User login
Progress in Management of Advanced Acute Lymphocytic Leukemia in Children
Incidence peaks in children aged 1-4 years, decreasing thereafter. Cases are highest among Native American/Alaskan Native and Hispanic children, and higher in White than Black children.4 ALL is seen more in patients with certain inherited conditions, including Down syndrome, ataxia telangiectasia, neurofibromatosis type 1, and Bloom syndrome.1
Treatment advances have improved remission rates and outcomes for patients. However, relapse is still a leading cause of death for patients of all ages.6 Prompt diagnosis and care are important to optimize outcomes, as treatment delay is associated with poorer survival.7
Pathophysiology
In ALL, abnormal, immature lymphocytes and progenitor B cells/T cells proliferate uncontrollably and eventually replace healthy cells in bone marrow and the lymphatic system. The loss of healthy cells leads to classic symptoms of cytopenia, splenomegaly, and hepatomegaly.1 B cells and T cells are descended from lymphoid stem cells (and are transformed by germline or somatic mutation into pathogenic cells, leading to symptom development and bone marrow dysfunction. Most pediatric patients have extensive bone marrow involvement at diagnosis, with > 25% blast cells in marrow (defined as M3 disease).4
Presentation
Patients usually present with signs and symptoms that are related to disease-associated anemia, thrombocytopenia, or neutropenia; these signs and symptoms may include fatigue or weakness, pale skin, bleeding or bruising easily, fever or infection, joint or extremity pain, B-cell symptoms such as night sweats or unintentional weight loss, and splenomegaly or hepatomegaly. Central nervous system (CNS) symptoms can include stroke-like symptoms due to leukemic cell invasion of CNS vasculature or neuropathies related to increased intracranial pressure. Sometimes, children may present with no symptoms other than joint or extremity pain.1,3,8
Classification
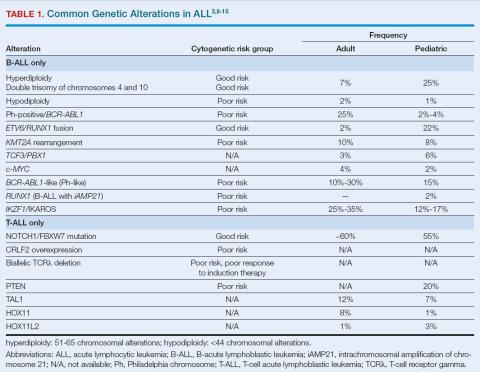
ALL is classified by whether it derives from B-cell or T-cell progenitor cells and, within these, by typical genetic alterations (Table 1).3,9-15 Some cytogenetics are associated with risk assessment as well. Well-identified B-ALL subtypes include Philadelphia (Ph) chromosome-positive, hyper- and hypodiploidy, and KMT2A rearranged, while newer classifications include Ph-like ALL and B-lymphoblastic leukemia with iAMP21. Provisional T-ALL subtypes include early T-cell precursor lymphoblastic leukemia and natural killer cell lymphoblastic leukemia.3
B-cell lineage is present in 88% of pediatric and 75%-80% of adult disease. T-ALL is found in about 12% of pediatric patients and 25% of adults.3,8 Familial syndromes associated with ALL are present in about 4% of pediatric patients, including autosomal dominant germline mutations in RUNX1 (T-cell ALL), ETV6 (B-ALL), PAX5 (B-ALL), IKZF1 (B-ALL and T-ALL), and TP53 (low-hypodiploid ALL).3 If a known-familial genotype is identified, families should be referred for genetic counseling and further testing if needed. If germline mutation is suspected, early identification is important; hereditary ALL can influence treatment choice and use of allogeneic transplantation or radiation.3
A third classification crucial to guiding treatment is Ph-positive vs Ph-negative or Ph-like, the latter strongly associated with abnormal B-cell development due to deletions in related genes.3,16 About 3% to 5% of pediatric patients and 25% of adults have Ph-positive ALL.17 The remission failure rate among pediatric patients treated with chemotherapy was 11% in one study, vs 2%-3% among patients with Ph-negative ALL.10
Diagnosis and Risk Stratification
Diagnosis is based on presentation and molecular features, requiring demonstration of ≥ 20% lymphoblasts in bone marrow biopsy or aspirate or ≥ 1,000 circulating lymphoblasts/mL in peripheral blood. Testing can include immunophenotyping using flow cytometry, molecular characterization of baseline leukemic clone, morphology using hematoxylin and eosin staining and Wright/Giemsa staining, and karyotyping.1,3 CNS involvement is assessed using a lumbar spinal tap.1
Risk stratification is based on molecular features (eg, high- and low-risk mutations, Table 1),3,9-15 which are assessed using fluorescence in-situ hybridization, broad-panel next-generation sequencing, and reverse-transcriptase polymerase chain reaction of bone marrow or peripheral blood.3 Other risk factors include age, CNS involvement, white blood cell (WBC) count, and response to initial induction or consolidation therapy.3
Pediatric patients are assigned standard or high risk based on factors identified by the Children’s Oncology Group and National Comprehensive Cancer Network (NCCN). Patients
