cycling37 and is well-tolerated with fewer interactions than other mood stabilizers (but cost and need for frequent dosing make it a second-line adjunct)
- supraphysiologic doses of thyroxine (≤0.4 mg/d, with T4 levels in the hyperthyroid range), which can improve response to mood-stabilizing regimens34 (but risks of inducing hyperthyroidism make this intervention third-line).
Related resources
- Dubovsky SL. Clinical guide to psychotropic medications. New York: WW Norton; 2005.
- Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin North Am 2005;28:349-70.
- Phillip Long, MD. Internet Mental Health. Online psychiatric diagnosis for the two-thirds of individuals with mental illness who do not seek treatment. www.mentalhealth.com/dis/p20-md02.html.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium • Lithobid, others
- Lorazepam • Ativan
- Memantine • Namenda
- Methylphenidate • Concerta, Ritalin, others
- Modafinil • Provigil
- Nimodipine • Nimotop
- Olanzapine/fluoxetine • Symbyax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Pregabalin • Lyrica
- Quetiapine • Seroquel
- Riluzole • Rilutek
- Selegiline • Eldepryl
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
- Verapamil • Calan, Isoptin, others
- Zonisamide • Zonegran
Dr. Mostert reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Dubovsky receives research/grant support from Eli Lilly and Company, Organon, Pfizer, UCB Pharma, anhd Forest Laboratories. He is a consultant to Oganon and Biovail Pharmaceuticals.
All phases of bipolar disorder can be difficult to treat, and patients remain symptomatic on average about half the time.1 Not all bipolar patients who experience continued illness and disability are treatment-resistant (Box 1), but when symptoms persist you may ask yourself: Was treatment suboptimal or simply ineffective?
Patients with severe symptoms may be satisfied with a substantial decrease in symptoms, but any residual symptoms cause ongoing distress and lower the threshold for recurrences.2 Finding the right combination of therapies for your patient is key to achieving an enduring response.
Future studies may tell us which treatments to combine and in what sequence for complex bipolar disorder, but—since most published studies exclude complex and comorbid cases—for now we must rely on limited controlled data and clinical experience. Using those resources, we offer comprehensive, practical recommendations for trouble-shooting (Box 2)3-6 and getting better results when bipolar disorder does not respond to standard treatment.
Some studies define treatment resistance as failure to respond to lithium, and in other settings it is viewed as failure to respond to ≥2 treatment courses. Because euthymia and normal functioning are important for long-term prognosis, we define treatment-resistance as failure to achieve both symptomatic and functional remission following an adequate course of therapy.
Effective strategies for treating bipolar disorder depend on:
- illness phase (later episodes are more difficult to treat than earlier ones)
- symptom complexity (mixed symptoms probably reflect more complex pathophysiology and are more likely to require combination therapies)
- predominant presentations (mania, depression, rapid and ultradian cycling)
- whether symptoms are acute or chronic.
Unfortunately, the findings of and strategies used in clinical trials of refractory bipolar disorder are difficult to extrapolate to everyday practice. Most studies exclude patients with a history of treatment resistance, severe symptoms, and important comorbidities such as substance abuse. In addition, the usual primary endpoint is response (≥50% reduction of symptoms) rather than remission (minimal symptoms and no longer meeting criteria for the disorder). Very few studies address functional remission, which is necessary to reduce the risk of symptomatic recurrence.
In clinical practice, when initial treatment for bipolar disorder fails to produce remission, systematically addressing 5 questions (Box 2) can help direct your next step.
Mania
When a patient with mania does not respond as expected, the next step depends on which antimanic agent you prescribed:
Lithium can take a month to become fully effective for mania, which is why a benzodiazepine or antipsychotic is often added acutely to reduce agitation. Do not mistake neurotoxic interactions between lithium and antipsychotics for increased mania.
Although data vary on lithium’s optimal serum level, adjust to approximately 0.8 to 1 mEq/L, if tolerated, when lower levels are not effective. Children and young adolescents may need higher serum levels (such as 1.5 mEq/L) because the difference between serum and brain lithium levels is greater in younger patients than in adults.
Consider the dosing schedule. Because lithium’s elimination half-life with repeated dosing is 24 hours, most adults can take any formulation once daily—which improves adherence and reduces adverse effects. Children eliminate lithium more rapidly and need more frequent dosing.
High loading doses result in more rapid control of agitation, probably as a result of sedation. In our experience, however, rapidly sedating patients may interfere with long-term adherence.
Carbamazepine, other anticonvulsants. Because they less sedating, carbamazepine and other anticonvulsants might not appear to be rapidly effective for bipolar mania. If you wait up to a month, however, any antimanic effect will be obvious.
Antipsychotics are rapidly effective for mania. Higher doses work faster but produce more side effects. After an acute response, some patients can be maintained on a second-generation antipsychotic (SGA), but others do better on a standard mood stabilizer such as lithium or valproate.
Calcium channel blockers. Verapamil has been effective mostly for lithium-responsive mania in 27 of 30 studies. Nimodipine has been useful for more complex bipolar syndromes in a few studies using patients as their own controls.
To be effective for bipolar disorder, however, calcium channel blockers require frequent, high dosing (such as verapamil, 120 mg 4 times daily, or nimodipine, 60 to 120 mg 6 times daily), which makes adherence difficult.
1 Is the patient taking anything that is making symptoms worse?
Antidepressants can induce mania, hypomania, and cycle acceleration in bipolar disorder, even when mood stabilizers are co-prescribed.3 Stimulants also may destabilize bipolar mood disorders; consider this possibility when patients taking stimulants for apparent attention-deficit/hyperactivity disorder at first appear to improve and then deteriorate.
Alcohol and cocaine can induce mania and depression. Cocaine is a potent kindling stimulus that could contribute to enduring mood instability.
2 Is the patient taking the medication?
Treatment adherence by bipolar patients may be as low as 35%.4 Ask outpatients what kinds of problems they have encountered taking medications, not whether they have such problems. Talk with the patient about adherence after each dosage increase, and be readily available. Prescribe extended-release pills for patients who have trouble keeping track of medications.
3 Is treatment adequate?
Adjust mood-stabilizer dosing until the patient responds or cannot tolerate the medication; complex cases often require combination treatment. Give the medication sufficient time to work; most mood stabilizers take ≥1 month to become fully effective.
4 Is another condition interfering with treatment?
Up to 70% of patients with refractory mood disorders have subclinical hypothyroidism. Look for:
- elevated thyroid stimulating hormone (TSH) with or without decreased thyroxine (T4)
- elevated TSH response to thyrotrop-inreleasing hormone (TRH).5
Also consider hypercalcemia from chronic lithium therapy,6 anemia, sleep apnea, posttraumatic stress disorder, substance use disorders, and personality disorders.
5 Am I ignoring psychotherapy?
Address psychosocial issues that influence the course of illness. Attend to patients’ important relationships, loss, negative thinking, and biological and social rhythms.
- consider augmentation first when a patient has had a partial response to a given medication
- switch when a patient cannot tolerate or shows no response to a therapeutic dose of a given medication.
Combinations of lithium and carbamazepine or valproate can be more effective than either drug alone, but therapeutic doses of each usually are needed. Carbamazepine has been used successfully to augment a partial response to nimodipine.9 In a small open-label trial, adding oxcarbazepine to lithium, valproate or antidepressants improved response in some patients with mild refractory mania.10
Switching among anticonvulsants can be useful because their actions and side effects differ. Clozapine in a wide range of doses can be very effective for refractory mania,11 but its use is difficult to monitor in highly agitated manic patients.
Other options. Electroconvulsive therapy (ECT) is the most effective treatment for mania, producing higher response rates than any antimanic drug.12 In a study of repetitive transcranial magnetic stimulation (rTMS), 8 of 9 patients with mania refractory to mood stabilizers had a sustained response after 1 month of right-sided rTMS treatment.13 Conversely, left-sided rTMS can aggravate mania.
Bipolar depression
Continuing controversy about the best way to treat bipolar depression makes it difficult to know if treatment has been suboptimal or a patient is treatment-resistant.
Antidepressants. No antidepressant is approved (or recommended) as monotherapy for bipolar depression, and most experts recommend against prescribing antidepressants without concomitant mood stabilizers. Even so:
- Clinicians prescribing monotherapy for bipolar disorder choose antidepressants twice as often as mood stabilizers.
- Antidepressants are prescribed more frequently in combination with mood stabilizers than as monotherapy, although empiric trials have shown most antidepressants are not effective for bipolar depression.14
In many cases, an antidepressant seems to help at first and then induces a recurrence of depression, often mixed with dysphoric hypomanic symptoms. The recurrent episode improves when the clinician increases the antidepressant dose or changes to another antidepressant, only to be followed by another recurrence that may be interpreted as an incomplete antidepressant response.
Antipsychotics. Quetiapine16 and a combination of olanzapine and fluoxetine17 are approved for treating bipolar depression. The studies supporting this indication lasted only 8 weeks, however, and excluded patients with the kinds of complicated and comorbid mood disorders commonly seen in clinical practice.
Many patients dropped out before the studies were completed, and “screen fails” (patients with the diagnosis who were not enrolled in the study) were not reported. In addition, “remitted” patients remained symptomatic.
Therefore, FDA approval of this indication does not guarantee these medications’ long-term efficacy or safety for bipolar depression or that they are useful in patients with complex forms of bipolar depression.
Recommended approach. Treatment resistance of bipolar depression to multiple mood stabilizers—with or without an antidepressant—or to an antipsychotic may manifest as lack of response, partial response, or initial good response followed by relapse or recurrence. Sometimes depression improves but irritability or mood lability worsen.
No reliable controlled studies have addressed complex refractory bipolar depression, but clinical experience suggests 1 approach for all of these responses:
Reconsider possible hypothyroidism. A low-normal T4—especially if decreased over time—and a mid-range or high-normal TSH—especially if increased—may indicate that subclinical hypothyroidism is inhibiting a response to mood stabilizers and antidepressants.18
Stop the antidepressant. If your patient is taking an antidepressant, it may be ineffective, creating mixed dysphoric hypomania, and/or driving another recurrence of depression. This is especially likely if the patient shows an initial prompt antidepressant response, but depression returns with irritability, insomnia, restlessness, or other subtle symptoms of dysphoric hypomania.
Withdraw the antidepressant gradually; for example, you might reduce the dose by 10% every few weeks so that the agent is discontinued across several months. Discontinuing an antidepressant too rapidly—even if it does not seem to be having any effect—can cause rebound depression that creates the mistaken impression that the antidepressant is needed.
Combine mood stabilizers. If a single mood stabilizer does not at least eliminate mood lability and other symptoms of activation, add a second agent. The combination of lithium and carbamazepine helps some depressed patients.20 Patients with considerable mood instability or psychotic symptoms may benefit from an adjunct antipsychotic.
Introduce mood stabilizers gradually. These medications may work more rapidly against mixed manic symptoms than they do against depression, especially when the dose is raised too quickly. The result is rapid control of irritability, hyperactivity, agitation, and related symptoms but an apparent increase in depression as mixed elements of elevated mood and energy are filtered out.
Add an antidepressant? If gradual adjustment of mood stabilizers eliminates mixed symptoms and mood fluctuations but the patient is still depressed, cautiously add an antidepressant. Antidepressants may be less likely to destabilize mood after all mixed elements have been treated completely.
