User login
Barriers to Establishing a PCS
Palliative care (PC) focuses on relieving distressing symptoms such as pain, dyspnea, fatigue, and depression; providing psychological, social, emotional, and spiritual support; and helping patients choose treatments consistent with their values.[1] Palliative care consultation services (PCSs) increase patient and family satisfaction,[2, 3] improve quality of life,[4] reduce resource utilization,[5] and decrease hospital expenditure.[2, 6] Hospitals that fund a PCS typically realize a sizable return on investment and good value, as these services provide better care at lower cost.[7] These benefits provide a strong rationale for all hospitals to establish a PCS. However, only 53% of acute care hospitals in California offer PC services, and only 37% have a hospital‐based PCS.[7] To increase access for patients with serious illness, it is necessary to understand the barriers that hinder the development of PCS. In this study, we asked leaders from hospitals without a PCS to describe these barriers and identify strategies that could overcome them and promote PCSs.
METHODS
In 2011, we surveyed all acute care hospitals in California to assess the prevalence of PCSs in the state. We defined a PCS as an interdisciplinary team that sees patients, identifies needs, makes treatment recommendations, facilitates patient and/or family decision making, and/or directly provides palliative care for patients with life‐threatening illness and their families. Hospitals that did not have a PCS were asked questions regarding plans to establish one (Is there an effort underway to establish a palliative care program in your hospital?), perceived barriers to starting one (What are 3 significant barriers or circumstances that have prevented your hospital from creating a palliative care program?), and ideas for overcoming barriers (What resources, training, policy changes would be most helpful in overcoming those barriers?). Questions that allowed for open‐ended responses were analyzed using a thematic approach.[8] Themes were initially reviewed by 1 researcher (C.J.B.), then refined and confirmed at each stage using an iterative process with other research team members (D.L.O., S.Z.P.) to reduce potential biases. Questions assessing hospital characteristics and status toward establishing a PCS provided a list of possible answers. Frequencies to these responses are reported accordingly.
RESULTS
Surveys were distributed to 376 acute care hospitals in California, of which 360 responded to the survey, resulting in a 96% response rate. Of the 360 hospitals surveyed, 46% (n=166) reported not offering any PCS. Out of the 166 that did not have PCS, 7 stated they had a PCS at some point in the last 5 years, but the program was discontinued. Hospitals without a PCS were largely for profit (75%, n=125), small with 150 beds (72%, n=120), and not affiliated with a system (63%, n=105). Overall, 34% (43/128) of hospitals reported that they had efforts underway to establish one, with 21% (9/43) expecting to start seeing patients within a year. Seventy‐two hospitals (56%, 72/128) reported that providers from local hospices aided them in providing their patients with PC, and that this approach met the needs of their patients. A total of 93 hospitals identified multiple barriers (n=186) to establishing a PCS, of which 162 responses could be categorized into 5 meaningful themes. Regarding strategies to overcome these barriers, 65 hospitals provided 72 responses that could be categorized into 5 meaningful themes (Table 1).
| Barriers and Strategies | Responses, % (n) |
|---|---|
| |
| Main barriers to establishing a PCS | 93 hospitals provided 186 barriers |
| Insufficient funding and/or resources | 31 (58) |
| Insufficient staff to support a PCS | 20 (37) |
| Perceived lack of need for a PCS | 14 (27) |
| Lack of support among nonpalliative care physicians | 13 (25) |
| Competing priorities | 8 (15) |
| Don't know/unsure | 14 (24) |
| Main strategies to overcome barriers to establishing a PCS | 65 hospitals provided 72 strategies |
| Reroute funding to establish a PCS | 28 (20) |
| Explain benefits of PCS to staff and community | 24 (17) |
| Provide a framework for how to establish a PCS | 21 (15) |
| Staff for a PCS | 18 (13) |
| Physician support | 10 (7) |
DISCUSSION
Despite citing obstacles to providing PCSs, one‐third of hospitals surveyed report that they are planning to establish a program. As an alternative, many hospitals without a PCS reported that they provide their patients with PC through partnerships with local hospice services. This approach may provide some hospitals with a practical alternative to having a PCS, especially in smaller institutions where budgets and the need for PC are proportionally small. Future surveys should account for this approach to providing PCS to patients. Sharing the strong evidence of return on investment from PCS[6, 7] with hospital leaders could help overcome the perceived barrier of cost and garner financial support. Training programs and technical assistance provided by the Palliative Care Leadership Center initiative and the Center to Advance Palliative Care have a proven track record in helping hospitals establish a PCS through mentored training,[9] and the End‐of‐Life Nursing Education Consortium has demonstrated effectiveness with nursing education.[10] These programs could provide the resources that many hospitals seek. Educating hospital leaders and clinicians about the evidence for PCSs improving care for patients with serious illness may further help to engender support for PCSs. One barrier that may be more difficult to overcome is the lack of trained PC clinicians. Efforts to educate and train generalist clinicians in primary PC may mitigate this shortfall.[1] Increasing the number of trained primary PC clinicians may also reduce fragmentation in patient care and reduce burden on specialist PC clinicians.[11] Specialty PC clinicians can also lend their expertise to hospitals seeking to start a PCS to achieve the goal of universal access to PCS.
Acknowledgements
The authors thank the Hospital Council of Northern and Central California, the Hospital Council of Southern California, and the Hospital Council of San Diego and Imperial Counties for their support in encouraging their members to participate. The authors also thank all of the respondents for their diligence and care in responding to the survey.
Disclosures
The California HealthCare Foundation provided funding to support the administration of the survey and analysis of findings, as well as limited dissemination of results though the foundation's communication venues. The authors report no conflicts of interest.
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2014.
- , , , et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180–190.
- , , , , . A systematic review of satisfaction with care at the end of life. J Am Geriatr Soc. 2008;56(1):124–129.
- . Health care system factors affecting end‐of‐life care. J Palliat Med. 2005;8(suppl 1):S79–S87.
- , , . Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs medical center. J Palliat Med. 2005;8(1):26–35.
- , , , et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790.
- , , . Two steps forward, one step back: changes in palliative care consultation services in California hospitals from 2007 to 2011. J Palliat Med. 2014;17(11):1214–1220.
- , . Using qualitative methods to explore key questions in palliative care. J Palliat Med. 2009;12(8):725–730.
- , , . Center to Advance Palliative Care palliative care consultation service metrics: consensus recommendations. J Palliat Med. 2008;11(10):1294–1298.
- , , , et al. Evaluation of the End‐of‐Life Nursing Education Consortium undergraduate faculty training program. J Palliat Med. 2005;8(1):107–114.
- , . Generalist plus specialist palliative care–creating a more sustainable model. N Engl J Med. 2013;368(13):1173–1175.
Palliative care (PC) focuses on relieving distressing symptoms such as pain, dyspnea, fatigue, and depression; providing psychological, social, emotional, and spiritual support; and helping patients choose treatments consistent with their values.[1] Palliative care consultation services (PCSs) increase patient and family satisfaction,[2, 3] improve quality of life,[4] reduce resource utilization,[5] and decrease hospital expenditure.[2, 6] Hospitals that fund a PCS typically realize a sizable return on investment and good value, as these services provide better care at lower cost.[7] These benefits provide a strong rationale for all hospitals to establish a PCS. However, only 53% of acute care hospitals in California offer PC services, and only 37% have a hospital‐based PCS.[7] To increase access for patients with serious illness, it is necessary to understand the barriers that hinder the development of PCS. In this study, we asked leaders from hospitals without a PCS to describe these barriers and identify strategies that could overcome them and promote PCSs.
METHODS
In 2011, we surveyed all acute care hospitals in California to assess the prevalence of PCSs in the state. We defined a PCS as an interdisciplinary team that sees patients, identifies needs, makes treatment recommendations, facilitates patient and/or family decision making, and/or directly provides palliative care for patients with life‐threatening illness and their families. Hospitals that did not have a PCS were asked questions regarding plans to establish one (Is there an effort underway to establish a palliative care program in your hospital?), perceived barriers to starting one (What are 3 significant barriers or circumstances that have prevented your hospital from creating a palliative care program?), and ideas for overcoming barriers (What resources, training, policy changes would be most helpful in overcoming those barriers?). Questions that allowed for open‐ended responses were analyzed using a thematic approach.[8] Themes were initially reviewed by 1 researcher (C.J.B.), then refined and confirmed at each stage using an iterative process with other research team members (D.L.O., S.Z.P.) to reduce potential biases. Questions assessing hospital characteristics and status toward establishing a PCS provided a list of possible answers. Frequencies to these responses are reported accordingly.
RESULTS
Surveys were distributed to 376 acute care hospitals in California, of which 360 responded to the survey, resulting in a 96% response rate. Of the 360 hospitals surveyed, 46% (n=166) reported not offering any PCS. Out of the 166 that did not have PCS, 7 stated they had a PCS at some point in the last 5 years, but the program was discontinued. Hospitals without a PCS were largely for profit (75%, n=125), small with 150 beds (72%, n=120), and not affiliated with a system (63%, n=105). Overall, 34% (43/128) of hospitals reported that they had efforts underway to establish one, with 21% (9/43) expecting to start seeing patients within a year. Seventy‐two hospitals (56%, 72/128) reported that providers from local hospices aided them in providing their patients with PC, and that this approach met the needs of their patients. A total of 93 hospitals identified multiple barriers (n=186) to establishing a PCS, of which 162 responses could be categorized into 5 meaningful themes. Regarding strategies to overcome these barriers, 65 hospitals provided 72 responses that could be categorized into 5 meaningful themes (Table 1).
| Barriers and Strategies | Responses, % (n) |
|---|---|
| |
| Main barriers to establishing a PCS | 93 hospitals provided 186 barriers |
| Insufficient funding and/or resources | 31 (58) |
| Insufficient staff to support a PCS | 20 (37) |
| Perceived lack of need for a PCS | 14 (27) |
| Lack of support among nonpalliative care physicians | 13 (25) |
| Competing priorities | 8 (15) |
| Don't know/unsure | 14 (24) |
| Main strategies to overcome barriers to establishing a PCS | 65 hospitals provided 72 strategies |
| Reroute funding to establish a PCS | 28 (20) |
| Explain benefits of PCS to staff and community | 24 (17) |
| Provide a framework for how to establish a PCS | 21 (15) |
| Staff for a PCS | 18 (13) |
| Physician support | 10 (7) |
DISCUSSION
Despite citing obstacles to providing PCSs, one‐third of hospitals surveyed report that they are planning to establish a program. As an alternative, many hospitals without a PCS reported that they provide their patients with PC through partnerships with local hospice services. This approach may provide some hospitals with a practical alternative to having a PCS, especially in smaller institutions where budgets and the need for PC are proportionally small. Future surveys should account for this approach to providing PCS to patients. Sharing the strong evidence of return on investment from PCS[6, 7] with hospital leaders could help overcome the perceived barrier of cost and garner financial support. Training programs and technical assistance provided by the Palliative Care Leadership Center initiative and the Center to Advance Palliative Care have a proven track record in helping hospitals establish a PCS through mentored training,[9] and the End‐of‐Life Nursing Education Consortium has demonstrated effectiveness with nursing education.[10] These programs could provide the resources that many hospitals seek. Educating hospital leaders and clinicians about the evidence for PCSs improving care for patients with serious illness may further help to engender support for PCSs. One barrier that may be more difficult to overcome is the lack of trained PC clinicians. Efforts to educate and train generalist clinicians in primary PC may mitigate this shortfall.[1] Increasing the number of trained primary PC clinicians may also reduce fragmentation in patient care and reduce burden on specialist PC clinicians.[11] Specialty PC clinicians can also lend their expertise to hospitals seeking to start a PCS to achieve the goal of universal access to PCS.
Acknowledgements
The authors thank the Hospital Council of Northern and Central California, the Hospital Council of Southern California, and the Hospital Council of San Diego and Imperial Counties for their support in encouraging their members to participate. The authors also thank all of the respondents for their diligence and care in responding to the survey.
Disclosures
The California HealthCare Foundation provided funding to support the administration of the survey and analysis of findings, as well as limited dissemination of results though the foundation's communication venues. The authors report no conflicts of interest.
Palliative care (PC) focuses on relieving distressing symptoms such as pain, dyspnea, fatigue, and depression; providing psychological, social, emotional, and spiritual support; and helping patients choose treatments consistent with their values.[1] Palliative care consultation services (PCSs) increase patient and family satisfaction,[2, 3] improve quality of life,[4] reduce resource utilization,[5] and decrease hospital expenditure.[2, 6] Hospitals that fund a PCS typically realize a sizable return on investment and good value, as these services provide better care at lower cost.[7] These benefits provide a strong rationale for all hospitals to establish a PCS. However, only 53% of acute care hospitals in California offer PC services, and only 37% have a hospital‐based PCS.[7] To increase access for patients with serious illness, it is necessary to understand the barriers that hinder the development of PCS. In this study, we asked leaders from hospitals without a PCS to describe these barriers and identify strategies that could overcome them and promote PCSs.
METHODS
In 2011, we surveyed all acute care hospitals in California to assess the prevalence of PCSs in the state. We defined a PCS as an interdisciplinary team that sees patients, identifies needs, makes treatment recommendations, facilitates patient and/or family decision making, and/or directly provides palliative care for patients with life‐threatening illness and their families. Hospitals that did not have a PCS were asked questions regarding plans to establish one (Is there an effort underway to establish a palliative care program in your hospital?), perceived barriers to starting one (What are 3 significant barriers or circumstances that have prevented your hospital from creating a palliative care program?), and ideas for overcoming barriers (What resources, training, policy changes would be most helpful in overcoming those barriers?). Questions that allowed for open‐ended responses were analyzed using a thematic approach.[8] Themes were initially reviewed by 1 researcher (C.J.B.), then refined and confirmed at each stage using an iterative process with other research team members (D.L.O., S.Z.P.) to reduce potential biases. Questions assessing hospital characteristics and status toward establishing a PCS provided a list of possible answers. Frequencies to these responses are reported accordingly.
RESULTS
Surveys were distributed to 376 acute care hospitals in California, of which 360 responded to the survey, resulting in a 96% response rate. Of the 360 hospitals surveyed, 46% (n=166) reported not offering any PCS. Out of the 166 that did not have PCS, 7 stated they had a PCS at some point in the last 5 years, but the program was discontinued. Hospitals without a PCS were largely for profit (75%, n=125), small with 150 beds (72%, n=120), and not affiliated with a system (63%, n=105). Overall, 34% (43/128) of hospitals reported that they had efforts underway to establish one, with 21% (9/43) expecting to start seeing patients within a year. Seventy‐two hospitals (56%, 72/128) reported that providers from local hospices aided them in providing their patients with PC, and that this approach met the needs of their patients. A total of 93 hospitals identified multiple barriers (n=186) to establishing a PCS, of which 162 responses could be categorized into 5 meaningful themes. Regarding strategies to overcome these barriers, 65 hospitals provided 72 responses that could be categorized into 5 meaningful themes (Table 1).
| Barriers and Strategies | Responses, % (n) |
|---|---|
| |
| Main barriers to establishing a PCS | 93 hospitals provided 186 barriers |
| Insufficient funding and/or resources | 31 (58) |
| Insufficient staff to support a PCS | 20 (37) |
| Perceived lack of need for a PCS | 14 (27) |
| Lack of support among nonpalliative care physicians | 13 (25) |
| Competing priorities | 8 (15) |
| Don't know/unsure | 14 (24) |
| Main strategies to overcome barriers to establishing a PCS | 65 hospitals provided 72 strategies |
| Reroute funding to establish a PCS | 28 (20) |
| Explain benefits of PCS to staff and community | 24 (17) |
| Provide a framework for how to establish a PCS | 21 (15) |
| Staff for a PCS | 18 (13) |
| Physician support | 10 (7) |
DISCUSSION
Despite citing obstacles to providing PCSs, one‐third of hospitals surveyed report that they are planning to establish a program. As an alternative, many hospitals without a PCS reported that they provide their patients with PC through partnerships with local hospice services. This approach may provide some hospitals with a practical alternative to having a PCS, especially in smaller institutions where budgets and the need for PC are proportionally small. Future surveys should account for this approach to providing PCS to patients. Sharing the strong evidence of return on investment from PCS[6, 7] with hospital leaders could help overcome the perceived barrier of cost and garner financial support. Training programs and technical assistance provided by the Palliative Care Leadership Center initiative and the Center to Advance Palliative Care have a proven track record in helping hospitals establish a PCS through mentored training,[9] and the End‐of‐Life Nursing Education Consortium has demonstrated effectiveness with nursing education.[10] These programs could provide the resources that many hospitals seek. Educating hospital leaders and clinicians about the evidence for PCSs improving care for patients with serious illness may further help to engender support for PCSs. One barrier that may be more difficult to overcome is the lack of trained PC clinicians. Efforts to educate and train generalist clinicians in primary PC may mitigate this shortfall.[1] Increasing the number of trained primary PC clinicians may also reduce fragmentation in patient care and reduce burden on specialist PC clinicians.[11] Specialty PC clinicians can also lend their expertise to hospitals seeking to start a PCS to achieve the goal of universal access to PCS.
Acknowledgements
The authors thank the Hospital Council of Northern and Central California, the Hospital Council of Southern California, and the Hospital Council of San Diego and Imperial Counties for their support in encouraging their members to participate. The authors also thank all of the respondents for their diligence and care in responding to the survey.
Disclosures
The California HealthCare Foundation provided funding to support the administration of the survey and analysis of findings, as well as limited dissemination of results though the foundation's communication venues. The authors report no conflicts of interest.
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2014.
- , , , et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180–190.
- , , , , . A systematic review of satisfaction with care at the end of life. J Am Geriatr Soc. 2008;56(1):124–129.
- . Health care system factors affecting end‐of‐life care. J Palliat Med. 2005;8(suppl 1):S79–S87.
- , , . Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs medical center. J Palliat Med. 2005;8(1):26–35.
- , , , et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790.
- , , . Two steps forward, one step back: changes in palliative care consultation services in California hospitals from 2007 to 2011. J Palliat Med. 2014;17(11):1214–1220.
- , . Using qualitative methods to explore key questions in palliative care. J Palliat Med. 2009;12(8):725–730.
- , , . Center to Advance Palliative Care palliative care consultation service metrics: consensus recommendations. J Palliat Med. 2008;11(10):1294–1298.
- , , , et al. Evaluation of the End‐of‐Life Nursing Education Consortium undergraduate faculty training program. J Palliat Med. 2005;8(1):107–114.
- , . Generalist plus specialist palliative care–creating a more sustainable model. N Engl J Med. 2013;368(13):1173–1175.
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2014.
- , , , et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180–190.
- , , , , . A systematic review of satisfaction with care at the end of life. J Am Geriatr Soc. 2008;56(1):124–129.
- . Health care system factors affecting end‐of‐life care. J Palliat Med. 2005;8(suppl 1):S79–S87.
- , , . Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs medical center. J Palliat Med. 2005;8(1):26–35.
- , , , et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790.
- , , . Two steps forward, one step back: changes in palliative care consultation services in California hospitals from 2007 to 2011. J Palliat Med. 2014;17(11):1214–1220.
- , . Using qualitative methods to explore key questions in palliative care. J Palliat Med. 2009;12(8):725–730.
- , , . Center to Advance Palliative Care palliative care consultation service metrics: consensus recommendations. J Palliat Med. 2008;11(10):1294–1298.
- , , , et al. Evaluation of the End‐of‐Life Nursing Education Consortium undergraduate faculty training program. J Palliat Med. 2005;8(1):107–114.
- , . Generalist plus specialist palliative care–creating a more sustainable model. N Engl J Med. 2013;368(13):1173–1175.
Severity of Symptoms
The frequency and severity of symptoms among older hospitalized patients with chronic illnesses can have a profound negative impact on their quality of life.1, 2 Nonetheless, research examining the prevalence and management of symptoms has focused predominantly on cancer patients.3 Few studies have included patients with other serious conditions such as heart failure (HF) and chronic obstructive pulmonary disease (COPD),3, 4 which are very common and are major causes of morbidity and mortality in the United States.5 One longitudinal assessment of symptom severity among a group of community‐based older adults diagnosed with COPD and HF reported high rates of moderate‐to‐severe pain, dyspnea, and anxiety at baseline and follow‐up, as long as 22 months later.6 Persistent symptoms over time can have an adverse effect on an individual's physical and emotional well‐being, and highlight opportunities to improve care.3, 7 Understanding patterns of symptom change over time is a key first step in developing systems to improve quality of care for people with chronic illness.
Among hospitalized patients, pain, dyspnea, anxiety, and depression cause the greatest symptom burden, accounting for 67% of all symptoms classified as moderate to severe.8 While assessment and management of symptoms may be the reason for admission to the hospital and the focus of inpatient care, this focus may not persist after discharge, leaving patients with significant symptoms that can diminish quality of life and contribute to readmission.9 We studied a cohort of older inpatients with serious illness over time in order to determine the prevalence, severity, burden, and predictors of symptoms during the course of hospitalization and at 2 weeks after discharge.
METHODS
Setting
The study was undertaken at a large academic medical center in San Francisco.
Subjects
Participants were patients 65 years or older admitted to the medicine or cardiology services with a primary diagnosis of cancer, COPD, or HF. Participants were required to be fully oriented and English‐speaking. Patients gave written informed consent to participate. The Committee on Human Research at the University of California, San Francisco, approved this study (H8695‐35172‐01).
Data Collection
Data collection was undertaken from March 2001 to December 2003. This study was part of a prospective, clinical trial that compared a proactive palliative medicine consultation with usual hospital care, and has been previously described.10 Upon study enrollment, all patients completed the Inpatient Care Survey. The survey asked participants about demographic information such as date of birth, sex, education level, race, and marital status. The survey instruments also included the Instrumental Activities of Daily Living (IADL) index and the Geriatric Depression Scale (GDS‐15). Each weekday during hospitalization, a trained research assistant asked patients to report their worst symptom level for pain, dyspnea, and anxiety in the past 24 hours using a 010 numeric rating scale, where 0 was none and 10 was the worst you can imagine. We further characterized scores into categories such that 0 was defined as none, 13 as mild, 46 as moderate, and 710 as severe. A follow‐up telephone survey, 2 weeks after discharge, reassessed patients' worst symptom levels in the past 24 hours for pain, dyspnea, and anxiety.
We also generated a composite score of symptoms to report a symptom burden score for these 3 symptoms. Using the categories of symptom severity, we assigned a score of 0 for none, 1 for mild, 2 for moderate, and 3 for severe. We summed the assigned scores for all 3 symptoms for each subject to generate a symptom burden score as follows: no symptom burden (0), mild symptom burden (13), moderate symptom burden (46), and severe symptom burden (79). In this scale, a moderate symptom burden would mean that a subject reported having at least 1 symptom at a moderate or severe level, with at least 1 other symptom present. A severe symptom burden would require the presence of all 3 symptoms, with at least 1 at a severe level.
We reviewed patient charts to assess severity of patient illness upon admission. For cancer, we recorded type; for COPD, we noted forced expiratory volume in 1 second (FEV1); and for HF, we recorded the ejection fraction. We also queried the National Death Index to get vital statistics on all subjects.
Data Preparation
The IADL asks patients to report whether they can perform 13 daily living skills without help, with some help, or were unable to complete tasks.11 Subjects who reported needing at least some help with any of the 13 items were categorized as dependent. The GDS‐15 is a widely used, validated 15‐item scale for assessing depressive mood in the elderly.12 Scores for the GDS‐15 range from 0 to 15, with higher scores indicating more depressive symptoms. Based on previous research, we categorized patients as either not depressed (05) or having probable depression (6 or more).12
Statistical Analysis
Because our clinical trial had no impact on care or symptoms, we combined intervention and usual care patients for this analysis of symptom severity. Descriptive statistics, such as frequencies, means, standard deviations (SDs), and 95% confidence intervals (CIs) were used to examine the distribution of measures. Chi‐square (2) analysis was undertaken to examine bivariate associations between categorical variables. Analysis of variance (ANOVA) was undertaken to examine associations between categorical and continuous variables. Multivariate logistic regression was used to examine predictors of symptom burden at follow‐up, including patient characteristics that were significant to P 0.10 in bivariate analysis. We used KaplanMeier survival curves to examine the relationship between primary diagnosis and mortality, and assessed statistical significance using log‐rank tests (MantelCox).13 The Statistical Package for the Social Sciences (SPSS) for Mac (version 17; SPSS Inc, Chicago, IL; March 11, 2009) was used to analyze these data.
RESULTS
Patient Characteristics
A total of 150 patients enrolled in the study. The mean length of stay was 5.4 days (SD: 5.6; range: 147 days). HF was the most common primary diagnosis (46.7%, n = 70) with 48% (n = 34) having an ejection fraction of 45% or less (mean = 43%; SD: 22); followed by cancer (30%, n = 45) with the most common type being prostate (18%, n = 8), lung (13%, n = 6), and breast (13%, n = 6); and COPD (23%, n = 35) with an average FEV1 of 1.5 L (SD: 0.94; range: 0.503.9). The mean age was 77 years (SD: 7.9; range: 6596 years). The majority of participants were men (56%, n = 83) and white (73%, n = 108), with the most being either married/partnered (43%, n = 64) or divorced/widowed (44%, n = 66). The IADL identified almost two‐thirds of participants as dependent (62%, n = 94). The GDS‐15 categorized three‐quarters of participants (n = 118) as not depressed. The only significant association between participant characteristics and their primary diagnosis was for the IADL index (Table 1), with significantly more (2 = 6.3; P = 0.04) patients with HF categorized as being dependent (72%).
| Characteristics | Primary Diagnosis | P | |||
|---|---|---|---|---|---|
| Cancer n = 44 | HF n = 70 | COPD n = 35 | |||
| |||||
| Length of stay | (Mean days) | 5.4 | 4.7 | 6.5 | 0.3 |
| Age | (Mean years) | 76 | 78 | 76 | 0.3 |
| Sex | |||||
| Female | 47% | 37% | 57% | 0.1 | |
| Marital status | 0.2 | ||||
| Single | 16 | 9 | 17 | ||
| Married/partnered | 51 | 45 | 29 | ||
| Divorced/widowed | 33 | 46 | 54 | ||
| Race | |||||
| White | 89 | 64 | 69 | 0.1 | |
| Black/African American | 7 | 21 | 23 | ||
| Asian or Pacific Islander | 5 | 10 | 9 | ||
| Other | 0 | 4 | 0 | ||
| IADL | |||||
| Dependent | 49 | 72 | 60 | 0.04 | |
| GDS‐15 | |||||
| Probable depression | 18 | 22 | 21 | 0.9 | |
Frequency and Severity of Symptoms
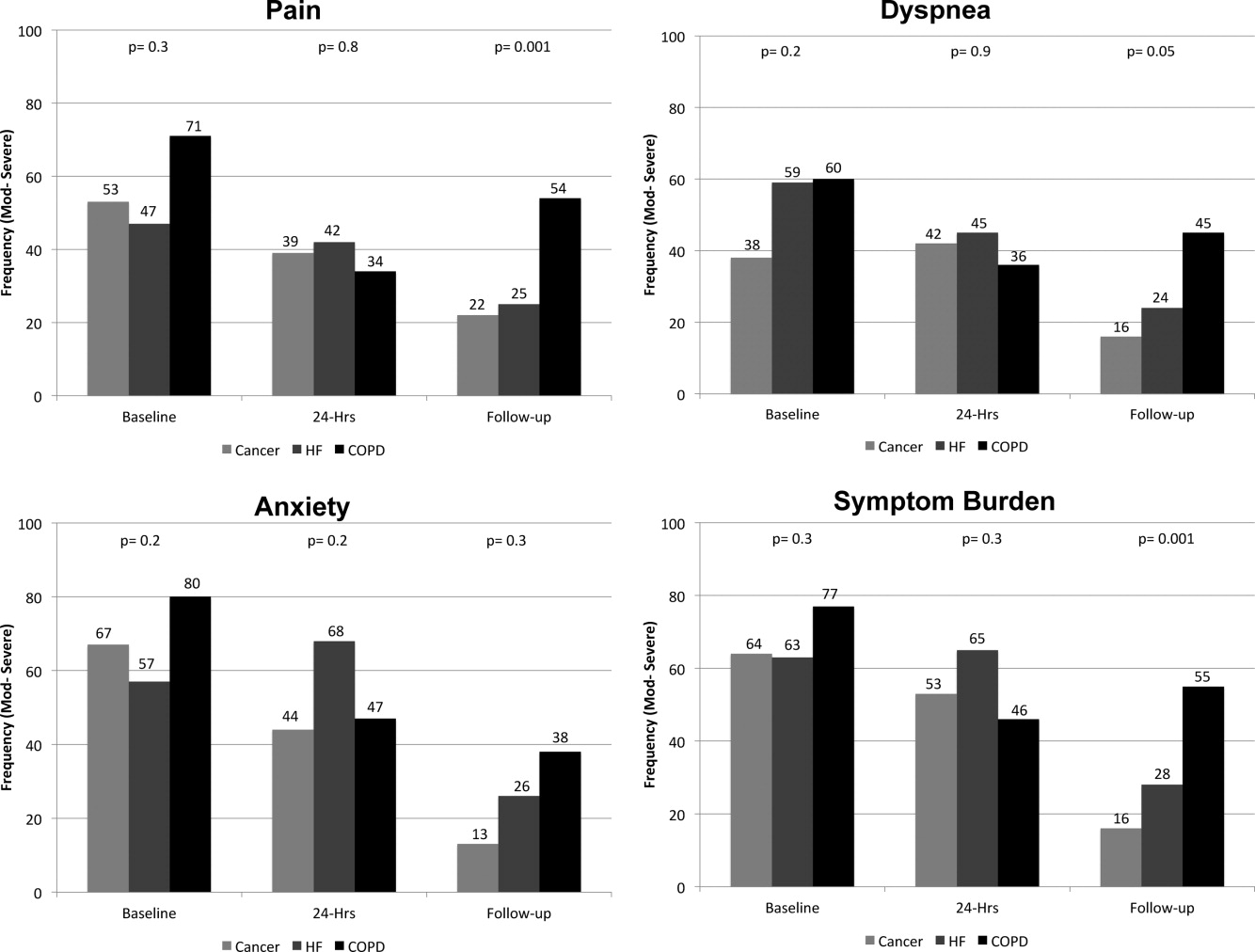
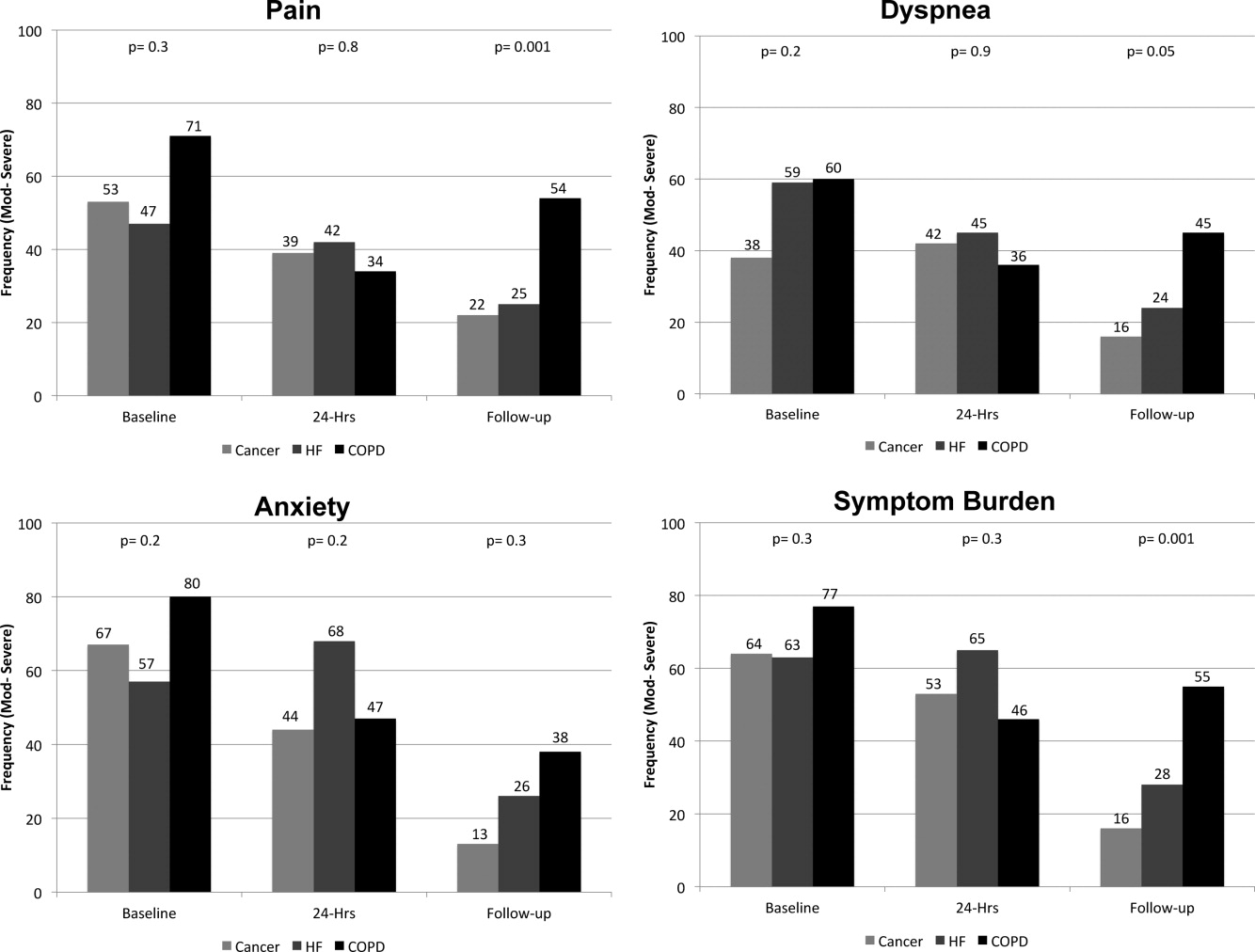
On average, the postdischarge follow‐up assessment was undertaken 24 days (median = 21.0; SD: 17.9; range: 7140 days) after the baseline assessment and 20 days after discharge (median = 15; SD: 17.0; range: 4139). At baseline, a large proportion of participants reported symptoms at a moderate‐to‐severe level for pain (54%, n = 81), dyspnea (53%, n = 79), and anxiety (63%, n = 94). The majority of patients (64%, n = 96) reported having 2 or more symptoms at a moderate‐to‐severe level and one quarter (27%, n = 41) had 3 symptoms at a moderate‐to‐severe level. While the frequency of moderate‐to‐severe symptoms decreased at the 24‐hour hospital assessment (pain = 42%, dyspnea = 45%, anxiety = 55%) and again at 2‐week follow‐up (pain = 28%, dyspnea = 27%, anxiety = 25%), a substantial symptom burden persisted with 30% (n = 36) of patients having moderate‐to‐severe levels at 2‐week follow‐up. Overall there were no differences between primary diagnosis and the frequency of symptoms at baseline or 24‐hour hospital assessment (Figure 1). However at follow‐up, those diagnosed with COPD were more likely to report moderate/severe pain (54%; 2 = 22.0; P < 0.001), dyspnea (45%; 2 = 9.3; P = 0.05), and overall symptom burden (55%; 2 = 25.9; P < 0.001) than those with cancer (pain = 22%, dyspnea = 16%, symptom burden = 16%) or HF (pain = 25%, dyspnea = 24%, symptom burden = 28%).
