User login
27-year-old man • muscle weakness • fatigue • electrolyte abnormalities • Dx?
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
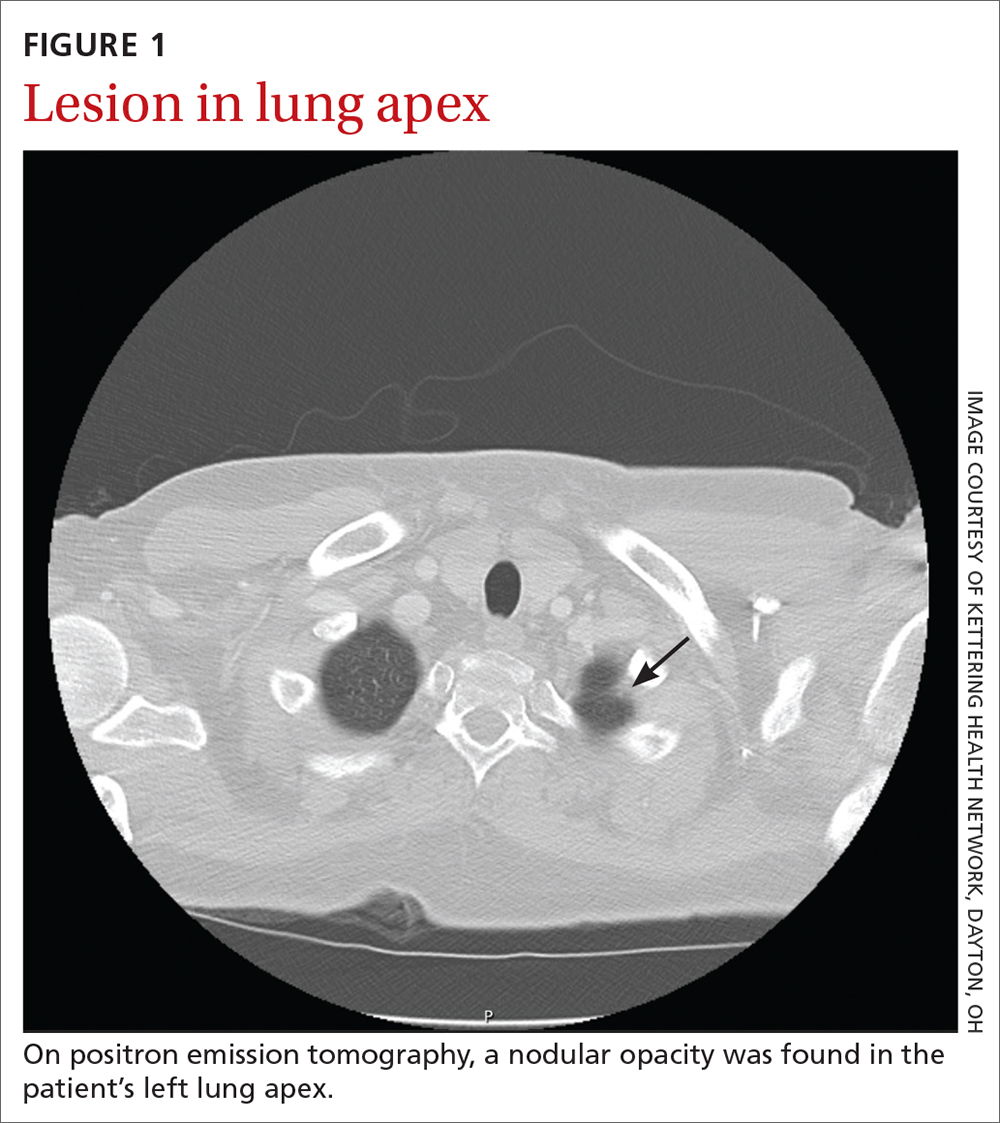
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).
THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
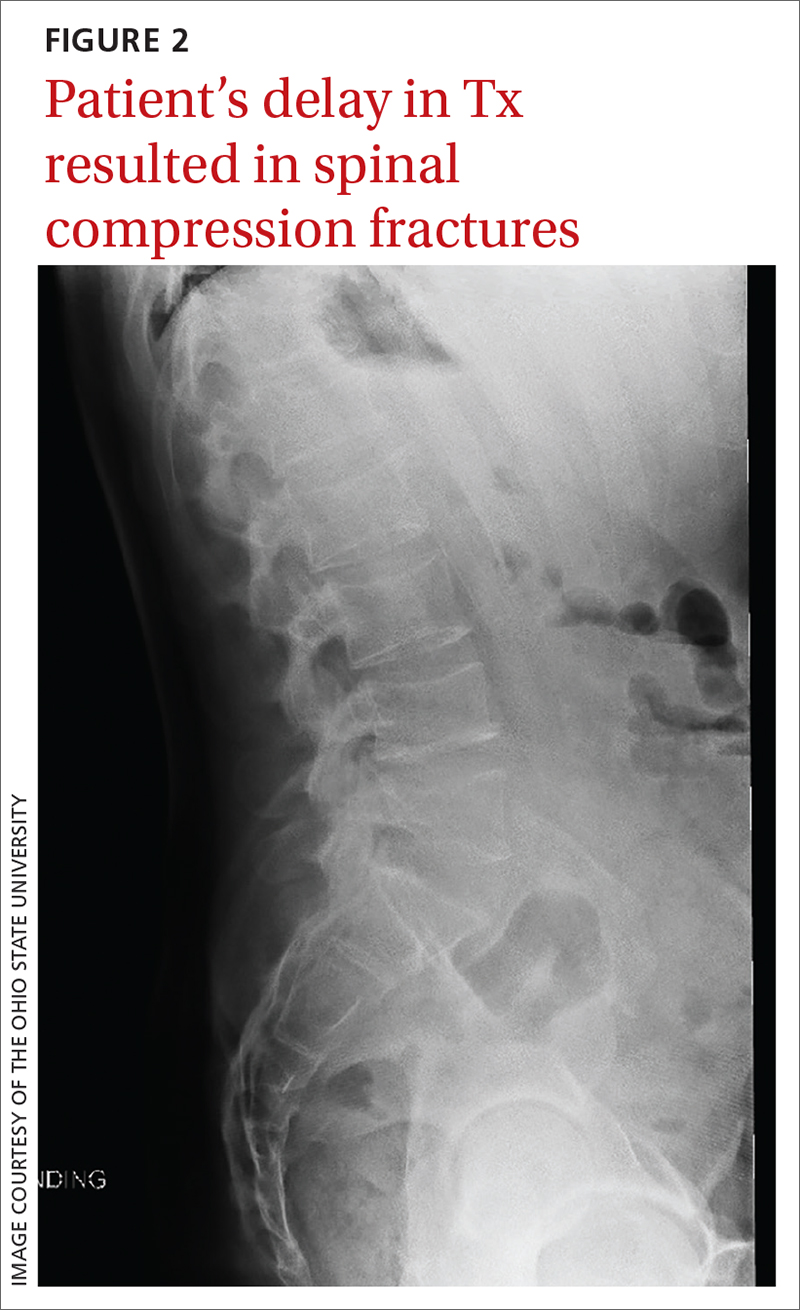
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2
Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).

THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2

Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).

THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2

Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2