Approximately 20% of bipolar patients are thought to experience rapid cycling, defined as ≥4 affective episodes/year separated by at least 2 weeks of euthymia between poles or with an immediate switch from one pole to the other.32 The prevalence of ultradian cycling—in which multiple brief affective episodes (usually subsyndromal or mixed) occur each day—is unclear.
Both cycling types probably represent stages in the evolution of bipolar mood disorders rather than distinct diagnoses. In many cases, mood cycling abates after months to years, but morbidity can be high and the wrong treatment may perpetuate mood cycling.
Complex mood cycling rarely responds to a single treatment, probably because its pathophysiology is complex. The need for polypharmacy may create the impression of treatment failure, but no one would expect a single medication to be sufficient for other complex illnesses such as cancer or AIDS.
21 Other medications have shown antidepressant effects in bipolar depression (Table).22-31 Although clinicians often use serotonin reuptake inhibitors, this practice has no empiric support in refractory bipolar depression—and our experience has not been particularly positive. Fluoxetine’s long half-life can perpetuate adverse effects long after the medication is withdrawn, and rebound depression is not uncommon when paroxetine or venlafaxine are withdrawn.
Some experts recommend discontinuing the antidepressant after depression remits to avoid driving more recurrences,3 but others do not think continuing antidepressants is risky. Apparently some patients do well with continued antidepressants, and others do not. In our experience, patients who have had mixed symptoms or mood lability are most likely to deteriorate with continued antidepressant treatment. Whenever depression returns after an initial and especially rapid response to an antidepressant, consider withdrawing the antidepressant and maximizing mood stabilizers first rather than changing or augmenting the antidepressant.
Treat seasonal symptoms. Many bipolar patients are most likely to be depressed in winter, and seasonal affective disorder is common in patients with a bipolar mood disorder. Their depression may respond to artificial bright light, usually given in the morning. Light therapy can help normalize the sleep-wake cycle, although it also can induce hypomania.
Other options. ECT is the most reliably effective treatment for bipolar depression. Because it treats both poles of the mood disorder, ECT also can be a useful maintenance treatment. A comparison of rTMS and placebo in 23 bipolar depressed patients failed to find any benefit of active treatment.32
Table
What now? Treatment options for refractory bipolar depression
| Treatment | Comment |
|---|---|
| Psychotherapy | Combine with somatic therapies for most patients with refractory mood disorders; adjunctive CBT, interpersonal and social rhythms therapy, or family-focused therapy speeded bipolar depression recovery in STEP-BD22 |
| Bupropion | Generally accepted as first-line antidepressant; the relatively low doses used may explain this agent’s lower risk of inducing mania compared with other antidepressants |
| MAO inhibitors | Can be combined with carbamazepine;23 tranylcypromine is best-studied antidepressant in bipolar depression and is especially useful for anergic states;24 selegiline also can be useful |
| Stimulants | Stimulants—such as methylphenidate, 15 to 30 mg/d—can be rapidly effective for lethargic, anergic depression (although evidence is limited); benefit wears off rapidly if mood is adversely affected |
| Pramipexole | Activating dopaminergic agent with rapid onset; investigational; has produced an antidepressant effect in patients with bipolar II depression when added to mood stabilizers25 |
| Modafinil | May be useful for residual fatigue in major depression and medication-induced sedation;26 improved depressive symptoms when used as an adjunct27 |
| Anticonvulsants | Anticonvulsants other than lamotrigine and carbamazepine-lithium combinations are considered later choices for bipolar depression; adjunctive zonisamide has been helpful in case series;28 gabapentin, pregabalin, and topiramate also can be useful adjuncts (although not supported by controlled studies in depression); adding levetiracetam may improve response29 |
| NMDA antagonist | Investigational; memantine30 was effective in a small controlled study, and riluzole (indicated for amyotrophic lateral sclerosis) was helpful in a small open study31 |
| CBT: cognitive-behavioral therapy; MAO: monoamine oxidase; NMDA: N-methyl-D-aspartate; STEP-BD: Systematic Treatment Enhancement Program for Bipolar Disorder | |
Rapid and ultradian cycling
No controlled studies have compared single-drug or combination therapies for rapid and ultradian cycling (Box 3).33 Thus, our recommendations for treating patients with cycling who have not responded to initial interventions are based on case series and clinical experience.
Reassess thyroid function. As many as 70% of patients with rapid cycling have subclinical hypothyroidism that contributes to mood instability.34 Thyroid replacement is indicated for any degree of hypothyroidism—even if medically unimportant—in patients with refractory mood disorders.
Slowly withdraw antidepressants. Most patients with rapid cycling are taking antidepressants. If your patient is experiencing depressive symptoms while taking an antidepressant, this means the antidepressant is not working and there is little point in continuing it. For patients being withdrawn from multiple antidepressants, rotate dose decrements to help you monitor the effect of each reduction.
Combine mood stabilizers. After optimizing the dose of a single mood stabilizer, add a second one from a different class. In an open trial, adding oxcarbazepine, up to 2,400 mg/d, helped approximately one-third of 20 patients with refractory mood cycling.10 Lithium is generally considered less effective than anticonvulsants in rapid cycling, but at least one study showed it was equivalent to carbamazepine for this problem.35 Lithium combined with other mood stabilizers may be more effective than lithium monotherapy in refractory bipolar states.
Other options to consider in combination with mood stabilizers:
- an antipsychotic, especially in the presence of psychotic symptoms, when mixed symptoms are present
- clozapine, which can be a highly effective adjunct for refractory mood cycling and mixed states36 (but is a later adjunct because of required monitoring, common adverse effects, and risk of interactions with carbamazepine and benzodiazepines)
- nimodipine, which has empiric support for complex mood cycling37 and is well-tolerated with fewer interactions than other mood stabilizers (but cost and need for frequent dosing make it a second-line adjunct)
- supraphysiologic doses of thyroxine (≤0.4 mg/d, with T4 levels in the hyperthyroid range), which can improve response to mood-stabilizing regimens34 (but risks of inducing hyperthyroidism make this intervention third-line).
Related resources
- Dubovsky SL. Clinical guide to psychotropic medications. New York: WW Norton; 2005.
- Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin North Am 2005;28:349-70.
- Phillip Long, MD. Internet Mental Health. Online psychiatric diagnosis for the two-thirds of individuals with mental illness who do not seek treatment. www.mentalhealth.com/dis/p20-md02.html.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium • Lithobid, others
- Lorazepam • Ativan
- Memantine • Namenda
- Methylphenidate • Concerta, Ritalin, others
- Modafinil • Provigil
- Nimodipine • Nimotop
- Olanzapine/fluoxetine • Symbyax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Pregabalin • Lyrica
- Quetiapine • Seroquel
- Riluzole • Rilutek
- Selegiline • Eldepryl
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
- Verapamil • Calan, Isoptin, others
- Zonisamide • Zonegran
Dr. Mostert reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Dubovsky receives research/grant support from Eli Lilly and Company, Organon, Pfizer, UCB Pharma, anhd Forest Laboratories. He is a consultant to Oganon and Biovail Pharmaceuticals.
1. Judd JL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003;61:261-9.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEPBD). Am J Psychiatry 2006;163:217-24.
3. Altschuler LL, Post RM, Leverich GS. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152:1130-8.
4. Osterberg L, Blaschke T. Drug therapy: adherence to medication. N Engl J Med 2005;353:487-97.
5. Kusalic M. Grade II and grade III hypothyroidism in rapid cycling bipolar patients. Biol Psychiatry 1992;25:177-81.
6. Franks RD, Dubovsky SL, Lifshitz M, et al. Long-term lithium carbonate therapy causes hyperparathyroidism. Arch Gen Psychiatry 1982;39:1074-7.
7. Allen MH, Hirschfeld RMA, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry 2006;163:272-5.
8. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially responsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
9. Pazzaglia P, Post RM, Ketter TA, et al. Nimodipine monotherapy and carbamazepine augmentation in patients with refractory recurrent affective illness. J Clin Psychopharmacol 1998;18:404-13.
10. Conway CR, Chibnall JT, Nelson LA, et al. An open-label trial of adjunctive oxcarbazepine for bipolar disorder. J Clin Psychopharmacol 2006;26:95-7.
11. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153:759-64.
12. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episodes: a review of 50 years’ experience. Am J Psychiatry 1994;151:169-76.
13. Michael N, Erfurth A. Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Disord 2004;78:253-7.
14. Baldessarini RJ, Leahy L, Arcona S, et al. Patterns of psychotropic drug prescription for U.S. patients with diagnoses of bipolar disorders. Psychiatr Serv 2007;58:85-91.
15. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007;356:1711-22.
16. Cookson J, Keck PE, Jr, Ketter TA, Macfadden W. Number needed to treat and time to response/remission for quetiapine monotherapy efficacy in acute bipolar depression: evidence from a large, randomized, placebo-controlled study. Int Clin Psychopharmacol 2007;22(2):93-100.
17. Tohen M, Vieta E, Calabrese JR, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
18. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry 2002;159:116-21.
19. Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet 2007;369:935-45.
20. Kishimoto A. The treatment of affective disorder with carbamazepine: prophylactic synergism of lithium and carbamazepine combination. Prog Neuropsychopharmacol Biol Psychiatry 1992;16:483-93.
21. McElroy SL, Zarate CA, Cookson J, et al. A 52-week, open-label continuation study of lamotrigine in the treatment of bipolar depression. J Clin Psychiatry 2004;65:204-10.
22. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry 2007;64:419-26.
23. Ketter TA, Post RM, Parekh PI, Worthington K. Addition of monoamine oxidase inhibitors to carbamazepine: preliminary evidence of safety and antidepressant efficacy in treatment-resistant depression. J Clin Psychiatry 1995;56:471-5.
24. Himmelhoch JM, Thase ME, Mallinger AG, Houck PR. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991;148:910-6.
25. Zarate CAJ, Payne JL, Singh J, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 2004;56:54-60.
26. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother 2007;41:1005-12.
27. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164:1242-9.
28. Anand A, Bukhari L, Jennings SA, et al. A preliminary open-label study of zonisamide treatment for bipolar depression in 10 patients. J Clin Psychiatry 2005;66:195-8.
29. Post RM, Altshuler LL, Frye MA, et al. Preliminary observations on the effectiveness of levetiracetam in the open adjunctive treatment of refractory bipolar disorder. J Clin Psychiatry 2005;66:370-4.
30. Zarate CAJ, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006;63:856-64.
31. Zarate CAJ, Quiroz JA, Singh JB, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 2005;57:430-2.
32. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord 2003;5:40-7.
33. Schneck CD. Treatment of rapid-cycling bipolar disorder. J Clin Psychiatry 2006;67(suppl 11):22-7.
34. Bauer MS, Whybrow PC, Winokur A. Rapid cycling bipolar affective disorder, I: Association with grade I hypothyroidism. Arch Gen Psychiatry 1990;47:427-32.
35. Okuma T, Yamashita I, Takahashi R, et al. Comparison of the antimanic efficacy of carbamazepine and lithium carbonate by double-blind controlled study. Pharmacopsychiatry 1990;23:143-50.