As symptom burden was our composite score for pain, dyspnea, and anxiety, we were interested in identifying variables in addition to primary diagnosis that might be associated with symptom burden at follow‐up. Bivariate analysis revealed that there was no significant association between symptom burden and age (2 = 1.5; P = 0.5), gender (2 = 1.3; P = 0.3), length of stay (2 = 0.4; P = 0.8), and (IADL) level of independence (2 = 0.3; P = 0.6). However, those with probable depression were more likely (2 = 11.9; P = 0.001) to have a moderate/severe symptom burden (62%, n = 13), compared to those with no depression (24%, n = 23). After adjusting for severity of symptom burden at baseline, multivariate logistic regression revealed that primary diagnosis (P = 0.01) and probable depression (OR = 4.9; 95% CI = 1.6, 14.9; P = 0.005) were associated with symptom severity. Patients with COPD had greater odds (OR = 7.0; 95% CI = 1.9, 26.2; P = 0.002) of moderate/severe symptom burden than those with cancer, while those with HF did not (OR = 2.3; 95% CI = 0.7, 7.7; P = 0.16). There was significant interaction between primary diagnosis and depression (P = 0.2).
Primary Diagnosis, Symptom Burden, and Survival Time
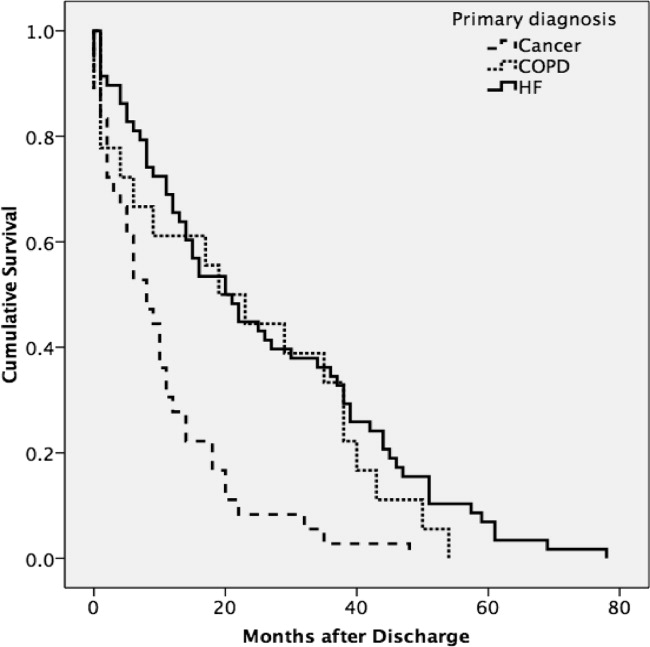
A total of 75% of patients were identified by the National Death Index to have died between hospital discharge and December 2007, of which 47% had died within 12 months after discharge. KaplanMeier survival curves (Figure 2) revealed a significant difference (MantelCox: 2 = 19.3; df = 1; P = 0.0001) in survival time, with patients diagnosed with COPD (median = 19.0 months; 95% CI = 6.5, 31.5) and HF (median = 20.0 months; 95% CI = 12.5, 27.5) having a longer survival than those with cancer (median = 8.0 months; 95% CI = 4.1, 11.9).
We also examined the relationship between symptom burden and survival time. KaplanMeier survival curves revealed no significant difference (MantelCox: 2 = 0.2; P = 0.6) in the survival time of patients classified with a symptom burden of none/moderate (median = 15.0 months; 95% CI = 8.8, 21.2) or moderate/severe (median = 14.0 months; 95% CI = 2.6, 25.4).
DISCUSSION
In our sample of older inpatients diagnosed with cancer, HF, and COPD, a large proportion reported moderate‐to‐severe levels of pain, dyspnea, and anxiety at baseline and follow‐up. When combined, these levels represent a considerable symptom burden, with over three‐quarters of participants reporting 2 to 3 symptoms at a moderate/severe level at baseline. While symptom scores decreased at 24‐hours and 2‐week follow‐up, symptom burden remained high, with almost half of the participants reporting 23 symptoms at a moderate‐to‐severe level at 24‐hour assessment and a large minority reporting moderate‐to‐severe symptoms at follow‐up. A higher percentage of patients with COPD reported moderate‐to‐severe pain, dyspnea, and overall symptom burden at follow‐up than participants with cancer or HF who reported a similar symptom burden. We also found that patients with probable depression were more likely to have a significant symptom burden at follow‐up. These findings highlight the need to routinely assess and treat symptoms over time, including depression, and especially in patients with COPD. While we found that hospital care was seemingly effective in improving symptoms, they persist at distressing levels in many patients.
Few studies have assessed the severity of symptoms over time. One study that did, examined symptom severity among community‐based elders diagnosed with HF and COPD.6 At baseline, these participants had a lower prevalence of moderate‐to‐severe symptoms than the hospitalized patients enrolled in our study, a finding that would be anticipated, as they may not have been as ill. However, symptom severity persisted in the community‐based subjects and, in some cases, worsened over the 22‐month assessment period for pain (HF = 20% vs 42%; COPD = 27% vs 20%), dyspnea (HF = 19% vs 29%; COPD = 66% vs 76%), and anxiety (HF = 2% vs 12%; COPD = 32% vs 23%).6 In contrast, while our subjects with a primary diagnosis of HF and COPD had a higher prevalence of moderate‐to‐severe symptoms at baseline, they did experience an improvement in the severity of pain, dyspnea, and anxiety at the 2‐week follow‐up assessment. However, despite a decrease in the prevalence of moderate‐to‐severe symptoms from baseline to follow‐up, a high symptom burden persisted for many patients, particularly for those diagnosed with COPD and those with probable depression at baseline. The severity of a patient's symptoms can have a profound negative effect on health status and quality of life.14 Findings from these studies suggest that symptoms are currently not being adequately managed, and highlight an urgent need to develop coordinated strategies and systems that focus on improving the management of symptoms, including depression, over time.6
We also found that subjects recruited for this study had advanced disease, evidenced by the fact that nearly half died within 12 months. We did not use specific prognostic indices or severity of illness criteria for recruiting subjects and simply approached patients admitted with one of the target diagnoses. Our study suggests that targeting these patients for routine symptom assessment and management, including for palliative care, would be a reasonable approach given the high symptom burden and relatively high mortality at 1 year.
Interpretation of these findings should be mitigated by the following limitations. Because of our setting, our findings may not be generalizable to all patients with cancer, HF, and COPD. However, our subjects were admitted to general medical and cardiology services, and had common conditions, and therefore are likely similar to those presenting to other hospitals. We relied on self‐report measures to assess severity of symptoms. Patient self‐report, while potentially subject to imprecision due to poor recall and social demand biases, is considered the gold standard for symptom assessment.15 Finally, 2‐week follow‐up is relatively short, and it is possible that symptoms may have improved had we assessed them over a longer period. The longitudinal study of elders in the community that followed subjects over 22 months found that, for many patients, symptoms worsened over time and nearly half of our subjects died at 12 months, suggesting that longer follow‐up would have been unlikely to show improvement in symptoms.6
A significant minority of participants reported a substantial, persistent symptom burden, yet all symptoms assessed in our study are potentially modifiable. Recognizing and treating symptoms can be achieved through the use of targeted interventions.6 Because symptoms can occur in clusters, successful treatment of 1 symptom may also help to improve other symptoms.1 The large number of participants reporting moderate‐to‐severe levels of symptom burden at 2 weeks after discharge highlights an unmet need for improved symptom control in the outpatient setting. Unfortunately, while evidence exists for managing pain in patients with cancer, such evidence‐based practices are lacking for the management of pain and other symptoms in patients with HF and COPD. Some symptoms may require specific, disease‐oriented management. However, many symptoms may be due to common comorbidities, such as pain from degenerative joint disease, that may likely respond to proven treatments.16
Our study confirmed the significant burden of symptoms experienced by patients with serious illness and demonstrated that patients with COPD report as much symptom burden as patients with cancer and HF, if not more. While symptom severity improved over the course of the hospitalization and follow‐up, a large percentage of patients reported significant symptom burden at follow‐up. Depression was also common in these patients. Because these symptoms diminish quality of life, routine assessment and management of these symptoms is critical for improving the quality of care provided to these patients. Additional research on the best approaches to manage symptoms, including medications, interventions, and structures of care, could further improve care.
Acknowledgements
The authors thank all the patients who participated in this study. They thank Joanne Batt, Wren Levenberg, and Emily Philipps for their expert help as research assistants. They also thank Harold Collard, MD, for providing valuable feedback on the manuscript. Data obtained from the National Death Index assisted us in meeting our study objectives. Steven Pantilat had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- ,,.Symptom clusters: the new frontier in symptom management research.J Natl Cancer Inst Monogr.2004(32):17–21.
- .Symptom burden: multiple symptoms and their impact as patient‐reported outcomes.J Natl Cancer Inst Monogr.2007(37):16–21.
- ,,.A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease.J Pain Symptom Manage.2006;31(1):58–69.
- ,,, et al.Comparing three life‐limiting diseases: does diagnosis matter or is sick, sick?J Pain Symptom Manage.2011;42(3):331–341.
- ,,,,,.Deaths: final data for 2006.Natl Vital Stat Rep.2009;57(14):1–134.
- ,,,,,.Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure.Arch Intern Med.2007;167(22):2503–2508.
- ,,,.Living with chronic obstructive pulmonary disease: a survey of patients' knowledge and attitudes.Respir Med.2009;103(7):1004–1012.
- ,,,,.The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment.J Pain Symptom Manage.1999;17(4):248–255.
- ,,, et al.Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure.JAMA.2010;303(17):1716–1722.
- ,,,.Hospital‐based palliative medicine consultation: a randomized controlled trial.Arch Intern Med.2010;170(22):2038–2040.
- ,,,,.Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,,.Screening for late life depression: cut‐off scores for the Geriatric Depression Scale and the Cornell Scale for Depression in Dementia among Japanese subjects.Int J Geriatr Psychiatry.2003;18(6):498–505.
- ,.Nonparametric estimation from incomplete observations.J Am Stat Assoc.1958;53(282):457–481.
- ,,.End stage chronic obstructive pulmonary disease.Pneumonol Alergol Pol.2009;77(2):173–179.
- ,,,.Relationship between social desirability and self‐report in chronic pain patients.Clin J Pain.1995;11(3):189–193.
- ,,,.Etiology and severity of pain among outpatients living with HF.J Card Fail.2010;16(8):S88.
The frequency and severity of symptoms among older hospitalized patients with chronic illnesses can have a profound negative impact on their quality of life.1, 2 Nonetheless, research examining the prevalence and management of symptoms has focused predominantly on cancer patients.3 Few studies have included patients with other serious conditions such as heart failure (HF) and chronic obstructive pulmonary disease (COPD),3, 4 which are very common and are major causes of morbidity and mortality in the United States.5 One longitudinal assessment of symptom severity among a group of community‐based older adults diagnosed with COPD and HF reported high rates of moderate‐to‐severe pain, dyspnea, and anxiety at baseline and follow‐up, as long as 22 months later.6 Persistent symptoms over time can have an adverse effect on an individual's physical and emotional well‐being, and highlight opportunities to improve care.3, 7 Understanding patterns of symptom change over time is a key first step in developing systems to improve quality of care for people with chronic illness.
Among hospitalized patients, pain, dyspnea, anxiety, and depression cause the greatest symptom burden, accounting for 67% of all symptoms classified as moderate to severe.8 While assessment and management of symptoms may be the reason for admission to the hospital and the focus of inpatient care, this focus may not persist after discharge, leaving patients with significant symptoms that can diminish quality of life and contribute to readmission.9 We studied a cohort of older inpatients with serious illness over time in order to determine the prevalence, severity, burden, and predictors of symptoms during the course of hospitalization and at 2 weeks after discharge.
METHODS
Setting
The study was undertaken at a large academic medical center in San Francisco.
Subjects
Participants were patients 65 years or older admitted to the medicine or cardiology services with a primary diagnosis of cancer, COPD, or HF. Participants were required to be fully oriented and English‐speaking. Patients gave written informed consent to participate. The Committee on Human Research at the University of California, San Francisco, approved this study (H8695‐35172‐01).
Data Collection
Data collection was undertaken from March 2001 to December 2003. This study was part of a prospective, clinical trial that compared a proactive palliative medicine consultation with usual hospital care, and has been previously described.10 Upon study enrollment, all patients completed the Inpatient Care Survey. The survey asked participants about demographic information such as date of birth, sex, education level, race, and marital status. The survey instruments also included the Instrumental Activities of Daily Living (IADL) index and the Geriatric Depression Scale (GDS‐15). Each weekday during hospitalization, a trained research assistant asked patients to report their worst symptom level for pain, dyspnea, and anxiety in the past 24 hours using a 010 numeric rating scale, where 0 was none and 10 was the worst you can imagine. We further characterized scores into categories such that 0 was defined as none, 13 as mild, 46 as moderate, and 710 as severe. A follow‐up telephone survey, 2 weeks after discharge, reassessed patients' worst symptom levels in the past 24 hours for pain, dyspnea, and anxiety.
We also generated a composite score of symptoms to report a symptom burden score for these 3 symptoms. Using the categories of symptom severity, we assigned a score of 0 for none, 1 for mild, 2 for moderate, and 3 for severe. We summed the assigned scores for all 3 symptoms for each subject to generate a symptom burden score as follows: no symptom burden (0), mild symptom burden (13), moderate symptom burden (46), and severe symptom burden (79). In this scale, a moderate symptom burden would mean that a subject reported having at least 1 symptom at a moderate or severe level, with at least 1 other symptom present. A severe symptom burden would require the presence of all 3 symptoms, with at least 1 at a severe level.
We reviewed patient charts to assess severity of patient illness upon admission. For cancer, we recorded type; for COPD, we noted forced expiratory volume in 1 second (FEV1); and for HF, we recorded the ejection fraction. We also queried the National Death Index to get vital statistics on all subjects.
Data Preparation
The IADL asks patients to report whether they can perform 13 daily living skills without help, with some help, or were unable to complete tasks.11 Subjects who reported needing at least some help with any of the 13 items were categorized as dependent. The GDS‐15 is a widely used, validated 15‐item scale for assessing depressive mood in the elderly.12 Scores for the GDS‐15 range from 0 to 15, with higher scores indicating more depressive symptoms. Based on previous research, we categorized patients as either not depressed (05) or having probable depression (6 or more).12
Statistical Analysis
Because our clinical trial had no impact on care or symptoms, we combined intervention and usual care patients for this analysis of symptom severity. Descriptive statistics, such as frequencies, means, standard deviations (SDs), and 95% confidence intervals (CIs) were used to examine the distribution of measures. Chi‐square (2) analysis was undertaken to examine bivariate associations between categorical variables. Analysis of variance (ANOVA) was undertaken to examine associations between categorical and continuous variables. Multivariate logistic regression was used to examine predictors of symptom burden at follow‐up, including patient characteristics that were significant to P 0.10 in bivariate analysis. We used KaplanMeier survival curves to examine the relationship between primary diagnosis and mortality, and assessed statistical significance using log‐rank tests (MantelCox).13 The Statistical Package for the Social Sciences (SPSS) for Mac (version 17; SPSS Inc, Chicago, IL; March 11, 2009) was used to analyze these data.
RESULTS
Patient Characteristics
A total of 150 patients enrolled in the study. The mean length of stay was 5.4 days (SD: 5.6; range: 147 days). HF was the most common primary diagnosis (46.7%, n = 70) with 48% (n = 34) having an ejection fraction of 45% or less (mean = 43%; SD: 22); followed by cancer (30%, n = 45) with the most common type being prostate (18%, n = 8), lung (13%, n = 6), and breast (13%, n = 6); and COPD (23%, n = 35) with an average FEV1 of 1.5 L (SD: 0.94; range: 0.503.9). The mean age was 77 years (SD: 7.9; range: 6596 years). The majority of participants were men (56%, n = 83) and white (73%, n = 108), with the most being either married/partnered (43%, n = 64) or divorced/widowed (44%, n = 66). The IADL identified almost two‐thirds of participants as dependent (62%, n = 94). The GDS‐15 categorized three‐quarters of participants (n = 118) as not depressed. The only significant association between participant characteristics and their primary diagnosis was for the IADL index (Table 1), with significantly more (2 = 6.3; P = 0.04) patients with HF categorized as being dependent (72%).
| Characteristics | Primary Diagnosis | P | |||
|---|---|---|---|---|---|
| Cancer n = 44 | HF n = 70 | COPD n = 35 | |||
| |||||
| Length of stay | (Mean days) | 5.4 | 4.7 | 6.5 | 0.3 |
| Age | (Mean years) | 76 | 78 | 76 | 0.3 |
| Sex | |||||
| Female | 47% | 37% | 57% | 0.1 | |
| Marital status | 0.2 | ||||
| Single | 16 | 9 | 17 | ||
| Married/partnered | 51 | 45 | 29 | ||
| Divorced/widowed | 33 | 46 | 54 | ||
| Race | |||||
| White | 89 | 64 | 69 | 0.1 | |
| Black/African American | 7 | 21 | 23 | ||
| Asian or Pacific Islander | 5 | 10 | 9 | ||
| Other | 0 | 4 | 0 | ||
| IADL | |||||
| Dependent | 49 | 72 | 60 | 0.04 | |
| GDS‐15 | |||||
| Probable depression | 18 | 22 | 21 | 0.9 | |
Frequency and Severity of Symptoms
On average, the postdischarge follow‐up assessment was undertaken 24 days (median = 21.0; SD: 17.9; range: 7140 days) after the baseline assessment and 20 days after discharge (median = 15; SD: 17.0; range: 4139). At baseline, a large proportion of participants reported symptoms at a moderate‐to‐severe level for pain (54%, n = 81), dyspnea (53%, n = 79), and anxiety (63%, n = 94). The majority of patients (64%, n = 96) reported having 2 or more symptoms at a moderate‐to‐severe level and one quarter (27%, n = 41) had 3 symptoms at a moderate‐to‐severe level. While the frequency of moderate‐to‐severe symptoms decreased at the 24‐hour hospital assessment (pain = 42%, dyspnea = 45%, anxiety = 55%) and again at 2‐week follow‐up (pain = 28%, dyspnea = 27%, anxiety = 25%), a substantial symptom burden persisted with 30% (n = 36) of patients having moderate‐to‐severe levels at 2‐week follow‐up. Overall there were no differences between primary diagnosis and the frequency of symptoms at baseline or 24‐hour hospital assessment (Figure 1). However at follow‐up, those diagnosed with COPD were more likely to report moderate/severe pain (54%; 2 = 22.0; P < 0.001), dyspnea (45%; 2 = 9.3; P = 0.05), and overall symptom burden (55%; 2 = 25.9; P < 0.001) than those with cancer (pain = 22%, dyspnea = 16%, symptom burden = 16%) or HF (pain = 25%, dyspnea = 24%, symptom burden = 28%).

As symptom burden was our composite score for pain, dyspnea, and anxiety, we were interested in identifying variables in addition to primary diagnosis that might be associated with symptom burden at follow‐up. Bivariate analysis revealed that there was no significant association between symptom burden and age (2 = 1.5; P = 0.5), gender (2 = 1.3; P = 0.3), length of stay (2 = 0.4; P = 0.8), and (IADL) level of independence (2 = 0.3; P = 0.6). However, those with probable depression were more likely (2 = 11.9; P = 0.001) to have a moderate/severe symptom burden (62%, n = 13), compared to those with no depression (24%, n = 23). After adjusting for severity of symptom burden at baseline, multivariate logistic regression revealed that primary diagnosis (P = 0.01) and probable depression (OR = 4.9; 95% CI = 1.6, 14.9; P = 0.005) were associated with symptom severity. Patients with COPD had greater odds (OR = 7.0; 95% CI = 1.9, 26.2; P = 0.002) of moderate/severe symptom burden than those with cancer, while those with HF did not (OR = 2.3; 95% CI = 0.7, 7.7; P = 0.16). There was significant interaction between primary diagnosis and depression (P = 0.2).
Primary Diagnosis, Symptom Burden, and Survival Time
A total of 75% of patients were identified by the National Death Index to have died between hospital discharge and December 2007, of which 47% had died within 12 months after discharge. KaplanMeier survival curves (Figure 2) revealed a significant difference (MantelCox: 2 = 19.3; df = 1; P = 0.0001) in survival time, with patients diagnosed with COPD (median = 19.0 months; 95% CI = 6.5, 31.5) and HF (median = 20.0 months; 95% CI = 12.5, 27.5) having a longer survival than those with cancer (median = 8.0 months; 95% CI = 4.1, 11.9).

We also examined the relationship between symptom burden and survival time. KaplanMeier survival curves revealed no significant difference (MantelCox: 2 = 0.2; P = 0.6) in the survival time of patients classified with a symptom burden of none/moderate (median = 15.0 months; 95% CI = 8.8, 21.2) or moderate/severe (median = 14.0 months; 95% CI = 2.6, 25.4).
DISCUSSION
In our sample of older inpatients diagnosed with cancer, HF, and COPD, a large proportion reported moderate‐to‐severe levels of pain, dyspnea, and anxiety at baseline and follow‐up. When combined, these levels represent a considerable symptom burden, with over three‐quarters of participants reporting 2 to 3 symptoms at a moderate/severe level at baseline. While symptom scores decreased at 24‐hours and 2‐week follow‐up, symptom burden remained high, with almost half of the participants reporting 23 symptoms at a moderate‐to‐severe level at 24‐hour assessment and a large minority reporting moderate‐to‐severe symptoms at follow‐up. A higher percentage of patients with COPD reported moderate‐to‐severe pain, dyspnea, and overall symptom burden at follow‐up than participants with cancer or HF who reported a similar symptom burden. We also found that patients with probable depression were more likely to have a significant symptom burden at follow‐up. These findings highlight the need to routinely assess and treat symptoms over time, including depression, and especially in patients with COPD. While we found that hospital care was seemingly effective in improving symptoms, they persist at distressing levels in many patients.
Few studies have assessed the severity of symptoms over time. One study that did, examined symptom severity among community‐based elders diagnosed with HF and COPD.6 At baseline, these participants had a lower prevalence of moderate‐to‐severe symptoms than the hospitalized patients enrolled in our study, a finding that would be anticipated, as they may not have been as ill. However, symptom severity persisted in the community‐based subjects and, in some cases, worsened over the 22‐month assessment period for pain (HF = 20% vs 42%; COPD = 27% vs 20%), dyspnea (HF = 19% vs 29%; COPD = 66% vs 76%), and anxiety (HF = 2% vs 12%; COPD = 32% vs 23%).6 In contrast, while our subjects with a primary diagnosis of HF and COPD had a higher prevalence of moderate‐to‐severe symptoms at baseline, they did experience an improvement in the severity of pain, dyspnea, and anxiety at the 2‐week follow‐up assessment. However, despite a decrease in the prevalence of moderate‐to‐severe symptoms from baseline to follow‐up, a high symptom burden persisted for many patients, particularly for those diagnosed with COPD and those with probable depression at baseline. The severity of a patient's symptoms can have a profound negative effect on health status and quality of life.14 Findings from these studies suggest that symptoms are currently not being adequately managed, and highlight an urgent need to develop coordinated strategies and systems that focus on improving the management of symptoms, including depression, over time.6
We also found that subjects recruited for this study had advanced disease, evidenced by the fact that nearly half died within 12 months. We did not use specific prognostic indices or severity of illness criteria for recruiting subjects and simply approached patients admitted with one of the target diagnoses. Our study suggests that targeting these patients for routine symptom assessment and management, including for palliative care, would be a reasonable approach given the high symptom burden and relatively high mortality at 1 year.
Interpretation of these findings should be mitigated by the following limitations. Because of our setting, our findings may not be generalizable to all patients with cancer, HF, and COPD. However, our subjects were admitted to general medical and cardiology services, and had common conditions, and therefore are likely similar to those presenting to other hospitals. We relied on self‐report measures to assess severity of symptoms. Patient self‐report, while potentially subject to imprecision due to poor recall and social demand biases, is considered the gold standard for symptom assessment.15 Finally, 2‐week follow‐up is relatively short, and it is possible that symptoms may have improved had we assessed them over a longer period. The longitudinal study of elders in the community that followed subjects over 22 months found that, for many patients, symptoms worsened over time and nearly half of our subjects died at 12 months, suggesting that longer follow‐up would have been unlikely to show improvement in symptoms.6
A significant minority of participants reported a substantial, persistent symptom burden, yet all symptoms assessed in our study are potentially modifiable. Recognizing and treating symptoms can be achieved through the use of targeted interventions.6 Because symptoms can occur in clusters, successful treatment of 1 symptom may also help to improve other symptoms.1 The large number of participants reporting moderate‐to‐severe levels of symptom burden at 2 weeks after discharge highlights an unmet need for improved symptom control in the outpatient setting. Unfortunately, while evidence exists for managing pain in patients with cancer, such evidence‐based practices are lacking for the management of pain and other symptoms in patients with HF and COPD. Some symptoms may require specific, disease‐oriented management. However, many symptoms may be due to common comorbidities, such as pain from degenerative joint disease, that may likely respond to proven treatments.16
Our study confirmed the significant burden of symptoms experienced by patients with serious illness and demonstrated that patients with COPD report as much symptom burden as patients with cancer and HF, if not more. While symptom severity improved over the course of the hospitalization and follow‐up, a large percentage of patients reported significant symptom burden at follow‐up. Depression was also common in these patients. Because these symptoms diminish quality of life, routine assessment and management of these symptoms is critical for improving the quality of care provided to these patients. Additional research on the best approaches to manage symptoms, including medications, interventions, and structures of care, could further improve care.
Acknowledgements
The authors thank all the patients who participated in this study. They thank Joanne Batt, Wren Levenberg, and Emily Philipps for their expert help as research assistants. They also thank Harold Collard, MD, for providing valuable feedback on the manuscript. Data obtained from the National Death Index assisted us in meeting our study objectives. Steven Pantilat had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The frequency and severity of symptoms among older hospitalized patients with chronic illnesses can have a profound negative impact on their quality of life.1, 2 Nonetheless, research examining the prevalence and management of symptoms has focused predominantly on cancer patients.3 Few studies have included patients with other serious conditions such as heart failure (HF) and chronic obstructive pulmonary disease (COPD),3, 4 which are very common and are major causes of morbidity and mortality in the United States.5 One longitudinal assessment of symptom severity among a group of community‐based older adults diagnosed with COPD and HF reported high rates of moderate‐to‐severe pain, dyspnea, and anxiety at baseline and follow‐up, as long as 22 months later.6 Persistent symptoms over time can have an adverse effect on an individual's physical and emotional well‐being, and highlight opportunities to improve care.3, 7 Understanding patterns of symptom change over time is a key first step in developing systems to improve quality of care for people with chronic illness.
Among hospitalized patients, pain, dyspnea, anxiety, and depression cause the greatest symptom burden, accounting for 67% of all symptoms classified as moderate to severe.8 While assessment and management of symptoms may be the reason for admission to the hospital and the focus of inpatient care, this focus may not persist after discharge, leaving patients with significant symptoms that can diminish quality of life and contribute to readmission.9 We studied a cohort of older inpatients with serious illness over time in order to determine the prevalence, severity, burden, and predictors of symptoms during the course of hospitalization and at 2 weeks after discharge.
METHODS
Setting
The study was undertaken at a large academic medical center in San Francisco.
Subjects
Participants were patients 65 years or older admitted to the medicine or cardiology services with a primary diagnosis of cancer, COPD, or HF. Participants were required to be fully oriented and English‐speaking. Patients gave written informed consent to participate. The Committee on Human Research at the University of California, San Francisco, approved this study (H8695‐35172‐01).
Data Collection
Data collection was undertaken from March 2001 to December 2003. This study was part of a prospective, clinical trial that compared a proactive palliative medicine consultation with usual hospital care, and has been previously described.10 Upon study enrollment, all patients completed the Inpatient Care Survey. The survey asked participants about demographic information such as date of birth, sex, education level, race, and marital status. The survey instruments also included the Instrumental Activities of Daily Living (IADL) index and the Geriatric Depression Scale (GDS‐15). Each weekday during hospitalization, a trained research assistant asked patients to report their worst symptom level for pain, dyspnea, and anxiety in the past 24 hours using a 010 numeric rating scale, where 0 was none and 10 was the worst you can imagine. We further characterized scores into categories such that 0 was defined as none, 13 as mild, 46 as moderate, and 710 as severe. A follow‐up telephone survey, 2 weeks after discharge, reassessed patients' worst symptom levels in the past 24 hours for pain, dyspnea, and anxiety.
We also generated a composite score of symptoms to report a symptom burden score for these 3 symptoms. Using the categories of symptom severity, we assigned a score of 0 for none, 1 for mild, 2 for moderate, and 3 for severe. We summed the assigned scores for all 3 symptoms for each subject to generate a symptom burden score as follows: no symptom burden (0), mild symptom burden (13), moderate symptom burden (46), and severe symptom burden (79). In this scale, a moderate symptom burden would mean that a subject reported having at least 1 symptom at a moderate or severe level, with at least 1 other symptom present. A severe symptom burden would require the presence of all 3 symptoms, with at least 1 at a severe level.
We reviewed patient charts to assess severity of patient illness upon admission. For cancer, we recorded type; for COPD, we noted forced expiratory volume in 1 second (FEV1); and for HF, we recorded the ejection fraction. We also queried the National Death Index to get vital statistics on all subjects.
Data Preparation
The IADL asks patients to report whether they can perform 13 daily living skills without help, with some help, or were unable to complete tasks.11 Subjects who reported needing at least some help with any of the 13 items were categorized as dependent. The GDS‐15 is a widely used, validated 15‐item scale for assessing depressive mood in the elderly.12 Scores for the GDS‐15 range from 0 to 15, with higher scores indicating more depressive symptoms. Based on previous research, we categorized patients as either not depressed (05) or having probable depression (6 or more).12
Statistical Analysis
Because our clinical trial had no impact on care or symptoms, we combined intervention and usual care patients for this analysis of symptom severity. Descriptive statistics, such as frequencies, means, standard deviations (SDs), and 95% confidence intervals (CIs) were used to examine the distribution of measures. Chi‐square (2) analysis was undertaken to examine bivariate associations between categorical variables. Analysis of variance (ANOVA) was undertaken to examine associations between categorical and continuous variables. Multivariate logistic regression was used to examine predictors of symptom burden at follow‐up, including patient characteristics that were significant to P 0.10 in bivariate analysis. We used KaplanMeier survival curves to examine the relationship between primary diagnosis and mortality, and assessed statistical significance using log‐rank tests (MantelCox).13 The Statistical Package for the Social Sciences (SPSS) for Mac (version 17; SPSS Inc, Chicago, IL; March 11, 2009) was used to analyze these data.
RESULTS
Patient Characteristics
A total of 150 patients enrolled in the study. The mean length of stay was 5.4 days (SD: 5.6; range: 147 days). HF was the most common primary diagnosis (46.7%, n = 70) with 48% (n = 34) having an ejection fraction of 45% or less (mean = 43%; SD: 22); followed by cancer (30%, n = 45) with the most common type being prostate (18%, n = 8), lung (13%, n = 6), and breast (13%, n = 6); and COPD (23%, n = 35) with an average FEV1 of 1.5 L (SD: 0.94; range: 0.503.9). The mean age was 77 years (SD: 7.9; range: 6596 years). The majority of participants were men (56%, n = 83) and white (73%, n = 108), with the most being either married/partnered (43%, n = 64) or divorced/widowed (44%, n = 66). The IADL identified almost two‐thirds of participants as dependent (62%, n = 94). The GDS‐15 categorized three‐quarters of participants (n = 118) as not depressed. The only significant association between participant characteristics and their primary diagnosis was for the IADL index (Table 1), with significantly more (2 = 6.3; P = 0.04) patients with HF categorized as being dependent (72%).
| Characteristics | Primary Diagnosis | P | |||
|---|---|---|---|---|---|
| Cancer n = 44 | HF n = 70 | COPD n = 35 | |||
| |||||
| Length of stay | (Mean days) | 5.4 | 4.7 | 6.5 | 0.3 |
| Age | (Mean years) | 76 | 78 | 76 | 0.3 |
| Sex | |||||
| Female | 47% | 37% | 57% | 0.1 | |
| Marital status | 0.2 | ||||
| Single | 16 | 9 | 17 | ||
| Married/partnered | 51 | 45 | 29 | ||
| Divorced/widowed | 33 | 46 | 54 | ||
| Race | |||||
| White | 89 | 64 | 69 | 0.1 | |
| Black/African American | 7 | 21 | 23 | ||
| Asian or Pacific Islander | 5 | 10 | 9 | ||
| Other | 0 | 4 | 0 | ||
| IADL | |||||
| Dependent | 49 | 72 | 60 | 0.04 | |
| GDS‐15 | |||||
| Probable depression | 18 | 22 | 21 | 0.9 | |
Frequency and Severity of Symptoms
On average, the postdischarge follow‐up assessment was undertaken 24 days (median = 21.0; SD: 17.9; range: 7140 days) after the baseline assessment and 20 days after discharge (median = 15; SD: 17.0; range: 4139). At baseline, a large proportion of participants reported symptoms at a moderate‐to‐severe level for pain (54%, n = 81), dyspnea (53%, n = 79), and anxiety (63%, n = 94). The majority of patients (64%, n = 96) reported having 2 or more symptoms at a moderate‐to‐severe level and one quarter (27%, n = 41) had 3 symptoms at a moderate‐to‐severe level. While the frequency of moderate‐to‐severe symptoms decreased at the 24‐hour hospital assessment (pain = 42%, dyspnea = 45%, anxiety = 55%) and again at 2‐week follow‐up (pain = 28%, dyspnea = 27%, anxiety = 25%), a substantial symptom burden persisted with 30% (n = 36) of patients having moderate‐to‐severe levels at 2‐week follow‐up. Overall there were no differences between primary diagnosis and the frequency of symptoms at baseline or 24‐hour hospital assessment (Figure 1). However at follow‐up, those diagnosed with COPD were more likely to report moderate/severe pain (54%; 2 = 22.0; P < 0.001), dyspnea (45%; 2 = 9.3; P = 0.05), and overall symptom burden (55%; 2 = 25.9; P < 0.001) than those with cancer (pain = 22%, dyspnea = 16%, symptom burden = 16%) or HF (pain = 25%, dyspnea = 24%, symptom burden = 28%).

As symptom burden was our composite score for pain, dyspnea, and anxiety, we were interested in identifying variables in addition to primary diagnosis that might be associated with symptom burden at follow‐up. Bivariate analysis revealed that there was no significant association between symptom burden and age (2 = 1.5; P = 0.5), gender (2 = 1.3; P = 0.3), length of stay (2 = 0.4; P = 0.8), and (IADL) level of independence (2 = 0.3; P = 0.6). However, those with probable depression were more likely (2 = 11.9; P = 0.001) to have a moderate/severe symptom burden (62%, n = 13), compared to those with no depression (24%, n = 23). After adjusting for severity of symptom burden at baseline, multivariate logistic regression revealed that primary diagnosis (P = 0.01) and probable depression (OR = 4.9; 95% CI = 1.6, 14.9; P = 0.005) were associated with symptom severity. Patients with COPD had greater odds (OR = 7.0; 95% CI = 1.9, 26.2; P = 0.002) of moderate/severe symptom burden than those with cancer, while those with HF did not (OR = 2.3; 95% CI = 0.7, 7.7; P = 0.16). There was significant interaction between primary diagnosis and depression (P = 0.2).
Primary Diagnosis, Symptom Burden, and Survival Time
A total of 75% of patients were identified by the National Death Index to have died between hospital discharge and December 2007, of which 47% had died within 12 months after discharge. KaplanMeier survival curves (Figure 2) revealed a significant difference (MantelCox: 2 = 19.3; df = 1; P = 0.0001) in survival time, with patients diagnosed with COPD (median = 19.0 months; 95% CI = 6.5, 31.5) and HF (median = 20.0 months; 95% CI = 12.5, 27.5) having a longer survival than those with cancer (median = 8.0 months; 95% CI = 4.1, 11.9).

We also examined the relationship between symptom burden and survival time. KaplanMeier survival curves revealed no significant difference (MantelCox: 2 = 0.2; P = 0.6) in the survival time of patients classified with a symptom burden of none/moderate (median = 15.0 months; 95% CI = 8.8, 21.2) or moderate/severe (median = 14.0 months; 95% CI = 2.6, 25.4).