aged 1 to < 10 years with WBC < 50 × 109/L are considered standard risk, and all others are considered high risk. Patients with ALL before age 1 have very high risk. All pediatric patients with T-ALL are considered high risk.3 Ph-positive, Ph-like, hypoploidy, failure to achieve remission with induction, and extramedullary disease are high-risk factors as well, whereas hyperploidy and certain mutations convey low risk.3
Newer treatment strategies for initial ALL diagnosis include targeted therapies. One goal of targeted therapy is avoidance of long-term toxicity, leading to improved survival outcomes. Well-studied targeted therapies include the tyrosine kinase inhibitors used in first-line and subsequent treatment of Ph-positive ALL.3
Treatment Options in Relapsed/Refractory ALL
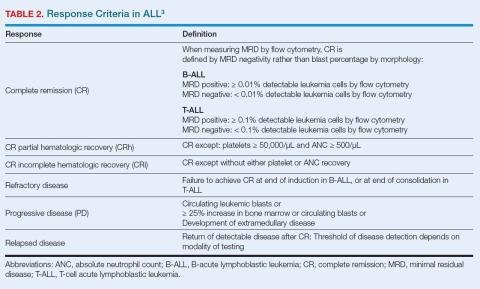
The initial treatment goal is complete remission (CR) defined as minimal residual disease (MRD) < 0.01% on flow cytometry (Table 2).3 Prognosis is dependent on time and location of relapse. Early relapse (< 18 months from diagnosis) predicts poor survival. Relapse in bone marrow is associated with poorer prognosis than relapse in CNS.11-18 Where possible, consolidation with allogeneic hematopoietic cell transplantation improves survival for patients with early relapse.6 Three approaches have advanced treatment options for relapsed/refractory (R/R) B-ALL, all based around common cell markers seen in B-ALL.
The CD22-directed antibody-drug conjugate inotuzumab ozogamicin is approved for adults with R/R B-ALL. In clinical trials, a higher percentage of patients had results below the MRD threshold, and longer progression-free survival and OS compared with standard care.19,20
Blinatumomab is a bispecific T-cell engager that binds to CD19 on the surface of B-ALL cells and to CD3 on T cells to trigger apoptosis.21 It was first approved for R/R ALL in adults or children, and is also now approved for treatment in remission with MRD ≥ 0.1%. Patients must demonstrate CD19-positive disease to qualify.15-22 For R/R ALL, blinatumomab improves OS and CR rates compared with standard chemotherapy.23
The use of CAR T-cell therapies has expanded greatly with increasing knowledge about their efficacy and safety. In R/R ALL, tisagenlecleucel (tisa-gen) is approved for treatment of patients aged ≤ 25 years, and brexucabtagene autoleucel (brexucel) is approved for treatment of adults.3,24,25 Patients undergoing the CAR T-cell process have apheresis to collect T cells, which are then manufactured before being reinfused into the patient. Depending on local capabilities, the time between T-cell harvest and reinfusion can extend to weeks.3,26,27 Cytoreduction with CAR T-cell therapy can allow previously ineligible patients (due to bulky disease) to undergo transplant. Patients treated in key clinical trials with tisa-gen or brexu-cel achieved high overall remission rates and improved event-free survival and OS rates compared with historical experience.25,28,29 Important toxicities with CAR T-cell therapy are cytokine release syndrome (CRS) and neurotoxicity, which can develop rapidly. NCCN recommends hospitalizing patients at the first sign of either adverse event. Patients can be managed with tocilizumab or steroids for low-grade CRS or steroids for neurotoxicity. The Society for Immunotherapy of Cancer, American Society of Clinical Oncology, and NCCN have guidelines on management of toxicities related to CAR T-cell therapy as well as management of symptoms and other adverse effects of CRS.5,23,24
Programs also incorporate telemedicine for symptom monitoring and follow-up.32-34 Centers providing CAR T-cell therapy must have a certified Risk Evaluation and Mitigation Strategy (REMS), which ensures adherence to specific guidelines for administration, adverse event management, and patient education.35,36 Overcoming technical, social, and financial barriers to CAR T-cell therapy is an ongoing challenge of great interest.37
R/R T-Cell Precursor ALL
Patients with R/R T-ALL have poor prognosis, partly due to limited treatment options. Nelarabine, a nucleoside analog, is the only approved treatment for R/R T-ALL, but has increasingly been used in first-line therapy added to multiagent chemotherapy as a consolidation and maintenance approach to pediatric disease.3,38,39 Four-year DSF in pediatric patients with newly diagnosed T-ALL undergoing treatment incorporating nelarabine was 88.9%.39 Treatment is associated with grade ≥ 3 neurotoxicity in > 10% of patients, and can include CNS toxicity as well as neuropathy.3
In a recently completed phase 2 trial (NCT03384654), daratumumab was added to standard chemotherapy (vincristine, prednisone, PEG-asparaginase, doxorubicin) for R/R T-ALL in pediatric (ages 1-17 years) and young adult patients (age ≥ 18 years).40 Among 24 pediatric patients, CR was 41.7% and overall response rate (ORR; ORR = CR + CRi) was 83% after 1 cycle of treatment. Ten (41.7%) pediatric patients achieved MRD-negative status as well. ORR was 60% in the 5 older patients. All pediatric patients had at least 1 grade ≥ 3 toxicity, but none of the adverse events led to discontinuation.40