36. Calabrese JR, Meltzer HY, Markovitz PJ. Clozapine prophylaxis in rapid cycling bipolar disorder. J Clin Psychopharmacol 1991;11:396-7.
37. Goodnick PJ. Nimodipine treatment of rapid cycling bipolar disorder. J Clin Psychiatry 1995;56:330.-
1. Judd JL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003;61:261-9.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEPBD). Am J Psychiatry 2006;163:217-24.
3. Altschuler LL, Post RM, Leverich GS. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152:1130-8.
4. Osterberg L, Blaschke T. Drug therapy: adherence to medication. N Engl J Med 2005;353:487-97.
5. Kusalic M. Grade II and grade III hypothyroidism in rapid cycling bipolar patients. Biol Psychiatry 1992;25:177-81.
6. Franks RD, Dubovsky SL, Lifshitz M, et al. Long-term lithium carbonate therapy causes hyperparathyroidism. Arch Gen Psychiatry 1982;39:1074-7.
7. Allen MH, Hirschfeld RMA, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry 2006;163:272-5.
8. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially responsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
9. Pazzaglia P, Post RM, Ketter TA, et al. Nimodipine monotherapy and carbamazepine augmentation in patients with refractory recurrent affective illness. J Clin Psychopharmacol 1998;18:404-13.
10. Conway CR, Chibnall JT, Nelson LA, et al. An open-label trial of adjunctive oxcarbazepine for bipolar disorder. J Clin Psychopharmacol 2006;26:95-7.
11. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153:759-64.
12. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episodes: a review of 50 years’ experience. Am J Psychiatry 1994;151:169-76.
13. Michael N, Erfurth A. Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Disord 2004;78:253-7.
14. Baldessarini RJ, Leahy L, Arcona S, et al. Patterns of psychotropic drug prescription for U.S. patients with diagnoses of bipolar disorders. Psychiatr Serv 2007;58:85-91.
15. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007;356:1711-22.
16. Cookson J, Keck PE, Jr, Ketter TA, Macfadden W. Number needed to treat and time to response/remission for quetiapine monotherapy efficacy in acute bipolar depression: evidence from a large, randomized, placebo-controlled study. Int Clin Psychopharmacol 2007;22(2):93-100.
17. Tohen M, Vieta E, Calabrese JR, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
18. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry 2002;159:116-21.
19. Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet 2007;369:935-45.
20. Kishimoto A. The treatment of affective disorder with carbamazepine: prophylactic synergism of lithium and carbamazepine combination. Prog Neuropsychopharmacol Biol Psychiatry 1992;16:483-93.
21. McElroy SL, Zarate CA, Cookson J, et al. A 52-week, open-label continuation study of lamotrigine in the treatment of bipolar depression. J Clin Psychiatry 2004;65:204-10.
22. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry 2007;64:419-26.
23. Ketter TA, Post RM, Parekh PI, Worthington K. Addition of monoamine oxidase inhibitors to carbamazepine: preliminary evidence of safety and antidepressant efficacy in treatment-resistant depression. J Clin Psychiatry 1995;56:471-5.
24. Himmelhoch JM, Thase ME, Mallinger AG, Houck PR. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991;148:910-6.
25. Zarate CAJ, Payne JL, Singh J, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 2004;56:54-60.
26. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother 2007;41:1005-12.
27. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164:1242-9.
28. Anand A, Bukhari L, Jennings SA, et al. A preliminary open-label study of zonisamide treatment for bipolar depression in 10 patients. J Clin Psychiatry 2005;66:195-8.
29. Post RM, Altshuler LL, Frye MA, et al. Preliminary observations on the effectiveness of levetiracetam in the open adjunctive treatment of refractory bipolar disorder. J Clin Psychiatry 2005;66:370-4.
30. Zarate CAJ, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006;63:856-64.
31. Zarate CAJ, Quiroz JA, Singh JB, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 2005;57:430-2.
32. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord 2003;5:40-7.
33. Schneck CD. Treatment of rapid-cycling bipolar disorder. J Clin Psychiatry 2006;67(suppl 11):22-7.
34. Bauer MS, Whybrow PC, Winokur A. Rapid cycling bipolar affective disorder, I: Association with grade I hypothyroidism. Arch Gen Psychiatry 1990;47:427-32.
35. Okuma T, Yamashita I, Takahashi R, et al. Comparison of the antimanic efficacy of carbamazepine and lithium carbonate by double-blind controlled study. Pharmacopsychiatry 1990;23:143-50.
36. Calabrese JR, Meltzer HY, Markovitz PJ. Clozapine prophylaxis in rapid cycling bipolar disorder. J Clin Psychopharmacol 1991;11:396-7.
37. Goodnick PJ. Nimodipine treatment of rapid cycling bipolar disorder. J Clin Psychiatry 1995;56:330.-
How to reduce mania risk when prescribing stimulants
Stimulants are most effective for childhood attention-deficit/hyperactivity disorder (ADHD),1 but they may induce mania or trigger a treatment-resistant course in children with comorbid bipolar disorder. To help you safely manage these complicated symptoms, this article offers a treatment algorithm and tips to:
- differentiate bipolar and ADHD symptoms
- identify patients at risk for stimulantinduced mania
- choose medications by a hierarchythat may reduce the risk of mood destabilization.
Bipolar mood symptoms emerge before age 20 in about 25% of persons with bipolar disorder (BP).3 Early-onset BP may be more severe than the adult-onset form, with more-affected family members and greater comorbidity with other disorders, especially ADHD.4
In one study, 91% of children with BP also met criteria for ADHD, and 19% of patients with ADHD also received a diagnosis of BP.5 Among 31 children ages 2 to 5 with BP, 80% met criteria for concurrent ADHD.6
Of 40 children age <5 presenting consecutively to a mental health clinic, 11 (28%) met criteria for mania, which was usually associated with euphoria.7 These 11 children also met criteria for ADHD.
A comparison study8 of children (mean age 12) found greater impairment, suicidality, irritability, and sadness in 43 with ADHD plus bipolar depression than in:
- 109 with ADHD plus major depressive disorder
- 128 without depression or mania.
Family prevalence of bipolar disorder and major depression was highest in the bipolar-ADHD group, which also had the highest rates of comorbid conduct disorder, oppositional defiant disorder, alcohol abuse, and agoraphobia. Average age of bipolar diagnosis was 6.3 years.
Adhd and/or bipolar disorder?
Some 70% to 90% of bipolar children and at least 30% to 40% of bipolar adolescents also have ADHD.2 This high comorbidity (Box 1)3-8 might mean that:
- one disorder predisposes to the other
- one is a precursor of the other
- they share common vulnerabilities or causes
- their symptoms overlap so much that patients with one disorder appear to meet criteria for the other.
Some experts contend that bipolar disorder and ADHD usually can be differentiated. Bipolar children score higher than those with ADHD on measures of anxiety/depression, aggression, and attention problems on the Child Behavior Checklist.9 Others believe ADHD symptoms that occur with bipolar disorder are a dimension of bipolar illness rather than a separate disorder.10
For every DSM-IV-TR diagnostic criterion for ADHD, a corresponding diagnostic criterion or common feature of bipolar disorder can be identified (Table 1). Mania and hypomania are obviously associated with hyperactivity and impulsivity, and tangential thinking and distractibility interfere with attention in many patients with bipolar disorder.
Though most ADHD symptoms can occur in bipolar patients, some features of bipolar illness are not characteristic of ADHD (Table 2). Children with ADHD can become hyper-focused on video games and television, for example, but they usually do not become engrossed in long, complicated books or preoccupied with other people, as can occur in bipolar disorder.
Table 1
How ADHD, bipolar symptoms overlap in three domains
| ADHD | Bipolar disorder |
|---|---|
| Inattention | |
| Fails to pay attention | Racing and tangential thoughts |
| Difficulty sustaining attention | Attention driven by racing thoughts, affective themes, and psychosis |
| Does not follow through | Direction of activity shifts with shifting mood |
| Difficulty organizing tasks | Disorganization, psychosis, excessive energy |
| Easily distracted | Distractibility |
| Hyperactivity | |
| Fidgets or squirms | Increased energy and activity |
| Runs about or climbs excessively | Hyperactivity, thrill-seeking |
| Difficulty engaging quietly in leisure activities | Increased energy, boredom |
| Often on the go | Increased energy, hyperactivity |
| Talks excessively | Rapid, pressured speech |
| Impulsivity | |
| Blurts out answers | Rapid, pressured, impulsive speech |
| Difficulty awaiting turn | Hyperactivity, increased energy, impatience, grandiosity |
| Interrupts or intrudes on others | Grandiosity, impatience, pressured speech, increased mental content |
Table 2
Bipolar features not seen in ADHD
|
A treatment hierarchy
Whether a bipolar patient’s attention problems are features of the primary condition or caused by comorbid ADHD may be unclear, but the treatment implications are important. All antidepressants can induce mania/hypomania and increase the risk of mixed states and mood cycling. Because stimulants have antidepressant properties and because some antidepressants are used to treat ADHD, a systematic approach is necessary when treating inattention in juvenile bipolar disorder.
A treatment hierarchy developed by the American Academy of Child and Adolescent Psychiatry Workgroup on Bipolar Disorder recommends beginning psychosocial approaches, such as training parents in behavior management techniques, and:
- treating bipolar disorder first in children who clearly have both ADHD and bipolar disorder
- adding ADHD treatment if ADHD symptoms persist and impair functioning.2
Who’s at risk for mood destabilization?
No data address differences between bipolar patients whose mood disorders deteriorate with stimulant use and those who remain stable. However, risk factors for mood destabilization that have been reported with antidepressants likely also apply to stimulants (Table 3) because stimulants’ adverse effects in bipolar disorder are probably related to their antidepressant properties.
For example, depressed patients who report that an antidepressant worked within hours to days may have bipolar disorder and be at risk for mood destabilization leading to treatment resistance.11 Antidepressant-induced mania also may be more likely:
- when depression is mixed with hypomanic symptoms such as racing thoughts, excessive talkativeness, aggression, irritability, distractibility, and increased drive12
- in patients with a history of antidepressant-induced mania, family history of bipolar disorder, or multiple antidepressant trials.13
Similarly, patients who report feeling better immediately after starting a stimulant—especially if they have evidence of elation, increased irritability, more aggression or impulsivity, decreased sleep, or related symptoms—may be developing stimulant-induced hypomania.
Table 3
Risk factors that may increase risk of stimulant-induced mania
|
| Source: Reference 25 |
Antidepressant-induced mania
Most studies of antidepressant-induced mania have examined outright mania, but hypomania and subsyndromal hypomanic syndromes also may cause significant morbidity and may worsen bipolar disorder’s course. A change in polarity may worsen a patient’s prognosis, but how do we know that antidepressants (or stimulants) caused it?
One suggested criterion is that mania or hypomania develops within 8 weeks of starting an antidepressant for the first time. A chart review of 51 bipolar patients who had extensive life charting found that 82% developed mania while taking an antidepressant—35% of them within 8 weeks.14 The authors attributed 50% of the risk of a first manic episode and/or cycle acceleration to antidepressants and 50% to spontaneous mood swings. They also noted that:
- an initial manic episode appeared to sensitize patients to subsequent manic episodes and rapid cycling
- mood stabilizers did not seem to prevent these outcomes.