DISCUSSION
In our sample of older inpatients diagnosed with cancer, HF, and COPD, a large proportion reported moderate‐to‐severe levels of pain, dyspnea, and anxiety at baseline and follow‐up. When combined, these levels represent a considerable symptom burden, with over three‐quarters of participants reporting 2 to 3 symptoms at a moderate/severe level at baseline. While symptom scores decreased at 24‐hours and 2‐week follow‐up, symptom burden remained high, with almost half of the participants reporting 23 symptoms at a moderate‐to‐severe level at 24‐hour assessment and a large minority reporting moderate‐to‐severe symptoms at follow‐up. A higher percentage of patients with COPD reported moderate‐to‐severe pain, dyspnea, and overall symptom burden at follow‐up than participants with cancer or HF who reported a similar symptom burden. We also found that patients with probable depression were more likely to have a significant symptom burden at follow‐up. These findings highlight the need to routinely assess and treat symptoms over time, including depression, and especially in patients with COPD. While we found that hospital care was seemingly effective in improving symptoms, they persist at distressing levels in many patients.
Few studies have assessed the severity of symptoms over time. One study that did, examined symptom severity among community‐based elders diagnosed with HF and COPD.6 At baseline, these participants had a lower prevalence of moderate‐to‐severe symptoms than the hospitalized patients enrolled in our study, a finding that would be anticipated, as they may not have been as ill. However, symptom severity persisted in the community‐based subjects and, in some cases, worsened over the 22‐month assessment period for pain (HF = 20% vs 42%; COPD = 27% vs 20%), dyspnea (HF = 19% vs 29%; COPD = 66% vs 76%), and anxiety (HF = 2% vs 12%; COPD = 32% vs 23%).6 In contrast, while our subjects with a primary diagnosis of HF and COPD had a higher prevalence of moderate‐to‐severe symptoms at baseline, they did experience an improvement in the severity of pain, dyspnea, and anxiety at the 2‐week follow‐up assessment. However, despite a decrease in the prevalence of moderate‐to‐severe symptoms from baseline to follow‐up, a high symptom burden persisted for many patients, particularly for those diagnosed with COPD and those with probable depression at baseline. The severity of a patient's symptoms can have a profound negative effect on health status and quality of life.14 Findings from these studies suggest that symptoms are currently not being adequately managed, and highlight an urgent need to develop coordinated strategies and systems that focus on improving the management of symptoms, including depression, over time.6
We also found that subjects recruited for this study had advanced disease, evidenced by the fact that nearly half died within 12 months. We did not use specific prognostic indices or severity of illness criteria for recruiting subjects and simply approached patients admitted with one of the target diagnoses. Our study suggests that targeting these patients for routine symptom assessment and management, including for palliative care, would be a reasonable approach given the high symptom burden and relatively high mortality at 1 year.
Interpretation of these findings should be mitigated by the following limitations. Because of our setting, our findings may not be generalizable to all patients with cancer, HF, and COPD. However, our subjects were admitted to general medical and cardiology services, and had common conditions, and therefore are likely similar to those presenting to other hospitals. We relied on self‐report measures to assess severity of symptoms. Patient self‐report, while potentially subject to imprecision due to poor recall and social demand biases, is considered the gold standard for symptom assessment.15 Finally, 2‐week follow‐up is relatively short, and it is possible that symptoms may have improved had we assessed them over a longer period. The longitudinal study of elders in the community that followed subjects over 22 months found that, for many patients, symptoms worsened over time and nearly half of our subjects died at 12 months, suggesting that longer follow‐up would have been unlikely to show improvement in symptoms.6
A significant minority of participants reported a substantial, persistent symptom burden, yet all symptoms assessed in our study are potentially modifiable. Recognizing and treating symptoms can be achieved through the use of targeted interventions.6 Because symptoms can occur in clusters, successful treatment of 1 symptom may also help to improve other symptoms.1 The large number of participants reporting moderate‐to‐severe levels of symptom burden at 2 weeks after discharge highlights an unmet need for improved symptom control in the outpatient setting. Unfortunately, while evidence exists for managing pain in patients with cancer, such evidence‐based practices are lacking for the management of pain and other symptoms in patients with HF and COPD. Some symptoms may require specific, disease‐oriented management. However, many symptoms may be due to common comorbidities, such as pain from degenerative joint disease, that may likely respond to proven treatments.16
Our study confirmed the significant burden of symptoms experienced by patients with serious illness and demonstrated that patients with COPD report as much symptom burden as patients with cancer and HF, if not more. While symptom severity improved over the course of the hospitalization and follow‐up, a large percentage of patients reported significant symptom burden at follow‐up. Depression was also common in these patients. Because these symptoms diminish quality of life, routine assessment and management of these symptoms is critical for improving the quality of care provided to these patients. Additional research on the best approaches to manage symptoms, including medications, interventions, and structures of care, could further improve care.
Acknowledgements
The authors thank all the patients who participated in this study. They thank Joanne Batt, Wren Levenberg, and Emily Philipps for their expert help as research assistants. They also thank Harold Collard, MD, for providing valuable feedback on the manuscript. Data obtained from the National Death Index assisted us in meeting our study objectives. Steven Pantilat had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
- ,,.Symptom clusters: the new frontier in symptom management research.J Natl Cancer Inst Monogr.2004(32):17–21.
- .Symptom burden: multiple symptoms and their impact as patient‐reported outcomes.J Natl Cancer Inst Monogr.2007(37):16–21.
- ,,.A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease.J Pain Symptom Manage.2006;31(1):58–69.
- ,,, et al.Comparing three life‐limiting diseases: does diagnosis matter or is sick, sick?J Pain Symptom Manage.2011;42(3):331–341.
- ,,,,,.Deaths: final data for 2006.Natl Vital Stat Rep.2009;57(14):1–134.
- ,,,,,.Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure.Arch Intern Med.2007;167(22):2503–2508.
- ,,,.Living with chronic obstructive pulmonary disease: a survey of patients' knowledge and attitudes.Respir Med.2009;103(7):1004–1012.
- ,,,,.The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment.J Pain Symptom Manage.1999;17(4):248–255.
- ,,, et al.Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure.JAMA.2010;303(17):1716–1722.
- ,,,.Hospital‐based palliative medicine consultation: a randomized controlled trial.Arch Intern Med.2010;170(22):2038–2040.
- ,,,,.Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,,.Screening for late life depression: cut‐off scores for the Geriatric Depression Scale and the Cornell Scale for Depression in Dementia among Japanese subjects.Int J Geriatr Psychiatry.2003;18(6):498–505.
- ,.Nonparametric estimation from incomplete observations.J Am Stat Assoc.1958;53(282):457–481.
- ,,.End stage chronic obstructive pulmonary disease.Pneumonol Alergol Pol.2009;77(2):173–179.
- ,,,.Relationship between social desirability and self‐report in chronic pain patients.Clin J Pain.1995;11(3):189–193.
- ,,,.Etiology and severity of pain among outpatients living with HF.J Card Fail.2010;16(8):S88.
- ,,.Symptom clusters: the new frontier in symptom management research.J Natl Cancer Inst Monogr.2004(32):17–21.
- .Symptom burden: multiple symptoms and their impact as patient‐reported outcomes.J Natl Cancer Inst Monogr.2007(37):16–21.
- ,,.A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease.J Pain Symptom Manage.2006;31(1):58–69.
- ,,, et al.Comparing three life‐limiting diseases: does diagnosis matter or is sick, sick?J Pain Symptom Manage.2011;42(3):331–341.
- ,,,,,.Deaths: final data for 2006.Natl Vital Stat Rep.2009;57(14):1–134.
- ,,,,,.Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure.Arch Intern Med.2007;167(22):2503–2508.
- ,,,.Living with chronic obstructive pulmonary disease: a survey of patients' knowledge and attitudes.Respir Med.2009;103(7):1004–1012.
- ,,,,.The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment.J Pain Symptom Manage.1999;17(4):248–255.
- ,,, et al.Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure.JAMA.2010;303(17):1716–1722.
- ,,,.Hospital‐based palliative medicine consultation: a randomized controlled trial.Arch Intern Med.2010;170(22):2038–2040.
- ,,,,.Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,,.Screening for late life depression: cut‐off scores for the Geriatric Depression Scale and the Cornell Scale for Depression in Dementia among Japanese subjects.Int J Geriatr Psychiatry.2003;18(6):498–505.
- ,.Nonparametric estimation from incomplete observations.J Am Stat Assoc.1958;53(282):457–481.
- ,,.End stage chronic obstructive pulmonary disease.Pneumonol Alergol Pol.2009;77(2):173–179.
- ,,,.Relationship between social desirability and self‐report in chronic pain patients.Clin J Pain.1995;11(3):189–193.
- ,,,.Etiology and severity of pain among outpatients living with HF.J Card Fail.2010;16(8):S88.
Copyright © 2012 Society of Hospital Medicine
Factors of Care Plan Discussions at Admission
Despite an ideal of dying at home, most Americans die in hospitals.1 Patients and families are clear about what they need from the healthcare system at the end of life: relief of distressing symptoms, the opportunity to communicate with physicians and others about death and dying, and the assurance that they will be attended to and comforted by their physicians as they approach death.2, 3 However, discussions about patient preferences for care occur infrequently,47 even though patients want to discuss care with their doctor,68 and physicians believe these discussions are their responsibility.9
The most prominent work in this area occurred in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) study, which focused on patients with advanced disease, often in the intensive care unit.4 Furthermore, few studies have focused on general medical patients, and healthcare has changed in important ways since SUPPORT's publication. First, the Patient Self‐Determination Act (PSDA) requires that all patients be asked about their care wishes at the time of admission and document the presence of an advanced directive.10, 11 Second, there is growing awareness of the need to improve palliative care for all hospitalized patients, with many advocating that hospitalization itself is a reason to ask about patient's preferences for care regardless of a patient's level of chronic or acute illness.12 Finally, emergence of hospitalists,1316 movement toward closed intensive care units,17, 18 and changes in residency training have increased segmentation in care of hospitalized patients.15, 18
To overcome limitations of previous literature and update our knowledge of how care discussions take place in the current healthcare environment, we analyzed data from a large study of patients admitted to general medicine services at 6 academic centers. Using this robust dataset, which included prospectively collected information about preferences for communication with their physician, we performed statistical analyses to understand which patient clinical, sociodemographic, and preference‐related factors, as well as factors related to their site of care, were associated with documentation that a code status discussion took place at the time of hospital admission.
PATIENTS AND METHODS
Sites
The Multicenter Hospitalist Study (MCHS) was a multicenter trial of general medical services that enrolled patients at 6 geographically diverse centers: The University of Chicago (which also served as the coordinating center), University of Iowa Hospitals and Clinics, University of California San Francisco, University of Wisconsin, University of New Mexico, and Brigham and Women's Hospital.19
Each site was selected to participate in the MCHS because patients on their general medicine service were admitted to hospitalist and nonhospitalist physicians in a random fashion (eg, based on predetermined call schedule based on day of the week). As teaching hospitals, house officers provided direct care to patients hospitalized at each center; nonteaching services were not present at the sites during the period of this study.
During the period of this study, each site complied with PSDA requirements for noting that patients had been informed about their right to create an advance directive, but no sites had a guideline or other program in place specifically intended to facilitate physician‐patient communication about care wishes. Two sites had active Hospice or Palliative Care services, and another 2 had Geriatrics Consultation services, but none had standard protocols mandating involvement of these consultants at the time of admission, the time when our key outcomes were documented.
Patients
Patients were eligible for inclusion in the MCHS if they were older than 18 years of age and were admitted at random to a hospitalist or nonhospitalist physician; we excluded patients from MCHS if they were admitted specifically under the care of their primary care physician or subspecialist (eg, admitted for chemotherapy) or were a prison inmate. Patients meeting these eligibility criteria were then approached for purposes of informed consent.
Data Collection
Data for this study were obtained from administrative data, patient interview, and chart abstraction as in previous work.14 Administrative data were drawn from cost‐accounting databases at each participating hospital; administrative data were used to provide cost and length of stay data, as well as information about patient insurance type, age, and sex.
We interviewed patients immediately after informed consent was obtained, with both taking place generally within 24 hours of admission. Interviews collected data about patient preferences for care and functional status,20 and other data not reliably available from administrative sources (such as housing situation).
Patient care plan before admission was taken from notes and orders written in the first 24 hours of hospitalization, as mentioned above. Using criteria we employed in previous work,21 a care discussion (CD) was defined as documentation of a discussion between patients (or family) and at least 1 physician (primary physician, hospitalist, consulting physician, or house officer) during the first 24 hours of hospitalization. CDs needed to specify that the person who wrote the note had actually spoken with the patient or their family for the purposes of determining preferences for care, and that this discussion resulted in a specific care plan. Thus, notations such as do not resuscitate/do not intubate, or spoke with family, questions answered, did not qualify as CDs, but a note stating the patient continues to want full efforts was counted as a CD.
Principal investigators at each site were responsible for training and overseeing interviewing and chart abstraction activities at each site, with central oversight of data quality provided by the central coordinating center. Upon receipt at the data coordinating center, all data were examined for missing, nonsensical, or outlier data with errors referred back to the participating sites for correction.
Statistical Analysis
For bivariable comparisons of patients with and without CDs, we used chi‐squared or Mann‐Whitney U‐tests, as appropriate.
Variables with P < 0.20 in bivariable comparisons were selected for initial inclusion in models. Then, using automated forward and stepwise selection techniques as well as manually entered variables, we fit multivariable generalized estimating equations permitting clustering of effects at the physician level to determine the independent association between the multiple factors tested and presence of a CD. In order to guard against the threat of multiple testing, we retained variables at a significance level of P < 0.01; variables were also retained because of observed confounding with other independent variables, or to maintain face validity of the model. All analyses were performed using SAS 9.0 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
Patient Sociodemographics (Table 1)
A total of 17,097 of 33,638 patients (50.8%) were interviewed and gave consent for chart abstraction. Of these patients, 1776 (10.3%) had a CD documented in the first 24 hours of hospitalization. Patients with documented CDs were older, more often white, had completed more years of education, were more likely to have lived in a nursing home prior to admission, and more likely to have been hospitalized in the last 12 months. The proportion of patients with CDs was highly variable across site of enrollment, from 2.8%‐24.9%.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P* |
|---|---|---|---|
| |||
| Age (Median, 95%CI)* | 56 (55, 56) | 69 (67, 71) | < 0.0001 |
| Female (n, %) | 8390 (54.8%) | 990 (55.7%) | 0.4312 |
| Race (n, %) | |||
| White | 6640 (43.3%) | 938 (52.8%) | < 0.0001 |
| African American | 4673 (30.5%) | 280 (15.8%) | |
| Asian | 532 (3.5%) | 167 (9.4%) | |
| American Indian | 325 (2.1%) | 26 (1.5%) | |
| Other | 1951 (12.7%) | 241 (13.6%) | |
| Refused/Don't know | 1200 (7.8%) | 124 (7.0%) | |
| Ethnicity (n, %) | |||
| Hispanic or Latino Ethnicity | 1724 (11.3%) | 183 (10.3%) | 0.0039 |
| Insurance type (n, %) | |||
| Charity | 481 (3.4%) | 14 (0.8%) | < 0.0001 |
| Indemnity | 3983 (28.2%) | 327 (19.3%) | |
| Medicaid | 2487 (17.6%) | 195 (11.5%) | |
| Medicare | 6418 (45.5%) | 1114 (65.9%) | |
| Other | 105 (0.7%) | 4 (0.2%) | |
| Self pay | 628 (4.5%) | 36 (2.1%) | |
| Self‐reported education (n, %) | |||
| Junior high school or less | 1297 (8.5%) | 217 (12.2%) | < 0.0001 |
| Some high school | 2146 (14.0%) | 182 (10.2%) | |
| High school graduate | 4435 (28.9%) | 465 (26.2%) | |
| Some college or junior college | 3521 (23.0%) | 347 (19.5%) | |
| College graduate | 1729 (11.3%) | 255 (14.4%) | |
| Post‐graduate | 1191 (7.8%) | 173 (9.7%) | |
| Refused/Don't know | 1002 (6.5%) | 137 (7.7%) | |
| Self reported income (n, %) | |||
| $2,500 or less | 1079 (7.0%) | 108 (6.1%) | 0.0002 |
| $2,501 to $5,000 | 424 (2.8%) | 33 (1.9%) | |
| $5,001 to $10,000 | 1436 (9.4%) | 211 (11.9%) | |
| $10,001 to $15,000 | 1080 (7.0%) | 141 (7.9%) | |
| $15,001 to $25,000 | 1054 (6.9%) | 134 (7.5%) | |
| $25,001 to $35,000 | 837 (5.5%) | 74 (4.2%) | |
| $35,001 to $50,000 | 882 (5.8%) | 94 (5.3%) | |
| $50,001 to $100,000 | 1027 (6.7%) | 125 (7.0%) | |
| $100,001 to $200,000 | 357 (2.3%) | 57 (3.2%) | |
| Over $200,000 | 245 (1.6%) | 34 (1.9%) | |
| Don't know/refused | 6900 (45.0%) | 765 (43.1%) | |
| Housing situation (n, %) | |||
| Own apartment or house | 11887 (77.6%) | 1264 (71.2%) | < 0.0001 |
| A relative or friend's apartment or house | 1804 (11.8%) | 217 (12.2%) | |
| A nursing home, group home, or long‐term care facility | 663 (4.3%) | 204 (11.5%) | |
| A homeless shelter | 258 (1.7%) | 27 (1.5%) | |
| Other | 709 (4.6%) | 64 (3.6%) | |
| Marital status (n, %) | |||
| Married | 4992 (32.6%) | 603 (34.0%) | < 0.0001 |
| Living as if married | 440 (2.9%) | 32 (1.8%) | |
| Divorced | 2027 (13.2%) | 199 (11.2%) | |
| Separated | 569 (3.7%) | 30 (1.7%) | |
| Widowed | 2577 (16.8%) | 487 (27.4%) | |
| Single | 4074 (26.6%) | 364 (20.5%) | |
| Refused | 642 (4.2%) | 61 (3.4%) | |
| Hospitalized in the last 12 months (n, %) | 7602 (49.6%) | 1011 (56.9%) | < 0.0001 |
| Site of enrollment (n, %) | |||
| A | 4602 (30.0%) | 135 (7.6%) | < 0.0001 |
| B | 1595 (10.4%) | 158 (8.9%) | |
| C | 3017 (19.7%) | 998 (56.2%) | |
| D | 2387 (15.6%) | 212 (11.9%) | |
| E | 2057 (13.4%) | 131 (7.4%) | |
| F | 1663 (10.9%) | 142 (8.0%) | |
Patient Self‐Reported Health Status and Comorbid Illness (Table 2)
Patients with CDs more often reported a lot of difficulties with bathing, eating, or dressing; household chores; and moderate activities. Patients with CDs were more likely to report accomplishing less than they would like due to their health. They were more likely to have cancer, depression, a history of stroke, and heart disease, but less likely to have diabetes or human immunodeficiency virus.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P** |
|---|---|---|---|
| |||
| Thinking back again to one month ago, did any impairment or health problem cause you to need help of other persons with personal care needs, such as eating, bathing, dressing, or getting around the home? (n, %) | |||
| No | 10673 (69.7%) | 973 (54.8%) | < 0.0001 |
| Yes, a little | 1933 (12.6%) | 268 (15.1%) | |
| Yes, a lot | 2127 (13.9%) | 487 (27.4%) | |
| Don't know | 588 (3.8%) | 48 (2.7%) | |
| Thinking back to one month ago, did any impairment or health problem cause you to need help in handling everyday household chores, necessary business, shopping, or getting around for other purposes? (n, %) | |||
| No | 7262 (47.4%) | 566 (31.9%) | < 0.0001 |
| Yes, a little | 2692 (17.6%) | 324 (18.2%) | |
| Yes, a lot | 4126 (26.9%) | 825 (46.5%) | |
| Don't know | 1241 (8.1%) | 61 (3.4%) | |
| As far as you know do you have any of the following health conditions at the present time? (n, %) | |||
| Cancer | |||
| No | 13281 (86.7%) | 1376 (77.5%) | < 0.0001 |
| Yes | 1751 (11.4%) | 351 (19.8%) | |
| Not sure | 289 (1.9%) | 49 (2.8%) | |
| Depression | |||
| No | 10269 (67.0%) | 1099 (61.9%) | < 0.0001 |
| Yes | 4730 (30.9%) | 624 (35.1%) | |
| Not sure | 322 (2.1%) | 53 (3.0%) | |
| Diabetes | |||
| No | 10902 (71.2%) | 1356 (76.4%) | < 0.0001 |
| Yes | 4132 (27.0%) | 394 (22.2%) | |
| Not sure | 287 (1.9%) | 26 (1.5%) | |
| Heart trouble | |||
| No | 10251 (66.9%) | 1080 (60.8%) | < 0.0001 |
| Yes | 4491 (29.3%) | 627 (35.3%) | |
| Not sure | 579 (3.8%) | 69 (3.9%) | |
| HIV or AIDS | |||
| No | 14300 (93.3%) | 1679 (94.5%) | 0.026 |
| Yes | 912 (6.0%) | 80 (4.5%) | |
| Not sure | 109 (0.7%) | 17 (1.0%) | |
| Stroke | |||
| No | 13344 (87.1%) | 1494 (84.1%) | 0.0005 |
| Yes | 1722 (11.2%) | 236 (13.3%) | |
| Not sure | 255 (1.7%) | 46 (2.6%) | |
Patient Preferences, Care Plan Documentation, and Care Coordination at Admission (Table 3)
Patients who had documented CDs were less likely to prefer my doctor give me choices regarding my care, and more often disagreed with the statement I prefer to leave care decisions to my physician. These patients were also more likely to have a durable power of attorney or living will in their chart, or have an alternate decision‐maker noted. The majority of patients without a documented CD (79.9%) had no notation of their care wishes, compared to 29.7% in patients with a documented CD. Patients with a documented CD were more likely to have a regular medical provider and a note in the chart from their primary care physician.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P* |
|---|---|---|---|
| |||
| I prefer my doctor give me choices regarding my care** (n, %) | |||
| Definitely agree | 11619 (75.8%) | 1247 (70.2%) | < 0.0001 |
| Somewhat agree | 1912 (12.5%) | 252 (14.2%) | |
| Somewhat disagree | 488 (3.2%) | 76 (4.3%) | |
| Definitely disagree | 414 (2.7%) | 87 (4.9%) | |
| Don't know | 888 (5.8%) | 114 (6.4%) | |
| I prefer to leave care decisions to my physician** (n, %) | |||
| Definitely agree | 5660 (36.9%) | 613 (34.5%) | < 0.0001 |
| Somewhat agree | 4539 (29.6%) | 493 (27.8%) | |
| Somewhat disagree | 2265 (14.8%) | 257 (14.5%) | |
| Definitely disagree | 1956 (12.8%) | 304 (17.1%) | |
| Don't know | 901 (5.9%) | 109 (6.1%) | |
| Documentation of care wishes before hospitalization (n, %) | |||
| No documentation | 12238 (79.9%) | 527 (29.7%) | < 0.0001 |
| Full support | 2624 (17.1%) | 742 (41.8%) | |
| Do not resuscitate or intubate (DNR/DNI) | 264 (1.7%) | 370 (20.8%) | |
| Hospice | 53 (0.3%) | 22 (1.2%) | |
| Other limitation (eg, no pressors) | 142 (0.9%) | 115 (6.5%) | |
| Had durable power of attorney in chart (n, %) | 286 (1.9%) | 133 (7.5%) | < 0.0001 |
| Had a living will in chart (n, %) | 266 (1.7%) | 142 (8.0%) | < 0.0001 |
| Alternate decision maker named in chart (n, %) | 2770 (18.1%) | 638 (35.9%) | < 0.0001 |
| Patient noted to be unable to participate in their care at admission (eg, confused, unable to respond) (n, %) | 1227 (8.0%) | 431 (24.3%) | < 0.0001 |
| Inpatient team documented discussion with primary care physician (n, %) | 627 (4.1%) | 136 (7.7%) | < 0.0001 |
| Do not have a regular medical provider** (n, %) | 3836 (25.0%) | 254 (14.3%) | < 0.0001 |
| Note from primary care physician in chart (n, %) | 148 (1.0%) | 39 (2.2%) | < 0.0001 |
Factors Associated with Documented Care Discussions (Table 4)
Using predictor variables presented in Tables 1‐3, we then constructed multivariable models seeking to understand factors independently associated with documentation of code status in the entire cohort, as well as among patients who had no preexisting care wishes.
| Entire Cohort (n = 17097) | Patients with No Documentation of Preadmission Wishes (n = 12765) | |||
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value | |
| Preadmission Code Status | ||||
| No documentation | Referent | NA | ||
| Full support | 3.22 (2.28, 4.55) | < 0.0001 | NA | |
| Do not resuscitate or intubate (DNR/DNI) | 11.32 (8.52, 15.04) | < 0.0001 | NA | |
| Hospice | 4.02 (2.33, 6.94) | < 0.0001 | NA | |
| Other limitation (eg, no pressors) | 10.13 (7.35, 13.96) | < 0.0001 | NA | |
| Insurance type | ||||
| Medicare | Referent | Referent | ||
| Charity care | 0.50 (0.30, 0.85) | 0.0099 | 0.56 (0.25, 1.25) | 0.1589 |
| Commercial | 0.81 (0.69, 0.95) | 0.0090 | 0.66 (0.52, 0.85) | 0.0009 |
| Medicaid | 0.69 (0.57, 0.82) | < 0.0001 | 0.49 (0.36, 0.67) | < 0.0001 |
| Other | 0.46 (0.18, 1.13) | 0.0912 | 0.60 (0.17, 2.12) | 0.4302 |
| Self pay | 0.70 (0.52, 0.95) | 0.0203 | 0.49 (0.29, 0.81) | 0.0060 |
| Any limitations in bathing, toileting, dressing or feeding self? | ||||
| No | Referent | Referent | ||
| Yes, a little | 1.25 (1.10, 1.42) | 0.0007 | 1.31 (1.03, 1.67) | 0.0272 |
| Yes, a lot | 1.25 (1.09, 1.43) | 0.0015 | 1.42 (1.11, 1.81) | 0.0055 |
| Unable to respond | 0.81 (0.59, 1.12) | 0.2006 | 0.80 (0.45, 1.41) | 0.4299 |
| Patient has a documented surrogate decision maker | 1.72 (1.47, 2.02) | < 0.0001 | 2.08 (1.62, 2.66) | < 0.0001 |
| Patient noted to be unable to participate in their care at admission (eg, confused, unable to respond) | 1.63 (1.37, 1.94) | < 0.0001 | 2.20 (1.60, 3.02) | < 0.0001 |
| Notation that team had spoken to primary care physician at admission | 1.65 (1.29, 2.11) | < 0.0001 | 1.45 (0.92, 2.28) | 0.1116 |
| History of cancer | ||||
| No | Referent | Referent | ||
| Yes | 1.31 (1.13, 1.51) | 0.0003 | 1.26 (0.96, 1.65) | 0.0960 |
| Not sure | 1.26 (0.87, 1.82) | 0.2162 | 1.80 (1.03, 3.15) | 0.0396 |
| History of diabetes | ||||
| No | Referent | Referent | ||
| Yes | 0.87 (0.75, 1.003) | 0.0543 | 0.79 (0.62, 0.997) | 0.0467 |
| Not sure | 0.61 (0.38, 0.99) | 0.0445 | 0.84 (0.43, 1.65) | 0.6183 |
| Housing situation | ||||
| Own house or apartment | Referent | Referent | ||
| Relative or friend's apartment or house | 1.22 (1.03, 1.45) | 0.0229 | 1.29 (0.97, 1.71) | 0.0783 |
| Nursing home, group home, or long‐term care facility | 1.42 (1.16, 1.74) | 0.0006 | 1.74 (1.27, 2.40) | 0.0007 |
| Homeless shelter | 1.12 (0.72, 1.73) | 0.6204 | 0.87 (0.46, 1.63) | 0.6559 |
| Other/Don't know | 1.02 (0.75, 1.40) | 0.8987 | 1.35 (0.78, 2.36) | 0.2859 |
| Age Group | ||||
| <50 | Referent | Referent | ||
| 5059 | 1.19 (0.99, 1.43) | 0.0647 | 1.18 (0.88, 1.59) | 0.2583 |
| 6069 | 1.18 (0.99, 1.40) | 0.0585 | 1.20 (0.88, 1.66) | 0.2549 |
| 7079 | 1.10 (0.91, 1.33) | 0.3178 | 1.19 (0.85, 1.67) | 0.3033 |
| 8089 | 1.23 (1.03, 1.47) | 0.0207 | 1.34 (0.96, 1.88) | 0.0879 |
| 90+ | 1.45 (1.12, 1.88) | 0.0045 | 1.44 (0.94, 2.20) | 0.0934 |
| Site of Enrollment | ||||
| A | Referent | Referent | ||
| B | 1.74 (1.16, 2.61) | 0.007 | 4.95 (2.90, 8.45) | < 0.0001 |
| C | 5.14 (3.42, 7.74) | < 0.0001 | 26.36 (17.28, 40.23) | < 0.0001 |
| D | 4.19 (2.64, 6.66) | < 0.0001 | 8.06 (4.63, 14.03) | < 0.0001 |
| E | 3.00 (1.82, 4.9) | < 0.0001 | 5.30 (2.71, 10.38) | < 0.0001 |
| F | 4.09 (2.69, 6.23) | < 0.0001 | 2.32 (1.32, 4.08) | 0.0037 |
In the entire cohort, insurance type was independently associated with likelihood of a care discussion, with patients with Medicare having greater adjusted odds ratio for a CD than patients with all other forms of insurance, even after adjusting for age. Patients who had functional limitations with bathing, toileting, and feeding; had a documented surrogate decision maker; were unable to participate in their care; had cancer; or did not live in their own home were more likely to have a documented CD. Subjects with diabetes were less likely to have a CD, although this was of borderline significance. Patients whose team had documented a CD with the patients' primary physician were also more likely to have a discussion noted. However, the magnitude of these predictors was small compared to the independent effects attributable to the site the patient was enrolled or whether the patient had any preexisting documentation. Whereas the adjusted odds ratio associated with clinical or functional measures (such as age, cancer) were generally between 1.5 and 2.5, the range of odds ratios associated with having any documentation of care wishes (compared to no documentation) were all greater than 3, and the odds ratios associated with site of enrollment were 1.7 or higher.
We observed similar findings in analyses limited to patients with no preexisting care documentation. While clinical, sociodemographic, and functional factors remained statistically associated with a CD (albeit with wider confidence intervals due to smaller sample sizes), the effect of the patient's site of enrollment became even more striking (Table 4).
DISCUSSION
In this multicenter study of hospitalized general medical patients, documentation of CDs were highly dependent on where patients received care and whether patients had previous documentation of a care plan. In contrast, although clinical, prognostic, and socioeconomic factors were also associated with whether physicians documented asking patients about their wishes for care, the influence of these factors was modest.
Improving communication between patients and their physicians during an episode of acute illness has been a long‐standing goal, with the Study to Understand Prognoses and Preferences for Outcomes of Treatment (SUPPORT) trial providing the most notable example of an effort to improve patient care through aligning patient wishes, prognosis, and aggressiveness for care. However, even the SUPPORT interventiona robust, well‐implemented, and highly labor‐intensive strategywas not able to achieve this goal. In their summary of SUPPORT study findings, the authors suggested that the likelihood of and effectiveness of communication in seriously ill patients may be powerfully influenced by patient and caregiver culture4; our findings may partially confirm SUPPORT's conclusions.
Preexisting documentation in our study would not have included mandated documentation that someone had given the patient information about advance directives (as mandated by the PSDA), but rather a specification for that advance care plan. This distinction means that preexisting documentation in our study represented a previous decision by the patient (or the patient and their physician) to have made a plan, and an association with hospital discussions may be because the first conversation is the hardest to undertake; subsequent discussions then represent confirmatory or clarifying discussions that may be less difficult to broach (particularly for less experienced trainees). A CD may have also been prompted when documentation was unclear, or when a change in prognosis took place (eg, a new diagnosis of metastatic cancer).22 Alternatively, a preexisting plan may serve as a reminder for clinicians to discuss code status, signify patients who are more willing to broach this subject, and either seem more approachable or bring up the topic themselves.
The influence of site on documentation and CD provides additional evidence that caregiver culture played a role in CDs. Although this variation may have been in part due to culture around documentation practices more generally, it is important to note that none of our participating centers had a policy for documentation of care wishes or patient‐doctor communication, or a policy mandating these discussions in any specific patient group. Furthermore, site‐related differences were seen even in patients with no preexisting documentation, and were seen after adjustment for other documentation or communication practices (eg, documenting a discussion with the patient's primary care provider), making it unlikely that documentation practices are solely responsible for our results. Persistence of variations in care documentation raises interesting questions, particularly when one considers recent data describing variations in end‐of‐life care between similar academic centers (one of which was a participating site in this trial).23 Given that the sites in our study represent diverse institutions yet share a number of characteristics, understanding the specific practices or aspects of medical culture that promote conversations may provide insights in how to improve this promotion elsewhere.
Our results would argue that mandates to document code status on admission may be unlikely to improve communication unless sites also develop an approach to using this newly documented information as a prompt for subsequent discussions. In nursing home settings, documentation of advance directives may reduce resource use, but it is unclear whether similar effects will be seen in hospital settings.24 It is also a challenge to insure that documentation of a care plan in the nursing home is communicated to the providers in the hospital.25 The PSDA was a first step in this direction, but its effects on improving communication are uncertain.26 Our results would confirm that the PSDA or systems to mandate documentation are not solutions in themselves, but are 2 steps in a larger process.
We do not want to discount our findings of less frequent CDs among patients of lower socioeconomic status, where gaps in quality of care, communication, and outcomes are well‐recognized.27 As such, our results delineate yet another area where practice can and should be improved for vulnerable patients. However, factors related to site of care and documentation may provide opportunities to improve care even more profoundly and within a fairly discrete (if complex) acute episode of care. Having said this, our results also demonstrate a potential pitfall of using code status documentation for risk‐adjustment, because such notation may be more dependent on local documentation patterns than clinical appropriateness.
Our study has a number of limitations. As an observational study, our findings are likely prone to biases related to unadjusted confounding due to comorbidity. The influence of comorbidity would seem to have been most important in biasing the effects of preexisting documentation, where documentation would be associated with more unaccounted comorbidity. However, there were no differences in documentation even after accounting for prognosis by adjusting for age, functional status, and a valid comorbidity score.28 As we have pointed out, our key outcome is based on documentation of communication and not actual communication, and as such may be biased in subtle ways not related to site of care or the items tested in our model. While we cannot directly eliminate the possibility of documentation biases in our results using statistical methods, it is important to point out that our chart abstraction protocol used highly specific criteria to detect these discussions, and therefore may under‐detect discussions which may have been documented in less detail. Our study did not examine whether documentation of CDs influenced subsequent care. However, previous studies have shown that advance care planning has only a minor influence on care.29 However, communication about preferences at the time of admission, when the need for specific care decisions may be more evident, may be more likely to influence hospital care. Our results show that previous documentation is associated with discussions early in an admission. Such discussion may affect care, even if the decision made is different than what was previously documented. In addition, patients who were included in our study (those able to provide consent and participate in an interview) may be healthier or more cognitively intact than a general population of hospitalized patients. However, how this would have affected our results is unclear. Being able to speak and consent for oneself are key facilitators to communication, but sicker patients who cannot consent or speak for themselves might also be more likely to have care planning decisions made based on illness severity; documentation in these patients may be more driven by whether such notes were required because of the involvement of home health services (or skilled nursing facilities). Finally, although our study is one of the largest examinations of in‐hospital communication to date and its implications for resident education are worth noting, the sites involved in the MCHS may not be representative of nonteaching hospitals, or community‐based teaching hospitals.