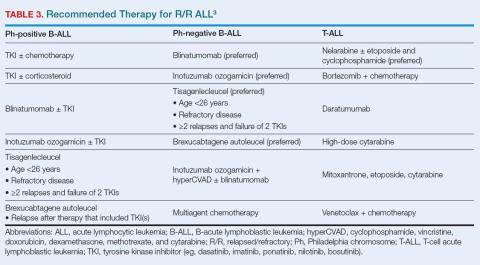
Success in achieving MRD-negative responses in patients treated for R/R ALL has increased interest in using targeted therapies for newly diagnosed patients. Recommended treatment approaches are summarized in Table 3.3
Long-Term Follow-Up and Survivorship
A study of > 500 pediatric patients followed for an average 23 years reassuringly found low prevalence of adverse outcomes related to disease or treatment. Major adverse outcomes such as death due to late relapse; secondary malignancy; or development of osteoporosis, cataracts, and diminished functional status were infrequent.41 Most prevalent were growth effects (short stature or growth hormone insufficiency), likely related to certain treatment approaches.41 Guidelines for long-term follow-up of pediatric patients are available from the Children’s Oncology Group.42
A 2017 systematic review concluded that the quality of life for survivors is diminished upon treatment, and persistently over time for some patients.43 In contrast, a 2022 comparison of long-term survivors (median 20.5 years since diagnosis) of pediatric ALL with healthy controls found that survivors had better quality of life in some domains, including general health, vitality, and mental health.44 Smaller percentages of survivors rated themselves happiest about sleep quality, absence of pain, and physical abilities.44
As therapy patterns and options evolve, continued follow-up is important to ensure patients derive optimal benefit from treatment and post-treatment life.
- Puckett Y, Chan O. Acute lymphocytic leukemia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated June 27, 2022. Accessed April 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459149/
- Cancer facts & figures 2023. American Cancer Society. 2023. Accessed April 10, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia. Version 1.2022. April 4, 2022. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Childhood acute lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 16, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: management of immunotherapy-related toxicities. Version 1.2023. March 10, 2023. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- DuVall AS, Sheade J, Anderson D, et al. Updates in the management of relapsed and refractory acute lymphoplastic leukemia: an urgent plea for new treatments is being answered! JCO Oncol Pract. 2022;18(7):479-487. doi:10.1200/OP.21.00843
- Baker JM, To T, Beyene J, Zagorski B, Greenberg ML, Sung L. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk Res. 2014;38(2):204-209. doi:10.1016/j.leukres.2013.11.014
- Acute adult lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 24, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, et al. Toward a NOTCH1/FBXW7/RAS/PTEN–based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2013;31(34):4333-4342. doi:10.1200/JCO.2012.48.5292
- Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966-1973. doi:10.1200/JCO.2011.39.7661
- Gao C, Liu SG, Zhang RD, et al. NOTCH1 mutations are associated with favourable long-term prognosis in paediatric T-cell acute lymphoblastic leukaemia: a retrospective study of patients treated on BCH-2003 and CCLG-2008 protocol in China. Br J Haematol. 2014;166(2):221-228. doi:10.1111/bjh.12866
- Yang YL, Hsiao CC, Chen HY, et al. Absence of biallelic TCRγ deletion predicts induction failure and poorer outcomes in childhood T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(6):846-851. doi:10.1002/pbc.24021
- Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3816-3823. doi:10.1200/JCO.2010.28.3390
- Bandapalli OR, Zimmermann M, Kox C, et al. NOTCH1 activation clinically antagonizes the unfavorable effect of PTEN inactivation in BFM-treated children with precursor T-cell acute lymphoblastic leukemia. Haematologica. 2013;98(6):928-936. doi:10.3324/haematol.2012.073585
- Palmi C, Savino AM, Silvestri D, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7(37):59260-59272. doi:10.18632/oncotarget.10610
- Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125-134. doi:10.1016/S1470-2045(08)70339-5
- Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. doi:10.1200/JCO.2010.30.1325
- Nguyen K, Devidas M, Cheng SC, et al.; Children’s Oncology Group. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22(12):2142-2150. doi:10.1038/leu.2008.251
- Besponsa. Prescribing information. Wyeth Pharmaceuticals Inc; 2017. BESPONSA® (inotuzumab ozogamicin) Dosing & Administration |Safety Info (pfizerpro.com)