A meta-analysis of 12 randomized, controlled, 4-to 12-week trials among 1,088 patients found antidepressants no more likely than placebo to induce mania in the short term.15 These trials did not, however, consider less-severe forms of overstimulation and were not designed to determine mania risk in bipolar depressed patients.
Post-mania cycling. Rapid and ultradian cycling and other forms of deterioration are more likely to occur after a manic or hypomanic episode than after a depressive episode.16
A longitudinal study17 indicated that antidepressant use did not predictably predate rapid cycling when depression was controlled. The authors, however, looked at the correlation between taking an antidepressant at study entry and rapid cycling over 1 year but did not examine whether antidepressants were started or stopped during the study.18 Rapid cycling prevalence declined from 19% to 5% during the study, but they did not determine whether withdrawing antidepressants was associated with this change.
In an earlier prospective study, rapid cycling was more severe while patients were taking antidepressants—despite the use of mood stabilizers—and cycling duration decreased when antidepressants were withdrawn.19
TCAs vs. newer agents. Tricyclic antidepressants (TCAs) are perceived as more likely to induce mania than are selective serotonin reuptake inhibitors (SSRIs) or bupropion. Comparing TCAs’ and newer antidepressants’ switch rates is difficult, however. Most antidepressant trials were designed to show efficacy and safety in unipolar, not bipolar, depression. Moreover, as exclusion criteria have improved with greater awareness of bipolar illness’ polymorphic manifestations, recent studies likely have enrolled fewer bipolar patients—who are most at risk to develop a manic switch—than did earlier TCA trials.
Bupropion, which has been used to treat ADHD, has been thought to have a low risk of inducing mania. In open observation, however, >50% of 11 patients with a history of developing mania with other antidepressants also had a manic switch on bupropion, even though they were taking mood stabilizers.20
Analysis of 155 antidepressant trials in 41 depressed patients found mania risk to be similar with bupropion, SSRIs, TCAs, monoamine oxidase inhibitors (MAOIs), and other newer antidepressants.21 Mania risk doubled when patients were not also taking mood stabilizers.
Going without mood stabilizers. Reports have emerged of patients with bipolar depression taking antidepressants such as fluoxetine and venlafaxine without a mood stabilizer for extended periods, without high rates of mania or mood cycling.22-24 These reports suggest that some bipolar depressed patients can tolerate antidepressants without a mood stabilizer, although we have no way to identify such patients in advance.
Cycle acceleration and treatment resistance may follow antidepressant-induced mania.25 In DSM-IV field trials, antidepressants appeared to have triggered rapid cycling in some 20% of bipolar patients.26 Mood stabilizers were not particularly effective in patients with treatment-resistant ultradian cycling, but withdrawing antidepressants improved outcome.27
Stimulant-induced mania
Compared with antidepressants, less information is available about stimulant-induced mania and rapid cycling.
Some carefully selected bipolar patients may tolerate ongoing stimulant treatment. For example, in 2 years of open experience with 5 bipolar type I and 3 bipolar type II adults, adding methylphenidate or amphetamine for residual depression or sedation was moderately helpful and did not lead to manic switching or drug misuse.28
On the other hand, affective symptoms worsened in nearly two-thirds of 31 children ages 2 to 5 when treated with stimulants or antidepressants without mood stabilizers. Most of the children also had ADHD, and valproate usually helped.6
In 40 patients, mean age 10, who entered the open-label phase of an 8-week trial of divalproex for manic and ADHD symptoms:
- Young Mania Rating Scale (YMRS) scores declined by≥50% in 32 (80%) by week 8, a greater initial response than usually reported in pediatric bipolar disorder with comorbid ADHD.
- ADHD symptoms, measured by Clinical Global Impressions (CGI) scores, did not change significantly.29
Thirty divalproex responders then received mixed amphetamine salts, 10 mg/d, or placebo plus divalproex, crossing over to the other treatment in a 4-week, double-blind trial. ADHD symptoms improved twice as much with the stimulant as with placebo, as measured by CGI scores, whereas YMRS scores did not differ significantly. Among 23 patients who continued the stimulant and divalproex for 12 more weeks, 45% required an increase in stimulant dosage and 1 relapsed into mania.
In this study, ADHD symptoms did not respond to mania treatment but did improve when a stimulant was added. This suggests either that patients had two disorders or that not all bipolar features remit at the same time. The trial’s low stimulant dosage and short duration provide insufficient evidence to support using stimulants over long periods in bipolar children.
LOng-term stimulant effects
Without long-term observations, some investigators have inferred stimulants’ impact on bipolar disorder. A poll of pediatric psychiatrists in the Netherlands, for example, found bipolar disorder in 39 children ages <13 (0.001%) in the previous year, compared with a prevalence of at least 1% in the United States.3 The authors concluded:
- Bipolar disorder emerges at younger ages in the United States than in the Netherlands.
- One reason may be that U.S. psychiatrists have a lower threshold for treating pediatric depression and hyperactivity with antidepressants and stimulants than Dutch psychiatrists do, evoking more-obvious bipolar symptoms at an earlier age.
Observations of 30 U.S. children with a manic episode and ADHD suggested that stimulants can induce manic symptoms:
- Mean age of ADHD onset was 5.5 years.
- Mean age of starting stimulants was 6.9 years.
- Mean age of hypomanic or manic symptom onset was 7.1 years.30
Similarly, in a survey of 34 adolescent manic inpatients, those who had taken stimulants had earlier mania onset (mean age 10.7) than did those who had not taken stimulants (mean age 13.9). Exposure to two stimulants was associated with earlier onset than exposure to one, but comorbid ADHD alone did not affect age of bipolar disorder onset.31
The same group10 reviewed charts of 80 consecutively hospitalized adolescents with a manic or mixed bipolar episode and found stimulant exposure was associated with relatively worse inpatient course, longer length of stay, more emergency medications, and more seclusion and restraint orders. Comorbid ADHD, mixed versus manic episode, and prior antidepressant exposure did not worsen the inpatient course.
A chart review by El-Mallakh et al32 found bipolar disorder was diagnosed at mean age 10.7 in 49 children exposed to antidepressants or stimulants, compared with mean age 12.7 in 44 unexposed children. The exposed group appeared to have tolerated stimulants longer than antidepressants before mania or hypomania emerged.33
In contrast, a retrospective review by Carlson et al34 of data from a longitudinal study of 75 boys with “hyperkinetic reaction of childhood” found that methylphenidate treatment did not appear more common in boys later diagnosed with bipolar disorder than in those without a bipolar diagnosis. This study had obvious methodologic limitations, lacking a hypothesis and focusing on a population with “minimal brain dysfunction.”
In a reanalysis of data from a 1-month methylphenidate titration trial, Galanter et al35 examined whether some 300 children ages 5 to 12 experienced manic symptoms, using the Diagnostic Interview Schedule for Children or the Child Behavior Checklist. At least during this brief trial, patients with and without manic symptoms showed no differences in response rates or adverse effects with stimulant therapy.
Drug treatment hierarchy
Mood stabilizers. Evidence supports starting all bipolar children with a mood stabilizer such as lithium or valproate (Algorithm). A few patients may tolerate stimulants without mood stabilizers, but the risk is high of inducing mania and precipitating a more complex and treatment-resistant disorder.
Carbamazepine can be effective, but it makes some youths aggressive or disorganized. Antipsychotics have not been tested in controlled trials in bipolar children and are not considered first-line treatments, especially as mood stabilizers. They can be effective for childhood mania, but outpatients needing ADHD treatment usually do not have severe manic syndromes.
Algorithm Reducing mania risk: Using stimulants in children with bipolar disorder
Combination therapy. Like many adults, bipolar children often require combinations of mood stabilizers. Kowatch et al36 found that 16 of 20 acutely ill bipolar children (mean age 11) responded to a combination of mood stabilizers after not responding to 8 weeks of a single mood stabilizer. Because bipolar disorder with comorbid ADHD suggests a complex pathophysiology, patients with both disorders may be more likely to require mood-stabilizer combinations than those with bipolar disorder alone.
The goal in treating bipolar disorder is to eliminate symptoms as completely as possible. In bipolar children with comorbid ADHD, be certain that subtle hypomanic symptoms—irritability, decreased sleep, hypersensitivity to interactions, psychosis—have remitted, as they could account for continued inattention. Persistent mood lability may indicate incomplete treatment of the mood disorder, which can increase sensitivity to destabilization by a stimulant.
If a child remains inattentive after the mood disorder is controlled, consider whether medications for the mood disorder are to blame. If medications are working well but causing cognitive side effects, a cholinesterase inhibitor may help.
Adding stimulants. If attention problems persist, consider cautiously adding a stimulant. Informed consent includes telling patients and families about the risks of mood destabilization with stimulants, even when used with mood stabilizers.
Increase stimulant dosage very slowly, and monitor the patient closely for emerging mood instability or subtle evidence of dysphoric hypomania. Address hypersensitivity to sounds, increased irritability, or other signs of activation with more-aggressive mood stabilization before assuming that these are ADHD symptoms that require a higher stimulant dosage.
Sustained-release stimulant preparations are probably second-line choices in patients with concomitant bipolar disorder. With long-acting stimulants, any worsening of the mood disorder will take longer to wear off. Antidepressants such as bupropion are potential alternatives to stimulants but are as likely to induce hypomania and mood cycling and may not be as effective.
Compared with stimulants, atomoxetine has a less-potent antidepressant effect and may be somewhat safer, but it is not as effective for ADHD and is longer-acting. Thus, atomoxetine could be a first-line alternative for comorbid ADHD, with stimulants being added if it is not effective. Clonidine can reduce hyperactivity but does not stabilize mood or improve attention.
When an antidepressant has brought bipolar depression into remission, discontinue it slowly to reduce the risk of rebound while continuing mood stabilizers to prevent recurrence. Because ADHD is not cyclical like bipolar depression, inattention returns for many patients when stimulants are withdrawn.
We do not yet know whether the risk of mood destabilization increases with long-term stimulant use, but discontinuation-induced refractoriness has not been reported with stimulants as it has with mood stabilizers and antidepressants. Thus, trying to withdraw stimulants once ADHD symptoms have remitted is prudent, while supplementing the regimen with behavioral treatments. If managing ADHD symptoms requires continued stimulant treatment, monitor the patient closely for mood destabilization.
Related resources
- American Academy of Child and Adolescent Psychiatry. Facts for families: Bipolar disorder in children and teens.
www.aacap.org/publications/factsFam/bipolar.htm. - National Institute of Mental Health. Database on ADHD.
www.nimh.nih.gov/publicat/adhd.cfm.
Drug brand names
- Amphetamine salts • Adderall
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Clonidine • Catapres
- Dexmethylphenidate • Focalin
- Fluoxetine • Prozac
- Lithium • Lithobid, others
- Methylphenidate • Concerta,
- Ritalin, others
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
Disclosures
Dr. Dubovsky receives research support from UCB Pharma, Forest Laboratories, and Solvay Pharmaceuticals, and is a speaker for Janssen Pharmaceutica and Forest Laboratories.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. AACAP. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(suppl 2):26S-49S.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder: child psychiatric workgroup on bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Reichart CG, Nolen W. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 2004;78:81-4.
4. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatric-onset bipolar disorder. Biol Psychiatry 2003;53:970-7.