Our results suggest that, although comorbid illness and socioeconomic status play an important role in determining which patients receive CDs at the time of admission, these factors are substantially less powerful than preexisting documentation practices and culture or care practices specific to their site of care. These results suggest that future work should consider organizational characteristics and culture as important targets for interventions to improve care planning in hospitalized patients.
- Committee on Care at the End of Life, Institute of Medicine.Approaching Death: Improving Care at the End of Life.Field MJ,Cassel CK, eds.Washington, DC:National Academy Press;1997.
- ,,,,,.Factors considered important at the end of life by patients, family, physicians, and other care providers.JAMA.2000;284(19):2476–2482.
- ,,,,,.In search of a good death: observations of patients, families, and providers.Ann Intern Med.2000;132(10):825–832.
- The SUPPORT Principal Investigators.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).JAMA.1995;274(20):1591–1598.
- ,.Choices about cardiopulmonary resuscitation in the hospital. When do physicians talk with patients?N Engl J Med.1984;310(17):1089–1093.
- ,,, et al.Patient preferences for communication with physicians about end‐of‐life decisions. SUPPORT Investigators. Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment.Ann Intern Med.1997;127(1):1–12.
- ,,,.Discussing cardiopulmonary resuscitation: a study of elderly outpatients.J Gen Intern Med.1988;3(4):317–321.
- ,,,,.Educating the elderly: cardiopulmonary resuscitation decisions before and after intervention.J Am Geriatr Soc.1991;39(4):372–377.
- ,,,.Factors influencing physicians in recommending in‐hospital cardiopulmonary resuscitation.Arch Intern Med.1993;153(17):1999–2003.
- Federal Register. 42 USC 1395‐1396. Patient Self‐Determination Act1990.
- ,,.Advance directives on admission. Clinical implications and analysis of the Patient Self‐Determination Act of 1990.JAMA.1991;266(3):402–405.
- ,,.A new doctor in the house: ethical issues in hospitalist systems.JAMA.1999;282(2):171–174.
- ,,,,,.Implementation of a hospitalist service at a community teaching hospital: improving clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of hospitalist physicians on an academic general medical service: results of a randomized trial.Ann Intern Med.2002;137:866–874.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,,.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279(19):1560–1565.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288(17):2151–2162.
- ,,, et al.Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery.JAMA.1999;281(14):1310–1317.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(Suppl 1):141–142.
- ,,.SF‐12: How to Score the SF‐12 Physical and Mental Health Summary Scales.2nd ed.Boston, MA:New England Medical Center, The Health Institute;1995.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, processes of care, and patient symptoms.Am J Med.2004;116(10):669–675.
- ,,,.Role of written advance directives in decision making: insights from qualitative and quantitative data.J Gen Intern Med.1998;13(7):439–446.
- ,,,,.Evaluating the efficiency of California providers in caring for patients with chronic illnesses.Health Aff (Millwood).2005 Jul‐Dec;Suppl Web Exclusives:W5–526–43.
- ,,, et al.Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial.JAMA.2000;283(11):1437–1444.
- ,.Meeting palliative care needs in post‐acute care settings: “to help them live until they die”.JAMA.2006;295(6):681–686.
- ,,, et al.Advance directives for seriously ill hospitalized patients: effectiveness with the patient self‐determination act and the SUPPORT intervention. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment.J Am Geriatr Soc.1997;45(4):500–507.
- Institute of Medicine.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care.Smedley BD,Stith AY,Nelson AR, eds.Washington, DC:National Academies Press;2003.
- ,,.Use of a self‐report‐generated Charlson Comorbidity Index for predicting mortality.Med Care.2005;43(6):607–615.
- ,,.Can clinical interventions change care at the end of life?Ann Intern Med.1997;126(5):381–388.
Despite an ideal of dying at home, most Americans die in hospitals.1 Patients and families are clear about what they need from the healthcare system at the end of life: relief of distressing symptoms, the opportunity to communicate with physicians and others about death and dying, and the assurance that they will be attended to and comforted by their physicians as they approach death.2, 3 However, discussions about patient preferences for care occur infrequently,47 even though patients want to discuss care with their doctor,68 and physicians believe these discussions are their responsibility.9
The most prominent work in this area occurred in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) study, which focused on patients with advanced disease, often in the intensive care unit.4 Furthermore, few studies have focused on general medical patients, and healthcare has changed in important ways since SUPPORT's publication. First, the Patient Self‐Determination Act (PSDA) requires that all patients be asked about their care wishes at the time of admission and document the presence of an advanced directive.10, 11 Second, there is growing awareness of the need to improve palliative care for all hospitalized patients, with many advocating that hospitalization itself is a reason to ask about patient's preferences for care regardless of a patient's level of chronic or acute illness.12 Finally, emergence of hospitalists,1316 movement toward closed intensive care units,17, 18 and changes in residency training have increased segmentation in care of hospitalized patients.15, 18
To overcome limitations of previous literature and update our knowledge of how care discussions take place in the current healthcare environment, we analyzed data from a large study of patients admitted to general medicine services at 6 academic centers. Using this robust dataset, which included prospectively collected information about preferences for communication with their physician, we performed statistical analyses to understand which patient clinical, sociodemographic, and preference‐related factors, as well as factors related to their site of care, were associated with documentation that a code status discussion took place at the time of hospital admission.
PATIENTS AND METHODS
Sites
The Multicenter Hospitalist Study (MCHS) was a multicenter trial of general medical services that enrolled patients at 6 geographically diverse centers: The University of Chicago (which also served as the coordinating center), University of Iowa Hospitals and Clinics, University of California San Francisco, University of Wisconsin, University of New Mexico, and Brigham and Women's Hospital.19
Each site was selected to participate in the MCHS because patients on their general medicine service were admitted to hospitalist and nonhospitalist physicians in a random fashion (eg, based on predetermined call schedule based on day of the week). As teaching hospitals, house officers provided direct care to patients hospitalized at each center; nonteaching services were not present at the sites during the period of this study.
During the period of this study, each site complied with PSDA requirements for noting that patients had been informed about their right to create an advance directive, but no sites had a guideline or other program in place specifically intended to facilitate physician‐patient communication about care wishes. Two sites had active Hospice or Palliative Care services, and another 2 had Geriatrics Consultation services, but none had standard protocols mandating involvement of these consultants at the time of admission, the time when our key outcomes were documented.
Patients
Patients were eligible for inclusion in the MCHS if they were older than 18 years of age and were admitted at random to a hospitalist or nonhospitalist physician; we excluded patients from MCHS if they were admitted specifically under the care of their primary care physician or subspecialist (eg, admitted for chemotherapy) or were a prison inmate. Patients meeting these eligibility criteria were then approached for purposes of informed consent.
Data Collection
Data for this study were obtained from administrative data, patient interview, and chart abstraction as in previous work.14 Administrative data were drawn from cost‐accounting databases at each participating hospital; administrative data were used to provide cost and length of stay data, as well as information about patient insurance type, age, and sex.
We interviewed patients immediately after informed consent was obtained, with both taking place generally within 24 hours of admission. Interviews collected data about patient preferences for care and functional status,20 and other data not reliably available from administrative sources (such as housing situation).
Patient care plan before admission was taken from notes and orders written in the first 24 hours of hospitalization, as mentioned above. Using criteria we employed in previous work,21 a care discussion (CD) was defined as documentation of a discussion between patients (or family) and at least 1 physician (primary physician, hospitalist, consulting physician, or house officer) during the first 24 hours of hospitalization. CDs needed to specify that the person who wrote the note had actually spoken with the patient or their family for the purposes of determining preferences for care, and that this discussion resulted in a specific care plan. Thus, notations such as do not resuscitate/do not intubate, or spoke with family, questions answered, did not qualify as CDs, but a note stating the patient continues to want full efforts was counted as a CD.
Principal investigators at each site were responsible for training and overseeing interviewing and chart abstraction activities at each site, with central oversight of data quality provided by the central coordinating center. Upon receipt at the data coordinating center, all data were examined for missing, nonsensical, or outlier data with errors referred back to the participating sites for correction.
Statistical Analysis
For bivariable comparisons of patients with and without CDs, we used chi‐squared or Mann‐Whitney U‐tests, as appropriate.
Variables with P < 0.20 in bivariable comparisons were selected for initial inclusion in models. Then, using automated forward and stepwise selection techniques as well as manually entered variables, we fit multivariable generalized estimating equations permitting clustering of effects at the physician level to determine the independent association between the multiple factors tested and presence of a CD. In order to guard against the threat of multiple testing, we retained variables at a significance level of P < 0.01; variables were also retained because of observed confounding with other independent variables, or to maintain face validity of the model. All analyses were performed using SAS 9.0 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
Patient Sociodemographics (Table 1)
A total of 17,097 of 33,638 patients (50.8%) were interviewed and gave consent for chart abstraction. Of these patients, 1776 (10.3%) had a CD documented in the first 24 hours of hospitalization. Patients with documented CDs were older, more often white, had completed more years of education, were more likely to have lived in a nursing home prior to admission, and more likely to have been hospitalized in the last 12 months. The proportion of patients with CDs was highly variable across site of enrollment, from 2.8%‐24.9%.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P* |
|---|---|---|---|
| |||
| Age (Median, 95%CI)* | 56 (55, 56) | 69 (67, 71) | < 0.0001 |
| Female (n, %) | 8390 (54.8%) | 990 (55.7%) | 0.4312 |
| Race (n, %) | |||
| White | 6640 (43.3%) | 938 (52.8%) | < 0.0001 |
| African American | 4673 (30.5%) | 280 (15.8%) | |
| Asian | 532 (3.5%) | 167 (9.4%) | |
| American Indian | 325 (2.1%) | 26 (1.5%) | |
| Other | 1951 (12.7%) | 241 (13.6%) | |
| Refused/Don't know | 1200 (7.8%) | 124 (7.0%) | |
| Ethnicity (n, %) | |||
| Hispanic or Latino Ethnicity | 1724 (11.3%) | 183 (10.3%) | 0.0039 |
| Insurance type (n, %) | |||
| Charity | 481 (3.4%) | 14 (0.8%) | < 0.0001 |
| Indemnity | 3983 (28.2%) | 327 (19.3%) | |
| Medicaid | 2487 (17.6%) | 195 (11.5%) | |
| Medicare | 6418 (45.5%) | 1114 (65.9%) | |
| Other | 105 (0.7%) | 4 (0.2%) | |
| Self pay | 628 (4.5%) | 36 (2.1%) | |
| Self‐reported education (n, %) | |||
| Junior high school or less | 1297 (8.5%) | 217 (12.2%) | < 0.0001 |
| Some high school | 2146 (14.0%) | 182 (10.2%) | |
| High school graduate | 4435 (28.9%) | 465 (26.2%) | |
| Some college or junior college | 3521 (23.0%) | 347 (19.5%) | |
| College graduate | 1729 (11.3%) | 255 (14.4%) | |
| Post‐graduate | 1191 (7.8%) | 173 (9.7%) | |
| Refused/Don't know | 1002 (6.5%) | 137 (7.7%) | |
| Self reported income (n, %) | |||
| $2,500 or less | 1079 (7.0%) | 108 (6.1%) | 0.0002 |
| $2,501 to $5,000 | 424 (2.8%) | 33 (1.9%) | |
| $5,001 to $10,000 | 1436 (9.4%) | 211 (11.9%) | |
| $10,001 to $15,000 | 1080 (7.0%) | 141 (7.9%) | |
| $15,001 to $25,000 | 1054 (6.9%) | 134 (7.5%) | |
| $25,001 to $35,000 | 837 (5.5%) | 74 (4.2%) | |
| $35,001 to $50,000 | 882 (5.8%) | 94 (5.3%) | |
| $50,001 to $100,000 | 1027 (6.7%) | 125 (7.0%) | |
| $100,001 to $200,000 | 357 (2.3%) | 57 (3.2%) | |
| Over $200,000 | 245 (1.6%) | 34 (1.9%) | |
| Don't know/refused | 6900 (45.0%) | 765 (43.1%) | |
| Housing situation (n, %) | |||
| Own apartment or house | 11887 (77.6%) | 1264 (71.2%) | < 0.0001 |
| A relative or friend's apartment or house | 1804 (11.8%) | 217 (12.2%) | |
| A nursing home, group home, or long‐term care facility | 663 (4.3%) | 204 (11.5%) | |
| A homeless shelter | 258 (1.7%) | 27 (1.5%) | |
| Other | 709 (4.6%) | 64 (3.6%) | |
| Marital status (n, %) | |||
| Married | 4992 (32.6%) | 603 (34.0%) | < 0.0001 |
| Living as if married | 440 (2.9%) | 32 (1.8%) | |
| Divorced | 2027 (13.2%) | 199 (11.2%) | |
| Separated | 569 (3.7%) | 30 (1.7%) | |
| Widowed | 2577 (16.8%) | 487 (27.4%) | |
| Single | 4074 (26.6%) | 364 (20.5%) | |
| Refused | 642 (4.2%) | 61 (3.4%) | |
| Hospitalized in the last 12 months (n, %) | 7602 (49.6%) | 1011 (56.9%) | < 0.0001 |
| Site of enrollment (n, %) | |||
| A | 4602 (30.0%) | 135 (7.6%) | < 0.0001 |
| B | 1595 (10.4%) | 158 (8.9%) | |
| C | 3017 (19.7%) | 998 (56.2%) | |
| D | 2387 (15.6%) | 212 (11.9%) | |
| E | 2057 (13.4%) | 131 (7.4%) | |
| F | 1663 (10.9%) | 142 (8.0%) | |
Patient Self‐Reported Health Status and Comorbid Illness (Table 2)
Patients with CDs more often reported a lot of difficulties with bathing, eating, or dressing; household chores; and moderate activities. Patients with CDs were more likely to report accomplishing less than they would like due to their health. They were more likely to have cancer, depression, a history of stroke, and heart disease, but less likely to have diabetes or human immunodeficiency virus.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P** |
|---|---|---|---|
| |||
| Thinking back again to one month ago, did any impairment or health problem cause you to need help of other persons with personal care needs, such as eating, bathing, dressing, or getting around the home? (n, %) | |||
| No | 10673 (69.7%) | 973 (54.8%) | < 0.0001 |
| Yes, a little | 1933 (12.6%) | 268 (15.1%) | |
| Yes, a lot | 2127 (13.9%) | 487 (27.4%) | |
| Don't know | 588 (3.8%) | 48 (2.7%) | |
| Thinking back to one month ago, did any impairment or health problem cause you to need help in handling everyday household chores, necessary business, shopping, or getting around for other purposes? (n, %) | |||
| No | 7262 (47.4%) | 566 (31.9%) | < 0.0001 |
| Yes, a little | 2692 (17.6%) | 324 (18.2%) | |
| Yes, a lot | 4126 (26.9%) | 825 (46.5%) | |
| Don't know | 1241 (8.1%) | 61 (3.4%) | |
| As far as you know do you have any of the following health conditions at the present time? (n, %) | |||
| Cancer | |||
| No | 13281 (86.7%) | 1376 (77.5%) | < 0.0001 |
| Yes | 1751 (11.4%) | 351 (19.8%) | |
| Not sure | 289 (1.9%) | 49 (2.8%) | |
| Depression | |||
| No | 10269 (67.0%) | 1099 (61.9%) | < 0.0001 |
| Yes | 4730 (30.9%) | 624 (35.1%) | |
| Not sure | 322 (2.1%) | 53 (3.0%) | |
| Diabetes | |||
| No | 10902 (71.2%) | 1356 (76.4%) | < 0.0001 |
| Yes | 4132 (27.0%) | 394 (22.2%) | |
| Not sure | 287 (1.9%) | 26 (1.5%) | |
| Heart trouble | |||
| No | 10251 (66.9%) | 1080 (60.8%) | < 0.0001 |
| Yes | 4491 (29.3%) | 627 (35.3%) | |
| Not sure | 579 (3.8%) | 69 (3.9%) | |
| HIV or AIDS | |||
| No | 14300 (93.3%) | 1679 (94.5%) | 0.026 |
| Yes | 912 (6.0%) | 80 (4.5%) | |
| Not sure | 109 (0.7%) | 17 (1.0%) | |
| Stroke | |||
| No | 13344 (87.1%) | 1494 (84.1%) | 0.0005 |
| Yes | 1722 (11.2%) | 236 (13.3%) | |
| Not sure | 255 (1.7%) | 46 (2.6%) | |
Patient Preferences, Care Plan Documentation, and Care Coordination at Admission (Table 3)
Patients who had documented CDs were less likely to prefer my doctor give me choices regarding my care, and more often disagreed with the statement I prefer to leave care decisions to my physician. These patients were also more likely to have a durable power of attorney or living will in their chart, or have an alternate decision‐maker noted. The majority of patients without a documented CD (79.9%) had no notation of their care wishes, compared to 29.7% in patients with a documented CD. Patients with a documented CD were more likely to have a regular medical provider and a note in the chart from their primary care physician.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P* |
|---|---|---|---|
| |||
| I prefer my doctor give me choices regarding my care** (n, %) | |||
| Definitely agree | 11619 (75.8%) | 1247 (70.2%) | < 0.0001 |
| Somewhat agree | 1912 (12.5%) | 252 (14.2%) | |
| Somewhat disagree | 488 (3.2%) | 76 (4.3%) | |
| Definitely disagree | 414 (2.7%) | 87 (4.9%) | |
| Don't know | 888 (5.8%) | 114 (6.4%) | |
| I prefer to leave care decisions to my physician** (n, %) | |||
| Definitely agree | 5660 (36.9%) | 613 (34.5%) | < 0.0001 |
| Somewhat agree | 4539 (29.6%) | 493 (27.8%) | |
| Somewhat disagree | 2265 (14.8%) | 257 (14.5%) | |
| Definitely disagree | 1956 (12.8%) | 304 (17.1%) | |
| Don't know | 901 (5.9%) | 109 (6.1%) | |
| Documentation of care wishes before hospitalization (n, %) | |||
| No documentation | 12238 (79.9%) | 527 (29.7%) | < 0.0001 |
| Full support | 2624 (17.1%) | 742 (41.8%) | |
| Do not resuscitate or intubate (DNR/DNI) | 264 (1.7%) | 370 (20.8%) | |
| Hospice | 53 (0.3%) | 22 (1.2%) | |
| Other limitation (eg, no pressors) | 142 (0.9%) | 115 (6.5%) | |
| Had durable power of attorney in chart (n, %) | 286 (1.9%) | 133 (7.5%) | < 0.0001 |
| Had a living will in chart (n, %) | 266 (1.7%) | 142 (8.0%) | < 0.0001 |
| Alternate decision maker named in chart (n, %) | 2770 (18.1%) | 638 (35.9%) | < 0.0001 |
| Patient noted to be unable to participate in their care at admission (eg, confused, unable to respond) (n, %) | 1227 (8.0%) | 431 (24.3%) | < 0.0001 |
| Inpatient team documented discussion with primary care physician (n, %) | 627 (4.1%) | 136 (7.7%) | < 0.0001 |
| Do not have a regular medical provider** (n, %) | 3836 (25.0%) | 254 (14.3%) | < 0.0001 |
| Note from primary care physician in chart (n, %) | 148 (1.0%) | 39 (2.2%) | < 0.0001 |
Factors Associated with Documented Care Discussions (Table 4)
Using predictor variables presented in Tables 1‐3, we then constructed multivariable models seeking to understand factors independently associated with documentation of code status in the entire cohort, as well as among patients who had no preexisting care wishes.
| Entire Cohort (n = 17097) | Patients with No Documentation of Preadmission Wishes (n = 12765) | |||
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value | |
| Preadmission Code Status | ||||
| No documentation | Referent | NA | ||
| Full support | 3.22 (2.28, 4.55) | < 0.0001 | NA | |
| Do not resuscitate or intubate (DNR/DNI) | 11.32 (8.52, 15.04) | < 0.0001 | NA | |
| Hospice | 4.02 (2.33, 6.94) | < 0.0001 | NA | |
| Other limitation (eg, no pressors) | 10.13 (7.35, 13.96) | < 0.0001 | NA | |
| Insurance type | ||||
| Medicare | Referent | Referent | ||
| Charity care | 0.50 (0.30, 0.85) | 0.0099 | 0.56 (0.25, 1.25) | 0.1589 |
| Commercial | 0.81 (0.69, 0.95) | 0.0090 | 0.66 (0.52, 0.85) | 0.0009 |
| Medicaid | 0.69 (0.57, 0.82) | < 0.0001 | 0.49 (0.36, 0.67) | < 0.0001 |
| Other | 0.46 (0.18, 1.13) | 0.0912 | 0.60 (0.17, 2.12) | 0.4302 |
| Self pay | 0.70 (0.52, 0.95) | 0.0203 | 0.49 (0.29, 0.81) | 0.0060 |
| Any limitations in bathing, toileting, dressing or feeding self? | ||||
| No | Referent | Referent | ||
| Yes, a little | 1.25 (1.10, 1.42) | 0.0007 | 1.31 (1.03, 1.67) | 0.0272 |
| Yes, a lot | 1.25 (1.09, 1.43) | 0.0015 | 1.42 (1.11, 1.81) | 0.0055 |
| Unable to respond | 0.81 (0.59, 1.12) | 0.2006 | 0.80 (0.45, 1.41) | 0.4299 |
| Patient has a documented surrogate decision maker | 1.72 (1.47, 2.02) | < 0.0001 | 2.08 (1.62, 2.66) | < 0.0001 |
| Patient noted to be unable to participate in their care at admission (eg, confused, unable to respond) | 1.63 (1.37, 1.94) | < 0.0001 | 2.20 (1.60, 3.02) | < 0.0001 |
| Notation that team had spoken to primary care physician at admission | 1.65 (1.29, 2.11) | < 0.0001 | 1.45 (0.92, 2.28) | 0.1116 |
| History of cancer | ||||
| No | Referent | Referent | ||
| Yes | 1.31 (1.13, 1.51) | 0.0003 | 1.26 (0.96, 1.65) | 0.0960 |
| Not sure | 1.26 (0.87, 1.82) | 0.2162 | 1.80 (1.03, 3.15) | 0.0396 |
| History of diabetes | ||||
| No | Referent | Referent | ||
| Yes | 0.87 (0.75, 1.003) | 0.0543 | 0.79 (0.62, 0.997) | 0.0467 |
| Not sure | 0.61 (0.38, 0.99) | 0.0445 | 0.84 (0.43, 1.65) | 0.6183 |
| Housing situation | ||||
| Own house or apartment | Referent | Referent | ||
| Relative or friend's apartment or house | 1.22 (1.03, 1.45) | 0.0229 | 1.29 (0.97, 1.71) | 0.0783 |
| Nursing home, group home, or long‐term care facility | 1.42 (1.16, 1.74) | 0.0006 | 1.74 (1.27, 2.40) | 0.0007 |
| Homeless shelter | 1.12 (0.72, 1.73) | 0.6204 | 0.87 (0.46, 1.63) | 0.6559 |
| Other/Don't know | 1.02 (0.75, 1.40) | 0.8987 | 1.35 (0.78, 2.36) | 0.2859 |
| Age Group | ||||
| <50 | Referent | Referent | ||
| 5059 | 1.19 (0.99, 1.43) | 0.0647 | 1.18 (0.88, 1.59) | 0.2583 |
| 6069 | 1.18 (0.99, 1.40) | 0.0585 | 1.20 (0.88, 1.66) | 0.2549 |
| 7079 | 1.10 (0.91, 1.33) | 0.3178 | 1.19 (0.85, 1.67) | 0.3033 |
| 8089 | 1.23 (1.03, 1.47) | 0.0207 | 1.34 (0.96, 1.88) | 0.0879 |
| 90+ | 1.45 (1.12, 1.88) | 0.0045 | 1.44 (0.94, 2.20) | 0.0934 |
| Site of Enrollment | ||||
| A | Referent | Referent | ||
| B | 1.74 (1.16, 2.61) | 0.007 | 4.95 (2.90, 8.45) | < 0.0001 |
| C | 5.14 (3.42, 7.74) | < 0.0001 | 26.36 (17.28, 40.23) | < 0.0001 |
| D | 4.19 (2.64, 6.66) | < 0.0001 | 8.06 (4.63, 14.03) | < 0.0001 |
| E | 3.00 (1.82, 4.9) | < 0.0001 | 5.30 (2.71, 10.38) | < 0.0001 |
| F | 4.09 (2.69, 6.23) | < 0.0001 | 2.32 (1.32, 4.08) | 0.0037 |
In the entire cohort, insurance type was independently associated with likelihood of a care discussion, with patients with Medicare having greater adjusted odds ratio for a CD than patients with all other forms of insurance, even after adjusting for age. Patients who had functional limitations with bathing, toileting, and feeding; had a documented surrogate decision maker; were unable to participate in their care; had cancer; or did not live in their own home were more likely to have a documented CD. Subjects with diabetes were less likely to have a CD, although this was of borderline significance. Patients whose team had documented a CD with the patients' primary physician were also more likely to have a discussion noted. However, the magnitude of these predictors was small compared to the independent effects attributable to the site the patient was enrolled or whether the patient had any preexisting documentation. Whereas the adjusted odds ratio associated with clinical or functional measures (such as age, cancer) were generally between 1.5 and 2.5, the range of odds ratios associated with having any documentation of care wishes (compared to no documentation) were all greater than 3, and the odds ratios associated with site of enrollment were 1.7 or higher.
We observed similar findings in analyses limited to patients with no preexisting care documentation. While clinical, sociodemographic, and functional factors remained statistically associated with a CD (albeit with wider confidence intervals due to smaller sample sizes), the effect of the patient's site of enrollment became even more striking (Table 4).
DISCUSSION
In this multicenter study of hospitalized general medical patients, documentation of CDs were highly dependent on where patients received care and whether patients had previous documentation of a care plan. In contrast, although clinical, prognostic, and socioeconomic factors were also associated with whether physicians documented asking patients about their wishes for care, the influence of these factors was modest.
Improving communication between patients and their physicians during an episode of acute illness has been a long‐standing goal, with the Study to Understand Prognoses and Preferences for Outcomes of Treatment (SUPPORT) trial providing the most notable example of an effort to improve patient care through aligning patient wishes, prognosis, and aggressiveness for care. However, even the SUPPORT interventiona robust, well‐implemented, and highly labor‐intensive strategywas not able to achieve this goal. In their summary of SUPPORT study findings, the authors suggested that the likelihood of and effectiveness of communication in seriously ill patients may be powerfully influenced by patient and caregiver culture4; our findings may partially confirm SUPPORT's conclusions.
Preexisting documentation in our study would not have included mandated documentation that someone had given the patient information about advance directives (as mandated by the PSDA), but rather a specification for that advance care plan. This distinction means that preexisting documentation in our study represented a previous decision by the patient (or the patient and their physician) to have made a plan, and an association with hospital discussions may be because the first conversation is the hardest to undertake; subsequent discussions then represent confirmatory or clarifying discussions that may be less difficult to broach (particularly for less experienced trainees). A CD may have also been prompted when documentation was unclear, or when a change in prognosis took place (eg, a new diagnosis of metastatic cancer).22 Alternatively, a preexisting plan may serve as a reminder for clinicians to discuss code status, signify patients who are more willing to broach this subject, and either seem more approachable or bring up the topic themselves.
The influence of site on documentation and CD provides additional evidence that caregiver culture played a role in CDs. Although this variation may have been in part due to culture around documentation practices more generally, it is important to note that none of our participating centers had a policy for documentation of care wishes or patient‐doctor communication, or a policy mandating these discussions in any specific patient group. Furthermore, site‐related differences were seen even in patients with no preexisting documentation, and were seen after adjustment for other documentation or communication practices (eg, documenting a discussion with the patient's primary care provider), making it unlikely that documentation practices are solely responsible for our results. Persistence of variations in care documentation raises interesting questions, particularly when one considers recent data describing variations in end‐of‐life care between similar academic centers (one of which was a participating site in this trial).23 Given that the sites in our study represent diverse institutions yet share a number of characteristics, understanding the specific practices or aspects of medical culture that promote conversations may provide insights in how to improve this promotion elsewhere.
Our results would argue that mandates to document code status on admission may be unlikely to improve communication unless sites also develop an approach to using this newly documented information as a prompt for subsequent discussions. In nursing home settings, documentation of advance directives may reduce resource use, but it is unclear whether similar effects will be seen in hospital settings.24 It is also a challenge to insure that documentation of a care plan in the nursing home is communicated to the providers in the hospital.25 The PSDA was a first step in this direction, but its effects on improving communication are uncertain.26 Our results would confirm that the PSDA or systems to mandate documentation are not solutions in themselves, but are 2 steps in a larger process.
We do not want to discount our findings of less frequent CDs among patients of lower socioeconomic status, where gaps in quality of care, communication, and outcomes are well‐recognized.27 As such, our results delineate yet another area where practice can and should be improved for vulnerable patients. However, factors related to site of care and documentation may provide opportunities to improve care even more profoundly and within a fairly discrete (if complex) acute episode of care. Having said this, our results also demonstrate a potential pitfall of using code status documentation for risk‐adjustment, because such notation may be more dependent on local documentation patterns than clinical appropriateness.
Our study has a number of limitations. As an observational study, our findings are likely prone to biases related to unadjusted confounding due to comorbidity. The influence of comorbidity would seem to have been most important in biasing the effects of preexisting documentation, where documentation would be associated with more unaccounted comorbidity. However, there were no differences in documentation even after accounting for prognosis by adjusting for age, functional status, and a valid comorbidity score.28 As we have pointed out, our key outcome is based on documentation of communication and not actual communication, and as such may be biased in subtle ways not related to site of care or the items tested in our model. While we cannot directly eliminate the possibility of documentation biases in our results using statistical methods, it is important to point out that our chart abstraction protocol used highly specific criteria to detect these discussions, and therefore may under‐detect discussions which may have been documented in less detail. Our study did not examine whether documentation of CDs influenced subsequent care. However, previous studies have shown that advance care planning has only a minor influence on care.29 However, communication about preferences at the time of admission, when the need for specific care decisions may be more evident, may be more likely to influence hospital care. Our results show that previous documentation is associated with discussions early in an admission. Such discussion may affect care, even if the decision made is different than what was previously documented. In addition, patients who were included in our study (those able to provide consent and participate in an interview) may be healthier or more cognitively intact than a general population of hospitalized patients. However, how this would have affected our results is unclear. Being able to speak and consent for oneself are key facilitators to communication, but sicker patients who cannot consent or speak for themselves might also be more likely to have care planning decisions made based on illness severity; documentation in these patients may be more driven by whether such notes were required because of the involvement of home health services (or skilled nursing facilities). Finally, although our study is one of the largest examinations of in‐hospital communication to date and its implications for resident education are worth noting, the sites involved in the MCHS may not be representative of nonteaching hospitals, or community‐based teaching hospitals.
Our results suggest that, although comorbid illness and socioeconomic status play an important role in determining which patients receive CDs at the time of admission, these factors are substantially less powerful than preexisting documentation practices and culture or care practices specific to their site of care. These results suggest that future work should consider organizational characteristics and culture as important targets for interventions to improve care planning in hospitalized patients.
Despite an ideal of dying at home, most Americans die in hospitals.1 Patients and families are clear about what they need from the healthcare system at the end of life: relief of distressing symptoms, the opportunity to communicate with physicians and others about death and dying, and the assurance that they will be attended to and comforted by their physicians as they approach death.2, 3 However, discussions about patient preferences for care occur infrequently,47 even though patients want to discuss care with their doctor,68 and physicians believe these discussions are their responsibility.9
The most prominent work in this area occurred in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) study, which focused on patients with advanced disease, often in the intensive care unit.4 Furthermore, few studies have focused on general medical patients, and healthcare has changed in important ways since SUPPORT's publication. First, the Patient Self‐Determination Act (PSDA) requires that all patients be asked about their care wishes at the time of admission and document the presence of an advanced directive.10, 11 Second, there is growing awareness of the need to improve palliative care for all hospitalized patients, with many advocating that hospitalization itself is a reason to ask about patient's preferences for care regardless of a patient's level of chronic or acute illness.12 Finally, emergence of hospitalists,1316 movement toward closed intensive care units,17, 18 and changes in residency training have increased segmentation in care of hospitalized patients.15, 18
To overcome limitations of previous literature and update our knowledge of how care discussions take place in the current healthcare environment, we analyzed data from a large study of patients admitted to general medicine services at 6 academic centers. Using this robust dataset, which included prospectively collected information about preferences for communication with their physician, we performed statistical analyses to understand which patient clinical, sociodemographic, and preference‐related factors, as well as factors related to their site of care, were associated with documentation that a code status discussion took place at the time of hospital admission.
PATIENTS AND METHODS
Sites
The Multicenter Hospitalist Study (MCHS) was a multicenter trial of general medical services that enrolled patients at 6 geographically diverse centers: The University of Chicago (which also served as the coordinating center), University of Iowa Hospitals and Clinics, University of California San Francisco, University of Wisconsin, University of New Mexico, and Brigham and Women's Hospital.19
Each site was selected to participate in the MCHS because patients on their general medicine service were admitted to hospitalist and nonhospitalist physicians in a random fashion (eg, based on predetermined call schedule based on day of the week). As teaching hospitals, house officers provided direct care to patients hospitalized at each center; nonteaching services were not present at the sites during the period of this study.
During the period of this study, each site complied with PSDA requirements for noting that patients had been informed about their right to create an advance directive, but no sites had a guideline or other program in place specifically intended to facilitate physician‐patient communication about care wishes. Two sites had active Hospice or Palliative Care services, and another 2 had Geriatrics Consultation services, but none had standard protocols mandating involvement of these consultants at the time of admission, the time when our key outcomes were documented.
Patients
Patients were eligible for inclusion in the MCHS if they were older than 18 years of age and were admitted at random to a hospitalist or nonhospitalist physician; we excluded patients from MCHS if they were admitted specifically under the care of their primary care physician or subspecialist (eg, admitted for chemotherapy) or were a prison inmate. Patients meeting these eligibility criteria were then approached for purposes of informed consent.
Data Collection
Data for this study were obtained from administrative data, patient interview, and chart abstraction as in previous work.14 Administrative data were drawn from cost‐accounting databases at each participating hospital; administrative data were used to provide cost and length of stay data, as well as information about patient insurance type, age, and sex.
We interviewed patients immediately after informed consent was obtained, with both taking place generally within 24 hours of admission. Interviews collected data about patient preferences for care and functional status,20 and other data not reliably available from administrative sources (such as housing situation).
Patient care plan before admission was taken from notes and orders written in the first 24 hours of hospitalization, as mentioned above. Using criteria we employed in previous work,21 a care discussion (CD) was defined as documentation of a discussion between patients (or family) and at least 1 physician (primary physician, hospitalist, consulting physician, or house officer) during the first 24 hours of hospitalization. CDs needed to specify that the person who wrote the note had actually spoken with the patient or their family for the purposes of determining preferences for care, and that this discussion resulted in a specific care plan. Thus, notations such as do not resuscitate/do not intubate, or spoke with family, questions answered, did not qualify as CDs, but a note stating the patient continues to want full efforts was counted as a CD.
Principal investigators at each site were responsible for training and overseeing interviewing and chart abstraction activities at each site, with central oversight of data quality provided by the central coordinating center. Upon receipt at the data coordinating center, all data were examined for missing, nonsensical, or outlier data with errors referred back to the participating sites for correction.
Statistical Analysis
For bivariable comparisons of patients with and without CDs, we used chi‐squared or Mann‐Whitney U‐tests, as appropriate.