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. doi:10.1056/NEJMoa1509277
- Lv M, Liu Y, Liu W, Xing Y, Zhang S. Immunotherapy for pediatric acute lymphoblastic leukemia: recent advances and future perspectives. Front Immunol. 2022;13:921894. doi:10.3389/fimmu.2022.921894
- Blincyto. Prescribing information. Amgen; 2022. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Blincyto/blincyto_pi_hcp_english.pdf
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/NEJMoa1609783
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. doi:10.1016/S0140-6736(21)01222-8
- Bhaskar ST, Dholaria BR, Singsayadeth S, Savani BN, Oluwole OO. Role of bridging therapy during chimeric antigen receptor T cell therapy. EJHaem. 2021;3(suppl 1):39-45. doi:10.1002/jha2.335
- Granroth G, Rosenthal A, McCallen M, et al. Supportive care for patients with lymphoma
undergoing CAR-T-cell therapy: the advanced practice provider’s perspective. Curr Oncol Rep. 2022;24(12):1863-1872. doi:10.1007/s11912-022-01330-z - Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphocytic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664-1669. doi:10.1200/JCO.22.00642
- Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. doi:10.1186/s13045-022-01379-0
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi:10.1136/jitc-2020-001511
- Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39(35):3978-3992. doi:10.1200/JCO.21.01992
- Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transpl. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
- LeBar K, Murawski S, Umayam S, Quinn V. The role of advanced practice providers and telemedicine in reinventing care: the transition of a CAR T-cell transplantation program to the outpatient setting. J Adv Pract Oncol. 2020;11(7):757-763. doi:10.6004/jadpro.2020.11.7.8
- Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy for aggressive B-cell lymphomas and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):e002056. doi:10.1136/jitc-2020-002056
- Kymriah. Prescribing information. Novartis Pharmaceuticals Corporation; 2022. https://www.fda.gov/media/107296/download
- Tecartus. Prescribing information. Kite Pharma, Inc; 2021. https://www.fda.gov/media/140409/download
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 2022;18(12):800-807. doi:10.1200/OP.22.00315
- Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135(3):159-166. doi:10.1182/blood.2019001557
- Summers RJ, Teachey DT. SOHO state of the art updates and next questions: novel approaches to pediatric T-cell ALL and T-lymphoblastic lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22(10):718-725. doi:10.1016/j.clml.2022.07.010
- Hogan LE, Bhatla T, Teachey DT, et al. Efficacy and safety of daratumumab (DARA) in pediatric and young adult patients (pts) with relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL): results from the phase 2 DELPHINUS study. J Clin Oncol. 2022;40(16 suppl):Abstract 10001. doi:10.1200/JCO.2022.40.16_suppl.10001
- Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study. Lancet Oncol. 2014;15(8):841-851. doi:10.1016/S1470-2045(14)70265-7
- Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Children’s Oncology Group. October 2018. Accessed April 10, 2023. http://www.survivorshipguidelines.org
- Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9). doi:10.1002/pbc.26489
- Chantziara S, Musoro J, Rowsell AC, et al; European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QLG) and Children’s Leukemia Group (CLG). Quality of life of long-term childhood acute lymphoblastic leukemia survivors: comparison with healthy controls. Psychooncology. 2022;31(12):2159-2168. doi:10.1002/pon.6060
Incidence peaks in children aged 1-4 years, decreasing thereafter. Cases are highest among Native American/Alaskan Native and Hispanic children, and higher in White than Black children.4 ALL is seen more in patients with certain inherited conditions, including Down syndrome, ataxia telangiectasia, neurofibromatosis type 1, and Bloom syndrome.1
Treatment advances have improved remission rates and outcomes for patients. However, relapse is still a leading cause of death for patients of all ages.6 Prompt diagnosis and care are important to optimize outcomes, as treatment delay is associated with poorer survival.7
Pathophysiology
In ALL, abnormal, immature lymphocytes and progenitor B cells/T cells proliferate uncontrollably and eventually replace healthy cells in bone marrow and the lymphatic system. The loss of healthy cells leads to classic symptoms of cytopenia, splenomegaly, and hepatomegaly.1 B cells and T cells are descended from lymphoid stem cells (and are transformed by germline or somatic mutation into pathogenic cells, leading to symptom development and bone marrow dysfunction. Most pediatric patients have extensive bone marrow involvement at diagnosis, with > 25% blast cells in marrow (defined as M3 disease).4