5. Geller B, Zimmerman B, Williams MB, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001;158:125-7.
6. Scheffer RE, Niskala Apps JA. The diagnosis of preschool bipolar disorder presenting with mania: open pharmacological treatment. J Affect Disord. 2004;82(suppl 1):S25-S34.
7. Dilsaver SC, Akiskal HS. Preschool-onset mania: incidence, phenomenology and family history. J Affect Disord 2004;82(suppl 1):S35-S43.
8. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82(suppl 1):S59-S69.
9. Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the Child Behavior Checklist in pediatric bipolar disorder. Biol Psychiatry 2003;53:1021-7.
10. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70:323-7.
11. Piver A. Ultrarapid response to an antidepressant: A clue to bipolarity? Can J Psychiatry 2003;48:427-8.
12. Bottlender R, Sato T, Kleindienst N, et al. Mixed depressive features predict maniform switch during treatment of depression in bipolar I disorder. J Affect Disord 2004;78:149-52.
13. Goldberg J, Truman CJ. Antidepressant-induced mania: an overview of current controversies. Bipolar Disord 2003;5:407-20.
14. Altshuler LL, Post RM, Leverich GS, et al. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152(8):1130-8.
15. Gijsman HJ, Geddes J, Rendell J, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004;161:1537-47.
16. Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988;145:844-8.
17. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder: demographics, diagnosis, family history and course. Arch Gen Psychiatry 1992;49:126-31.
18. Wehr TA. Can antidepressants induce rapid cycling? Arch Gen Psychiatry 1993;50(6):495-6.
19. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145:179-84.
20. Fogelson DL, Bystritsky A, Pasnau R. Bupropion in the treatment of bipolar disorders: the same old story. J Clin Psychiatry 1992;53:443-6.
21. Goldberg J, Ernst CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry 2002;63:985-91.
22. Amsterdam JD, Shults J, Brunswick DJ, Hundert M. Short-term fluoxetine monotherapy for bipolar type II or bipolar NOS major depression—low manic switch rate. Bipolar Disord 2004;6:75-81.
23. Simpson SG, DePaulo JR. Fluoxetine treatment of bipolar II depression. J Clin Psychopharmacol 1991;11:52-4.
24. Amsterdam JD, Garcia-Espana F. Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 2000;59:225-9.
25. Goldberg J. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract 2003;9:181-94.
26. Bauer M, Calabrese JR, Dunner DL. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry 1994;151:506-15.
27. Prien RF, Kupfer DJ, Mansky PA. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984;41:1096-1104.
28. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6:416-20.
29. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
30. Kowatch RA, Suppes T, Carmody T, et al. Effect size of lithium, divalproex sodium, and carbamezepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2000;39:713-20.
31. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
32. El-Mallakh RS, Cicero D, Holman J, Robertson J. Antidepressant exposure in children diagnosed with bipolar disorder. Bipolar Disord 2001;3(suppl 1):35-9.
33. Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry 2003;66:317-22.
34. Carlson G, Loney J, Salisbury H, et al. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharmacol 2000;10:175-84.
35. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13:123-36.
36. Kowatch RA, Sethuraman G, Hume JH, Kromelis M, Weinberg WA. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry. 2003;53:978-84.
Stimulants are most effective for childhood attention-deficit/hyperactivity disorder (ADHD),1 but they may induce mania or trigger a treatment-resistant course in children with comorbid bipolar disorder. To help you safely manage these complicated symptoms, this article offers a treatment algorithm and tips to:
- differentiate bipolar and ADHD symptoms
- identify patients at risk for stimulantinduced mania
- choose medications by a hierarchythat may reduce the risk of mood destabilization.
Bipolar mood symptoms emerge before age 20 in about 25% of persons with bipolar disorder (BP).3 Early-onset BP may be more severe than the adult-onset form, with more-affected family members and greater comorbidity with other disorders, especially ADHD.4
In one study, 91% of children with BP also met criteria for ADHD, and 19% of patients with ADHD also received a diagnosis of BP.5 Among 31 children ages 2 to 5 with BP, 80% met criteria for concurrent ADHD.6
Of 40 children age <5 presenting consecutively to a mental health clinic, 11 (28%) met criteria for mania, which was usually associated with euphoria.7 These 11 children also met criteria for ADHD.
A comparison study8 of children (mean age 12) found greater impairment, suicidality, irritability, and sadness in 43 with ADHD plus bipolar depression than in:
- 109 with ADHD plus major depressive disorder
- 128 without depression or mania.
Family prevalence of bipolar disorder and major depression was highest in the bipolar-ADHD group, which also had the highest rates of comorbid conduct disorder, oppositional defiant disorder, alcohol abuse, and agoraphobia. Average age of bipolar diagnosis was 6.3 years.
Adhd and/or bipolar disorder?
Some 70% to 90% of bipolar children and at least 30% to 40% of bipolar adolescents also have ADHD.2 This high comorbidity (Box 1)3-8 might mean that:
- one disorder predisposes to the other
- one is a precursor of the other
- they share common vulnerabilities or causes
- their symptoms overlap so much that patients with one disorder appear to meet criteria for the other.
Some experts contend that bipolar disorder and ADHD usually can be differentiated. Bipolar children score higher than those with ADHD on measures of anxiety/depression, aggression, and attention problems on the Child Behavior Checklist.9 Others believe ADHD symptoms that occur with bipolar disorder are a dimension of bipolar illness rather than a separate disorder.10
For every DSM-IV-TR diagnostic criterion for ADHD, a corresponding diagnostic criterion or common feature of bipolar disorder can be identified (Table 1). Mania and hypomania are obviously associated with hyperactivity and impulsivity, and tangential thinking and distractibility interfere with attention in many patients with bipolar disorder.
Though most ADHD symptoms can occur in bipolar patients, some features of bipolar illness are not characteristic of ADHD (Table 2). Children with ADHD can become hyper-focused on video games and television, for example, but they usually do not become engrossed in long, complicated books or preoccupied with other people, as can occur in bipolar disorder.
Table 1
How ADHD, bipolar symptoms overlap in three domains
| ADHD | Bipolar disorder |
|---|---|
| Inattention | |
| Fails to pay attention | Racing and tangential thoughts |
| Difficulty sustaining attention | Attention driven by racing thoughts, affective themes, and psychosis |
| Does not follow through | Direction of activity shifts with shifting mood |
| Difficulty organizing tasks | Disorganization, psychosis, excessive energy |
| Easily distracted | Distractibility |
| Hyperactivity | |
| Fidgets or squirms | Increased energy and activity |
| Runs about or climbs excessively | Hyperactivity, thrill-seeking |
| Difficulty engaging quietly in leisure activities | Increased energy, boredom |
| Often on the go | Increased energy, hyperactivity |
| Talks excessively | Rapid, pressured speech |
| Impulsivity | |
| Blurts out answers | Rapid, pressured, impulsive speech |
| Difficulty awaiting turn | Hyperactivity, increased energy, impatience, grandiosity |
| Interrupts or intrudes on others | Grandiosity, impatience, pressured speech, increased mental content |
Table 2
Bipolar features not seen in ADHD
|
A treatment hierarchy
Whether a bipolar patient’s attention problems are features of the primary condition or caused by comorbid ADHD may be unclear, but the treatment implications are important. All antidepressants can induce mania/hypomania and increase the risk of mixed states and mood cycling. Because stimulants have antidepressant properties and because some antidepressants are used to treat ADHD, a systematic approach is necessary when treating inattention in juvenile bipolar disorder.
A treatment hierarchy developed by the American Academy of Child and Adolescent Psychiatry Workgroup on Bipolar Disorder recommends beginning psychosocial approaches, such as training parents in behavior management techniques, and:
- treating bipolar disorder first in children who clearly have both ADHD and bipolar disorder
- adding ADHD treatment if ADHD symptoms persist and impair functioning.2
Who’s at risk for mood destabilization?
No data address differences between bipolar patients whose mood disorders deteriorate with stimulant use and those who remain stable. However, risk factors for mood destabilization that have been reported with antidepressants likely also apply to stimulants (Table 3) because stimulants’ adverse effects in bipolar disorder are probably related to their antidepressant properties.
For example, depressed patients who report that an antidepressant worked within hours to days may have bipolar disorder and be at risk for mood destabilization leading to treatment resistance.11 Antidepressant-induced mania also may be more likely:
- when depression is mixed with hypomanic symptoms such as racing thoughts, excessive talkativeness, aggression, irritability, distractibility, and increased drive12
- in patients with a history of antidepressant-induced mania, family history of bipolar disorder, or multiple antidepressant trials.13
Similarly, patients who report feeling better immediately after starting a stimulant—especially if they have evidence of elation, increased irritability, more aggression or impulsivity, decreased sleep, or related symptoms—may be developing stimulant-induced hypomania.
Table 3
Risk factors that may increase risk of stimulant-induced mania
|
| Source: Reference 25 |
Antidepressant-induced mania
Most studies of antidepressant-induced mania have examined outright mania, but hypomania and subsyndromal hypomanic syndromes also may cause significant morbidity and may worsen bipolar disorder’s course. A change in polarity may worsen a patient’s prognosis, but how do we know that antidepressants (or stimulants) caused it?
One suggested criterion is that mania or hypomania develops within 8 weeks of starting an antidepressant for the first time. A chart review of 51 bipolar patients who had extensive life charting found that 82% developed mania while taking an antidepressant—35% of them within 8 weeks.14 The authors attributed 50% of the risk of a first manic episode and/or cycle acceleration to antidepressants and 50% to spontaneous mood swings. They also noted that:
- an initial manic episode appeared to sensitize patients to subsequent manic episodes and rapid cycling
- mood stabilizers did not seem to prevent these outcomes.
A meta-analysis of 12 randomized, controlled, 4-to 12-week trials among 1,088 patients found antidepressants no more likely than placebo to induce mania in the short term.15 These trials did not, however, consider less-severe forms of overstimulation and were not designed to determine mania risk in bipolar depressed patients.
Post-mania cycling. Rapid and ultradian cycling and other forms of deterioration are more likely to occur after a manic or hypomanic episode than after a depressive episode.16
A longitudinal study17 indicated that antidepressant use did not predictably predate rapid cycling when depression was controlled. The authors, however, looked at the correlation between taking an antidepressant at study entry and rapid cycling over 1 year but did not examine whether antidepressants were started or stopped during the study.18 Rapid cycling prevalence declined from 19% to 5% during the study, but they did not determine whether withdrawing antidepressants was associated with this change.
In an earlier prospective study, rapid cycling was more severe while patients were taking antidepressants—despite the use of mood stabilizers—and cycling duration decreased when antidepressants were withdrawn.19
TCAs vs. newer agents. Tricyclic antidepressants (TCAs) are perceived as more likely to induce mania than are selective serotonin reuptake inhibitors (SSRIs) or bupropion. Comparing TCAs’ and newer antidepressants’ switch rates is difficult, however. Most antidepressant trials were designed to show efficacy and safety in unipolar, not bipolar, depression. Moreover, as exclusion criteria have improved with greater awareness of bipolar illness’ polymorphic manifestations, recent studies likely have enrolled fewer bipolar patients—who are most at risk to develop a manic switch—than did earlier TCA trials.