Variables with P < 0.20 in bivariable comparisons were selected for initial inclusion in models. Then, using automated forward and stepwise selection techniques as well as manually entered variables, we fit multivariable generalized estimating equations permitting clustering of effects at the physician level to determine the independent association between the multiple factors tested and presence of a CD. In order to guard against the threat of multiple testing, we retained variables at a significance level of P < 0.01; variables were also retained because of observed confounding with other independent variables, or to maintain face validity of the model. All analyses were performed using SAS 9.0 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
Patient Sociodemographics (Table 1)
A total of 17,097 of 33,638 patients (50.8%) were interviewed and gave consent for chart abstraction. Of these patients, 1776 (10.3%) had a CD documented in the first 24 hours of hospitalization. Patients with documented CDs were older, more often white, had completed more years of education, were more likely to have lived in a nursing home prior to admission, and more likely to have been hospitalized in the last 12 months. The proportion of patients with CDs was highly variable across site of enrollment, from 2.8%‐24.9%.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P* |
|---|---|---|---|
| |||
| Age (Median, 95%CI)* | 56 (55, 56) | 69 (67, 71) | < 0.0001 |
| Female (n, %) | 8390 (54.8%) | 990 (55.7%) | 0.4312 |
| Race (n, %) | |||
| White | 6640 (43.3%) | 938 (52.8%) | < 0.0001 |
| African American | 4673 (30.5%) | 280 (15.8%) | |
| Asian | 532 (3.5%) | 167 (9.4%) | |
| American Indian | 325 (2.1%) | 26 (1.5%) | |
| Other | 1951 (12.7%) | 241 (13.6%) | |
| Refused/Don't know | 1200 (7.8%) | 124 (7.0%) | |
| Ethnicity (n, %) | |||
| Hispanic or Latino Ethnicity | 1724 (11.3%) | 183 (10.3%) | 0.0039 |
| Insurance type (n, %) | |||
| Charity | 481 (3.4%) | 14 (0.8%) | < 0.0001 |
| Indemnity | 3983 (28.2%) | 327 (19.3%) | |
| Medicaid | 2487 (17.6%) | 195 (11.5%) | |
| Medicare | 6418 (45.5%) | 1114 (65.9%) | |
| Other | 105 (0.7%) | 4 (0.2%) | |
| Self pay | 628 (4.5%) | 36 (2.1%) | |
| Self‐reported education (n, %) | |||
| Junior high school or less | 1297 (8.5%) | 217 (12.2%) | < 0.0001 |
| Some high school | 2146 (14.0%) | 182 (10.2%) | |
| High school graduate | 4435 (28.9%) | 465 (26.2%) | |
| Some college or junior college | 3521 (23.0%) | 347 (19.5%) | |
| College graduate | 1729 (11.3%) | 255 (14.4%) | |
| Post‐graduate | 1191 (7.8%) | 173 (9.7%) | |
| Refused/Don't know | 1002 (6.5%) | 137 (7.7%) | |
| Self reported income (n, %) | |||
| $2,500 or less | 1079 (7.0%) | 108 (6.1%) | 0.0002 |
| $2,501 to $5,000 | 424 (2.8%) | 33 (1.9%) | |
| $5,001 to $10,000 | 1436 (9.4%) | 211 (11.9%) | |
| $10,001 to $15,000 | 1080 (7.0%) | 141 (7.9%) | |
| $15,001 to $25,000 | 1054 (6.9%) | 134 (7.5%) | |
| $25,001 to $35,000 | 837 (5.5%) | 74 (4.2%) | |
| $35,001 to $50,000 | 882 (5.8%) | 94 (5.3%) | |
| $50,001 to $100,000 | 1027 (6.7%) | 125 (7.0%) | |
| $100,001 to $200,000 | 357 (2.3%) | 57 (3.2%) | |
| Over $200,000 | 245 (1.6%) | 34 (1.9%) | |
| Don't know/refused | 6900 (45.0%) | 765 (43.1%) | |
| Housing situation (n, %) | |||
| Own apartment or house | 11887 (77.6%) | 1264 (71.2%) | < 0.0001 |
| A relative or friend's apartment or house | 1804 (11.8%) | 217 (12.2%) | |
| A nursing home, group home, or long‐term care facility | 663 (4.3%) | 204 (11.5%) | |
| A homeless shelter | 258 (1.7%) | 27 (1.5%) | |
| Other | 709 (4.6%) | 64 (3.6%) | |
| Marital status (n, %) | |||
| Married | 4992 (32.6%) | 603 (34.0%) | < 0.0001 |
| Living as if married | 440 (2.9%) | 32 (1.8%) | |
| Divorced | 2027 (13.2%) | 199 (11.2%) | |
| Separated | 569 (3.7%) | 30 (1.7%) | |
| Widowed | 2577 (16.8%) | 487 (27.4%) | |
| Single | 4074 (26.6%) | 364 (20.5%) | |
| Refused | 642 (4.2%) | 61 (3.4%) | |
| Hospitalized in the last 12 months (n, %) | 7602 (49.6%) | 1011 (56.9%) | < 0.0001 |
| Site of enrollment (n, %) | |||
| A | 4602 (30.0%) | 135 (7.6%) | < 0.0001 |
| B | 1595 (10.4%) | 158 (8.9%) | |
| C | 3017 (19.7%) | 998 (56.2%) | |
| D | 2387 (15.6%) | 212 (11.9%) | |
| E | 2057 (13.4%) | 131 (7.4%) | |
| F | 1663 (10.9%) | 142 (8.0%) | |
Patient Self‐Reported Health Status and Comorbid Illness (Table 2)
Patients with CDs more often reported a lot of difficulties with bathing, eating, or dressing; household chores; and moderate activities. Patients with CDs were more likely to report accomplishing less than they would like due to their health. They were more likely to have cancer, depression, a history of stroke, and heart disease, but less likely to have diabetes or human immunodeficiency virus.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P** |
|---|---|---|---|
| |||
| Thinking back again to one month ago, did any impairment or health problem cause you to need help of other persons with personal care needs, such as eating, bathing, dressing, or getting around the home? (n, %) | |||
| No | 10673 (69.7%) | 973 (54.8%) | < 0.0001 |
| Yes, a little | 1933 (12.6%) | 268 (15.1%) | |
| Yes, a lot | 2127 (13.9%) | 487 (27.4%) | |
| Don't know | 588 (3.8%) | 48 (2.7%) | |
| Thinking back to one month ago, did any impairment or health problem cause you to need help in handling everyday household chores, necessary business, shopping, or getting around for other purposes? (n, %) | |||
| No | 7262 (47.4%) | 566 (31.9%) | < 0.0001 |
| Yes, a little | 2692 (17.6%) | 324 (18.2%) | |
| Yes, a lot | 4126 (26.9%) | 825 (46.5%) | |
| Don't know | 1241 (8.1%) | 61 (3.4%) | |
| As far as you know do you have any of the following health conditions at the present time? (n, %) | |||
| Cancer | |||
| No | 13281 (86.7%) | 1376 (77.5%) | < 0.0001 |
| Yes | 1751 (11.4%) | 351 (19.8%) | |
| Not sure | 289 (1.9%) | 49 (2.8%) | |
| Depression | |||
| No | 10269 (67.0%) | 1099 (61.9%) | < 0.0001 |
| Yes | 4730 (30.9%) | 624 (35.1%) | |
| Not sure | 322 (2.1%) | 53 (3.0%) | |
| Diabetes | |||
| No | 10902 (71.2%) | 1356 (76.4%) | < 0.0001 |
| Yes | 4132 (27.0%) | 394 (22.2%) | |
| Not sure | 287 (1.9%) | 26 (1.5%) | |
| Heart trouble | |||
| No | 10251 (66.9%) | 1080 (60.8%) | < 0.0001 |
| Yes | 4491 (29.3%) | 627 (35.3%) | |
| Not sure | 579 (3.8%) | 69 (3.9%) | |
| HIV or AIDS | |||
| No | 14300 (93.3%) | 1679 (94.5%) | 0.026 |
| Yes | 912 (6.0%) | 80 (4.5%) | |
| Not sure | 109 (0.7%) | 17 (1.0%) | |
| Stroke | |||
| No | 13344 (87.1%) | 1494 (84.1%) | 0.0005 |
| Yes | 1722 (11.2%) | 236 (13.3%) | |
| Not sure | 255 (1.7%) | 46 (2.6%) | |
Patient Preferences, Care Plan Documentation, and Care Coordination at Admission (Table 3)
Patients who had documented CDs were less likely to prefer my doctor give me choices regarding my care, and more often disagreed with the statement I prefer to leave care decisions to my physician. These patients were also more likely to have a durable power of attorney or living will in their chart, or have an alternate decision‐maker noted. The majority of patients without a documented CD (79.9%) had no notation of their care wishes, compared to 29.7% in patients with a documented CD. Patients with a documented CD were more likely to have a regular medical provider and a note in the chart from their primary care physician.
| Value | No Documented CD (n = 15321, 89.7%) | Documented CD (n = 1776, 10.3%) | P* |
|---|---|---|---|
| |||
| I prefer my doctor give me choices regarding my care** (n, %) | |||
| Definitely agree | 11619 (75.8%) | 1247 (70.2%) | < 0.0001 |
| Somewhat agree | 1912 (12.5%) | 252 (14.2%) | |
| Somewhat disagree | 488 (3.2%) | 76 (4.3%) | |
| Definitely disagree | 414 (2.7%) | 87 (4.9%) | |
| Don't know | 888 (5.8%) | 114 (6.4%) | |
| I prefer to leave care decisions to my physician** (n, %) | |||
| Definitely agree | 5660 (36.9%) | 613 (34.5%) | < 0.0001 |
| Somewhat agree | 4539 (29.6%) | 493 (27.8%) | |
| Somewhat disagree | 2265 (14.8%) | 257 (14.5%) | |
| Definitely disagree | 1956 (12.8%) | 304 (17.1%) | |
| Don't know | 901 (5.9%) | 109 (6.1%) | |
| Documentation of care wishes before hospitalization (n, %) | |||
| No documentation | 12238 (79.9%) | 527 (29.7%) | < 0.0001 |
| Full support | 2624 (17.1%) | 742 (41.8%) | |
| Do not resuscitate or intubate (DNR/DNI) | 264 (1.7%) | 370 (20.8%) | |
| Hospice | 53 (0.3%) | 22 (1.2%) | |
| Other limitation (eg, no pressors) | 142 (0.9%) | 115 (6.5%) | |
| Had durable power of attorney in chart (n, %) | 286 (1.9%) | 133 (7.5%) | < 0.0001 |
| Had a living will in chart (n, %) | 266 (1.7%) | 142 (8.0%) | < 0.0001 |
| Alternate decision maker named in chart (n, %) | 2770 (18.1%) | 638 (35.9%) | < 0.0001 |
| Patient noted to be unable to participate in their care at admission (eg, confused, unable to respond) (n, %) | 1227 (8.0%) | 431 (24.3%) | < 0.0001 |
| Inpatient team documented discussion with primary care physician (n, %) | 627 (4.1%) | 136 (7.7%) | < 0.0001 |
| Do not have a regular medical provider** (n, %) | 3836 (25.0%) | 254 (14.3%) | < 0.0001 |
| Note from primary care physician in chart (n, %) | 148 (1.0%) | 39 (2.2%) | < 0.0001 |
Factors Associated with Documented Care Discussions (Table 4)
Using predictor variables presented in Tables 1‐3, we then constructed multivariable models seeking to understand factors independently associated with documentation of code status in the entire cohort, as well as among patients who had no preexisting care wishes.
| Entire Cohort (n = 17097) | Patients with No Documentation of Preadmission Wishes (n = 12765) | |||
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value | |
| Preadmission Code Status | ||||
| No documentation | Referent | NA | ||
| Full support | 3.22 (2.28, 4.55) | < 0.0001 | NA | |
| Do not resuscitate or intubate (DNR/DNI) | 11.32 (8.52, 15.04) | < 0.0001 | NA | |
| Hospice | 4.02 (2.33, 6.94) | < 0.0001 | NA | |
| Other limitation (eg, no pressors) | 10.13 (7.35, 13.96) | < 0.0001 | NA | |
| Insurance type | ||||
| Medicare | Referent | Referent | ||
| Charity care | 0.50 (0.30, 0.85) | 0.0099 | 0.56 (0.25, 1.25) | 0.1589 |
| Commercial | 0.81 (0.69, 0.95) | 0.0090 | 0.66 (0.52, 0.85) | 0.0009 |
| Medicaid | 0.69 (0.57, 0.82) | < 0.0001 | 0.49 (0.36, 0.67) | < 0.0001 |
| Other | 0.46 (0.18, 1.13) | 0.0912 | 0.60 (0.17, 2.12) | 0.4302 |
| Self pay | 0.70 (0.52, 0.95) | 0.0203 | 0.49 (0.29, 0.81) | 0.0060 |
| Any limitations in bathing, toileting, dressing or feeding self? | ||||
| No | Referent | Referent | ||
| Yes, a little | 1.25 (1.10, 1.42) | 0.0007 | 1.31 (1.03, 1.67) | 0.0272 |
| Yes, a lot | 1.25 (1.09, 1.43) | 0.0015 | 1.42 (1.11, 1.81) | 0.0055 |
| Unable to respond | 0.81 (0.59, 1.12) | 0.2006 | 0.80 (0.45, 1.41) | 0.4299 |
| Patient has a documented surrogate decision maker | 1.72 (1.47, 2.02) | < 0.0001 | 2.08 (1.62, 2.66) | < 0.0001 |
| Patient noted to be unable to participate in their care at admission (eg, confused, unable to respond) | 1.63 (1.37, 1.94) | < 0.0001 | 2.20 (1.60, 3.02) | < 0.0001 |
| Notation that team had spoken to primary care physician at admission | 1.65 (1.29, 2.11) | < 0.0001 | 1.45 (0.92, 2.28) | 0.1116 |
| History of cancer | ||||
| No | Referent | Referent | ||
| Yes | 1.31 (1.13, 1.51) | 0.0003 | 1.26 (0.96, 1.65) | 0.0960 |
| Not sure | 1.26 (0.87, 1.82) | 0.2162 | 1.80 (1.03, 3.15) | 0.0396 |
| History of diabetes | ||||
| No | Referent | Referent | ||
| Yes | 0.87 (0.75, 1.003) | 0.0543 | 0.79 (0.62, 0.997) | 0.0467 |
| Not sure | 0.61 (0.38, 0.99) | 0.0445 | 0.84 (0.43, 1.65) | 0.6183 |
| Housing situation | ||||
| Own house or apartment | Referent | Referent | ||
| Relative or friend's apartment or house | 1.22 (1.03, 1.45) | 0.0229 | 1.29 (0.97, 1.71) | 0.0783 |
| Nursing home, group home, or long‐term care facility | 1.42 (1.16, 1.74) | 0.0006 | 1.74 (1.27, 2.40) | 0.0007 |
| Homeless shelter | 1.12 (0.72, 1.73) | 0.6204 | 0.87 (0.46, 1.63) | 0.6559 |
| Other/Don't know | 1.02 (0.75, 1.40) | 0.8987 | 1.35 (0.78, 2.36) | 0.2859 |
| Age Group | ||||
| <50 | Referent | Referent | ||
| 5059 | 1.19 (0.99, 1.43) | 0.0647 | 1.18 (0.88, 1.59) | 0.2583 |
| 6069 | 1.18 (0.99, 1.40) | 0.0585 | 1.20 (0.88, 1.66) | 0.2549 |
| 7079 | 1.10 (0.91, 1.33) | 0.3178 | 1.19 (0.85, 1.67) | 0.3033 |
| 8089 | 1.23 (1.03, 1.47) | 0.0207 | 1.34 (0.96, 1.88) | 0.0879 |
| 90+ | 1.45 (1.12, 1.88) | 0.0045 | 1.44 (0.94, 2.20) | 0.0934 |
| Site of Enrollment | ||||
| A | Referent | Referent | ||
| B | 1.74 (1.16, 2.61) | 0.007 | 4.95 (2.90, 8.45) | < 0.0001 |
| C | 5.14 (3.42, 7.74) | < 0.0001 | 26.36 (17.28, 40.23) | < 0.0001 |
| D | 4.19 (2.64, 6.66) | < 0.0001 | 8.06 (4.63, 14.03) | < 0.0001 |
| E | 3.00 (1.82, 4.9) | < 0.0001 | 5.30 (2.71, 10.38) | < 0.0001 |
| F | 4.09 (2.69, 6.23) | < 0.0001 | 2.32 (1.32, 4.08) | 0.0037 |
In the entire cohort, insurance type was independently associated with likelihood of a care discussion, with patients with Medicare having greater adjusted odds ratio for a CD than patients with all other forms of insurance, even after adjusting for age. Patients who had functional limitations with bathing, toileting, and feeding; had a documented surrogate decision maker; were unable to participate in their care; had cancer; or did not live in their own home were more likely to have a documented CD. Subjects with diabetes were less likely to have a CD, although this was of borderline significance. Patients whose team had documented a CD with the patients' primary physician were also more likely to have a discussion noted. However, the magnitude of these predictors was small compared to the independent effects attributable to the site the patient was enrolled or whether the patient had any preexisting documentation. Whereas the adjusted odds ratio associated with clinical or functional measures (such as age, cancer) were generally between 1.5 and 2.5, the range of odds ratios associated with having any documentation of care wishes (compared to no documentation) were all greater than 3, and the odds ratios associated with site of enrollment were 1.7 or higher.
We observed similar findings in analyses limited to patients with no preexisting care documentation. While clinical, sociodemographic, and functional factors remained statistically associated with a CD (albeit with wider confidence intervals due to smaller sample sizes), the effect of the patient's site of enrollment became even more striking (Table 4).
DISCUSSION
In this multicenter study of hospitalized general medical patients, documentation of CDs were highly dependent on where patients received care and whether patients had previous documentation of a care plan. In contrast, although clinical, prognostic, and socioeconomic factors were also associated with whether physicians documented asking patients about their wishes for care, the influence of these factors was modest.
Improving communication between patients and their physicians during an episode of acute illness has been a long‐standing goal, with the Study to Understand Prognoses and Preferences for Outcomes of Treatment (SUPPORT) trial providing the most notable example of an effort to improve patient care through aligning patient wishes, prognosis, and aggressiveness for care. However, even the SUPPORT interventiona robust, well‐implemented, and highly labor‐intensive strategywas not able to achieve this goal. In their summary of SUPPORT study findings, the authors suggested that the likelihood of and effectiveness of communication in seriously ill patients may be powerfully influenced by patient and caregiver culture4; our findings may partially confirm SUPPORT's conclusions.
Preexisting documentation in our study would not have included mandated documentation that someone had given the patient information about advance directives (as mandated by the PSDA), but rather a specification for that advance care plan. This distinction means that preexisting documentation in our study represented a previous decision by the patient (or the patient and their physician) to have made a plan, and an association with hospital discussions may be because the first conversation is the hardest to undertake; subsequent discussions then represent confirmatory or clarifying discussions that may be less difficult to broach (particularly for less experienced trainees). A CD may have also been prompted when documentation was unclear, or when a change in prognosis took place (eg, a new diagnosis of metastatic cancer).22 Alternatively, a preexisting plan may serve as a reminder for clinicians to discuss code status, signify patients who are more willing to broach this subject, and either seem more approachable or bring up the topic themselves.
The influence of site on documentation and CD provides additional evidence that caregiver culture played a role in CDs. Although this variation may have been in part due to culture around documentation practices more generally, it is important to note that none of our participating centers had a policy for documentation of care wishes or patient‐doctor communication, or a policy mandating these discussions in any specific patient group. Furthermore, site‐related differences were seen even in patients with no preexisting documentation, and were seen after adjustment for other documentation or communication practices (eg, documenting a discussion with the patient's primary care provider), making it unlikely that documentation practices are solely responsible for our results. Persistence of variations in care documentation raises interesting questions, particularly when one considers recent data describing variations in end‐of‐life care between similar academic centers (one of which was a participating site in this trial).23 Given that the sites in our study represent diverse institutions yet share a number of characteristics, understanding the specific practices or aspects of medical culture that promote conversations may provide insights in how to improve this promotion elsewhere.
Our results would argue that mandates to document code status on admission may be unlikely to improve communication unless sites also develop an approach to using this newly documented information as a prompt for subsequent discussions. In nursing home settings, documentation of advance directives may reduce resource use, but it is unclear whether similar effects will be seen in hospital settings.24 It is also a challenge to insure that documentation of a care plan in the nursing home is communicated to the providers in the hospital.25 The PSDA was a first step in this direction, but its effects on improving communication are uncertain.26 Our results would confirm that the PSDA or systems to mandate documentation are not solutions in themselves, but are 2 steps in a larger process.
We do not want to discount our findings of less frequent CDs among patients of lower socioeconomic status, where gaps in quality of care, communication, and outcomes are well‐recognized.27 As such, our results delineate yet another area where practice can and should be improved for vulnerable patients. However, factors related to site of care and documentation may provide opportunities to improve care even more profoundly and within a fairly discrete (if complex) acute episode of care. Having said this, our results also demonstrate a potential pitfall of using code status documentation for risk‐adjustment, because such notation may be more dependent on local documentation patterns than clinical appropriateness.
Our study has a number of limitations. As an observational study, our findings are likely prone to biases related to unadjusted confounding due to comorbidity. The influence of comorbidity would seem to have been most important in biasing the effects of preexisting documentation, where documentation would be associated with more unaccounted comorbidity. However, there were no differences in documentation even after accounting for prognosis by adjusting for age, functional status, and a valid comorbidity score.28 As we have pointed out, our key outcome is based on documentation of communication and not actual communication, and as such may be biased in subtle ways not related to site of care or the items tested in our model. While we cannot directly eliminate the possibility of documentation biases in our results using statistical methods, it is important to point out that our chart abstraction protocol used highly specific criteria to detect these discussions, and therefore may under‐detect discussions which may have been documented in less detail. Our study did not examine whether documentation of CDs influenced subsequent care. However, previous studies have shown that advance care planning has only a minor influence on care.29 However, communication about preferences at the time of admission, when the need for specific care decisions may be more evident, may be more likely to influence hospital care. Our results show that previous documentation is associated with discussions early in an admission. Such discussion may affect care, even if the decision made is different than what was previously documented. In addition, patients who were included in our study (those able to provide consent and participate in an interview) may be healthier or more cognitively intact than a general population of hospitalized patients. However, how this would have affected our results is unclear. Being able to speak and consent for oneself are key facilitators to communication, but sicker patients who cannot consent or speak for themselves might also be more likely to have care planning decisions made based on illness severity; documentation in these patients may be more driven by whether such notes were required because of the involvement of home health services (or skilled nursing facilities). Finally, although our study is one of the largest examinations of in‐hospital communication to date and its implications for resident education are worth noting, the sites involved in the MCHS may not be representative of nonteaching hospitals, or community‐based teaching hospitals.
Our results suggest that, although comorbid illness and socioeconomic status play an important role in determining which patients receive CDs at the time of admission, these factors are substantially less powerful than preexisting documentation practices and culture or care practices specific to their site of care. These results suggest that future work should consider organizational characteristics and culture as important targets for interventions to improve care planning in hospitalized patients.
- Committee on Care at the End of Life, Institute of Medicine.Approaching Death: Improving Care at the End of Life.Field MJ,Cassel CK, eds.Washington, DC:National Academy Press;1997.
- ,,,,,.Factors considered important at the end of life by patients, family, physicians, and other care providers.JAMA.2000;284(19):2476–2482.
- ,,,,,.In search of a good death: observations of patients, families, and providers.Ann Intern Med.2000;132(10):825–832.
- The SUPPORT Principal Investigators.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).JAMA.1995;274(20):1591–1598.
- ,.Choices about cardiopulmonary resuscitation in the hospital. When do physicians talk with patients?N Engl J Med.1984;310(17):1089–1093.
- ,,, et al.Patient preferences for communication with physicians about end‐of‐life decisions. SUPPORT Investigators. Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment.Ann Intern Med.1997;127(1):1–12.
- ,,,.Discussing cardiopulmonary resuscitation: a study of elderly outpatients.J Gen Intern Med.1988;3(4):317–321.
- ,,,,.Educating the elderly: cardiopulmonary resuscitation decisions before and after intervention.J Am Geriatr Soc.1991;39(4):372–377.
- ,,,.Factors influencing physicians in recommending in‐hospital cardiopulmonary resuscitation.Arch Intern Med.1993;153(17):1999–2003.
- Federal Register. 42 USC 1395‐1396. Patient Self‐Determination Act1990.
- ,,.Advance directives on admission. Clinical implications and analysis of the Patient Self‐Determination Act of 1990.JAMA.1991;266(3):402–405.
- ,,.A new doctor in the house: ethical issues in hospitalist systems.JAMA.1999;282(2):171–174.
- ,,,,,.Implementation of a hospitalist service at a community teaching hospital: improving clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of hospitalist physicians on an academic general medical service: results of a randomized trial.Ann Intern Med.2002;137:866–874.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,,.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279(19):1560–1565.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288(17):2151–2162.
- ,,, et al.Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery.JAMA.1999;281(14):1310–1317.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(Suppl 1):141–142.
- ,,.SF‐12: How to Score the SF‐12 Physical and Mental Health Summary Scales.2nd ed.Boston, MA:New England Medical Center, The Health Institute;1995.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, processes of care, and patient symptoms.Am J Med.2004;116(10):669–675.
- ,,,.Role of written advance directives in decision making: insights from qualitative and quantitative data.J Gen Intern Med.1998;13(7):439–446.
- ,,,,.Evaluating the efficiency of California providers in caring for patients with chronic illnesses.Health Aff (Millwood).2005 Jul‐Dec;Suppl Web Exclusives:W5–526–43.
- ,,, et al.Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial.JAMA.2000;283(11):1437–1444.
- ,.Meeting palliative care needs in post‐acute care settings: “to help them live until they die”.JAMA.2006;295(6):681–686.
- ,,, et al.Advance directives for seriously ill hospitalized patients: effectiveness with the patient self‐determination act and the SUPPORT intervention. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment.J Am Geriatr Soc.1997;45(4):500–507.
- Institute of Medicine.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care.Smedley BD,Stith AY,Nelson AR, eds.Washington, DC:National Academies Press;2003.
- ,,.Use of a self‐report‐generated Charlson Comorbidity Index for predicting mortality.Med Care.2005;43(6):607–615.
- ,,.Can clinical interventions change care at the end of life?Ann Intern Med.1997;126(5):381–388.
- Committee on Care at the End of Life, Institute of Medicine.Approaching Death: Improving Care at the End of Life.Field MJ,Cassel CK, eds.Washington, DC:National Academy Press;1997.
- ,,,,,.Factors considered important at the end of life by patients, family, physicians, and other care providers.JAMA.2000;284(19):2476–2482.
- ,,,,,.In search of a good death: observations of patients, families, and providers.Ann Intern Med.2000;132(10):825–832.
- The SUPPORT Principal Investigators.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).JAMA.1995;274(20):1591–1598.
- ,.Choices about cardiopulmonary resuscitation in the hospital. When do physicians talk with patients?N Engl J Med.1984;310(17):1089–1093.
- ,,, et al.Patient preferences for communication with physicians about end‐of‐life decisions. SUPPORT Investigators. Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment.Ann Intern Med.1997;127(1):1–12.
- ,,,.Discussing cardiopulmonary resuscitation: a study of elderly outpatients.J Gen Intern Med.1988;3(4):317–321.
- ,,,,.Educating the elderly: cardiopulmonary resuscitation decisions before and after intervention.J Am Geriatr Soc.1991;39(4):372–377.
- ,,,.Factors influencing physicians in recommending in‐hospital cardiopulmonary resuscitation.Arch Intern Med.1993;153(17):1999–2003.
- Federal Register. 42 USC 1395‐1396. Patient Self‐Determination Act1990.
- ,,.Advance directives on admission. Clinical implications and analysis of the Patient Self‐Determination Act of 1990.JAMA.1991;266(3):402–405.
- ,,.A new doctor in the house: ethical issues in hospitalist systems.JAMA.1999;282(2):171–174.
- ,,,,,.Implementation of a hospitalist service at a community teaching hospital: improving clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of hospitalist physicians on an academic general medical service: results of a randomized trial.Ann Intern Med.2002;137:866–874.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,,.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279(19):1560–1565.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288(17):2151–2162.
- ,,, et al.Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery.JAMA.1999;281(14):1310–1317.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(Suppl 1):141–142.
- ,,.SF‐12: How to Score the SF‐12 Physical and Mental Health Summary Scales.2nd ed.Boston, MA:New England Medical Center, The Health Institute;1995.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, processes of care, and patient symptoms.Am J Med.2004;116(10):669–675.
- ,,,.Role of written advance directives in decision making: insights from qualitative and quantitative data.J Gen Intern Med.1998;13(7):439–446.
- ,,,,.Evaluating the efficiency of California providers in caring for patients with chronic illnesses.Health Aff (Millwood).2005 Jul‐Dec;Suppl Web Exclusives:W5–526–43.
- ,,, et al.Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial.JAMA.2000;283(11):1437–1444.
- ,.Meeting palliative care needs in post‐acute care settings: “to help them live until they die”.JAMA.2006;295(6):681–686.
- ,,, et al.Advance directives for seriously ill hospitalized patients: effectiveness with the patient self‐determination act and the SUPPORT intervention. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment.J Am Geriatr Soc.1997;45(4):500–507.
- Institute of Medicine.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care.Smedley BD,Stith AY,Nelson AR, eds.Washington, DC:National Academies Press;2003.
- ,,.Use of a self‐report‐generated Charlson Comorbidity Index for predicting mortality.Med Care.2005;43(6):607–615.
- ,,.Can clinical interventions change care at the end of life?Ann Intern Med.1997;126(5):381–388.
Copyright © 2008 Society of Hospital Medicine
Discussing Resuscitation Preferences
Mrs. G is a 58‐year‐old woman with metastatic pancreatic cancer, diagnosed 8 months prior to admission when she presented to her primary care doctor with abdominal pain and weight loss. The cancer was locally advanced and metastatic to the liver at the time of diagnosis, and she underwent first‐line palliative chemotherapy with gemcitabine without objective or symptomatic improvement. She is now admitted to the hospitalist service with intractable abdominal pain, uncontrolled on her outpatient doses of opioids. On the day after admission, her pain is well controlled on morphine via patient‐controlled anesthesia. The hospitalist decides to talk with her about her about resuscitation status. At this point, she is full code.
Dr. S sits down with her and says, There's something I need to talk with you about that I talk with all of my patients about. And that is, if your heart were to stop, and mind you, I'm not expecting this to happen anytime soon, do you want us to do everything possible to bring you back?
Dr. S pauses to catch his breath, and then Mrs. G says, Well, I guess so, if you thought you could bring me back.
He continues, We are not always successful at bringing people back, but let me explain what this would entail: we could do chest compressions, administer shocks to your heart, put a breathing tube down into the back of your throat, hook you up to a machine that helps you breathe
Mrs. G glances up with a confused look. Dr. S says, You know, Mrs. G, I've started this conversation all wrong. I'm going to step out for a few minutes, collect my thoughts, and come back to talk with you again. Is that OK? Mrs. G smiles and says, Of course.
The doctor reenters the room about 20 minutes later, pulls up a chair next to her bed, and in a relaxed tone of voice says, Tell me what you understand about your illness.
Mrs. G says, I understand that I have cancer in my pancreas and liver and that I may not last very long, perhaps months if that. My oncologist offered me more chemotherapy, but I decided against it because it didn't seem that it would offer me much. And the first chemotherapy was really hard.
Dr. S then asks, As you look ahead, what worries you most?
Mrs. G replies, I really worry that I will die in terrible pain. That scares me terribly. I also worry about my husband and how he's going to cope with my worsening condition and with my death, let alone my pain.
Dr. S then says, That sounds really frightening. Tell me more about that. She continues, Well, I really want to spend the time I have left with my husband and familymy children and grandchildrenand I want to have some good time with them. But I keep worrying that it won't be able to be that way. She starts crying. You know, I'm so scared about going home and being in terrible pain again and having my husband and family be terrified
She pauses for a moment. The doctor doesn't say anything.
She starts up again, But I don't want to end up in the hospital again.I don't want to end up on machines like my mother‐in‐law did. She looks up at Dr. S, expecting a response.
Dr. S takes her hand and says, You know, I realize this is not easy to talk about, but it's really important for me to hear about your worries so that I can know how to help. The good news is we have many good methods to control your pain and that we can help you to be comfortable and to remain at home.
Thank you. Thank you so much.
As these conversations illustrate, discussing preferences regarding resuscitation is a challenging and important task for physicians. Understanding patients' wishes at the end of life allows clinicians to provide the type of care patients want, to avoid unwanted interventions, and to promote patient autonomy and dignity. Despite the frequency with which physicians have these conversations, they typically fall short when discussing code status with patients. Clinicians fail to address patients' concerns, monopolize conversations, and commonly misunderstand patients' resuscitation preferences.13 Often these discussions do not occur at all; more than 70% of seriously ill patients have never discussed advance directives with their physician.2, 4 The multicenter SUPPORT study, which demonstrated serious problems in the care of seriously ill hospitalized patients, documented that only 47% of physicians knew when their patients preferred do‐not‐resuscitate status.5
Hospitalists frequently conduct resuscitation discussions. Patients who are admitted to the hospital are usually seriously ill, and hospitalists need to assess rapidly whether an individual patient would want a resuscitation attempt if he or she had a cardiopulmonary arrest in the hospital. They need to build trust and rapport quickly with patients they have never met. Despite this challenge, hospitalists are in a good position to discuss resuscitation preferences.6 Patients may be more willing to discuss these issues in inpatient rather than outpatient settings because their acute deterioration may encourage self‐reflection.6 Furthermore, the time and productivity pressures of office practice often make it difficult for primary care physicians, who often know the patient and family best, to address advance directives. Although studies have documented that patients are interested in talking to their primary physicians about these matters,7, 8 these conversations do not occur with regularity. Preliminary research has raised the possibility that cancer patients may actually prefer to discuss these issues in an inpatient setting with a hospital‐based provider rather than with their oncologists.9 Studies have not addressed the question of whether patients with diseases other than cancer would prefer to discuss these issues with a hospitalist or their outpatient subspecialist.
Given that more than half of all Americans die in hospitals, hospitalists care for many people who are terminally ill and will need to assess preferences for cardiopulmonary resuscitation (CPR) and other treatments. Hospitalists need to be competent and compassionate in their approach to patients and their families. In this articles we review clinician barriers to holding these conversations, offer a variety of approaches to enhance these discussions, and review communication techniques that can be used to improve understanding.
Clinician Barriers to Discussing Resuscitation
Clinicians' own barriers may lead to infrequent and inadequate conversations about resuscitation. Understanding these barriers may allow providers to overcome them and facilitate better and more frequent communication. A discussion of patient barriers is beyond the scope of this article.
Unresolved Feelings about Death and Dying
When discussing resuscitation, or code status, physicians are discussing the possibility of death with a patient. In the first scenario above, the clinician lists the many procedures that could be done if the patient's heart were to stop without using the words die or death. The clinician never explicitly acknowledges that the patient has a serious illness that could lead to her death. Medical culture is focused on cure and on warding off death until the last possible moment. Because clinicians work in this culture, many have unresolved feelings of personal failure that are triggered when treating a dying patient.10 Also, the death of a patient can lead to anxieties about the clinician's own mortality and raise uncomfortable feelings of loss, related to the patient or to memories of deaths of the clinician's loved ones. In an attempt to avoid these feelings, whether conscious or not, physicians may resist talking to patients about death.10
Fear of Taking Away Hope
Clinicians fear that patients will lose hope if they are too honest about prognosis and acknowledge the inevitability of death. This concern may be true for a small minority of patients with advanced terminal illness who are solely focused on continued treatment and in such denial that they never consider the possibility of death. Most patients, however, understand on some level that they are getting sicker and may die, but expect clinicians to initiate discussions about death and dying.8, 11 Clinicians should understand that patients can have hope about many things beyond cure of their illness. For example, they can hope for good control of their symptoms so they can spend meaningful time with family and friends, heal troubled relationships, create a legacy, and say good‐bye. As in other developmental stages throughout life, the process of dying can be a time of emotional and spiritual growth and provide an opportunity to deepen relationships and find greater meaning.12 Despite their fears, physicians are much less likely to take away hope than they think. In fact, they can carry out the important actions of helping patients to refocus on more attainable goals and helping to return hope to what may be perceived by all as a hopeless situation.13, 14
Inadequate Training
There have been many educational interventions in both outpatient and inpatient settings to encourage physicians to discuss advance directives with patients.1521 In most of these studies, clinicians were sent reminders, but did not receive training or feedback to improve their communication skills. Although these interventions have led to modest increases in the number of advance directives, little is known about the quality of the conversations between clinician and patient.