Presentation
Patients usually present with signs and symptoms that are related to disease-associated anemia, thrombocytopenia, or neutropenia; these signs and symptoms may include fatigue or weakness, pale skin, bleeding or bruising easily, fever or infection, joint or extremity pain, B-cell symptoms such as night sweats or unintentional weight loss, and splenomegaly or hepatomegaly. Central nervous system (CNS) symptoms can include stroke-like symptoms due to leukemic cell invasion of CNS vasculature or neuropathies related to increased intracranial pressure. Sometimes, children may present with no symptoms other than joint or extremity pain.1,3,8
Classification
ALL is classified by whether it derives from B-cell or T-cell progenitor cells and, within these, by typical genetic alterations (Table 1).3,9-15 Some cytogenetics are associated with risk assessment as well. Well-identified B-ALL subtypes include Philadelphia (Ph) chromosome-positive, hyper- and hypodiploidy, and KMT2A rearranged, while newer classifications include Ph-like ALL and B-lymphoblastic leukemia with iAMP21. Provisional T-ALL subtypes include early T-cell precursor lymphoblastic leukemia and natural killer cell lymphoblastic leukemia.3
B-cell lineage is present in 88% of pediatric and 75%-80% of adult disease. T-ALL is found in about 12% of pediatric patients and 25% of adults.3,8 Familial syndromes associated with ALL are present in about 4% of pediatric patients, including autosomal dominant germline mutations in RUNX1 (T-cell ALL), ETV6 (B-ALL), PAX5 (B-ALL), IKZF1 (B-ALL and T-ALL), and TP53 (low-hypodiploid ALL).3 If a known-familial genotype is identified, families should be referred for genetic counseling and further testing if needed. If germline mutation is suspected, early identification is important; hereditary ALL can influence treatment choice and use of allogeneic transplantation or radiation.3
A third classification crucial to guiding treatment is Ph-positive vs Ph-negative or Ph-like, the latter strongly associated with abnormal B-cell development due to deletions in related genes.3,16 About 3% to 5% of pediatric patients and 25% of adults have Ph-positive ALL.17 The remission failure rate among pediatric patients treated with chemotherapy was 11% in one study, vs 2%-3% among patients with Ph-negative ALL.10
Diagnosis and Risk Stratification
Diagnosis is based on presentation and molecular features, requiring demonstration of ≥ 20% lymphoblasts in bone marrow biopsy or aspirate or ≥ 1,000 circulating lymphoblasts/mL in peripheral blood. Testing can include immunophenotyping using flow cytometry, molecular characterization of baseline leukemic clone, morphology using hematoxylin and eosin staining and Wright/Giemsa staining, and karyotyping.1,3 CNS involvement is assessed using a lumbar spinal tap.1
Risk stratification is based on molecular features (eg, high- and low-risk mutations, Table 1),3,9-15 which are assessed using fluorescence in-situ hybridization, broad-panel next-generation sequencing, and reverse-transcriptase polymerase chain reaction of bone marrow or peripheral blood.3 Other risk factors include age, CNS involvement, white blood cell (WBC) count, and response to initial induction or consolidation therapy.3
Pediatric patients are assigned standard or high risk based on factors identified by the Children’s Oncology Group and National Comprehensive Cancer Network (NCCN). Patients
aged 1 to < 10 years with WBC < 50 × 109/L are considered standard risk, and all others are considered high risk. Patients with ALL before age 1 have very high risk. All pediatric patients with T-ALL are considered high risk.3 Ph-positive, Ph-like, hypoploidy, failure to achieve remission with induction, and extramedullary disease are high-risk factors as well, whereas hyperploidy and certain mutations convey low risk.3
Newer treatment strategies for initial ALL diagnosis include targeted therapies. One goal of targeted therapy is avoidance of long-term toxicity, leading to improved survival outcomes. Well-studied targeted therapies include the tyrosine kinase inhibitors used in first-line and subsequent treatment of Ph-positive ALL.3
Treatment Options in Relapsed/Refractory ALL
The initial treatment goal is complete remission (CR) defined as minimal residual disease (MRD) < 0.01% on flow cytometry (Table 2).3 Prognosis is dependent on time and location of relapse. Early relapse (< 18 months from diagnosis) predicts poor survival. Relapse in bone marrow is associated with poorer prognosis than relapse in CNS.11-18 Where possible, consolidation with allogeneic hematopoietic cell transplantation improves survival for patients with early relapse.6 Three approaches have advanced treatment options for relapsed/refractory (R/R) B-ALL, all based around common cell markers seen in B-ALL.
