User login
Reproductive planning for women after solid-organ transplant
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
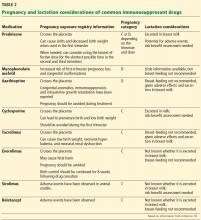
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
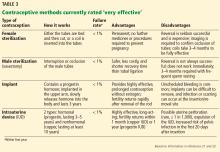
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
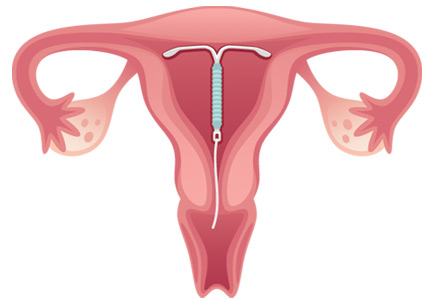
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
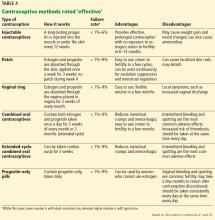
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
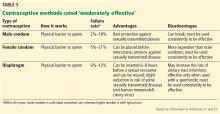
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
KEY POINTS
- The number of solid-organ transplants in US women of childbearing age has increased over the past 20 years.
- Women should wait at least 1 year after receiving a solid-organ transplant before attempting to become pregnant, and then should do so only when cleared by the transplant team and obstetrician, with close monitoring.
- The various types of contraception can be grouped by their effectiveness and by the medical eligibility criteria set by the US Centers for Disease Control and Prevention.
- Transplant recipients of childbearing age should use 2 contraceptive methods concurrently, one of which should be condoms.