There are acknowledged deficits in undergraduate22 and graduate medical education in discussing preferences and goals of care with patients.23, 24 A national survey of medical education deans showed that two‐thirds believed insufficient time was given to palliative care education including communication skills.25 Reflecting this lack of training, medical students and residents feel unprepared to take care of dying patients.26 In one survey, hospitalists reported that although palliative care was very important to their practice, they had not received enough training in palliative care knowledge and skills.27
Traditional Ethical Frameworks
Another difficulty arises from myths about the ethical perspectives that inform medical decision making and obtaining informed consent. Although these perspectives highlight the importance of patient autonomy and the right of the individual to choose medical treatments, they do not require physicians to describe every possible treatment if, in the clinician's judgment, a particular treatment would not benefit a patient. Physicians do have an obligation to use their medical knowledge and judgment in offering treatments and discussing side effects.28 In an attempt to honor a patient's autonomy when discussing advance directivesand possibly out of fear of coercing patientsclinicians sometimes offer a menu of treatment options without exploring the patient's underlying goals for these treatments. This approach can become meaningless out of context if the patient does not understand the probability that these interventions will work or the interrelatedness of the interventions. For example, when given a list of choices regarding resuscitation, a patient may say, I would like chest compressions and a chest tube, but no shocks please. Such a statement makes little sense clinically. Instead, physicians should have meaningful conversations with their patients in which they describe treatment options in the context of patient goals and values and help patients come to decisions in a shared process.
Practical Concerns
Outpatient providers find that time constraints and the competing demands that occur in caring for patients with multiple chronic health problems make it difficult to discuss advance directives.29 Hospitalists are also subject to productivity pressures and may feel similarly stressed for time. Outpatient providers spend about 5 minutes on each of these conversations,1 and medicine residents in the inpatient setting spend about 10 minutes.30 However, many of the conversations studied were inadequate; thus, it is unknown how long it takes to have an effective conversation. Hospitalists should keep in mind that they need not have these discussions every day with each patient and that having these conversations may end up saving time in the long run if they have a clearer sense of a patient's wishes and goals.
Laying Groundwork for the Discussion
The decision regarding resuscitation should be seen in the context of the patient's goals and values and overall health status. To address code status effectively, it is imperative first to elicit the person's view of his/her illness and then gently correct any misunderstandings. A patient who thinks her/his life will go on indefinitely may feel no need to consider her/his own mortality or alternatives to full resuscitation status. Alternatively, a patient who senses his/her mortality may have already thought about resuscitation and have clear preferences. A key first step in the conversation is to understand a patient's values and goals and comprehension of his/her illness. As in the second discussion above, a clinician can begin a discussion by saying, Tell me what you understand about your illness.
Discussing prognosis with patients can be difficult as physicians struggle with uncertainty. In the most comprehensive study to date of prognostication, physicians overestimated patient survival on average by a factor of 5.31 Nonetheless, there are compelling reasons to discuss prognosis. Failure to do so often results in patients spending their last days in the hospital receiving more aggressive treatments than they might choose if they understood their prognoses.32 Further, patients are denied the opportunity to address issues of life closure, such as spending time with family, thinking about legacy, and settling financial affairs. Physicians also fear they will take away hope with prognostication and believe patients expect greater accuracy than they can provide.33
Physicians can improve their prognostication skills by considering patients' functional status and clinical signs and symptoms and by using validated scales. The Karnofsky Performance Score (KPS) and the Eastern Cooperative Oncology Group (ECOG) Performance Status have been shown to correlate with survival,3440 and the Palliative Prognostic Score (PaP) has been validated in both cancer and noncancer patients.41, 4446 The PaP uses a combination of the KPS, clinical signs and symptoms, and the clinician's clinical prediction of survival. In addition, clinicians can be honest with patients about prognostic uncertainties and give prognoses in ranges, such as days to weeks, or weeks to months.
How to Broach the Subject of Resuscitation
In the first scenario presented in this article, the hospitalist says, There's something I need to talk with you about that I talk with all of my patients about. Although many clinicians begin resuscitation discussions this way, the question is problematic because often it is untrue. Most clinicians do not discuss code status with all patients who are admitted to the hospital. A better option would be to say, When I take care of patients with advanced cancer, I like to talk with them about their wishes regarding resuscitation. Is that all right? Better yet would be to ask a general question such as As you look ahead, what worries you most? or As you look to the future, what do you hope for? These and other useful questions appear in Table 1. These questions allow patients to bring up their concerns, show that the clinician cares about them, and often segue into a discussion of patients' hopes and worries about their own death and dying process. These questions often allow patients to bring up important goals that bear directly on the issue of resuscitation. For example, in the second scenario at the beginning of the article, the patient says she wants to have quality time with her family at home and does not want life‐sustaining technologies. Such a patient may not want resuscitation. When discussing CPR, it can also be helpful to state explicitly that resuscitation is used when a patient has died, rather than to use euphemisms such as, If your heart were to stop. The clinician can ask explicitly, If you were to die, would you want? There are other strategies for introducing the subject of resuscitation if these questions do not work. If a patient seems uncomfortable with the conversation, the clinician can address this discomfort directly by saying, This conversation seems to make you uncomfortable. Other strategies for exploring these issues include inquiring if the person has ever discussed resuscitation with his/her family or another physician, or asking if anyone else in the family has been very sick. Additionally, clinicians can ask questions about surrogate decision making. If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? This can then lead into a discussion of whether the patient has spoken to that person about his/her preferences, and if so, what was the content of that conversation. Another useful question is, Is there any state that would be worse than death for you? This question focuses on outcomes and allows the physician to put the issue of resuscitation into perspective for a patient.
| When I take care of patients with advanced cancer [or heart disease or lung disease, etc.], I like to talk with them about their wishes for care if they were to get very sick and even die suddenly. Is that all right? |
| As you look ahead, what worries you most? |
| As you look to the future, what do you hope for? |
| Has a close friend or family member ever been really sick? |
| If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? |
| Is there any state that would be worse than death for you? |
Knowing the Facts about Cardiopulmonary Resuscitation
In general, about 1 in 7 patients who have undergone CPR in the hospital survive to hospital discharge. Two literature reviews, from 1989 and 1998, of hospitalized patients who underwent CPR in the hospital reported surprisingly similar statistics. Immediate survival in both series was 41%, and survival to hospital discharge was 13%‐14%. Factors that increased survival included arrest due to coronary artery disease or drug overdose/adverse reaction, and location of arrest in the intensive care unit (ICU). Factors that decreased survival included sepsis at time of arrest, cancer, whether localized or metastatic, dementia, acute renal failure, bed‐bound status, and residence in a nursing home. Neither age nor sex was associated with survival.47, 48 In a meta‐analysis of outcome studies of CPR in metastatic cancer patients, none of 117 patients survived to hospital discharge.49
Most people get their information about CPR survival rates from the mass media, which portray CPR as a very successful procedure. For instance, on television, the sole source of information on CPR for most patients, the rate of survival to hospital discharge is 64%, much higher than the 1314% cited in the medical literature.50 Thus, it is no surprise that a patient with metastatic cancer or another life‐limiting illness would assume a positive outcome with CPR.
Knowledge of the facts about CPR survival rates is key when a physician discusses code status with a patient because these data influence patients' decisions. Patients who have a realistic understanding of their chances are less likely to prefer resuscitation.51, 52 Offering patients information about the success rate for CPR in their particular situation is critical in helping them reach a decision consistent with their values and goals. This information can be given quantitatively or qualitatively depending on the clinician assessment of what the patient would prefer.
Avoiding Stark Dichotomies in Resuscitation Discussions
In clinical practice there are 3 ways in which physicians can present resuscitation decisions as black and white and ignore the shades of gray. First, clinicians may present the choice between resuscitation and do not resuscitate (DNR) as a choice between life and death. In the first scenario above, the physician states If your heart were to stop, do you want us to do everything to bring you back? implying he would be able to save the patient's life with resuscitation attempts. When discussing resuscitation, clinicians should avoid language that suggests such a stark dichotomy. The reality is that most patients die despite resuscitation attempts. In fact, a patient is actually choosing between certain death (without resuscitation) and likely death (if resuscitation is attempted). For a patient with a serious, life‐limiting illness, it may be more effective to frame the conversation in terms of how that person envisions the end of life, and not whether death will eventually occur.
Second, clinicians and patients sometimes equate DNR with doing nothing or giving up. Clinicians fail to discuss other treatment options or alternatives, such as continuing ongoing aggressive medical treatments with DNR status or pursuing palliative care. Performing resuscitation is equated with activity and treatment, whereas withholding resuscitation is seen as passivity and giving up. To the patient, this can feel like abandonment, as if the doctor is withholding a treatment and not offering anything in its place. Examples of positive phrases that demonstrate the physician will continue to offer excellent care include: We will continue maximal medical therapy. However, if you die despite these treatments, we will let you die peacefully and won't attempt to revive you; We'll continue the intravenous antibiotics, but we won't plan to move you to the ICU if things worsen53; and We will work hard to treat your pain and other symptoms and to get you home. In addition, hospitalists must ensure, when signing out to physician colleagues, nurses, respiratory therapists, and others, that DNR orders are not overinterpreted to mean no treatment. Although a DNR order states that in the event of a cardiac arrest, no attempt at resuscitation will be made, it should have no bearing on other appropriate and desired interventions, including antibiotics, chemotherapy, and artificial nutrition; treatment for pain and other symptoms; and even monitoring in an ICU. This misunderstanding of DNR status is common among health care providers and has led many to argue that DNR orders should be part of a more comprehensive treatment plan that outlines where the patient's wishes for treatment fall on the spectrum from otherwise aggressive measures to comfort care.54, 55 Physicians who have a clear understanding of a DNR order will be able to reassure a patient that they will continue to receive desired care, but that if the patient dies, no attempt to resuscitate will be made.
Third, it is important to remind patients who choose full resuscitation status that additional decisions will need to be made if resuscitation is successful. Most patients who survive cardiopulmonary arrest end up worse off clinically and spend time in an ICU with life‐support measures in place, such as mechanical ventilation and vasopressors. Even if they survive, there will likely be a period during which they are unable to speak for themselves. This situation puts the burden of decision making on their surrogates or an appointed durable power of attorney for health care (DPOA‐HC). It is important to ask patients ahead of time whether there are conditions under which they would not want ongoing life‐sustaining measures. For example, a person might opt for discontinuation of life‐support measures if the physician and family agreed that there was only a minimal chance of cognitive recovery existed and that ongoing support was only prolonging inevitable death. To clarify the patient's wishes in this situation, you might ask, Are there conditions that would be worse than death? Encouraging the patient to share his/her wishes in this situation with a surrogate or DPOA‐HC will help to ensure those wishes are respected.
Communication Techniques
When discussing advance directives, it is important to give patients the chance to describe their life goals and their values to establish a context for understanding the role of life‐sustaining treatments. One useful method to elicit these goals and values is to ask open‐ended questions, followed by periods of silence so the patient has time to express himself/herself. In the second scenario, above, the physician used open‐ended questions in several instances: Tell me what you understand about your illness and As you look ahead, what worries you most? Tulsky and colleagues documented that medical residents spoke 76% of the time in discussions with patients about code status.30 In an ideal case, this ratio should be reversed or at least be even, allowing patients the time to explain their thoughts. Acknowledging patients' emotions by stating simply, You seem [angry/sad/perplexed], and waiting for an answer can help patients feel they are being understood. Making empathic statements is another powerful communication technique that conveys understanding.56 Examples include, That must be really sad for you, and It must be frightening to be in so much pain. As noted, silence can also be a powerful tool. Clinicians tend to be uncomfortable with silence and so fill the gaps with words. Allowing for silence enables patients to digest what they have heard, encourages them to continue speaking, and shows them the clinician wants to hear what they have to say. When giving information about any medical issues and especially about CPR, it is important to explain concepts in lay terms and to avoid medical jargon.57 Additionally, nonverbal communication techniques such as making eye contact, head nodding, and leaning in toward the patient all help in communicating engagement in the conversation. Having the conversation in a quiet and private place and sitting at the same level as the patient or family is also important. It is always a good idea to check in with patients to assess their understanding. Simply asking, Do you have any questions about what I said? or Does that all make sense? gives patients the opportunity to ask for clarification. Attempting to summarize what a patient has said can also help to clarify misunderstandings. Useful phrases include, Let me see if I've gotten this right or I want to make sure that I understood what you're telling me, followed by the clinician's synopsis of important points discussed.58 A summary of important communication techniques can be found in Table 2.
| Ask open‐ended questions followed by periods of silence |
| Tell me what you understand about your illness. |
| As you look ahead, what worries you most? |
| Acknowledge emotion |
| You seem [angry/sad/perplexed]. |
| Make empathetic statements |
| That must be really hard for you. |
| It must be terrible to be in so much pain. |
| Use nonverbal communication techniques such as eye contact, head nodding, leaning in toward the patient, sitting down, and sitting at patient's level |
| Allow for silence |
| Assess patient's understanding |
| Do you have any questions about what I said? |
| Does that all make sense? |
| Confirm your own understanding |
| Let me see if I've gotten this right. |
| I want to make sure I understand what you've been telling me. |
| Avoid medical jargon |
| Use the I wish statement |
| I wish there were more chemotherapy we could give you that would make a difference. |
| Use the Hope for the best, prepare for the worst statement |
| I think we should hope that the chemotherapy works but prepare for the possibility that it might not. |
There are 2 additional statements that can be very useful when patients and families are struggling with the reality of severe illness and are still hoping for longer life and cure. The first is the I wish statement, in which the clinician allies himself or herself with the patient's or family's wishes by stating, I wish it were different. I wish there were more chemotherapy we could give you that would make a difference.59 Occasionally, when tension is developing because the clinician does not believe an intervention is warranted but the patient desires it nonetheless, the I wish statement can be a powerful way of realigning with the patient. For example, responding to a patient who says, I want chemo to cure my cancer, with the statement There is no chemotherapy to help you can seem antagonistic. In contrast, saying, I wish there were a treatment that would make your cancer go away aligns the clinician with the patient and supports the patient. Another advantage of I wish statements is that they are truethe clinician does wish that there was an effective treatment and would gladly provide it if available. In general, I wish statements are more effective than I'm sorry statements, because the latter can be interpreted as the clinician taking responsibility for the situation. When a provider says, I'm sorry to give you this bad news, the patient may feel the need to say, That's OK. On the other hand, saying, I'm so sorry that your mother is dying, is very human and unlikely to be interpreted as the clinician taking responsibility for the death.
A similar technique is to use the statement Hope for the best, prepare for the worst when speaking with patients and families.60 For example, a physician could say, I really wish your mother could get better, and we should still hope for that; at the same time, we need to prepare for what will happen if she doesn't get better. Once again, this phrase both allows the patient or family to continue hoping things will improve and the clinician to support this hope, while simultaneously beginning the process of planning for the more likely outcome. Over time, the patient and family often move toward accepting that the patient is dying. Finally, trying to help the patient or family maintain hope in the face of illness and death is challenging but important: If your mother can't get better, are there other things you can hope for? Helping to identify tangible and realistic goals, such as being free from pain, seeing an important family member one last time, or getting home can provide hope at a difficult time.
Giving a Recommendation
Most patients with serious illness and their families want help making complex and ethically charged decisions. When clinicians ask patients to make decisions unilaterally, patients often feel anxious, sometimes for weeks.61 Families are often paralyzed when faced with the very difficult decision of whether to withdraw life‐sustaining interventions from a family member with an advanced terminal illness. Even if they understand on an intellectual level that ceasing to provide potentially curative or life‐prolonging therapies is the best choice, they are not yet able to accept this decision on an emotional level and ultimately may feel responsible and then guilty for the patient's death. Physicians need to carry some of the burden of making these difficult decisions. One way to relieve family members of some of this guilt is to recommend a plan of care based on substituted judgment, that is, on what the patient said she or he wanted or what the family thinks the patient would have wanted.6264 In addition, clinicians should use their medical expertise, experience, and understanding of the situation to make recommendations. The patient or family can then accept or reject the physician's advice, which maintains patient autonomy, yet not have to explicitly instruct the clinician to withdraw or limit life‐sustaining interventions.
The preceding discussion and recommendations can guide scenarios like those presented at the beginning of this article. In the second conversation, the clinician had just told the patient that he could help her to achieve her goal of pain control and of returning and staying home.
Dr. S says, I want to make sure I've understood what you've said. To summarize, you've told me how important it is to you to have your pain controlled, to have some good time with your husband and family at home, and not to come back to the hospital. Is that right?
Mrs. G: Yes, that's right.
Dr. S: And how is your pain today?
Mrs. G: So much better. I'm hoping I can go home soon.
Dr. S: That should be possible. In the next day or so, we will be getting you back onto medications that you can take by mouth. But before you go home, we need to figure out how we can support you and your family at home: get you ongoing help with pain control and any other issues that come up and support for your husband and family as well.
Mrs. G: Yes, my family really needs support.
Dr. S: Have you had any experience with hospice before? I'm thinking that that would be the best way to get you the support you and your family need.
Mrs. G: Yes. When my sister died, she had home hospice. They were very good and helpful, especially to her husband, my brother‐in‐law. Yes, I would like that, as I just don't want to come back to the hospital. Hopefully, they can help my husband adjust to things.
Dr. S continues: I think they will help your husband a lot, both before and also after your death. He pauses.
Mrs. G: That's good. I worry about him so much.
Dr. S: As you know, one of the important goals of hospice care is to keep you at home so you don't have to return to the hospital. And when the time comes, to help you die peacefully at home. Mrs. G nods. What this also means is that they would not use CPR, that is, not do chest compressions, when you were actively dying.
Mrs. G: Yes, I want to be able to go home and be at peace. The last thing I would want is someone thumping on my chest as I was dying. She smiles.
Dr. S.: Do you have any other thoughts or questions?
Mrs. G: Well, yes. I'm wondering if we can set up a time to talk with my husband about all of this. I think it would be helpful for him to talk with you. Would that be possible?
Dr. S: I would be happy to talk with your husband. When is he coming in?
They negotiate a time.
Mrs. G: I want to thank you for taking the time to talk with me. I am really grateful.
CONCLUSIONS
The decision about cardiopulmonary resuscitation is part of a larger conversation with a patient about how she or he wants to spend the rest of his/her life. Importantly, the decision should be made in context, rather than in isolation. Given the understanding that develops between physician and patient in the conversation above, it is not necessary to describe all the specific treatments that occur during CPR because the physician has already established that the patient does not want to return to the hospital, and she understands that she has a terminal condition and is dying. Through exploring a patient's goals and values, a clinician can discover a patient's preferences for care generally and come up with a comprehensive plan that addresses the particular individual's medical, social, and emotional needs. For physicians, few interventions are as important or rewarding as relieving patients' suffering and helping them attain their goals during and at the end of life.
- ,,,.Opening the black box: how do physicians communicate about advance directives?Ann Intern Med.1998;129:441–449.
- ,,, et al.Physician understanding of patient resuscitation preferences: insights and clinical implications.J Am Geriatr Soc.2000;48:S44–S51.
- ,,,,.Patient knowledge and physician predictions of treatment preferences after discussion of advance directives.J Gen Intern Med.1998;13:447–454.
- ,,.The value of disease severity in predicting patient readiness to address end‐of‐life issues.Arch Intern Med.2003;163:609–612.
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).The SUPPORT Principal Investigators.JAMA.1995;274:1591–1598.
- ,,,,,.Can we talk? Inpatient discussions about advance directives in a community hospital. Attending physicians' attitudes, their inpatients' wishes and reported evidence.Arch Intern Med.1994;154:2299–2308.
- ,,,,.Advance directives for medical care—a case for greater use.N Engl J Med.1991;324:889–895.
- ,,.Patient attitudes to discussing life‐sustaining treatment.Arch Intern Med.1986;146:1613–1615.
- ,.Paradoxes in cancer patients' advance care planning.J Palliat Med.2000;3(1):27–35.
- ,,.The inner life of physicians and care of the seriously ill.JAMA.2001;286:3007–3014.
- ,,.The discussion about advance directives; patient and physician opinions regarding when and how it should be conducted.Arch Intern Med.1995;155:1025–1030.
- .Psychological considerations, growth, and transcendence at the end of life: the art of the possible.JAMA.2001;285:2898–2905.
- .Care of dying patients: beyond symptom management.West J Med.1999;171(4):253–256.
- ,,,.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,,,,,.Promoting advance directives among elderly primary care patients.J Gen Intern Med.2004;19:944–951.
- ,,,,.Implementing advance directives in the primary care setting.Arch Intern Med.1994;154:2321–2327.
- ,.Will outpatients complete living wills? A comparison of two interventions.J Gen Intern Med.1991;6:41–46.
- ,,, et al.Enhancement of proxy appointments for older persons: physician counselling in ambulatory settings.J Am Geriatr Soc.1996;44(1):37–43.
- ,,,.Strategies to promote the use of advance directives in a residency outpatient practice.J Gen Intern Med.1996;11:657–663.
- ,,,,,.Promoting inpatient directives about life‐sustaining treatments in a community hospital. Results of a 3‐year time‐series intervention trial.Arch Intern Med.1995;155:2317–2323.
- ,,,,.An educational intervention in the surgical ICU to improve ethical decisions.Surgery.1995;118(2):294–299.
- ,,.The status of medical education in end‐of‐life care. A national report.J Gen Intern Med.2003;18:685–695.
- ,.ACGME Requirements for end‐of‐life training in selected residency and fellowship programs: a status report.Acad Med.2002;77(4):299–304.
- ,,,.End‐of‐life care education in internal medicine residency programs: an interinstitutional study.J Palliat Med.2002;5:487–496.
- ,,,,,.End‐of‐life care in the curriculum: a national study of medical education deans.Acad Med.2004;79:760–768.
- ,,,,.Assessing medical students' training in end‐of‐life communication: a survey of interns at one urban teaching hospital.Acad Med.2003;78:530–537.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national surveyAm J Med.2001;111(3):247–254.
- ,.Legal and ethical myths about informed consent.Arch Intern Med.1996;156:2521–2526.
- ,,.Physician reluctance to discuss advance directives: an empiric investigation of potential barriers.Arch Intern Med.1994;154:2311–2318.
- ,,.How do medical residents discuss resuscitation with patients?J Gen Intern Med.1995;10:436–442.
- ,,,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.Br Med J.2000;320:469–473.
- .Death Foretold; Prophecy and Prognosis in Medical Care.Chicago:University of Chicago Press,1999.
- ,.Attitude and self‐reported practice regarding prognostication in a national sample of internists.Arch Intern Med.1998;158:2389–2395.
- ,,, et al.Prognostic factors for survival in patients treated in phase I clinical trials.Cancer.1994;74:1965–1973.
- ,,, et al.Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric studyCancer.1995;75:2613–2622.
- ,,.Evaluation of patients with advanced cancer using Karnovsky performance status.Cancer.1980;45:2220–2224.
- ,,,.The Karnofsky Performance Status Scale. An exam of its reliability and validity in a research setting.Cancer.1984;53:2002–2007.
- .A demographic and prognostic approach to defining the end of life.J Palliat Med.2005;8(suppl 1):s12–s21.
- .Estimating length of survival in end‐stage cancer: a review of the literature.J Pain Symptom Manage.1995;10:548–555.
- ,.Prognostic factors, survival, and advanced cancer.J Palliat Care.1992;1992(8):4.
- ,,.A new palliative prognostic score: a first step for the staging of terminally ill cancer patients.J Pain Symp Management1999;17(4):231–239.
- ,,.Survival prediction in terminal cancer patients: A systematic review of the medical literature.Palliat Med.2000;14:363–374.
- ,,.Clinical symptoms and length of survival in patients with terminal cancer.Arch Intern Med.1988;148:1586–1591.
- ,,.Successful validation of the palliative prognostic score in terminally ill cancer patients.J Pain Symptom Manage.1999;17:240–247.
- ,,.The use of the palliative prognostic score in patients with diagnoses other than cancer.J Pain Symptom Manage.2003;26:883–885.
- ,,.Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer.J Clin Oncol.2004;22:4823–4828.
- ,,,.Survival after in‐hospital cardiopulmonary resuscitation: a meta‐analysis.J Gen Intern Med,1998;13(12):805–16.
- .Informing the patient about cardiopulmonary resuscitation: when the risks outweigh the benefits.J Gen Intern Med.1989;4:349–355.
- .Resuscitation of patients with metastatic cancer: Is transient benefit still futile?Arch Intern Med.1991;151:235–239.
- ,,.Cardiopulmonary resuscitation on television—miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- ,,,.Resuscitation decision making in the elderly: the value of outcome data.J Gen Intern Med.1993;8:295–300.
- .Discussing do‐not‐resuscitate status.J Clin Oncol.2001;19:1576–1581.
- .Do‐not‐resuscitate decisions in a community hospital. Incidence, implications, and outcomes.JAMA.1986;256:1164–1169.
- ,,,.A prospective study of the efficacy of the physician order form for life‐sustaining treatment.J Am Geriatr Soc.1998;46:1170–1171.
- ,,, for theACPAE‐o‐LCCP.Discussing palliative care with patients.Ann Intern Med.1999;130:744–749.
- ,,,,.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55(3):164–177.
- ,,, et al.“Let me see if I have this right…”: words that help build empathy.Ann Intern Med.2001;135(3):221–227.
- ,,.“I wish things were different”: expressing wishes in response to loss, futility, and unrealistic hopes.Ann Intern Med.2001;135:551–555.
- ,,.Hope for the best, and prepare for the worst.Ann Intern Med.2003;138:439–443.
- ,,,.When the treatment goal is not cure: are cancer patients equipped to make informed decisions?J Clin Oncol.2002;20:503–513.
- ,,.Beyond autonomy: Diversifying end‐of‐life decision‐making approaches to serve patients and families.J Am Geriatr Soc.2005;53:1046–1050.
- ,,,.Measuring patients' desire for autonomy: decision making and information‐seeking preferences among medical patients.J Gen Intern Med.1989;4(1):23–30.
- ,.Physician recommendations and patient autonomy: finding a balance between physician power and patient choice.Ann Intern Med.1996;125:763–769.
Mrs. G is a 58‐year‐old woman with metastatic pancreatic cancer, diagnosed 8 months prior to admission when she presented to her primary care doctor with abdominal pain and weight loss. The cancer was locally advanced and metastatic to the liver at the time of diagnosis, and she underwent first‐line palliative chemotherapy with gemcitabine without objective or symptomatic improvement. She is now admitted to the hospitalist service with intractable abdominal pain, uncontrolled on her outpatient doses of opioids. On the day after admission, her pain is well controlled on morphine via patient‐controlled anesthesia. The hospitalist decides to talk with her about her about resuscitation status. At this point, she is full code.
Dr. S sits down with her and says, There's something I need to talk with you about that I talk with all of my patients about. And that is, if your heart were to stop, and mind you, I'm not expecting this to happen anytime soon, do you want us to do everything possible to bring you back?
Dr. S pauses to catch his breath, and then Mrs. G says, Well, I guess so, if you thought you could bring me back.
He continues, We are not always successful at bringing people back, but let me explain what this would entail: we could do chest compressions, administer shocks to your heart, put a breathing tube down into the back of your throat, hook you up to a machine that helps you breathe
Mrs. G glances up with a confused look. Dr. S says, You know, Mrs. G, I've started this conversation all wrong. I'm going to step out for a few minutes, collect my thoughts, and come back to talk with you again. Is that OK? Mrs. G smiles and says, Of course.
The doctor reenters the room about 20 minutes later, pulls up a chair next to her bed, and in a relaxed tone of voice says, Tell me what you understand about your illness.
Mrs. G says, I understand that I have cancer in my pancreas and liver and that I may not last very long, perhaps months if that. My oncologist offered me more chemotherapy, but I decided against it because it didn't seem that it would offer me much. And the first chemotherapy was really hard.
Dr. S then asks, As you look ahead, what worries you most?
Mrs. G replies, I really worry that I will die in terrible pain. That scares me terribly. I also worry about my husband and how he's going to cope with my worsening condition and with my death, let alone my pain.
Dr. S then says, That sounds really frightening. Tell me more about that. She continues, Well, I really want to spend the time I have left with my husband and familymy children and grandchildrenand I want to have some good time with them. But I keep worrying that it won't be able to be that way. She starts crying. You know, I'm so scared about going home and being in terrible pain again and having my husband and family be terrified
She pauses for a moment. The doctor doesn't say anything.
She starts up again, But I don't want to end up in the hospital again.I don't want to end up on machines like my mother‐in‐law did. She looks up at Dr. S, expecting a response.
Dr. S takes her hand and says, You know, I realize this is not easy to talk about, but it's really important for me to hear about your worries so that I can know how to help. The good news is we have many good methods to control your pain and that we can help you to be comfortable and to remain at home.
Thank you. Thank you so much.
As these conversations illustrate, discussing preferences regarding resuscitation is a challenging and important task for physicians. Understanding patients' wishes at the end of life allows clinicians to provide the type of care patients want, to avoid unwanted interventions, and to promote patient autonomy and dignity. Despite the frequency with which physicians have these conversations, they typically fall short when discussing code status with patients. Clinicians fail to address patients' concerns, monopolize conversations, and commonly misunderstand patients' resuscitation preferences.13 Often these discussions do not occur at all; more than 70% of seriously ill patients have never discussed advance directives with their physician.2, 4 The multicenter SUPPORT study, which demonstrated serious problems in the care of seriously ill hospitalized patients, documented that only 47% of physicians knew when their patients preferred do‐not‐resuscitate status.5
Hospitalists frequently conduct resuscitation discussions. Patients who are admitted to the hospital are usually seriously ill, and hospitalists need to assess rapidly whether an individual patient would want a resuscitation attempt if he or she had a cardiopulmonary arrest in the hospital. They need to build trust and rapport quickly with patients they have never met. Despite this challenge, hospitalists are in a good position to discuss resuscitation preferences.6 Patients may be more willing to discuss these issues in inpatient rather than outpatient settings because their acute deterioration may encourage self‐reflection.6 Furthermore, the time and productivity pressures of office practice often make it difficult for primary care physicians, who often know the patient and family best, to address advance directives. Although studies have documented that patients are interested in talking to their primary physicians about these matters,7, 8 these conversations do not occur with regularity. Preliminary research has raised the possibility that cancer patients may actually prefer to discuss these issues in an inpatient setting with a hospital‐based provider rather than with their oncologists.9 Studies have not addressed the question of whether patients with diseases other than cancer would prefer to discuss these issues with a hospitalist or their outpatient subspecialist.
Given that more than half of all Americans die in hospitals, hospitalists care for many people who are terminally ill and will need to assess preferences for cardiopulmonary resuscitation (CPR) and other treatments. Hospitalists need to be competent and compassionate in their approach to patients and their families. In this articles we review clinician barriers to holding these conversations, offer a variety of approaches to enhance these discussions, and review communication techniques that can be used to improve understanding.
Clinician Barriers to Discussing Resuscitation
Clinicians' own barriers may lead to infrequent and inadequate conversations about resuscitation. Understanding these barriers may allow providers to overcome them and facilitate better and more frequent communication. A discussion of patient barriers is beyond the scope of this article.
Unresolved Feelings about Death and Dying
When discussing resuscitation, or code status, physicians are discussing the possibility of death with a patient. In the first scenario above, the clinician lists the many procedures that could be done if the patient's heart were to stop without using the words die or death. The clinician never explicitly acknowledges that the patient has a serious illness that could lead to her death. Medical culture is focused on cure and on warding off death until the last possible moment. Because clinicians work in this culture, many have unresolved feelings of personal failure that are triggered when treating a dying patient.10 Also, the death of a patient can lead to anxieties about the clinician's own mortality and raise uncomfortable feelings of loss, related to the patient or to memories of deaths of the clinician's loved ones. In an attempt to avoid these feelings, whether conscious or not, physicians may resist talking to patients about death.10
Fear of Taking Away Hope
Clinicians fear that patients will lose hope if they are too honest about prognosis and acknowledge the inevitability of death. This concern may be true for a small minority of patients with advanced terminal illness who are solely focused on continued treatment and in such denial that they never consider the possibility of death. Most patients, however, understand on some level that they are getting sicker and may die, but expect clinicians to initiate discussions about death and dying.8, 11 Clinicians should understand that patients can have hope about many things beyond cure of their illness. For example, they can hope for good control of their symptoms so they can spend meaningful time with family and friends, heal troubled relationships, create a legacy, and say good‐bye. As in other developmental stages throughout life, the process of dying can be a time of emotional and spiritual growth and provide an opportunity to deepen relationships and find greater meaning.12 Despite their fears, physicians are much less likely to take away hope than they think. In fact, they can carry out the important actions of helping patients to refocus on more attainable goals and helping to return hope to what may be perceived by all as a hopeless situation.13, 14
Inadequate Training
There have been many educational interventions in both outpatient and inpatient settings to encourage physicians to discuss advance directives with patients.1521 In most of these studies, clinicians were sent reminders, but did not receive training or feedback to improve their communication skills. Although these interventions have led to modest increases in the number of advance directives, little is known about the quality of the conversations between clinician and patient.
There are acknowledged deficits in undergraduate22 and graduate medical education in discussing preferences and goals of care with patients.23, 24 A national survey of medical education deans showed that two‐thirds believed insufficient time was given to palliative care education including communication skills.25 Reflecting this lack of training, medical students and residents feel unprepared to take care of dying patients.26 In one survey, hospitalists reported that although palliative care was very important to their practice, they had not received enough training in palliative care knowledge and skills.27
Traditional Ethical Frameworks
Another difficulty arises from myths about the ethical perspectives that inform medical decision making and obtaining informed consent. Although these perspectives highlight the importance of patient autonomy and the right of the individual to choose medical treatments, they do not require physicians to describe every possible treatment if, in the clinician's judgment, a particular treatment would not benefit a patient. Physicians do have an obligation to use their medical knowledge and judgment in offering treatments and discussing side effects.28 In an attempt to honor a patient's autonomy when discussing advance directivesand possibly out of fear of coercing patientsclinicians sometimes offer a menu of treatment options without exploring the patient's underlying goals for these treatments. This approach can become meaningless out of context if the patient does not understand the probability that these interventions will work or the interrelatedness of the interventions. For example, when given a list of choices regarding resuscitation, a patient may say, I would like chest compressions and a chest tube, but no shocks please. Such a statement makes little sense clinically. Instead, physicians should have meaningful conversations with their patients in which they describe treatment options in the context of patient goals and values and help patients come to decisions in a shared process.
Practical Concerns
Outpatient providers find that time constraints and the competing demands that occur in caring for patients with multiple chronic health problems make it difficult to discuss advance directives.29 Hospitalists are also subject to productivity pressures and may feel similarly stressed for time. Outpatient providers spend about 5 minutes on each of these conversations,1 and medicine residents in the inpatient setting spend about 10 minutes.30 However, many of the conversations studied were inadequate; thus, it is unknown how long it takes to have an effective conversation. Hospitalists should keep in mind that they need not have these discussions every day with each patient and that having these conversations may end up saving time in the long run if they have a clearer sense of a patient's wishes and goals.