Bupropion, which has been used to treat ADHD, has been thought to have a low risk of inducing mania. In open observation, however, >50% of 11 patients with a history of developing mania with other antidepressants also had a manic switch on bupropion, even though they were taking mood stabilizers.20
Analysis of 155 antidepressant trials in 41 depressed patients found mania risk to be similar with bupropion, SSRIs, TCAs, monoamine oxidase inhibitors (MAOIs), and other newer antidepressants.21 Mania risk doubled when patients were not also taking mood stabilizers.
Going without mood stabilizers. Reports have emerged of patients with bipolar depression taking antidepressants such as fluoxetine and venlafaxine without a mood stabilizer for extended periods, without high rates of mania or mood cycling.22-24 These reports suggest that some bipolar depressed patients can tolerate antidepressants without a mood stabilizer, although we have no way to identify such patients in advance.
Cycle acceleration and treatment resistance may follow antidepressant-induced mania.25 In DSM-IV field trials, antidepressants appeared to have triggered rapid cycling in some 20% of bipolar patients.26 Mood stabilizers were not particularly effective in patients with treatment-resistant ultradian cycling, but withdrawing antidepressants improved outcome.27
Stimulant-induced mania
Compared with antidepressants, less information is available about stimulant-induced mania and rapid cycling.
Some carefully selected bipolar patients may tolerate ongoing stimulant treatment. For example, in 2 years of open experience with 5 bipolar type I and 3 bipolar type II adults, adding methylphenidate or amphetamine for residual depression or sedation was moderately helpful and did not lead to manic switching or drug misuse.28
On the other hand, affective symptoms worsened in nearly two-thirds of 31 children ages 2 to 5 when treated with stimulants or antidepressants without mood stabilizers. Most of the children also had ADHD, and valproate usually helped.6
In 40 patients, mean age 10, who entered the open-label phase of an 8-week trial of divalproex for manic and ADHD symptoms:
- Young Mania Rating Scale (YMRS) scores declined by≥50% in 32 (80%) by week 8, a greater initial response than usually reported in pediatric bipolar disorder with comorbid ADHD.
- ADHD symptoms, measured by Clinical Global Impressions (CGI) scores, did not change significantly.29
Thirty divalproex responders then received mixed amphetamine salts, 10 mg/d, or placebo plus divalproex, crossing over to the other treatment in a 4-week, double-blind trial. ADHD symptoms improved twice as much with the stimulant as with placebo, as measured by CGI scores, whereas YMRS scores did not differ significantly. Among 23 patients who continued the stimulant and divalproex for 12 more weeks, 45% required an increase in stimulant dosage and 1 relapsed into mania.
In this study, ADHD symptoms did not respond to mania treatment but did improve when a stimulant was added. This suggests either that patients had two disorders or that not all bipolar features remit at the same time. The trial’s low stimulant dosage and short duration provide insufficient evidence to support using stimulants over long periods in bipolar children.
LOng-term stimulant effects
Without long-term observations, some investigators have inferred stimulants’ impact on bipolar disorder. A poll of pediatric psychiatrists in the Netherlands, for example, found bipolar disorder in 39 children ages <13 (0.001%) in the previous year, compared with a prevalence of at least 1% in the United States.3 The authors concluded:
- Bipolar disorder emerges at younger ages in the United States than in the Netherlands.
- One reason may be that U.S. psychiatrists have a lower threshold for treating pediatric depression and hyperactivity with antidepressants and stimulants than Dutch psychiatrists do, evoking more-obvious bipolar symptoms at an earlier age.
Observations of 30 U.S. children with a manic episode and ADHD suggested that stimulants can induce manic symptoms:
- Mean age of ADHD onset was 5.5 years.
- Mean age of starting stimulants was 6.9 years.
- Mean age of hypomanic or manic symptom onset was 7.1 years.30
Similarly, in a survey of 34 adolescent manic inpatients, those who had taken stimulants had earlier mania onset (mean age 10.7) than did those who had not taken stimulants (mean age 13.9). Exposure to two stimulants was associated with earlier onset than exposure to one, but comorbid ADHD alone did not affect age of bipolar disorder onset.31
The same group10 reviewed charts of 80 consecutively hospitalized adolescents with a manic or mixed bipolar episode and found stimulant exposure was associated with relatively worse inpatient course, longer length of stay, more emergency medications, and more seclusion and restraint orders. Comorbid ADHD, mixed versus manic episode, and prior antidepressant exposure did not worsen the inpatient course.
A chart review by El-Mallakh et al32 found bipolar disorder was diagnosed at mean age 10.7 in 49 children exposed to antidepressants or stimulants, compared with mean age 12.7 in 44 unexposed children. The exposed group appeared to have tolerated stimulants longer than antidepressants before mania or hypomania emerged.33
In contrast, a retrospective review by Carlson et al34 of data from a longitudinal study of 75 boys with “hyperkinetic reaction of childhood” found that methylphenidate treatment did not appear more common in boys later diagnosed with bipolar disorder than in those without a bipolar diagnosis. This study had obvious methodologic limitations, lacking a hypothesis and focusing on a population with “minimal brain dysfunction.”
In a reanalysis of data from a 1-month methylphenidate titration trial, Galanter et al35 examined whether some 300 children ages 5 to 12 experienced manic symptoms, using the Diagnostic Interview Schedule for Children or the Child Behavior Checklist. At least during this brief trial, patients with and without manic symptoms showed no differences in response rates or adverse effects with stimulant therapy.
Drug treatment hierarchy
Mood stabilizers. Evidence supports starting all bipolar children with a mood stabilizer such as lithium or valproate (Algorithm). A few patients may tolerate stimulants without mood stabilizers, but the risk is high of inducing mania and precipitating a more complex and treatment-resistant disorder.
Carbamazepine can be effective, but it makes some youths aggressive or disorganized. Antipsychotics have not been tested in controlled trials in bipolar children and are not considered first-line treatments, especially as mood stabilizers. They can be effective for childhood mania, but outpatients needing ADHD treatment usually do not have severe manic syndromes.
Algorithm Reducing mania risk: Using stimulants in children with bipolar disorder
Combination therapy. Like many adults, bipolar children often require combinations of mood stabilizers. Kowatch et al36 found that 16 of 20 acutely ill bipolar children (mean age 11) responded to a combination of mood stabilizers after not responding to 8 weeks of a single mood stabilizer. Because bipolar disorder with comorbid ADHD suggests a complex pathophysiology, patients with both disorders may be more likely to require mood-stabilizer combinations than those with bipolar disorder alone.
The goal in treating bipolar disorder is to eliminate symptoms as completely as possible. In bipolar children with comorbid ADHD, be certain that subtle hypomanic symptoms—irritability, decreased sleep, hypersensitivity to interactions, psychosis—have remitted, as they could account for continued inattention. Persistent mood lability may indicate incomplete treatment of the mood disorder, which can increase sensitivity to destabilization by a stimulant.
If a child remains inattentive after the mood disorder is controlled, consider whether medications for the mood disorder are to blame. If medications are working well but causing cognitive side effects, a cholinesterase inhibitor may help.
Adding stimulants. If attention problems persist, consider cautiously adding a stimulant. Informed consent includes telling patients and families about the risks of mood destabilization with stimulants, even when used with mood stabilizers.
Increase stimulant dosage very slowly, and monitor the patient closely for emerging mood instability or subtle evidence of dysphoric hypomania. Address hypersensitivity to sounds, increased irritability, or other signs of activation with more-aggressive mood stabilization before assuming that these are ADHD symptoms that require a higher stimulant dosage.
Sustained-release stimulant preparations are probably second-line choices in patients with concomitant bipolar disorder. With long-acting stimulants, any worsening of the mood disorder will take longer to wear off. Antidepressants such as bupropion are potential alternatives to stimulants but are as likely to induce hypomania and mood cycling and may not be as effective.
Compared with stimulants, atomoxetine has a less-potent antidepressant effect and may be somewhat safer, but it is not as effective for ADHD and is longer-acting. Thus, atomoxetine could be a first-line alternative for comorbid ADHD, with stimulants being added if it is not effective. Clonidine can reduce hyperactivity but does not stabilize mood or improve attention.
When an antidepressant has brought bipolar depression into remission, discontinue it slowly to reduce the risk of rebound while continuing mood stabilizers to prevent recurrence. Because ADHD is not cyclical like bipolar depression, inattention returns for many patients when stimulants are withdrawn.
We do not yet know whether the risk of mood destabilization increases with long-term stimulant use, but discontinuation-induced refractoriness has not been reported with stimulants as it has with mood stabilizers and antidepressants. Thus, trying to withdraw stimulants once ADHD symptoms have remitted is prudent, while supplementing the regimen with behavioral treatments. If managing ADHD symptoms requires continued stimulant treatment, monitor the patient closely for mood destabilization.
Related resources
- American Academy of Child and Adolescent Psychiatry. Facts for families: Bipolar disorder in children and teens.
www.aacap.org/publications/factsFam/bipolar.htm. - National Institute of Mental Health. Database on ADHD.
www.nimh.nih.gov/publicat/adhd.cfm.
Drug brand names
- Amphetamine salts • Adderall
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Clonidine • Catapres
- Dexmethylphenidate • Focalin
- Fluoxetine • Prozac
- Lithium • Lithobid, others
- Methylphenidate • Concerta,
- Ritalin, others
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
Disclosures
Dr. Dubovsky receives research support from UCB Pharma, Forest Laboratories, and Solvay Pharmaceuticals, and is a speaker for Janssen Pharmaceutica and Forest Laboratories.
Stimulants are most effective for childhood attention-deficit/hyperactivity disorder (ADHD),1 but they may induce mania or trigger a treatment-resistant course in children with comorbid bipolar disorder. To help you safely manage these complicated symptoms, this article offers a treatment algorithm and tips to:
- differentiate bipolar and ADHD symptoms
- identify patients at risk for stimulantinduced mania
- choose medications by a hierarchythat may reduce the risk of mood destabilization.
Bipolar mood symptoms emerge before age 20 in about 25% of persons with bipolar disorder (BP).3 Early-onset BP may be more severe than the adult-onset form, with more-affected family members and greater comorbidity with other disorders, especially ADHD.4
In one study, 91% of children with BP also met criteria for ADHD, and 19% of patients with ADHD also received a diagnosis of BP.5 Among 31 children ages 2 to 5 with BP, 80% met criteria for concurrent ADHD.6
Of 40 children age <5 presenting consecutively to a mental health clinic, 11 (28%) met criteria for mania, which was usually associated with euphoria.7 These 11 children also met criteria for ADHD.
A comparison study8 of children (mean age 12) found greater impairment, suicidality, irritability, and sadness in 43 with ADHD plus bipolar depression than in:
- 109 with ADHD plus major depressive disorder
- 128 without depression or mania.
Family prevalence of bipolar disorder and major depression was highest in the bipolar-ADHD group, which also had the highest rates of comorbid conduct disorder, oppositional defiant disorder, alcohol abuse, and agoraphobia. Average age of bipolar diagnosis was 6.3 years.
Adhd and/or bipolar disorder?
Some 70% to 90% of bipolar children and at least 30% to 40% of bipolar adolescents also have ADHD.2 This high comorbidity (Box 1)3-8 might mean that:
- one disorder predisposes to the other
- one is a precursor of the other
- they share common vulnerabilities or causes
- their symptoms overlap so much that patients with one disorder appear to meet criteria for the other.