The CD22-directed antibody-drug conjugate inotuzumab ozogamicin is approved for adults with R/R B-ALL. In clinical trials, a higher percentage of patients had results below the MRD threshold, and longer progression-free survival and OS compared with standard care.19,20
Blinatumomab is a bispecific T-cell engager that binds to CD19 on the surface of B-ALL cells and to CD3 on T cells to trigger apoptosis.21 It was first approved for R/R ALL in adults or children, and is also now approved for treatment in remission with MRD ≥ 0.1%. Patients must demonstrate CD19-positive disease to qualify.15-22 For R/R ALL, blinatumomab improves OS and CR rates compared with standard chemotherapy.23
The use of CAR T-cell therapies has expanded greatly with increasing knowledge about their efficacy and safety. In R/R ALL, tisagenlecleucel (tisa-gen) is approved for treatment of patients aged ≤ 25 years, and brexucabtagene autoleucel (brexucel) is approved for treatment of adults.3,24,25 Patients undergoing the CAR T-cell process have apheresis to collect T cells, which are then manufactured before being reinfused into the patient. Depending on local capabilities, the time between T-cell harvest and reinfusion can extend to weeks.3,26,27 Cytoreduction with CAR T-cell therapy can allow previously ineligible patients (due to bulky disease) to undergo transplant. Patients treated in key clinical trials with tisa-gen or brexu-cel achieved high overall remission rates and improved event-free survival and OS rates compared with historical experience.25,28,29 Important toxicities with CAR T-cell therapy are cytokine release syndrome (CRS) and neurotoxicity, which can develop rapidly. NCCN recommends hospitalizing patients at the first sign of either adverse event. Patients can be managed with tocilizumab or steroids for low-grade CRS or steroids for neurotoxicity. The Society for Immunotherapy of Cancer, American Society of Clinical Oncology, and NCCN have guidelines on management of toxicities related to CAR T-cell therapy as well as management of symptoms and other adverse effects of CRS.5,23,24
Programs also incorporate telemedicine for symptom monitoring and follow-up.32-34 Centers providing CAR T-cell therapy must have a certified Risk Evaluation and Mitigation Strategy (REMS), which ensures adherence to specific guidelines for administration, adverse event management, and patient education.35,36 Overcoming technical, social, and financial barriers to CAR T-cell therapy is an ongoing challenge of great interest.37
R/R T-Cell Precursor ALL
Patients with R/R T-ALL have poor prognosis, partly due to limited treatment options. Nelarabine, a nucleoside analog, is the only approved treatment for R/R T-ALL, but has increasingly been used in first-line therapy added to multiagent chemotherapy as a consolidation and maintenance approach to pediatric disease.3,38,39 Four-year DSF in pediatric patients with newly diagnosed T-ALL undergoing treatment incorporating nelarabine was 88.9%.39 Treatment is associated with grade ≥ 3 neurotoxicity in > 10% of patients, and can include CNS toxicity as well as neuropathy.3
In a recently completed phase 2 trial (NCT03384654), daratumumab was added to standard chemotherapy (vincristine, prednisone, PEG-asparaginase, doxorubicin) for R/R T-ALL in pediatric (ages 1-17 years) and young adult patients (age ≥ 18 years).40 Among 24 pediatric patients, CR was 41.7% and overall response rate (ORR; ORR = CR + CRi) was 83% after 1 cycle of treatment. Ten (41.7%) pediatric patients achieved MRD-negative status as well. ORR was 60% in the 5 older patients. All pediatric patients had at least 1 grade ≥ 3 toxicity, but none of the adverse events led to discontinuation.40
Success in achieving MRD-negative responses in patients treated for R/R ALL has increased interest in using targeted therapies for newly diagnosed patients. Recommended treatment approaches are summarized in Table 3.3
Long-Term Follow-Up and Survivorship
A study of > 500 pediatric patients followed for an average 23 years reassuringly found low prevalence of adverse outcomes related to disease or treatment. Major adverse outcomes such as death due to late relapse; secondary malignancy; or development of osteoporosis, cataracts, and diminished functional status were infrequent.41 Most prevalent were growth effects (short stature or growth hormone insufficiency), likely related to certain treatment approaches.41 Guidelines for long-term follow-up of pediatric patients are available from the Children’s Oncology Group.42
A 2017 systematic review concluded that the quality of life for survivors is diminished upon treatment, and persistently over time for some patients.43 In contrast, a 2022 comparison of long-term survivors (median 20.5 years since diagnosis) of pediatric ALL with healthy controls found that survivors had better quality of life in some domains, including general health, vitality, and mental health.44 Smaller percentages of survivors rated themselves happiest about sleep quality, absence of pain, and physical abilities.44
As therapy patterns and options evolve, continued follow-up is important to ensure patients derive optimal benefit from treatment and post-treatment life.