Laying Groundwork for the Discussion
The decision regarding resuscitation should be seen in the context of the patient's goals and values and overall health status. To address code status effectively, it is imperative first to elicit the person's view of his/her illness and then gently correct any misunderstandings. A patient who thinks her/his life will go on indefinitely may feel no need to consider her/his own mortality or alternatives to full resuscitation status. Alternatively, a patient who senses his/her mortality may have already thought about resuscitation and have clear preferences. A key first step in the conversation is to understand a patient's values and goals and comprehension of his/her illness. As in the second discussion above, a clinician can begin a discussion by saying, Tell me what you understand about your illness.
Discussing prognosis with patients can be difficult as physicians struggle with uncertainty. In the most comprehensive study to date of prognostication, physicians overestimated patient survival on average by a factor of 5.31 Nonetheless, there are compelling reasons to discuss prognosis. Failure to do so often results in patients spending their last days in the hospital receiving more aggressive treatments than they might choose if they understood their prognoses.32 Further, patients are denied the opportunity to address issues of life closure, such as spending time with family, thinking about legacy, and settling financial affairs. Physicians also fear they will take away hope with prognostication and believe patients expect greater accuracy than they can provide.33
Physicians can improve their prognostication skills by considering patients' functional status and clinical signs and symptoms and by using validated scales. The Karnofsky Performance Score (KPS) and the Eastern Cooperative Oncology Group (ECOG) Performance Status have been shown to correlate with survival,3440 and the Palliative Prognostic Score (PaP) has been validated in both cancer and noncancer patients.41, 4446 The PaP uses a combination of the KPS, clinical signs and symptoms, and the clinician's clinical prediction of survival. In addition, clinicians can be honest with patients about prognostic uncertainties and give prognoses in ranges, such as days to weeks, or weeks to months.
How to Broach the Subject of Resuscitation
In the first scenario presented in this article, the hospitalist says, There's something I need to talk with you about that I talk with all of my patients about. Although many clinicians begin resuscitation discussions this way, the question is problematic because often it is untrue. Most clinicians do not discuss code status with all patients who are admitted to the hospital. A better option would be to say, When I take care of patients with advanced cancer, I like to talk with them about their wishes regarding resuscitation. Is that all right? Better yet would be to ask a general question such as As you look ahead, what worries you most? or As you look to the future, what do you hope for? These and other useful questions appear in Table 1. These questions allow patients to bring up their concerns, show that the clinician cares about them, and often segue into a discussion of patients' hopes and worries about their own death and dying process. These questions often allow patients to bring up important goals that bear directly on the issue of resuscitation. For example, in the second scenario at the beginning of the article, the patient says she wants to have quality time with her family at home and does not want life‐sustaining technologies. Such a patient may not want resuscitation. When discussing CPR, it can also be helpful to state explicitly that resuscitation is used when a patient has died, rather than to use euphemisms such as, If your heart were to stop. The clinician can ask explicitly, If you were to die, would you want? There are other strategies for introducing the subject of resuscitation if these questions do not work. If a patient seems uncomfortable with the conversation, the clinician can address this discomfort directly by saying, This conversation seems to make you uncomfortable. Other strategies for exploring these issues include inquiring if the person has ever discussed resuscitation with his/her family or another physician, or asking if anyone else in the family has been very sick. Additionally, clinicians can ask questions about surrogate decision making. If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? This can then lead into a discussion of whether the patient has spoken to that person about his/her preferences, and if so, what was the content of that conversation. Another useful question is, Is there any state that would be worse than death for you? This question focuses on outcomes and allows the physician to put the issue of resuscitation into perspective for a patient.
| When I take care of patients with advanced cancer [or heart disease or lung disease, etc.], I like to talk with them about their wishes for care if they were to get very sick and even die suddenly. Is that all right? |
| As you look ahead, what worries you most? |
| As you look to the future, what do you hope for? |
| Has a close friend or family member ever been really sick? |
| If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? |
| Is there any state that would be worse than death for you? |
Knowing the Facts about Cardiopulmonary Resuscitation
In general, about 1 in 7 patients who have undergone CPR in the hospital survive to hospital discharge. Two literature reviews, from 1989 and 1998, of hospitalized patients who underwent CPR in the hospital reported surprisingly similar statistics. Immediate survival in both series was 41%, and survival to hospital discharge was 13%‐14%. Factors that increased survival included arrest due to coronary artery disease or drug overdose/adverse reaction, and location of arrest in the intensive care unit (ICU). Factors that decreased survival included sepsis at time of arrest, cancer, whether localized or metastatic, dementia, acute renal failure, bed‐bound status, and residence in a nursing home. Neither age nor sex was associated with survival.47, 48 In a meta‐analysis of outcome studies of CPR in metastatic cancer patients, none of 117 patients survived to hospital discharge.49
Most people get their information about CPR survival rates from the mass media, which portray CPR as a very successful procedure. For instance, on television, the sole source of information on CPR for most patients, the rate of survival to hospital discharge is 64%, much higher than the 1314% cited in the medical literature.50 Thus, it is no surprise that a patient with metastatic cancer or another life‐limiting illness would assume a positive outcome with CPR.
Knowledge of the facts about CPR survival rates is key when a physician discusses code status with a patient because these data influence patients' decisions. Patients who have a realistic understanding of their chances are less likely to prefer resuscitation.51, 52 Offering patients information about the success rate for CPR in their particular situation is critical in helping them reach a decision consistent with their values and goals. This information can be given quantitatively or qualitatively depending on the clinician assessment of what the patient would prefer.
Avoiding Stark Dichotomies in Resuscitation Discussions
In clinical practice there are 3 ways in which physicians can present resuscitation decisions as black and white and ignore the shades of gray. First, clinicians may present the choice between resuscitation and do not resuscitate (DNR) as a choice between life and death. In the first scenario above, the physician states If your heart were to stop, do you want us to do everything to bring you back? implying he would be able to save the patient's life with resuscitation attempts. When discussing resuscitation, clinicians should avoid language that suggests such a stark dichotomy. The reality is that most patients die despite resuscitation attempts. In fact, a patient is actually choosing between certain death (without resuscitation) and likely death (if resuscitation is attempted). For a patient with a serious, life‐limiting illness, it may be more effective to frame the conversation in terms of how that person envisions the end of life, and not whether death will eventually occur.
Second, clinicians and patients sometimes equate DNR with doing nothing or giving up. Clinicians fail to discuss other treatment options or alternatives, such as continuing ongoing aggressive medical treatments with DNR status or pursuing palliative care. Performing resuscitation is equated with activity and treatment, whereas withholding resuscitation is seen as passivity and giving up. To the patient, this can feel like abandonment, as if the doctor is withholding a treatment and not offering anything in its place. Examples of positive phrases that demonstrate the physician will continue to offer excellent care include: We will continue maximal medical therapy. However, if you die despite these treatments, we will let you die peacefully and won't attempt to revive you; We'll continue the intravenous antibiotics, but we won't plan to move you to the ICU if things worsen53; and We will work hard to treat your pain and other symptoms and to get you home. In addition, hospitalists must ensure, when signing out to physician colleagues, nurses, respiratory therapists, and others, that DNR orders are not overinterpreted to mean no treatment. Although a DNR order states that in the event of a cardiac arrest, no attempt at resuscitation will be made, it should have no bearing on other appropriate and desired interventions, including antibiotics, chemotherapy, and artificial nutrition; treatment for pain and other symptoms; and even monitoring in an ICU. This misunderstanding of DNR status is common among health care providers and has led many to argue that DNR orders should be part of a more comprehensive treatment plan that outlines where the patient's wishes for treatment fall on the spectrum from otherwise aggressive measures to comfort care.54, 55 Physicians who have a clear understanding of a DNR order will be able to reassure a patient that they will continue to receive desired care, but that if the patient dies, no attempt to resuscitate will be made.
Third, it is important to remind patients who choose full resuscitation status that additional decisions will need to be made if resuscitation is successful. Most patients who survive cardiopulmonary arrest end up worse off clinically and spend time in an ICU with life‐support measures in place, such as mechanical ventilation and vasopressors. Even if they survive, there will likely be a period during which they are unable to speak for themselves. This situation puts the burden of decision making on their surrogates or an appointed durable power of attorney for health care (DPOA‐HC). It is important to ask patients ahead of time whether there are conditions under which they would not want ongoing life‐sustaining measures. For example, a person might opt for discontinuation of life‐support measures if the physician and family agreed that there was only a minimal chance of cognitive recovery existed and that ongoing support was only prolonging inevitable death. To clarify the patient's wishes in this situation, you might ask, Are there conditions that would be worse than death? Encouraging the patient to share his/her wishes in this situation with a surrogate or DPOA‐HC will help to ensure those wishes are respected.
Communication Techniques
When discussing advance directives, it is important to give patients the chance to describe their life goals and their values to establish a context for understanding the role of life‐sustaining treatments. One useful method to elicit these goals and values is to ask open‐ended questions, followed by periods of silence so the patient has time to express himself/herself. In the second scenario, above, the physician used open‐ended questions in several instances: Tell me what you understand about your illness and As you look ahead, what worries you most? Tulsky and colleagues documented that medical residents spoke 76% of the time in discussions with patients about code status.30 In an ideal case, this ratio should be reversed or at least be even, allowing patients the time to explain their thoughts. Acknowledging patients' emotions by stating simply, You seem [angry/sad/perplexed], and waiting for an answer can help patients feel they are being understood. Making empathic statements is another powerful communication technique that conveys understanding.56 Examples include, That must be really sad for you, and It must be frightening to be in so much pain. As noted, silence can also be a powerful tool. Clinicians tend to be uncomfortable with silence and so fill the gaps with words. Allowing for silence enables patients to digest what they have heard, encourages them to continue speaking, and shows them the clinician wants to hear what they have to say. When giving information about any medical issues and especially about CPR, it is important to explain concepts in lay terms and to avoid medical jargon.57 Additionally, nonverbal communication techniques such as making eye contact, head nodding, and leaning in toward the patient all help in communicating engagement in the conversation. Having the conversation in a quiet and private place and sitting at the same level as the patient or family is also important. It is always a good idea to check in with patients to assess their understanding. Simply asking, Do you have any questions about what I said? or Does that all make sense? gives patients the opportunity to ask for clarification. Attempting to summarize what a patient has said can also help to clarify misunderstandings. Useful phrases include, Let me see if I've gotten this right or I want to make sure that I understood what you're telling me, followed by the clinician's synopsis of important points discussed.58 A summary of important communication techniques can be found in Table 2.
| Ask open‐ended questions followed by periods of silence |
| Tell me what you understand about your illness. |
| As you look ahead, what worries you most? |
| Acknowledge emotion |
| You seem [angry/sad/perplexed]. |
| Make empathetic statements |
| That must be really hard for you. |
| It must be terrible to be in so much pain. |
| Use nonverbal communication techniques such as eye contact, head nodding, leaning in toward the patient, sitting down, and sitting at patient's level |
| Allow for silence |
| Assess patient's understanding |
| Do you have any questions about what I said? |
| Does that all make sense? |
| Confirm your own understanding |
| Let me see if I've gotten this right. |
| I want to make sure I understand what you've been telling me. |
| Avoid medical jargon |
| Use the I wish statement |
| I wish there were more chemotherapy we could give you that would make a difference. |
| Use the Hope for the best, prepare for the worst statement |
| I think we should hope that the chemotherapy works but prepare for the possibility that it might not. |
There are 2 additional statements that can be very useful when patients and families are struggling with the reality of severe illness and are still hoping for longer life and cure. The first is the I wish statement, in which the clinician allies himself or herself with the patient's or family's wishes by stating, I wish it were different. I wish there were more chemotherapy we could give you that would make a difference.59 Occasionally, when tension is developing because the clinician does not believe an intervention is warranted but the patient desires it nonetheless, the I wish statement can be a powerful way of realigning with the patient. For example, responding to a patient who says, I want chemo to cure my cancer, with the statement There is no chemotherapy to help you can seem antagonistic. In contrast, saying, I wish there were a treatment that would make your cancer go away aligns the clinician with the patient and supports the patient. Another advantage of I wish statements is that they are truethe clinician does wish that there was an effective treatment and would gladly provide it if available. In general, I wish statements are more effective than I'm sorry statements, because the latter can be interpreted as the clinician taking responsibility for the situation. When a provider says, I'm sorry to give you this bad news, the patient may feel the need to say, That's OK. On the other hand, saying, I'm so sorry that your mother is dying, is very human and unlikely to be interpreted as the clinician taking responsibility for the death.
A similar technique is to use the statement Hope for the best, prepare for the worst when speaking with patients and families.60 For example, a physician could say, I really wish your mother could get better, and we should still hope for that; at the same time, we need to prepare for what will happen if she doesn't get better. Once again, this phrase both allows the patient or family to continue hoping things will improve and the clinician to support this hope, while simultaneously beginning the process of planning for the more likely outcome. Over time, the patient and family often move toward accepting that the patient is dying. Finally, trying to help the patient or family maintain hope in the face of illness and death is challenging but important: If your mother can't get better, are there other things you can hope for? Helping to identify tangible and realistic goals, such as being free from pain, seeing an important family member one last time, or getting home can provide hope at a difficult time.
Giving a Recommendation
Most patients with serious illness and their families want help making complex and ethically charged decisions. When clinicians ask patients to make decisions unilaterally, patients often feel anxious, sometimes for weeks.61 Families are often paralyzed when faced with the very difficult decision of whether to withdraw life‐sustaining interventions from a family member with an advanced terminal illness. Even if they understand on an intellectual level that ceasing to provide potentially curative or life‐prolonging therapies is the best choice, they are not yet able to accept this decision on an emotional level and ultimately may feel responsible and then guilty for the patient's death. Physicians need to carry some of the burden of making these difficult decisions. One way to relieve family members of some of this guilt is to recommend a plan of care based on substituted judgment, that is, on what the patient said she or he wanted or what the family thinks the patient would have wanted.6264 In addition, clinicians should use their medical expertise, experience, and understanding of the situation to make recommendations. The patient or family can then accept or reject the physician's advice, which maintains patient autonomy, yet not have to explicitly instruct the clinician to withdraw or limit life‐sustaining interventions.
The preceding discussion and recommendations can guide scenarios like those presented at the beginning of this article. In the second conversation, the clinician had just told the patient that he could help her to achieve her goal of pain control and of returning and staying home.
Dr. S says, I want to make sure I've understood what you've said. To summarize, you've told me how important it is to you to have your pain controlled, to have some good time with your husband and family at home, and not to come back to the hospital. Is that right?
Mrs. G: Yes, that's right.
Dr. S: And how is your pain today?
Mrs. G: So much better. I'm hoping I can go home soon.
Dr. S: That should be possible. In the next day or so, we will be getting you back onto medications that you can take by mouth. But before you go home, we need to figure out how we can support you and your family at home: get you ongoing help with pain control and any other issues that come up and support for your husband and family as well.
Mrs. G: Yes, my family really needs support.
Dr. S: Have you had any experience with hospice before? I'm thinking that that would be the best way to get you the support you and your family need.
Mrs. G: Yes. When my sister died, she had home hospice. They were very good and helpful, especially to her husband, my brother‐in‐law. Yes, I would like that, as I just don't want to come back to the hospital. Hopefully, they can help my husband adjust to things.
Dr. S continues: I think they will help your husband a lot, both before and also after your death. He pauses.
Mrs. G: That's good. I worry about him so much.
Dr. S: As you know, one of the important goals of hospice care is to keep you at home so you don't have to return to the hospital. And when the time comes, to help you die peacefully at home. Mrs. G nods. What this also means is that they would not use CPR, that is, not do chest compressions, when you were actively dying.
Mrs. G: Yes, I want to be able to go home and be at peace. The last thing I would want is someone thumping on my chest as I was dying. She smiles.
Dr. S.: Do you have any other thoughts or questions?
Mrs. G: Well, yes. I'm wondering if we can set up a time to talk with my husband about all of this. I think it would be helpful for him to talk with you. Would that be possible?
Dr. S: I would be happy to talk with your husband. When is he coming in?
They negotiate a time.
Mrs. G: I want to thank you for taking the time to talk with me. I am really grateful.
CONCLUSIONS
The decision about cardiopulmonary resuscitation is part of a larger conversation with a patient about how she or he wants to spend the rest of his/her life. Importantly, the decision should be made in context, rather than in isolation. Given the understanding that develops between physician and patient in the conversation above, it is not necessary to describe all the specific treatments that occur during CPR because the physician has already established that the patient does not want to return to the hospital, and she understands that she has a terminal condition and is dying. Through exploring a patient's goals and values, a clinician can discover a patient's preferences for care generally and come up with a comprehensive plan that addresses the particular individual's medical, social, and emotional needs. For physicians, few interventions are as important or rewarding as relieving patients' suffering and helping them attain their goals during and at the end of life.
Mrs. G is a 58‐year‐old woman with metastatic pancreatic cancer, diagnosed 8 months prior to admission when she presented to her primary care doctor with abdominal pain and weight loss. The cancer was locally advanced and metastatic to the liver at the time of diagnosis, and she underwent first‐line palliative chemotherapy with gemcitabine without objective or symptomatic improvement. She is now admitted to the hospitalist service with intractable abdominal pain, uncontrolled on her outpatient doses of opioids. On the day after admission, her pain is well controlled on morphine via patient‐controlled anesthesia. The hospitalist decides to talk with her about her about resuscitation status. At this point, she is full code.
Dr. S sits down with her and says, There's something I need to talk with you about that I talk with all of my patients about. And that is, if your heart were to stop, and mind you, I'm not expecting this to happen anytime soon, do you want us to do everything possible to bring you back?
Dr. S pauses to catch his breath, and then Mrs. G says, Well, I guess so, if you thought you could bring me back.
He continues, We are not always successful at bringing people back, but let me explain what this would entail: we could do chest compressions, administer shocks to your heart, put a breathing tube down into the back of your throat, hook you up to a machine that helps you breathe
Mrs. G glances up with a confused look. Dr. S says, You know, Mrs. G, I've started this conversation all wrong. I'm going to step out for a few minutes, collect my thoughts, and come back to talk with you again. Is that OK? Mrs. G smiles and says, Of course.
The doctor reenters the room about 20 minutes later, pulls up a chair next to her bed, and in a relaxed tone of voice says, Tell me what you understand about your illness.
Mrs. G says, I understand that I have cancer in my pancreas and liver and that I may not last very long, perhaps months if that. My oncologist offered me more chemotherapy, but I decided against it because it didn't seem that it would offer me much. And the first chemotherapy was really hard.
Dr. S then asks, As you look ahead, what worries you most?
Mrs. G replies, I really worry that I will die in terrible pain. That scares me terribly. I also worry about my husband and how he's going to cope with my worsening condition and with my death, let alone my pain.
Dr. S then says, That sounds really frightening. Tell me more about that. She continues, Well, I really want to spend the time I have left with my husband and familymy children and grandchildrenand I want to have some good time with them. But I keep worrying that it won't be able to be that way. She starts crying. You know, I'm so scared about going home and being in terrible pain again and having my husband and family be terrified
She pauses for a moment. The doctor doesn't say anything.
She starts up again, But I don't want to end up in the hospital again.I don't want to end up on machines like my mother‐in‐law did. She looks up at Dr. S, expecting a response.
Dr. S takes her hand and says, You know, I realize this is not easy to talk about, but it's really important for me to hear about your worries so that I can know how to help. The good news is we have many good methods to control your pain and that we can help you to be comfortable and to remain at home.
Thank you. Thank you so much.
As these conversations illustrate, discussing preferences regarding resuscitation is a challenging and important task for physicians. Understanding patients' wishes at the end of life allows clinicians to provide the type of care patients want, to avoid unwanted interventions, and to promote patient autonomy and dignity. Despite the frequency with which physicians have these conversations, they typically fall short when discussing code status with patients. Clinicians fail to address patients' concerns, monopolize conversations, and commonly misunderstand patients' resuscitation preferences.13 Often these discussions do not occur at all; more than 70% of seriously ill patients have never discussed advance directives with their physician.2, 4 The multicenter SUPPORT study, which demonstrated serious problems in the care of seriously ill hospitalized patients, documented that only 47% of physicians knew when their patients preferred do‐not‐resuscitate status.5
Hospitalists frequently conduct resuscitation discussions. Patients who are admitted to the hospital are usually seriously ill, and hospitalists need to assess rapidly whether an individual patient would want a resuscitation attempt if he or she had a cardiopulmonary arrest in the hospital. They need to build trust and rapport quickly with patients they have never met. Despite this challenge, hospitalists are in a good position to discuss resuscitation preferences.6 Patients may be more willing to discuss these issues in inpatient rather than outpatient settings because their acute deterioration may encourage self‐reflection.6 Furthermore, the time and productivity pressures of office practice often make it difficult for primary care physicians, who often know the patient and family best, to address advance directives. Although studies have documented that patients are interested in talking to their primary physicians about these matters,7, 8 these conversations do not occur with regularity. Preliminary research has raised the possibility that cancer patients may actually prefer to discuss these issues in an inpatient setting with a hospital‐based provider rather than with their oncologists.9 Studies have not addressed the question of whether patients with diseases other than cancer would prefer to discuss these issues with a hospitalist or their outpatient subspecialist.
Given that more than half of all Americans die in hospitals, hospitalists care for many people who are terminally ill and will need to assess preferences for cardiopulmonary resuscitation (CPR) and other treatments. Hospitalists need to be competent and compassionate in their approach to patients and their families. In this articles we review clinician barriers to holding these conversations, offer a variety of approaches to enhance these discussions, and review communication techniques that can be used to improve understanding.
Clinician Barriers to Discussing Resuscitation
Clinicians' own barriers may lead to infrequent and inadequate conversations about resuscitation. Understanding these barriers may allow providers to overcome them and facilitate better and more frequent communication. A discussion of patient barriers is beyond the scope of this article.
Unresolved Feelings about Death and Dying
When discussing resuscitation, or code status, physicians are discussing the possibility of death with a patient. In the first scenario above, the clinician lists the many procedures that could be done if the patient's heart were to stop without using the words die or death. The clinician never explicitly acknowledges that the patient has a serious illness that could lead to her death. Medical culture is focused on cure and on warding off death until the last possible moment. Because clinicians work in this culture, many have unresolved feelings of personal failure that are triggered when treating a dying patient.10 Also, the death of a patient can lead to anxieties about the clinician's own mortality and raise uncomfortable feelings of loss, related to the patient or to memories of deaths of the clinician's loved ones. In an attempt to avoid these feelings, whether conscious or not, physicians may resist talking to patients about death.10
Fear of Taking Away Hope
Clinicians fear that patients will lose hope if they are too honest about prognosis and acknowledge the inevitability of death. This concern may be true for a small minority of patients with advanced terminal illness who are solely focused on continued treatment and in such denial that they never consider the possibility of death. Most patients, however, understand on some level that they are getting sicker and may die, but expect clinicians to initiate discussions about death and dying.8, 11 Clinicians should understand that patients can have hope about many things beyond cure of their illness. For example, they can hope for good control of their symptoms so they can spend meaningful time with family and friends, heal troubled relationships, create a legacy, and say good‐bye. As in other developmental stages throughout life, the process of dying can be a time of emotional and spiritual growth and provide an opportunity to deepen relationships and find greater meaning.12 Despite their fears, physicians are much less likely to take away hope than they think. In fact, they can carry out the important actions of helping patients to refocus on more attainable goals and helping to return hope to what may be perceived by all as a hopeless situation.13, 14
Inadequate Training
There have been many educational interventions in both outpatient and inpatient settings to encourage physicians to discuss advance directives with patients.1521 In most of these studies, clinicians were sent reminders, but did not receive training or feedback to improve their communication skills. Although these interventions have led to modest increases in the number of advance directives, little is known about the quality of the conversations between clinician and patient.
There are acknowledged deficits in undergraduate22 and graduate medical education in discussing preferences and goals of care with patients.23, 24 A national survey of medical education deans showed that two‐thirds believed insufficient time was given to palliative care education including communication skills.25 Reflecting this lack of training, medical students and residents feel unprepared to take care of dying patients.26 In one survey, hospitalists reported that although palliative care was very important to their practice, they had not received enough training in palliative care knowledge and skills.27
Traditional Ethical Frameworks
Another difficulty arises from myths about the ethical perspectives that inform medical decision making and obtaining informed consent. Although these perspectives highlight the importance of patient autonomy and the right of the individual to choose medical treatments, they do not require physicians to describe every possible treatment if, in the clinician's judgment, a particular treatment would not benefit a patient. Physicians do have an obligation to use their medical knowledge and judgment in offering treatments and discussing side effects.28 In an attempt to honor a patient's autonomy when discussing advance directivesand possibly out of fear of coercing patientsclinicians sometimes offer a menu of treatment options without exploring the patient's underlying goals for these treatments. This approach can become meaningless out of context if the patient does not understand the probability that these interventions will work or the interrelatedness of the interventions. For example, when given a list of choices regarding resuscitation, a patient may say, I would like chest compressions and a chest tube, but no shocks please. Such a statement makes little sense clinically. Instead, physicians should have meaningful conversations with their patients in which they describe treatment options in the context of patient goals and values and help patients come to decisions in a shared process.
Practical Concerns
Outpatient providers find that time constraints and the competing demands that occur in caring for patients with multiple chronic health problems make it difficult to discuss advance directives.29 Hospitalists are also subject to productivity pressures and may feel similarly stressed for time. Outpatient providers spend about 5 minutes on each of these conversations,1 and medicine residents in the inpatient setting spend about 10 minutes.30 However, many of the conversations studied were inadequate; thus, it is unknown how long it takes to have an effective conversation. Hospitalists should keep in mind that they need not have these discussions every day with each patient and that having these conversations may end up saving time in the long run if they have a clearer sense of a patient's wishes and goals.
Laying Groundwork for the Discussion
The decision regarding resuscitation should be seen in the context of the patient's goals and values and overall health status. To address code status effectively, it is imperative first to elicit the person's view of his/her illness and then gently correct any misunderstandings. A patient who thinks her/his life will go on indefinitely may feel no need to consider her/his own mortality or alternatives to full resuscitation status. Alternatively, a patient who senses his/her mortality may have already thought about resuscitation and have clear preferences. A key first step in the conversation is to understand a patient's values and goals and comprehension of his/her illness. As in the second discussion above, a clinician can begin a discussion by saying, Tell me what you understand about your illness.
Discussing prognosis with patients can be difficult as physicians struggle with uncertainty. In the most comprehensive study to date of prognostication, physicians overestimated patient survival on average by a factor of 5.31 Nonetheless, there are compelling reasons to discuss prognosis. Failure to do so often results in patients spending their last days in the hospital receiving more aggressive treatments than they might choose if they understood their prognoses.32 Further, patients are denied the opportunity to address issues of life closure, such as spending time with family, thinking about legacy, and settling financial affairs. Physicians also fear they will take away hope with prognostication and believe patients expect greater accuracy than they can provide.33
Physicians can improve their prognostication skills by considering patients' functional status and clinical signs and symptoms and by using validated scales. The Karnofsky Performance Score (KPS) and the Eastern Cooperative Oncology Group (ECOG) Performance Status have been shown to correlate with survival,3440 and the Palliative Prognostic Score (PaP) has been validated in both cancer and noncancer patients.41, 4446 The PaP uses a combination of the KPS, clinical signs and symptoms, and the clinician's clinical prediction of survival. In addition, clinicians can be honest with patients about prognostic uncertainties and give prognoses in ranges, such as days to weeks, or weeks to months.
How to Broach the Subject of Resuscitation
In the first scenario presented in this article, the hospitalist says, There's something I need to talk with you about that I talk with all of my patients about. Although many clinicians begin resuscitation discussions this way, the question is problematic because often it is untrue. Most clinicians do not discuss code status with all patients who are admitted to the hospital. A better option would be to say, When I take care of patients with advanced cancer, I like to talk with them about their wishes regarding resuscitation. Is that all right? Better yet would be to ask a general question such as As you look ahead, what worries you most? or As you look to the future, what do you hope for? These and other useful questions appear in Table 1. These questions allow patients to bring up their concerns, show that the clinician cares about them, and often segue into a discussion of patients' hopes and worries about their own death and dying process. These questions often allow patients to bring up important goals that bear directly on the issue of resuscitation. For example, in the second scenario at the beginning of the article, the patient says she wants to have quality time with her family at home and does not want life‐sustaining technologies. Such a patient may not want resuscitation. When discussing CPR, it can also be helpful to state explicitly that resuscitation is used when a patient has died, rather than to use euphemisms such as, If your heart were to stop. The clinician can ask explicitly, If you were to die, would you want? There are other strategies for introducing the subject of resuscitation if these questions do not work. If a patient seems uncomfortable with the conversation, the clinician can address this discomfort directly by saying, This conversation seems to make you uncomfortable. Other strategies for exploring these issues include inquiring if the person has ever discussed resuscitation with his/her family or another physician, or asking if anyone else in the family has been very sick. Additionally, clinicians can ask questions about surrogate decision making. If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? This can then lead into a discussion of whether the patient has spoken to that person about his/her preferences, and if so, what was the content of that conversation. Another useful question is, Is there any state that would be worse than death for you? This question focuses on outcomes and allows the physician to put the issue of resuscitation into perspective for a patient.
| When I take care of patients with advanced cancer [or heart disease or lung disease, etc.], I like to talk with them about their wishes for care if they were to get very sick and even die suddenly. Is that all right? |
| As you look ahead, what worries you most? |
| As you look to the future, what do you hope for? |
| Has a close friend or family member ever been really sick? |
| If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? |
| Is there any state that would be worse than death for you? |
Knowing the Facts about Cardiopulmonary Resuscitation
In general, about 1 in 7 patients who have undergone CPR in the hospital survive to hospital discharge. Two literature reviews, from 1989 and 1998, of hospitalized patients who underwent CPR in the hospital reported surprisingly similar statistics. Immediate survival in both series was 41%, and survival to hospital discharge was 13%‐14%. Factors that increased survival included arrest due to coronary artery disease or drug overdose/adverse reaction, and location of arrest in the intensive care unit (ICU). Factors that decreased survival included sepsis at time of arrest, cancer, whether localized or metastatic, dementia, acute renal failure, bed‐bound status, and residence in a nursing home. Neither age nor sex was associated with survival.47, 48 In a meta‐analysis of outcome studies of CPR in metastatic cancer patients, none of 117 patients survived to hospital discharge.49
Most people get their information about CPR survival rates from the mass media, which portray CPR as a very successful procedure. For instance, on television, the sole source of information on CPR for most patients, the rate of survival to hospital discharge is 64%, much higher than the 1314% cited in the medical literature.50 Thus, it is no surprise that a patient with metastatic cancer or another life‐limiting illness would assume a positive outcome with CPR.
Knowledge of the facts about CPR survival rates is key when a physician discusses code status with a patient because these data influence patients' decisions. Patients who have a realistic understanding of their chances are less likely to prefer resuscitation.51, 52 Offering patients information about the success rate for CPR in their particular situation is critical in helping them reach a decision consistent with their values and goals. This information can be given quantitatively or qualitatively depending on the clinician assessment of what the patient would prefer.
Avoiding Stark Dichotomies in Resuscitation Discussions
In clinical practice there are 3 ways in which physicians can present resuscitation decisions as black and white and ignore the shades of gray. First, clinicians may present the choice between resuscitation and do not resuscitate (DNR) as a choice between life and death. In the first scenario above, the physician states If your heart were to stop, do you want us to do everything to bring you back? implying he would be able to save the patient's life with resuscitation attempts. When discussing resuscitation, clinicians should avoid language that suggests such a stark dichotomy. The reality is that most patients die despite resuscitation attempts. In fact, a patient is actually choosing between certain death (without resuscitation) and likely death (if resuscitation is attempted). For a patient with a serious, life‐limiting illness, it may be more effective to frame the conversation in terms of how that person envisions the end of life, and not whether death will eventually occur.
Second, clinicians and patients sometimes equate DNR with doing nothing or giving up. Clinicians fail to discuss other treatment options or alternatives, such as continuing ongoing aggressive medical treatments with DNR status or pursuing palliative care. Performing resuscitation is equated with activity and treatment, whereas withholding resuscitation is seen as passivity and giving up. To the patient, this can feel like abandonment, as if the doctor is withholding a treatment and not offering anything in its place. Examples of positive phrases that demonstrate the physician will continue to offer excellent care include: We will continue maximal medical therapy. However, if you die despite these treatments, we will let you die peacefully and won't attempt to revive you; We'll continue the intravenous antibiotics, but we won't plan to move you to the ICU if things worsen53; and We will work hard to treat your pain and other symptoms and to get you home. In addition, hospitalists must ensure, when signing out to physician colleagues, nurses, respiratory therapists, and others, that DNR orders are not overinterpreted to mean no treatment. Although a DNR order states that in the event of a cardiac arrest, no attempt at resuscitation will be made, it should have no bearing on other appropriate and desired interventions, including antibiotics, chemotherapy, and artificial nutrition; treatment for pain and other symptoms; and even monitoring in an ICU. This misunderstanding of DNR status is common among health care providers and has led many to argue that DNR orders should be part of a more comprehensive treatment plan that outlines where the patient's wishes for treatment fall on the spectrum from otherwise aggressive measures to comfort care.54, 55 Physicians who have a clear understanding of a DNR order will be able to reassure a patient that they will continue to receive desired care, but that if the patient dies, no attempt to resuscitate will be made.
Third, it is important to remind patients who choose full resuscitation status that additional decisions will need to be made if resuscitation is successful. Most patients who survive cardiopulmonary arrest end up worse off clinically and spend time in an ICU with life‐support measures in place, such as mechanical ventilation and vasopressors. Even if they survive, there will likely be a period during which they are unable to speak for themselves. This situation puts the burden of decision making on their surrogates or an appointed durable power of attorney for health care (DPOA‐HC). It is important to ask patients ahead of time whether there are conditions under which they would not want ongoing life‐sustaining measures. For example, a person might opt for discontinuation of life‐support measures if the physician and family agreed that there was only a minimal chance of cognitive recovery existed and that ongoing support was only prolonging inevitable death. To clarify the patient's wishes in this situation, you might ask, Are there conditions that would be worse than death? Encouraging the patient to share his/her wishes in this situation with a surrogate or DPOA‐HC will help to ensure those wishes are respected.