Some experts contend that bipolar disorder and ADHD usually can be differentiated. Bipolar children score higher than those with ADHD on measures of anxiety/depression, aggression, and attention problems on the Child Behavior Checklist.9 Others believe ADHD symptoms that occur with bipolar disorder are a dimension of bipolar illness rather than a separate disorder.10
For every DSM-IV-TR diagnostic criterion for ADHD, a corresponding diagnostic criterion or common feature of bipolar disorder can be identified (Table 1). Mania and hypomania are obviously associated with hyperactivity and impulsivity, and tangential thinking and distractibility interfere with attention in many patients with bipolar disorder.
Though most ADHD symptoms can occur in bipolar patients, some features of bipolar illness are not characteristic of ADHD (Table 2). Children with ADHD can become hyper-focused on video games and television, for example, but they usually do not become engrossed in long, complicated books or preoccupied with other people, as can occur in bipolar disorder.
Table 1
How ADHD, bipolar symptoms overlap in three domains
| ADHD | Bipolar disorder |
|---|---|
| Inattention | |
| Fails to pay attention | Racing and tangential thoughts |
| Difficulty sustaining attention | Attention driven by racing thoughts, affective themes, and psychosis |
| Does not follow through | Direction of activity shifts with shifting mood |
| Difficulty organizing tasks | Disorganization, psychosis, excessive energy |
| Easily distracted | Distractibility |
| Hyperactivity | |
| Fidgets or squirms | Increased energy and activity |
| Runs about or climbs excessively | Hyperactivity, thrill-seeking |
| Difficulty engaging quietly in leisure activities | Increased energy, boredom |
| Often on the go | Increased energy, hyperactivity |
| Talks excessively | Rapid, pressured speech |
| Impulsivity | |
| Blurts out answers | Rapid, pressured, impulsive speech |
| Difficulty awaiting turn | Hyperactivity, increased energy, impatience, grandiosity |
| Interrupts or intrudes on others | Grandiosity, impatience, pressured speech, increased mental content |
Table 2
Bipolar features not seen in ADHD
|
A treatment hierarchy
Whether a bipolar patient’s attention problems are features of the primary condition or caused by comorbid ADHD may be unclear, but the treatment implications are important. All antidepressants can induce mania/hypomania and increase the risk of mixed states and mood cycling. Because stimulants have antidepressant properties and because some antidepressants are used to treat ADHD, a systematic approach is necessary when treating inattention in juvenile bipolar disorder.
A treatment hierarchy developed by the American Academy of Child and Adolescent Psychiatry Workgroup on Bipolar Disorder recommends beginning psychosocial approaches, such as training parents in behavior management techniques, and:
- treating bipolar disorder first in children who clearly have both ADHD and bipolar disorder
- adding ADHD treatment if ADHD symptoms persist and impair functioning.2
Who’s at risk for mood destabilization?
No data address differences between bipolar patients whose mood disorders deteriorate with stimulant use and those who remain stable. However, risk factors for mood destabilization that have been reported with antidepressants likely also apply to stimulants (Table 3) because stimulants’ adverse effects in bipolar disorder are probably related to their antidepressant properties.
For example, depressed patients who report that an antidepressant worked within hours to days may have bipolar disorder and be at risk for mood destabilization leading to treatment resistance.11 Antidepressant-induced mania also may be more likely:
- when depression is mixed with hypomanic symptoms such as racing thoughts, excessive talkativeness, aggression, irritability, distractibility, and increased drive12
- in patients with a history of antidepressant-induced mania, family history of bipolar disorder, or multiple antidepressant trials.13
Similarly, patients who report feeling better immediately after starting a stimulant—especially if they have evidence of elation, increased irritability, more aggression or impulsivity, decreased sleep, or related symptoms—may be developing stimulant-induced hypomania.
Table 3
Risk factors that may increase risk of stimulant-induced mania
|
| Source: Reference 25 |
Antidepressant-induced mania
Most studies of antidepressant-induced mania have examined outright mania, but hypomania and subsyndromal hypomanic syndromes also may cause significant morbidity and may worsen bipolar disorder’s course. A change in polarity may worsen a patient’s prognosis, but how do we know that antidepressants (or stimulants) caused it?
One suggested criterion is that mania or hypomania develops within 8 weeks of starting an antidepressant for the first time. A chart review of 51 bipolar patients who had extensive life charting found that 82% developed mania while taking an antidepressant—35% of them within 8 weeks.14 The authors attributed 50% of the risk of a first manic episode and/or cycle acceleration to antidepressants and 50% to spontaneous mood swings. They also noted that:
- an initial manic episode appeared to sensitize patients to subsequent manic episodes and rapid cycling
- mood stabilizers did not seem to prevent these outcomes.
A meta-analysis of 12 randomized, controlled, 4-to 12-week trials among 1,088 patients found antidepressants no more likely than placebo to induce mania in the short term.15 These trials did not, however, consider less-severe forms of overstimulation and were not designed to determine mania risk in bipolar depressed patients.
Post-mania cycling. Rapid and ultradian cycling and other forms of deterioration are more likely to occur after a manic or hypomanic episode than after a depressive episode.16
A longitudinal study17 indicated that antidepressant use did not predictably predate rapid cycling when depression was controlled. The authors, however, looked at the correlation between taking an antidepressant at study entry and rapid cycling over 1 year but did not examine whether antidepressants were started or stopped during the study.18 Rapid cycling prevalence declined from 19% to 5% during the study, but they did not determine whether withdrawing antidepressants was associated with this change.
In an earlier prospective study, rapid cycling was more severe while patients were taking antidepressants—despite the use of mood stabilizers—and cycling duration decreased when antidepressants were withdrawn.19
TCAs vs. newer agents. Tricyclic antidepressants (TCAs) are perceived as more likely to induce mania than are selective serotonin reuptake inhibitors (SSRIs) or bupropion. Comparing TCAs’ and newer antidepressants’ switch rates is difficult, however. Most antidepressant trials were designed to show efficacy and safety in unipolar, not bipolar, depression. Moreover, as exclusion criteria have improved with greater awareness of bipolar illness’ polymorphic manifestations, recent studies likely have enrolled fewer bipolar patients—who are most at risk to develop a manic switch—than did earlier TCA trials.
Bupropion, which has been used to treat ADHD, has been thought to have a low risk of inducing mania. In open observation, however, >50% of 11 patients with a history of developing mania with other antidepressants also had a manic switch on bupropion, even though they were taking mood stabilizers.20
Analysis of 155 antidepressant trials in 41 depressed patients found mania risk to be similar with bupropion, SSRIs, TCAs, monoamine oxidase inhibitors (MAOIs), and other newer antidepressants.21 Mania risk doubled when patients were not also taking mood stabilizers.
Going without mood stabilizers. Reports have emerged of patients with bipolar depression taking antidepressants such as fluoxetine and venlafaxine without a mood stabilizer for extended periods, without high rates of mania or mood cycling.22-24 These reports suggest that some bipolar depressed patients can tolerate antidepressants without a mood stabilizer, although we have no way to identify such patients in advance.
Cycle acceleration and treatment resistance may follow antidepressant-induced mania.25 In DSM-IV field trials, antidepressants appeared to have triggered rapid cycling in some 20% of bipolar patients.26 Mood stabilizers were not particularly effective in patients with treatment-resistant ultradian cycling, but withdrawing antidepressants improved outcome.27
Stimulant-induced mania
Compared with antidepressants, less information is available about stimulant-induced mania and rapid cycling.
Some carefully selected bipolar patients may tolerate ongoing stimulant treatment. For example, in 2 years of open experience with 5 bipolar type I and 3 bipolar type II adults, adding methylphenidate or amphetamine for residual depression or sedation was moderately helpful and did not lead to manic switching or drug misuse.28
On the other hand, affective symptoms worsened in nearly two-thirds of 31 children ages 2 to 5 when treated with stimulants or antidepressants without mood stabilizers. Most of the children also had ADHD, and valproate usually helped.6
In 40 patients, mean age 10, who entered the open-label phase of an 8-week trial of divalproex for manic and ADHD symptoms:
- Young Mania Rating Scale (YMRS) scores declined by≥50% in 32 (80%) by week 8, a greater initial response than usually reported in pediatric bipolar disorder with comorbid ADHD.
- ADHD symptoms, measured by Clinical Global Impressions (CGI) scores, did not change significantly.29
Thirty divalproex responders then received mixed amphetamine salts, 10 mg/d, or placebo plus divalproex, crossing over to the other treatment in a 4-week, double-blind trial. ADHD symptoms improved twice as much with the stimulant as with placebo, as measured by CGI scores, whereas YMRS scores did not differ significantly. Among 23 patients who continued the stimulant and divalproex for 12 more weeks, 45% required an increase in stimulant dosage and 1 relapsed into mania.
In this study, ADHD symptoms did not respond to mania treatment but did improve when a stimulant was added. This suggests either that patients had two disorders or that not all bipolar features remit at the same time. The trial’s low stimulant dosage and short duration provide insufficient evidence to support using stimulants over long periods in bipolar children.
LOng-term stimulant effects
Without long-term observations, some investigators have inferred stimulants’ impact on bipolar disorder. A poll of pediatric psychiatrists in the Netherlands, for example, found bipolar disorder in 39 children ages <13 (0.001%) in the previous year, compared with a prevalence of at least 1% in the United States.3 The authors concluded:
- Bipolar disorder emerges at younger ages in the United States than in the Netherlands.
- One reason may be that U.S. psychiatrists have a lower threshold for treating pediatric depression and hyperactivity with antidepressants and stimulants than Dutch psychiatrists do, evoking more-obvious bipolar symptoms at an earlier age.
Observations of 30 U.S. children with a manic episode and ADHD suggested that stimulants can induce manic symptoms:
- Mean age of ADHD onset was 5.5 years.
- Mean age of starting stimulants was 6.9 years.
- Mean age of hypomanic or manic symptom onset was 7.1 years.30
Similarly, in a survey of 34 adolescent manic inpatients, those who had taken stimulants had earlier mania onset (mean age 10.7) than did those who had not taken stimulants (mean age 13.9). Exposure to two stimulants was associated with earlier onset than exposure to one, but comorbid ADHD alone did not affect age of bipolar disorder onset.31
The same group10 reviewed charts of 80 consecutively hospitalized adolescents with a manic or mixed bipolar episode and found stimulant exposure was associated with relatively worse inpatient course, longer length of stay, more emergency medications, and more seclusion and restraint orders. Comorbid ADHD, mixed versus manic episode, and prior antidepressant exposure did not worsen the inpatient course.
A chart review by El-Mallakh et al32 found bipolar disorder was diagnosed at mean age 10.7 in 49 children exposed to antidepressants or stimulants, compared with mean age 12.7 in 44 unexposed children. The exposed group appeared to have tolerated stimulants longer than antidepressants before mania or hypomania emerged.33
In contrast, a retrospective review by Carlson et al34 of data from a longitudinal study of 75 boys with “hyperkinetic reaction of childhood” found that methylphenidate treatment did not appear more common in boys later diagnosed with bipolar disorder than in those without a bipolar diagnosis. This study had obvious methodologic limitations, lacking a hypothesis and focusing on a population with “minimal brain dysfunction.”