Incidence peaks in children aged 1-4 years, decreasing thereafter. Cases are highest among Native American/Alaskan Native and Hispanic children, and higher in White than Black children.4 ALL is seen more in patients with certain inherited conditions, including Down syndrome, ataxia telangiectasia, neurofibromatosis type 1, and Bloom syndrome.1
Treatment advances have improved remission rates and outcomes for patients. However, relapse is still a leading cause of death for patients of all ages.6 Prompt diagnosis and care are important to optimize outcomes, as treatment delay is associated with poorer survival.7
Pathophysiology
In ALL, abnormal, immature lymphocytes and progenitor B cells/T cells proliferate uncontrollably and eventually replace healthy cells in bone marrow and the lymphatic system. The loss of healthy cells leads to classic symptoms of cytopenia, splenomegaly, and hepatomegaly.1 B cells and T cells are descended from lymphoid stem cells (and are transformed by germline or somatic mutation into pathogenic cells, leading to symptom development and bone marrow dysfunction. Most pediatric patients have extensive bone marrow involvement at diagnosis, with > 25% blast cells in marrow (defined as M3 disease).4
Presentation
Patients usually present with signs and symptoms that are related to disease-associated anemia, thrombocytopenia, or neutropenia; these signs and symptoms may include fatigue or weakness, pale skin, bleeding or bruising easily, fever or infection, joint or extremity pain, B-cell symptoms such as night sweats or unintentional weight loss, and splenomegaly or hepatomegaly. Central nervous system (CNS) symptoms can include stroke-like symptoms due to leukemic cell invasion of CNS vasculature or neuropathies related to increased intracranial pressure. Sometimes, children may present with no symptoms other than joint or extremity pain.1,3,8
Classification
ALL is classified by whether it derives from B-cell or T-cell progenitor cells and, within these, by typical genetic alterations (Table 1).3,9-15 Some cytogenetics are associated with risk assessment as well. Well-identified B-ALL subtypes include Philadelphia (Ph) chromosome-positive, hyper- and hypodiploidy, and KMT2A rearranged, while newer classifications include Ph-like ALL and B-lymphoblastic leukemia with iAMP21. Provisional T-ALL subtypes include early T-cell precursor lymphoblastic leukemia and natural killer cell lymphoblastic leukemia.3
B-cell lineage is present in 88% of pediatric and 75%-80% of adult disease. T-ALL is found in about 12% of pediatric patients and 25% of adults.3,8 Familial syndromes associated with ALL are present in about 4% of pediatric patients, including autosomal dominant germline mutations in RUNX1 (T-cell ALL), ETV6 (B-ALL), PAX5 (B-ALL), IKZF1 (B-ALL and T-ALL), and TP53 (low-hypodiploid ALL).3 If a known-familial genotype is identified, families should be referred for genetic counseling and further testing if needed. If germline mutation is suspected, early identification is important; hereditary ALL can influence treatment choice and use of allogeneic transplantation or radiation.3
A third classification crucial to guiding treatment is Ph-positive vs Ph-negative or Ph-like, the latter strongly associated with abnormal B-cell development due to deletions in related genes.3,16 About 3% to 5% of pediatric patients and 25% of adults have Ph-positive ALL.17 The remission failure rate among pediatric patients treated with chemotherapy was 11% in one study, vs 2%-3% among patients with Ph-negative ALL.10
Diagnosis and Risk Stratification
Diagnosis is based on presentation and molecular features, requiring demonstration of ≥ 20% lymphoblasts in bone marrow biopsy or aspirate or ≥ 1,000 circulating lymphoblasts/mL in peripheral blood. Testing can include immunophenotyping using flow cytometry, molecular characterization of baseline leukemic clone, morphology using hematoxylin and eosin staining and Wright/Giemsa staining, and karyotyping.1,3 CNS involvement is assessed using a lumbar spinal tap.1
Risk stratification is based on molecular features (eg, high- and low-risk mutations, Table 1),3,9-15 which are assessed using fluorescence in-situ hybridization, broad-panel next-generation sequencing, and reverse-transcriptase polymerase chain reaction of bone marrow or peripheral blood.3 Other risk factors include age, CNS involvement, white blood cell (WBC) count, and response to initial induction or consolidation therapy.3
Pediatric patients are assigned standard or high risk based on factors identified by the Children’s Oncology Group and National Comprehensive Cancer Network (NCCN). Patients
aged 1 to < 10 years with WBC < 50 × 109/L are considered standard risk, and all others are considered high risk. Patients with ALL before age 1 have very high risk. All pediatric patients with T-ALL are considered high risk.3 Ph-positive, Ph-like, hypoploidy, failure to achieve remission with induction, and extramedullary disease are high-risk factors as well, whereas hyperploidy and certain mutations convey low risk.3
Newer treatment strategies for initial ALL diagnosis include targeted therapies. One goal of targeted therapy is avoidance of long-term toxicity, leading to improved survival outcomes. Well-studied targeted therapies include the tyrosine kinase inhibitors used in first-line and subsequent treatment of Ph-positive ALL.3
Treatment Options in Relapsed/Refractory ALL
The initial treatment goal is complete remission (CR) defined as minimal residual disease (MRD) < 0.01% on flow cytometry (Table 2).3 Prognosis is dependent on time and location of relapse. Early relapse (< 18 months from diagnosis) predicts poor survival. Relapse in bone marrow is associated with poorer prognosis than relapse in CNS.11-18 Where possible, consolidation with allogeneic hematopoietic cell transplantation improves survival for patients with early relapse.6 Three approaches have advanced treatment options for relapsed/refractory (R/R) B-ALL, all based around common cell markers seen in B-ALL.