Communication Techniques
When discussing advance directives, it is important to give patients the chance to describe their life goals and their values to establish a context for understanding the role of life‐sustaining treatments. One useful method to elicit these goals and values is to ask open‐ended questions, followed by periods of silence so the patient has time to express himself/herself. In the second scenario, above, the physician used open‐ended questions in several instances: Tell me what you understand about your illness and As you look ahead, what worries you most? Tulsky and colleagues documented that medical residents spoke 76% of the time in discussions with patients about code status.30 In an ideal case, this ratio should be reversed or at least be even, allowing patients the time to explain their thoughts. Acknowledging patients' emotions by stating simply, You seem [angry/sad/perplexed], and waiting for an answer can help patients feel they are being understood. Making empathic statements is another powerful communication technique that conveys understanding.56 Examples include, That must be really sad for you, and It must be frightening to be in so much pain. As noted, silence can also be a powerful tool. Clinicians tend to be uncomfortable with silence and so fill the gaps with words. Allowing for silence enables patients to digest what they have heard, encourages them to continue speaking, and shows them the clinician wants to hear what they have to say. When giving information about any medical issues and especially about CPR, it is important to explain concepts in lay terms and to avoid medical jargon.57 Additionally, nonverbal communication techniques such as making eye contact, head nodding, and leaning in toward the patient all help in communicating engagement in the conversation. Having the conversation in a quiet and private place and sitting at the same level as the patient or family is also important. It is always a good idea to check in with patients to assess their understanding. Simply asking, Do you have any questions about what I said? or Does that all make sense? gives patients the opportunity to ask for clarification. Attempting to summarize what a patient has said can also help to clarify misunderstandings. Useful phrases include, Let me see if I've gotten this right or I want to make sure that I understood what you're telling me, followed by the clinician's synopsis of important points discussed.58 A summary of important communication techniques can be found in Table 2.
| Ask open‐ended questions followed by periods of silence |
| Tell me what you understand about your illness. |
| As you look ahead, what worries you most? |
| Acknowledge emotion |
| You seem [angry/sad/perplexed]. |
| Make empathetic statements |
| That must be really hard for you. |
| It must be terrible to be in so much pain. |
| Use nonverbal communication techniques such as eye contact, head nodding, leaning in toward the patient, sitting down, and sitting at patient's level |
| Allow for silence |
| Assess patient's understanding |
| Do you have any questions about what I said? |
| Does that all make sense? |
| Confirm your own understanding |
| Let me see if I've gotten this right. |
| I want to make sure I understand what you've been telling me. |
| Avoid medical jargon |
| Use the I wish statement |
| I wish there were more chemotherapy we could give you that would make a difference. |
| Use the Hope for the best, prepare for the worst statement |
| I think we should hope that the chemotherapy works but prepare for the possibility that it might not. |
There are 2 additional statements that can be very useful when patients and families are struggling with the reality of severe illness and are still hoping for longer life and cure. The first is the I wish statement, in which the clinician allies himself or herself with the patient's or family's wishes by stating, I wish it were different. I wish there were more chemotherapy we could give you that would make a difference.59 Occasionally, when tension is developing because the clinician does not believe an intervention is warranted but the patient desires it nonetheless, the I wish statement can be a powerful way of realigning with the patient. For example, responding to a patient who says, I want chemo to cure my cancer, with the statement There is no chemotherapy to help you can seem antagonistic. In contrast, saying, I wish there were a treatment that would make your cancer go away aligns the clinician with the patient and supports the patient. Another advantage of I wish statements is that they are truethe clinician does wish that there was an effective treatment and would gladly provide it if available. In general, I wish statements are more effective than I'm sorry statements, because the latter can be interpreted as the clinician taking responsibility for the situation. When a provider says, I'm sorry to give you this bad news, the patient may feel the need to say, That's OK. On the other hand, saying, I'm so sorry that your mother is dying, is very human and unlikely to be interpreted as the clinician taking responsibility for the death.
A similar technique is to use the statement Hope for the best, prepare for the worst when speaking with patients and families.60 For example, a physician could say, I really wish your mother could get better, and we should still hope for that; at the same time, we need to prepare for what will happen if she doesn't get better. Once again, this phrase both allows the patient or family to continue hoping things will improve and the clinician to support this hope, while simultaneously beginning the process of planning for the more likely outcome. Over time, the patient and family often move toward accepting that the patient is dying. Finally, trying to help the patient or family maintain hope in the face of illness and death is challenging but important: If your mother can't get better, are there other things you can hope for? Helping to identify tangible and realistic goals, such as being free from pain, seeing an important family member one last time, or getting home can provide hope at a difficult time.
Giving a Recommendation
Most patients with serious illness and their families want help making complex and ethically charged decisions. When clinicians ask patients to make decisions unilaterally, patients often feel anxious, sometimes for weeks.61 Families are often paralyzed when faced with the very difficult decision of whether to withdraw life‐sustaining interventions from a family member with an advanced terminal illness. Even if they understand on an intellectual level that ceasing to provide potentially curative or life‐prolonging therapies is the best choice, they are not yet able to accept this decision on an emotional level and ultimately may feel responsible and then guilty for the patient's death. Physicians need to carry some of the burden of making these difficult decisions. One way to relieve family members of some of this guilt is to recommend a plan of care based on substituted judgment, that is, on what the patient said she or he wanted or what the family thinks the patient would have wanted.6264 In addition, clinicians should use their medical expertise, experience, and understanding of the situation to make recommendations. The patient or family can then accept or reject the physician's advice, which maintains patient autonomy, yet not have to explicitly instruct the clinician to withdraw or limit life‐sustaining interventions.
The preceding discussion and recommendations can guide scenarios like those presented at the beginning of this article. In the second conversation, the clinician had just told the patient that he could help her to achieve her goal of pain control and of returning and staying home.
Dr. S says, I want to make sure I've understood what you've said. To summarize, you've told me how important it is to you to have your pain controlled, to have some good time with your husband and family at home, and not to come back to the hospital. Is that right?
Mrs. G: Yes, that's right.
Dr. S: And how is your pain today?
Mrs. G: So much better. I'm hoping I can go home soon.
Dr. S: That should be possible. In the next day or so, we will be getting you back onto medications that you can take by mouth. But before you go home, we need to figure out how we can support you and your family at home: get you ongoing help with pain control and any other issues that come up and support for your husband and family as well.
Mrs. G: Yes, my family really needs support.
Dr. S: Have you had any experience with hospice before? I'm thinking that that would be the best way to get you the support you and your family need.
Mrs. G: Yes. When my sister died, she had home hospice. They were very good and helpful, especially to her husband, my brother‐in‐law. Yes, I would like that, as I just don't want to come back to the hospital. Hopefully, they can help my husband adjust to things.
Dr. S continues: I think they will help your husband a lot, both before and also after your death. He pauses.
Mrs. G: That's good. I worry about him so much.
Dr. S: As you know, one of the important goals of hospice care is to keep you at home so you don't have to return to the hospital. And when the time comes, to help you die peacefully at home. Mrs. G nods. What this also means is that they would not use CPR, that is, not do chest compressions, when you were actively dying.
Mrs. G: Yes, I want to be able to go home and be at peace. The last thing I would want is someone thumping on my chest as I was dying. She smiles.
Dr. S.: Do you have any other thoughts or questions?
Mrs. G: Well, yes. I'm wondering if we can set up a time to talk with my husband about all of this. I think it would be helpful for him to talk with you. Would that be possible?
Dr. S: I would be happy to talk with your husband. When is he coming in?
They negotiate a time.
Mrs. G: I want to thank you for taking the time to talk with me. I am really grateful.
CONCLUSIONS
The decision about cardiopulmonary resuscitation is part of a larger conversation with a patient about how she or he wants to spend the rest of his/her life. Importantly, the decision should be made in context, rather than in isolation. Given the understanding that develops between physician and patient in the conversation above, it is not necessary to describe all the specific treatments that occur during CPR because the physician has already established that the patient does not want to return to the hospital, and she understands that she has a terminal condition and is dying. Through exploring a patient's goals and values, a clinician can discover a patient's preferences for care generally and come up with a comprehensive plan that addresses the particular individual's medical, social, and emotional needs. For physicians, few interventions are as important or rewarding as relieving patients' suffering and helping them attain their goals during and at the end of life.
- ,,,.Opening the black box: how do physicians communicate about advance directives?Ann Intern Med.1998;129:441–449.
- ,,, et al.Physician understanding of patient resuscitation preferences: insights and clinical implications.J Am Geriatr Soc.2000;48:S44–S51.
- ,,,,.Patient knowledge and physician predictions of treatment preferences after discussion of advance directives.J Gen Intern Med.1998;13:447–454.
- ,,.The value of disease severity in predicting patient readiness to address end‐of‐life issues.Arch Intern Med.2003;163:609–612.
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).The SUPPORT Principal Investigators.JAMA.1995;274:1591–1598.
- ,,,,,.Can we talk? Inpatient discussions about advance directives in a community hospital. Attending physicians' attitudes, their inpatients' wishes and reported evidence.Arch Intern Med.1994;154:2299–2308.
- ,,,,.Advance directives for medical care—a case for greater use.N Engl J Med.1991;324:889–895.
- ,,.Patient attitudes to discussing life‐sustaining treatment.Arch Intern Med.1986;146:1613–1615.
- ,.Paradoxes in cancer patients' advance care planning.J Palliat Med.2000;3(1):27–35.
- ,,.The inner life of physicians and care of the seriously ill.JAMA.2001;286:3007–3014.
- ,,.The discussion about advance directives; patient and physician opinions regarding when and how it should be conducted.Arch Intern Med.1995;155:1025–1030.
- .Psychological considerations, growth, and transcendence at the end of life: the art of the possible.JAMA.2001;285:2898–2905.
- .Care of dying patients: beyond symptom management.West J Med.1999;171(4):253–256.
- ,,,.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,,,,,.Promoting advance directives among elderly primary care patients.J Gen Intern Med.2004;19:944–951.
- ,,,,.Implementing advance directives in the primary care setting.Arch Intern Med.1994;154:2321–2327.
- ,.Will outpatients complete living wills? A comparison of two interventions.J Gen Intern Med.1991;6:41–46.
- ,,, et al.Enhancement of proxy appointments for older persons: physician counselling in ambulatory settings.J Am Geriatr Soc.1996;44(1):37–43.
- ,,,.Strategies to promote the use of advance directives in a residency outpatient practice.J Gen Intern Med.1996;11:657–663.
- ,,,,,.Promoting inpatient directives about life‐sustaining treatments in a community hospital. Results of a 3‐year time‐series intervention trial.Arch Intern Med.1995;155:2317–2323.
- ,,,,.An educational intervention in the surgical ICU to improve ethical decisions.Surgery.1995;118(2):294–299.
- ,,.The status of medical education in end‐of‐life care. A national report.J Gen Intern Med.2003;18:685–695.
- ,.ACGME Requirements for end‐of‐life training in selected residency and fellowship programs: a status report.Acad Med.2002;77(4):299–304.
- ,,,.End‐of‐life care education in internal medicine residency programs: an interinstitutional study.J Palliat Med.2002;5:487–496.
- ,,,,,.End‐of‐life care in the curriculum: a national study of medical education deans.Acad Med.2004;79:760–768.
- ,,,,.Assessing medical students' training in end‐of‐life communication: a survey of interns at one urban teaching hospital.Acad Med.2003;78:530–537.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national surveyAm J Med.2001;111(3):247–254.
- ,.Legal and ethical myths about informed consent.Arch Intern Med.1996;156:2521–2526.
- ,,.Physician reluctance to discuss advance directives: an empiric investigation of potential barriers.Arch Intern Med.1994;154:2311–2318.
- ,,.How do medical residents discuss resuscitation with patients?J Gen Intern Med.1995;10:436–442.
- ,,,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.Br Med J.2000;320:469–473.
- .Death Foretold; Prophecy and Prognosis in Medical Care.Chicago:University of Chicago Press,1999.
- ,.Attitude and self‐reported practice regarding prognostication in a national sample of internists.Arch Intern Med.1998;158:2389–2395.
- ,,, et al.Prognostic factors for survival in patients treated in phase I clinical trials.Cancer.1994;74:1965–1973.
- ,,, et al.Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric studyCancer.1995;75:2613–2622.
- ,,.Evaluation of patients with advanced cancer using Karnovsky performance status.Cancer.1980;45:2220–2224.
- ,,,.The Karnofsky Performance Status Scale. An exam of its reliability and validity in a research setting.Cancer.1984;53:2002–2007.
- .A demographic and prognostic approach to defining the end of life.J Palliat Med.2005;8(suppl 1):s12–s21.
- .Estimating length of survival in end‐stage cancer: a review of the literature.J Pain Symptom Manage.1995;10:548–555.
- ,.Prognostic factors, survival, and advanced cancer.J Palliat Care.1992;1992(8):4.
- ,,.A new palliative prognostic score: a first step for the staging of terminally ill cancer patients.J Pain Symp Management1999;17(4):231–239.
- ,,.Survival prediction in terminal cancer patients: A systematic review of the medical literature.Palliat Med.2000;14:363–374.
- ,,.Clinical symptoms and length of survival in patients with terminal cancer.Arch Intern Med.1988;148:1586–1591.
- ,,.Successful validation of the palliative prognostic score in terminally ill cancer patients.J Pain Symptom Manage.1999;17:240–247.
- ,,.The use of the palliative prognostic score in patients with diagnoses other than cancer.J Pain Symptom Manage.2003;26:883–885.
- ,,.Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer.J Clin Oncol.2004;22:4823–4828.
- ,,,.Survival after in‐hospital cardiopulmonary resuscitation: a meta‐analysis.J Gen Intern Med,1998;13(12):805–16.
- .Informing the patient about cardiopulmonary resuscitation: when the risks outweigh the benefits.J Gen Intern Med.1989;4:349–355.
- .Resuscitation of patients with metastatic cancer: Is transient benefit still futile?Arch Intern Med.1991;151:235–239.
- ,,.Cardiopulmonary resuscitation on television—miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- ,,,.Resuscitation decision making in the elderly: the value of outcome data.J Gen Intern Med.1993;8:295–300.
- .Discussing do‐not‐resuscitate status.J Clin Oncol.2001;19:1576–1581.
- .Do‐not‐resuscitate decisions in a community hospital. Incidence, implications, and outcomes.JAMA.1986;256:1164–1169.
- ,,,.A prospective study of the efficacy of the physician order form for life‐sustaining treatment.J Am Geriatr Soc.1998;46:1170–1171.
- ,,, for theACPAE‐o‐LCCP.Discussing palliative care with patients.Ann Intern Med.1999;130:744–749.
- ,,,,.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55(3):164–177.
- ,,, et al.“Let me see if I have this right…”: words that help build empathy.Ann Intern Med.2001;135(3):221–227.
- ,,.“I wish things were different”: expressing wishes in response to loss, futility, and unrealistic hopes.Ann Intern Med.2001;135:551–555.
- ,,.Hope for the best, and prepare for the worst.Ann Intern Med.2003;138:439–443.
- ,,,.When the treatment goal is not cure: are cancer patients equipped to make informed decisions?J Clin Oncol.2002;20:503–513.
- ,,.Beyond autonomy: Diversifying end‐of‐life decision‐making approaches to serve patients and families.J Am Geriatr Soc.2005;53:1046–1050.
- ,,,.Measuring patients' desire for autonomy: decision making and information‐seeking preferences among medical patients.J Gen Intern Med.1989;4(1):23–30.
- ,.Physician recommendations and patient autonomy: finding a balance between physician power and patient choice.Ann Intern Med.1996;125:763–769.
- ,,,.Opening the black box: how do physicians communicate about advance directives?Ann Intern Med.1998;129:441–449.
- ,,, et al.Physician understanding of patient resuscitation preferences: insights and clinical implications.J Am Geriatr Soc.2000;48:S44–S51.
- ,,,,.Patient knowledge and physician predictions of treatment preferences after discussion of advance directives.J Gen Intern Med.1998;13:447–454.
- ,,.The value of disease severity in predicting patient readiness to address end‐of‐life issues.Arch Intern Med.2003;163:609–612.
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).The SUPPORT Principal Investigators.JAMA.1995;274:1591–1598.
- ,,,,,.Can we talk? Inpatient discussions about advance directives in a community hospital. Attending physicians' attitudes, their inpatients' wishes and reported evidence.Arch Intern Med.1994;154:2299–2308.
- ,,,,.Advance directives for medical care—a case for greater use.N Engl J Med.1991;324:889–895.
- ,,.Patient attitudes to discussing life‐sustaining treatment.Arch Intern Med.1986;146:1613–1615.
- ,.Paradoxes in cancer patients' advance care planning.J Palliat Med.2000;3(1):27–35.
- ,,.The inner life of physicians and care of the seriously ill.JAMA.2001;286:3007–3014.
- ,,.The discussion about advance directives; patient and physician opinions regarding when and how it should be conducted.Arch Intern Med.1995;155:1025–1030.
- .Psychological considerations, growth, and transcendence at the end of life: the art of the possible.JAMA.2001;285:2898–2905.
- .Care of dying patients: beyond symptom management.West J Med.1999;171(4):253–256.
- ,,,.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,,,,,.Promoting advance directives among elderly primary care patients.J Gen Intern Med.2004;19:944–951.
- ,,,,.Implementing advance directives in the primary care setting.Arch Intern Med.1994;154:2321–2327.
- ,.Will outpatients complete living wills? A comparison of two interventions.J Gen Intern Med.1991;6:41–46.
- ,,, et al.Enhancement of proxy appointments for older persons: physician counselling in ambulatory settings.J Am Geriatr Soc.1996;44(1):37–43.
- ,,,.Strategies to promote the use of advance directives in a residency outpatient practice.J Gen Intern Med.1996;11:657–663.
- ,,,,,.Promoting inpatient directives about life‐sustaining treatments in a community hospital. Results of a 3‐year time‐series intervention trial.Arch Intern Med.1995;155:2317–2323.
- ,,,,.An educational intervention in the surgical ICU to improve ethical decisions.Surgery.1995;118(2):294–299.
- ,,.The status of medical education in end‐of‐life care. A national report.J Gen Intern Med.2003;18:685–695.
- ,.ACGME Requirements for end‐of‐life training in selected residency and fellowship programs: a status report.Acad Med.2002;77(4):299–304.
- ,,,.End‐of‐life care education in internal medicine residency programs: an interinstitutional study.J Palliat Med.2002;5:487–496.
- ,,,,,.End‐of‐life care in the curriculum: a national study of medical education deans.Acad Med.2004;79:760–768.
- ,,,,.Assessing medical students' training in end‐of‐life communication: a survey of interns at one urban teaching hospital.Acad Med.2003;78:530–537.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national surveyAm J Med.2001;111(3):247–254.
- ,.Legal and ethical myths about informed consent.Arch Intern Med.1996;156:2521–2526.
- ,,.Physician reluctance to discuss advance directives: an empiric investigation of potential barriers.Arch Intern Med.1994;154:2311–2318.
- ,,.How do medical residents discuss resuscitation with patients?J Gen Intern Med.1995;10:436–442.
- ,,,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.Br Med J.2000;320:469–473.
- .Death Foretold; Prophecy and Prognosis in Medical Care.Chicago:University of Chicago Press,1999.
- ,.Attitude and self‐reported practice regarding prognostication in a national sample of internists.Arch Intern Med.1998;158:2389–2395.
- ,,, et al.Prognostic factors for survival in patients treated in phase I clinical trials.Cancer.1994;74:1965–1973.
- ,,, et al.Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric studyCancer.1995;75:2613–2622.
- ,,.Evaluation of patients with advanced cancer using Karnovsky performance status.Cancer.1980;45:2220–2224.
- ,,,.The Karnofsky Performance Status Scale. An exam of its reliability and validity in a research setting.Cancer.1984;53:2002–2007.
- .A demographic and prognostic approach to defining the end of life.J Palliat Med.2005;8(suppl 1):s12–s21.
- .Estimating length of survival in end‐stage cancer: a review of the literature.J Pain Symptom Manage.1995;10:548–555.
- ,.Prognostic factors, survival, and advanced cancer.J Palliat Care.1992;1992(8):4.
- ,,.A new palliative prognostic score: a first step for the staging of terminally ill cancer patients.J Pain Symp Management1999;17(4):231–239.
- ,,.Survival prediction in terminal cancer patients: A systematic review of the medical literature.Palliat Med.2000;14:363–374.
- ,,.Clinical symptoms and length of survival in patients with terminal cancer.Arch Intern Med.1988;148:1586–1591.
- ,,.Successful validation of the palliative prognostic score in terminally ill cancer patients.J Pain Symptom Manage.1999;17:240–247.
- ,,.The use of the palliative prognostic score in patients with diagnoses other than cancer.J Pain Symptom Manage.2003;26:883–885.
- ,,.Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer.J Clin Oncol.2004;22:4823–4828.
- ,,,.Survival after in‐hospital cardiopulmonary resuscitation: a meta‐analysis.J Gen Intern Med,1998;13(12):805–16.
- .Informing the patient about cardiopulmonary resuscitation: when the risks outweigh the benefits.J Gen Intern Med.1989;4:349–355.
- .Resuscitation of patients with metastatic cancer: Is transient benefit still futile?Arch Intern Med.1991;151:235–239.
- ,,.Cardiopulmonary resuscitation on television—miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- ,,,.Resuscitation decision making in the elderly: the value of outcome data.J Gen Intern Med.1993;8:295–300.
- .Discussing do‐not‐resuscitate status.J Clin Oncol.2001;19:1576–1581.
- .Do‐not‐resuscitate decisions in a community hospital. Incidence, implications, and outcomes.JAMA.1986;256:1164–1169.
- ,,,.A prospective study of the efficacy of the physician order form for life‐sustaining treatment.J Am Geriatr Soc.1998;46:1170–1171.
- ,,, for theACPAE‐o‐LCCP.Discussing palliative care with patients.Ann Intern Med.1999;130:744–749.
- ,,,,.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55(3):164–177.
- ,,, et al.“Let me see if I have this right…”: words that help build empathy.Ann Intern Med.2001;135(3):221–227.
- ,,.“I wish things were different”: expressing wishes in response to loss, futility, and unrealistic hopes.Ann Intern Med.2001;135:551–555.
- ,,.Hope for the best, and prepare for the worst.Ann Intern Med.2003;138:439–443.
- ,,,.When the treatment goal is not cure: are cancer patients equipped to make informed decisions?J Clin Oncol.2002;20:503–513.
- ,,.Beyond autonomy: Diversifying end‐of‐life decision‐making approaches to serve patients and families.J Am Geriatr Soc.2005;53:1046–1050.
- ,,,.Measuring patients' desire for autonomy: decision making and information‐seeking preferences among medical patients.J Gen Intern Med.1989;4(1):23–30.
- ,.Physician recommendations and patient autonomy: finding a balance between physician power and patient choice.Ann Intern Med.1996;125:763–769.
Editorial
It is right and fitting that an article focused on palliative care appears in the inaugural issue of the Journal of Hospital Medicine (JHM).1 Both hospital medicine and palliative care are rapidly growing fields expanding in response to quality and economic imperatives. Both fields recognize the need to develop systems to care for seriously ill patients and to work within interdisciplinary teams. In fact, a natural and mutually beneficial relationship should exist between these two fields. For palliative care, hospital medicine and hospitalists offer the physicians and systems approach to care that could guarantee access to high‐quality palliative care for all hospitalized patients. In addition, hospitalists offer the promise of increasing the number of hospital‐based palliative care programs as the presence of a hospitalist program is strongly associated with having or starting such a program.2, 3 For hospital medicine and hospitalists, palliative care offers a compassionate and high‐quality response to the challenge of caring for seriously and terminally ill patients and their families. By each embracing the other, both fields could find willing and eager partners in the quest to provide the highest possible quality of care for hospitalized patients.
In this first issue of JHM, Dr. Meier offers hospitalists an intriguing and attractive picture of palliative care. She describes how the growth of palliative care is driven by the needs of an ever‐larger group of patients living with chronic and life‐threatening illness and evidence of high quality and satisfaction for these patients who have many physical, emotional, psychological, and spiritual concerns. Dr. Meier also demonstrates how hospital‐based palliative care can coordinate with hospices to provide the continuity of care for terminally ill patients that is often elusive at hospital discharge. Finally, Dr. Meier provides a practical list of resources for clinicians seeking further training in the field. No doubt hospitalists will appreciate this list as the core competencies in hospital medicine, published as a supplement to this issue of JHM, include palliative care, pain management, communication, and discharge planning.
As Dr. Meier states in her article Palliative Care in Hospitals, many types of clinicians can provide palliative care in hospitals, including general internists, nurses, geriatricians, oncologists, hospitalists, and others, yet hospitalists are likely to emerge as the predominant providers of palliative care to hospitalized patients.4 That 75% of Americans die in institutionalized settings, where hospitalists are becoming the dominant providers of care, will drive this prediction.5 In addition, hospitalists are increasingly leading efforts in quality improvement, patient satisfaction, and patient safety.6 Of necessity these initiatives will involve the sickest hospitalized patients and will look to palliative care as a proven response for improving quality and increasing satisfaction.
Hospital medicine and palliative care have other aspects in common that make a melding of the two fields beneficial. Both fields recognize and emphasize the need for interdisciplinary care; good communication between members of the health care team and between health care providers and patients; and timely, effective, and responsible discharge planning. Finally, both fields often rely on multiple sources of funding including professional fee billing and support from the hospital for the added value that programs provide. Sharing so many issues in common should help hospital medicine and palliative care form strong links.
For these links to take hold and for the benefits of this partnership to bear fruit, members of both fields, and especially those with a foot in each, need to reach out. For hospitalists this means getting educated in palliative care, an area for which hospitalists recognize they are underprepared.7 Each hospitalist must be able to provide primary, basic palliative care to each patient.8 Some hospitalists will discover the rewards of palliative care and seek further training and even board certification. These hospitalists can start or join palliative care teams in their institutions. Finally, some hospitalists will become experts in palliative care and join or lead palliative care programs at tertiary care centers. In turn, palliative care providers must reach out to hospitalists. Palliative care clinicians should seek out hospitalists at their institutions and hospices should contact hospitalists at their local hospitals. These programs need to invite hospitalists to participate in the palliative care team and suggest how their services can help the patients of hospitalists. This natural alliance can come about only if both sides reach out.
A partnership between palliative care and hospital medicine will be good for patients and their families as well as for each field, as hospitalists enable realization of the goal of providing palliative care to every patient in the United States. In addition, this partnership will be good for hospitalists who embrace this work. Palliative care can connect us to the humanism and compassion that brought so many of us to medicine and can serve as an antidote to burnout. Furthermore, by caring for patients with life‐threatening illnesses we remember that our time is limited and that each day is a gift. We recognize the importance of making the most of our time regardless of how long we have and of choosing carefully how and with whom we spend our time.
In this first issue of JHM, Dr. Meier makes a strong argument for the need and continued growth of palliative care in hospitals, lays out a strategy for achieving this growth through education and program development, and in doing so, opens the door to hope for the future. Through palliative care we can offer patients hope for healing when cure is not possible, for comfort in the face of suffering, and for what can still be despite all that cannot. The possibility that hospitalists could provide all patients access to palliative care is cause enough for hope. The knowledge that hospitalists will play a major role in making this possibility a reality and may become the predominant providers of palliative care can make that hope a reality.
- .Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- ,.Prevalence and structure of palliative care services in California hospitals.Arch Intern Med.2003;163:1084–1088.
- ,,,,,.Evaluation of the California Hospital Initiative in Palliative Services (CHIPS).Arch Intern Med. In press.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.Am J Med.2001;111:10S–14S.
- Field MJ,Cassell CK, Eds.Approaching death: improving care at the end of life.Washington, DC:National Academy Press,1997.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- .Secondary and tertiary palliative care in US hospitals.JAMA.2002;287:875–881.
It is right and fitting that an article focused on palliative care appears in the inaugural issue of the Journal of Hospital Medicine (JHM).1 Both hospital medicine and palliative care are rapidly growing fields expanding in response to quality and economic imperatives. Both fields recognize the need to develop systems to care for seriously ill patients and to work within interdisciplinary teams. In fact, a natural and mutually beneficial relationship should exist between these two fields. For palliative care, hospital medicine and hospitalists offer the physicians and systems approach to care that could guarantee access to high‐quality palliative care for all hospitalized patients. In addition, hospitalists offer the promise of increasing the number of hospital‐based palliative care programs as the presence of a hospitalist program is strongly associated with having or starting such a program.2, 3 For hospital medicine and hospitalists, palliative care offers a compassionate and high‐quality response to the challenge of caring for seriously and terminally ill patients and their families. By each embracing the other, both fields could find willing and eager partners in the quest to provide the highest possible quality of care for hospitalized patients.
In this first issue of JHM, Dr. Meier offers hospitalists an intriguing and attractive picture of palliative care. She describes how the growth of palliative care is driven by the needs of an ever‐larger group of patients living with chronic and life‐threatening illness and evidence of high quality and satisfaction for these patients who have many physical, emotional, psychological, and spiritual concerns. Dr. Meier also demonstrates how hospital‐based palliative care can coordinate with hospices to provide the continuity of care for terminally ill patients that is often elusive at hospital discharge. Finally, Dr. Meier provides a practical list of resources for clinicians seeking further training in the field. No doubt hospitalists will appreciate this list as the core competencies in hospital medicine, published as a supplement to this issue of JHM, include palliative care, pain management, communication, and discharge planning.
As Dr. Meier states in her article Palliative Care in Hospitals, many types of clinicians can provide palliative care in hospitals, including general internists, nurses, geriatricians, oncologists, hospitalists, and others, yet hospitalists are likely to emerge as the predominant providers of palliative care to hospitalized patients.4 That 75% of Americans die in institutionalized settings, where hospitalists are becoming the dominant providers of care, will drive this prediction.5 In addition, hospitalists are increasingly leading efforts in quality improvement, patient satisfaction, and patient safety.6 Of necessity these initiatives will involve the sickest hospitalized patients and will look to palliative care as a proven response for improving quality and increasing satisfaction.
Hospital medicine and palliative care have other aspects in common that make a melding of the two fields beneficial. Both fields recognize and emphasize the need for interdisciplinary care; good communication between members of the health care team and between health care providers and patients; and timely, effective, and responsible discharge planning. Finally, both fields often rely on multiple sources of funding including professional fee billing and support from the hospital for the added value that programs provide. Sharing so many issues in common should help hospital medicine and palliative care form strong links.
For these links to take hold and for the benefits of this partnership to bear fruit, members of both fields, and especially those with a foot in each, need to reach out. For hospitalists this means getting educated in palliative care, an area for which hospitalists recognize they are underprepared.7 Each hospitalist must be able to provide primary, basic palliative care to each patient.8 Some hospitalists will discover the rewards of palliative care and seek further training and even board certification. These hospitalists can start or join palliative care teams in their institutions. Finally, some hospitalists will become experts in palliative care and join or lead palliative care programs at tertiary care centers. In turn, palliative care providers must reach out to hospitalists. Palliative care clinicians should seek out hospitalists at their institutions and hospices should contact hospitalists at their local hospitals. These programs need to invite hospitalists to participate in the palliative care team and suggest how their services can help the patients of hospitalists. This natural alliance can come about only if both sides reach out.
A partnership between palliative care and hospital medicine will be good for patients and their families as well as for each field, as hospitalists enable realization of the goal of providing palliative care to every patient in the United States. In addition, this partnership will be good for hospitalists who embrace this work. Palliative care can connect us to the humanism and compassion that brought so many of us to medicine and can serve as an antidote to burnout. Furthermore, by caring for patients with life‐threatening illnesses we remember that our time is limited and that each day is a gift. We recognize the importance of making the most of our time regardless of how long we have and of choosing carefully how and with whom we spend our time.
In this first issue of JHM, Dr. Meier makes a strong argument for the need and continued growth of palliative care in hospitals, lays out a strategy for achieving this growth through education and program development, and in doing so, opens the door to hope for the future. Through palliative care we can offer patients hope for healing when cure is not possible, for comfort in the face of suffering, and for what can still be despite all that cannot. The possibility that hospitalists could provide all patients access to palliative care is cause enough for hope. The knowledge that hospitalists will play a major role in making this possibility a reality and may become the predominant providers of palliative care can make that hope a reality.
It is right and fitting that an article focused on palliative care appears in the inaugural issue of the Journal of Hospital Medicine (JHM).1 Both hospital medicine and palliative care are rapidly growing fields expanding in response to quality and economic imperatives. Both fields recognize the need to develop systems to care for seriously ill patients and to work within interdisciplinary teams. In fact, a natural and mutually beneficial relationship should exist between these two fields. For palliative care, hospital medicine and hospitalists offer the physicians and systems approach to care that could guarantee access to high‐quality palliative care for all hospitalized patients. In addition, hospitalists offer the promise of increasing the number of hospital‐based palliative care programs as the presence of a hospitalist program is strongly associated with having or starting such a program.2, 3 For hospital medicine and hospitalists, palliative care offers a compassionate and high‐quality response to the challenge of caring for seriously and terminally ill patients and their families. By each embracing the other, both fields could find willing and eager partners in the quest to provide the highest possible quality of care for hospitalized patients.
In this first issue of JHM, Dr. Meier offers hospitalists an intriguing and attractive picture of palliative care. She describes how the growth of palliative care is driven by the needs of an ever‐larger group of patients living with chronic and life‐threatening illness and evidence of high quality and satisfaction for these patients who have many physical, emotional, psychological, and spiritual concerns. Dr. Meier also demonstrates how hospital‐based palliative care can coordinate with hospices to provide the continuity of care for terminally ill patients that is often elusive at hospital discharge. Finally, Dr. Meier provides a practical list of resources for clinicians seeking further training in the field. No doubt hospitalists will appreciate this list as the core competencies in hospital medicine, published as a supplement to this issue of JHM, include palliative care, pain management, communication, and discharge planning.
As Dr. Meier states in her article Palliative Care in Hospitals, many types of clinicians can provide palliative care in hospitals, including general internists, nurses, geriatricians, oncologists, hospitalists, and others, yet hospitalists are likely to emerge as the predominant providers of palliative care to hospitalized patients.4 That 75% of Americans die in institutionalized settings, where hospitalists are becoming the dominant providers of care, will drive this prediction.5 In addition, hospitalists are increasingly leading efforts in quality improvement, patient satisfaction, and patient safety.6 Of necessity these initiatives will involve the sickest hospitalized patients and will look to palliative care as a proven response for improving quality and increasing satisfaction.
Hospital medicine and palliative care have other aspects in common that make a melding of the two fields beneficial. Both fields recognize and emphasize the need for interdisciplinary care; good communication between members of the health care team and between health care providers and patients; and timely, effective, and responsible discharge planning. Finally, both fields often rely on multiple sources of funding including professional fee billing and support from the hospital for the added value that programs provide. Sharing so many issues in common should help hospital medicine and palliative care form strong links.
For these links to take hold and for the benefits of this partnership to bear fruit, members of both fields, and especially those with a foot in each, need to reach out. For hospitalists this means getting educated in palliative care, an area for which hospitalists recognize they are underprepared.7 Each hospitalist must be able to provide primary, basic palliative care to each patient.8 Some hospitalists will discover the rewards of palliative care and seek further training and even board certification. These hospitalists can start or join palliative care teams in their institutions. Finally, some hospitalists will become experts in palliative care and join or lead palliative care programs at tertiary care centers. In turn, palliative care providers must reach out to hospitalists. Palliative care clinicians should seek out hospitalists at their institutions and hospices should contact hospitalists at their local hospitals. These programs need to invite hospitalists to participate in the palliative care team and suggest how their services can help the patients of hospitalists. This natural alliance can come about only if both sides reach out.
A partnership between palliative care and hospital medicine will be good for patients and their families as well as for each field, as hospitalists enable realization of the goal of providing palliative care to every patient in the United States. In addition, this partnership will be good for hospitalists who embrace this work. Palliative care can connect us to the humanism and compassion that brought so many of us to medicine and can serve as an antidote to burnout. Furthermore, by caring for patients with life‐threatening illnesses we remember that our time is limited and that each day is a gift. We recognize the importance of making the most of our time regardless of how long we have and of choosing carefully how and with whom we spend our time.
In this first issue of JHM, Dr. Meier makes a strong argument for the need and continued growth of palliative care in hospitals, lays out a strategy for achieving this growth through education and program development, and in doing so, opens the door to hope for the future. Through palliative care we can offer patients hope for healing when cure is not possible, for comfort in the face of suffering, and for what can still be despite all that cannot. The possibility that hospitalists could provide all patients access to palliative care is cause enough for hope. The knowledge that hospitalists will play a major role in making this possibility a reality and may become the predominant providers of palliative care can make that hope a reality.
- .Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- ,.Prevalence and structure of palliative care services in California hospitals.Arch Intern Med.2003;163:1084–1088.
- ,,,,,.Evaluation of the California Hospital Initiative in Palliative Services (CHIPS).Arch Intern Med. In press.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.Am J Med.2001;111:10S–14S.
- Field MJ,Cassell CK, Eds.Approaching death: improving care at the end of life.Washington, DC:National Academy Press,1997.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- .Secondary and tertiary palliative care in US hospitals.JAMA.2002;287:875–881.
- .Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- ,.Prevalence and structure of palliative care services in California hospitals.Arch Intern Med.2003;163:1084–1088.
- ,,,,,.Evaluation of the California Hospital Initiative in Palliative Services (CHIPS).Arch Intern Med. In press.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.Am J Med.2001;111:10S–14S.
- Field MJ,Cassell CK, Eds.Approaching death: improving care at the end of life.Washington, DC:National Academy Press,1997.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- .Secondary and tertiary palliative care in US hospitals.JAMA.2002;287:875–881.