In a reanalysis of data from a 1-month methylphenidate titration trial, Galanter et al35 examined whether some 300 children ages 5 to 12 experienced manic symptoms, using the Diagnostic Interview Schedule for Children or the Child Behavior Checklist. At least during this brief trial, patients with and without manic symptoms showed no differences in response rates or adverse effects with stimulant therapy.
Drug treatment hierarchy
Mood stabilizers. Evidence supports starting all bipolar children with a mood stabilizer such as lithium or valproate (Algorithm). A few patients may tolerate stimulants without mood stabilizers, but the risk is high of inducing mania and precipitating a more complex and treatment-resistant disorder.
Carbamazepine can be effective, but it makes some youths aggressive or disorganized. Antipsychotics have not been tested in controlled trials in bipolar children and are not considered first-line treatments, especially as mood stabilizers. They can be effective for childhood mania, but outpatients needing ADHD treatment usually do not have severe manic syndromes.
Algorithm Reducing mania risk: Using stimulants in children with bipolar disorder
Combination therapy. Like many adults, bipolar children often require combinations of mood stabilizers. Kowatch et al36 found that 16 of 20 acutely ill bipolar children (mean age 11) responded to a combination of mood stabilizers after not responding to 8 weeks of a single mood stabilizer. Because bipolar disorder with comorbid ADHD suggests a complex pathophysiology, patients with both disorders may be more likely to require mood-stabilizer combinations than those with bipolar disorder alone.
The goal in treating bipolar disorder is to eliminate symptoms as completely as possible. In bipolar children with comorbid ADHD, be certain that subtle hypomanic symptoms—irritability, decreased sleep, hypersensitivity to interactions, psychosis—have remitted, as they could account for continued inattention. Persistent mood lability may indicate incomplete treatment of the mood disorder, which can increase sensitivity to destabilization by a stimulant.
If a child remains inattentive after the mood disorder is controlled, consider whether medications for the mood disorder are to blame. If medications are working well but causing cognitive side effects, a cholinesterase inhibitor may help.
Adding stimulants. If attention problems persist, consider cautiously adding a stimulant. Informed consent includes telling patients and families about the risks of mood destabilization with stimulants, even when used with mood stabilizers.
Increase stimulant dosage very slowly, and monitor the patient closely for emerging mood instability or subtle evidence of dysphoric hypomania. Address hypersensitivity to sounds, increased irritability, or other signs of activation with more-aggressive mood stabilization before assuming that these are ADHD symptoms that require a higher stimulant dosage.
Sustained-release stimulant preparations are probably second-line choices in patients with concomitant bipolar disorder. With long-acting stimulants, any worsening of the mood disorder will take longer to wear off. Antidepressants such as bupropion are potential alternatives to stimulants but are as likely to induce hypomania and mood cycling and may not be as effective.
Compared with stimulants, atomoxetine has a less-potent antidepressant effect and may be somewhat safer, but it is not as effective for ADHD and is longer-acting. Thus, atomoxetine could be a first-line alternative for comorbid ADHD, with stimulants being added if it is not effective. Clonidine can reduce hyperactivity but does not stabilize mood or improve attention.
When an antidepressant has brought bipolar depression into remission, discontinue it slowly to reduce the risk of rebound while continuing mood stabilizers to prevent recurrence. Because ADHD is not cyclical like bipolar depression, inattention returns for many patients when stimulants are withdrawn.
We do not yet know whether the risk of mood destabilization increases with long-term stimulant use, but discontinuation-induced refractoriness has not been reported with stimulants as it has with mood stabilizers and antidepressants. Thus, trying to withdraw stimulants once ADHD symptoms have remitted is prudent, while supplementing the regimen with behavioral treatments. If managing ADHD symptoms requires continued stimulant treatment, monitor the patient closely for mood destabilization.
Related resources
- American Academy of Child and Adolescent Psychiatry. Facts for families: Bipolar disorder in children and teens.
www.aacap.org/publications/factsFam/bipolar.htm. - National Institute of Mental Health. Database on ADHD.
www.nimh.nih.gov/publicat/adhd.cfm.
Drug brand names
- Amphetamine salts • Adderall
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Clonidine • Catapres
- Dexmethylphenidate • Focalin
- Fluoxetine • Prozac
- Lithium • Lithobid, others
- Methylphenidate • Concerta,
- Ritalin, others
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
Disclosures
Dr. Dubovsky receives research support from UCB Pharma, Forest Laboratories, and Solvay Pharmaceuticals, and is a speaker for Janssen Pharmaceutica and Forest Laboratories.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. AACAP. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(suppl 2):26S-49S.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder: child psychiatric workgroup on bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Reichart CG, Nolen W. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 2004;78:81-4.
4. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatric-onset bipolar disorder. Biol Psychiatry 2003;53:970-7.
5. Geller B, Zimmerman B, Williams MB, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001;158:125-7.
6. Scheffer RE, Niskala Apps JA. The diagnosis of preschool bipolar disorder presenting with mania: open pharmacological treatment. J Affect Disord. 2004;82(suppl 1):S25-S34.
7. Dilsaver SC, Akiskal HS. Preschool-onset mania: incidence, phenomenology and family history. J Affect Disord 2004;82(suppl 1):S35-S43.
8. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82(suppl 1):S59-S69.
9. Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the Child Behavior Checklist in pediatric bipolar disorder. Biol Psychiatry 2003;53:1021-7.
10. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70:323-7.
11. Piver A. Ultrarapid response to an antidepressant: A clue to bipolarity? Can J Psychiatry 2003;48:427-8.
12. Bottlender R, Sato T, Kleindienst N, et al. Mixed depressive features predict maniform switch during treatment of depression in bipolar I disorder. J Affect Disord 2004;78:149-52.
13. Goldberg J, Truman CJ. Antidepressant-induced mania: an overview of current controversies. Bipolar Disord 2003;5:407-20.
14. Altshuler LL, Post RM, Leverich GS, et al. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152(8):1130-8.
15. Gijsman HJ, Geddes J, Rendell J, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004;161:1537-47.
16. Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988;145:844-8.
17. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder: demographics, diagnosis, family history and course. Arch Gen Psychiatry 1992;49:126-31.
18. Wehr TA. Can antidepressants induce rapid cycling? Arch Gen Psychiatry 1993;50(6):495-6.
19. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145:179-84.
20. Fogelson DL, Bystritsky A, Pasnau R. Bupropion in the treatment of bipolar disorders: the same old story. J Clin Psychiatry 1992;53:443-6.
21. Goldberg J, Ernst CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry 2002;63:985-91.
22. Amsterdam JD, Shults J, Brunswick DJ, Hundert M. Short-term fluoxetine monotherapy for bipolar type II or bipolar NOS major depression—low manic switch rate. Bipolar Disord 2004;6:75-81.
23. Simpson SG, DePaulo JR. Fluoxetine treatment of bipolar II depression. J Clin Psychopharmacol 1991;11:52-4.
24. Amsterdam JD, Garcia-Espana F. Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 2000;59:225-9.
25. Goldberg J. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract 2003;9:181-94.
26. Bauer M, Calabrese JR, Dunner DL. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry 1994;151:506-15.
27. Prien RF, Kupfer DJ, Mansky PA. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984;41:1096-1104.
28. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6:416-20.
29. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
30. Kowatch RA, Suppes T, Carmody T, et al. Effect size of lithium, divalproex sodium, and carbamezepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2000;39:713-20.
31. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
32. El-Mallakh RS, Cicero D, Holman J, Robertson J. Antidepressant exposure in children diagnosed with bipolar disorder. Bipolar Disord 2001;3(suppl 1):35-9.
33. Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry 2003;66:317-22.
34. Carlson G, Loney J, Salisbury H, et al. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharmacol 2000;10:175-84.
35. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13:123-36.
36. Kowatch RA, Sethuraman G, Hume JH, Kromelis M, Weinberg WA. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry. 2003;53:978-84.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. AACAP. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(suppl 2):26S-49S.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder: child psychiatric workgroup on bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Reichart CG, Nolen W. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 2004;78:81-4.
4. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatric-onset bipolar disorder. Biol Psychiatry 2003;53:970-7.
5. Geller B, Zimmerman B, Williams MB, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001;158:125-7.
6. Scheffer RE, Niskala Apps JA. The diagnosis of preschool bipolar disorder presenting with mania: open pharmacological treatment. J Affect Disord. 2004;82(suppl 1):S25-S34.
7. Dilsaver SC, Akiskal HS. Preschool-onset mania: incidence, phenomenology and family history. J Affect Disord 2004;82(suppl 1):S35-S43.
8. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82(suppl 1):S59-S69.
9. Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the Child Behavior Checklist in pediatric bipolar disorder. Biol Psychiatry 2003;53:1021-7.
10. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70:323-7.
11. Piver A. Ultrarapid response to an antidepressant: A clue to bipolarity? Can J Psychiatry 2003;48:427-8.
12. Bottlender R, Sato T, Kleindienst N, et al. Mixed depressive features predict maniform switch during treatment of depression in bipolar I disorder. J Affect Disord 2004;78:149-52.
13. Goldberg J, Truman CJ. Antidepressant-induced mania: an overview of current controversies. Bipolar Disord 2003;5:407-20.
14. Altshuler LL, Post RM, Leverich GS, et al. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152(8):1130-8.
15. Gijsman HJ, Geddes J, Rendell J, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004;161:1537-47.
16. Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988;145:844-8.
17. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder: demographics, diagnosis, family history and course. Arch Gen Psychiatry 1992;49:126-31.
18. Wehr TA. Can antidepressants induce rapid cycling? Arch Gen Psychiatry 1993;50(6):495-6.
19. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145:179-84.
20. Fogelson DL, Bystritsky A, Pasnau R. Bupropion in the treatment of bipolar disorders: the same old story. J Clin Psychiatry 1992;53:443-6.
21. Goldberg J, Ernst CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry 2002;63:985-91.
22. Amsterdam JD, Shults J, Brunswick DJ, Hundert M. Short-term fluoxetine monotherapy for bipolar type II or bipolar NOS major depression—low manic switch rate. Bipolar Disord 2004;6:75-81.
23. Simpson SG, DePaulo JR. Fluoxetine treatment of bipolar II depression. J Clin Psychopharmacol 1991;11:52-4.
24. Amsterdam JD, Garcia-Espana F. Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 2000;59:225-9.
25. Goldberg J. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract 2003;9:181-94.
26. Bauer M, Calabrese JR, Dunner DL. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry 1994;151:506-15.
27. Prien RF, Kupfer DJ, Mansky PA. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984;41:1096-1104.
28. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6:416-20.
29. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
30. Kowatch RA, Suppes T, Carmody T, et al. Effect size of lithium, divalproex sodium, and carbamezepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2000;39:713-20.
31. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
32. El-Mallakh RS, Cicero D, Holman J, Robertson J. Antidepressant exposure in children diagnosed with bipolar disorder. Bipolar Disord 2001;3(suppl 1):35-9.
33. Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry 2003;66:317-22.
34. Carlson G, Loney J, Salisbury H, et al. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharmacol 2000;10:175-84.
35. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13:123-36.
36. Kowatch RA, Sethuraman G, Hume JH, Kromelis M, Weinberg WA. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry. 2003;53:978-84.