User login
Benzodiazepine and Z-hypnotic stewardship
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
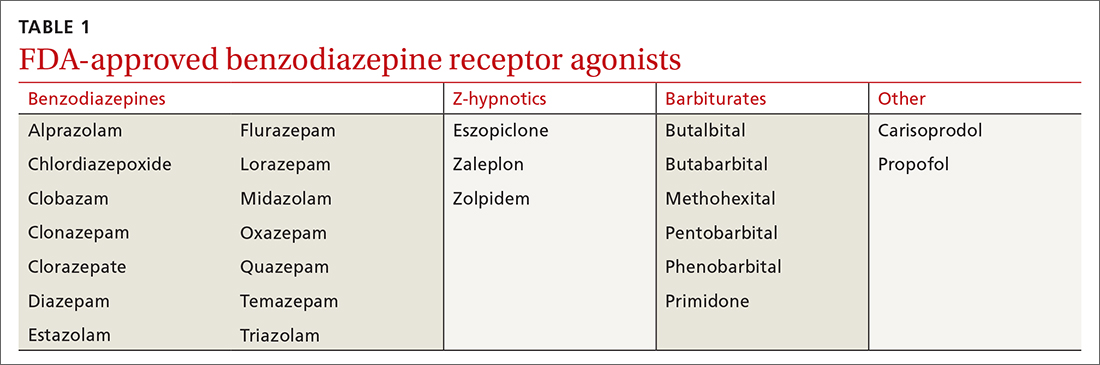
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1
With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1

With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1

With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
PRACTICE RECOMMENDATIONS
› Recommend cognitive behavioral therapy as first-line treatment for anxiety and insomnia. A
› Limit benzodiazepine prescribing to ≤ 2 to 4 weeks for anxiety and insomnia. B
› Taper benzodiazepines slowly and flexibly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series