The CD22-directed antibody-drug conjugate inotuzumab ozogamicin is approved for adults with R/R B-ALL. In clinical trials, a higher percentage of patients had results below the MRD threshold, and longer progression-free survival and OS compared with standard care.19,20
Blinatumomab is a bispecific T-cell engager that binds to CD19 on the surface of B-ALL cells and to CD3 on T cells to trigger apoptosis.21 It was first approved for R/R ALL in adults or children, and is also now approved for treatment in remission with MRD ≥ 0.1%. Patients must demonstrate CD19-positive disease to qualify.15-22 For R/R ALL, blinatumomab improves OS and CR rates compared with standard chemotherapy.23
The use of CAR T-cell therapies has expanded greatly with increasing knowledge about their efficacy and safety. In R/R ALL, tisagenlecleucel (tisa-gen) is approved for treatment of patients aged ≤ 25 years, and brexucabtagene autoleucel (brexucel) is approved for treatment of adults.3,24,25 Patients undergoing the CAR T-cell process have apheresis to collect T cells, which are then manufactured before being reinfused into the patient. Depending on local capabilities, the time between T-cell harvest and reinfusion can extend to weeks.3,26,27 Cytoreduction with CAR T-cell therapy can allow previously ineligible patients (due to bulky disease) to undergo transplant. Patients treated in key clinical trials with tisa-gen or brexu-cel achieved high overall remission rates and improved event-free survival and OS rates compared with historical experience.25,28,29 Important toxicities with CAR T-cell therapy are cytokine release syndrome (CRS) and neurotoxicity, which can develop rapidly. NCCN recommends hospitalizing patients at the first sign of either adverse event. Patients can be managed with tocilizumab or steroids for low-grade CRS or steroids for neurotoxicity. The Society for Immunotherapy of Cancer, American Society of Clinical Oncology, and NCCN have guidelines on management of toxicities related to CAR T-cell therapy as well as management of symptoms and other adverse effects of CRS.5,23,24
Programs also incorporate telemedicine for symptom monitoring and follow-up.32-34 Centers providing CAR T-cell therapy must have a certified Risk Evaluation and Mitigation Strategy (REMS), which ensures adherence to specific guidelines for administration, adverse event management, and patient education.35,36 Overcoming technical, social, and financial barriers to CAR T-cell therapy is an ongoing challenge of great interest.37
R/R T-Cell Precursor ALL
Patients with R/R T-ALL have poor prognosis, partly due to limited treatment options. Nelarabine, a nucleoside analog, is the only approved treatment for R/R T-ALL, but has increasingly been used in first-line therapy added to multiagent chemotherapy as a consolidation and maintenance approach to pediatric disease.3,38,39 Four-year DSF in pediatric patients with newly diagnosed T-ALL undergoing treatment incorporating nelarabine was 88.9%.39 Treatment is associated with grade ≥ 3 neurotoxicity in > 10% of patients, and can include CNS toxicity as well as neuropathy.3
In a recently completed phase 2 trial (NCT03384654), daratumumab was added to standard chemotherapy (vincristine, prednisone, PEG-asparaginase, doxorubicin) for R/R T-ALL in pediatric (ages 1-17 years) and young adult patients (age ≥ 18 years).40 Among 24 pediatric patients, CR was 41.7% and overall response rate (ORR; ORR = CR + CRi) was 83% after 1 cycle of treatment. Ten (41.7%) pediatric patients achieved MRD-negative status as well. ORR was 60% in the 5 older patients. All pediatric patients had at least 1 grade ≥ 3 toxicity, but none of the adverse events led to discontinuation.40
Success in achieving MRD-negative responses in patients treated for R/R ALL has increased interest in using targeted therapies for newly diagnosed patients. Recommended treatment approaches are summarized in Table 3.3
Long-Term Follow-Up and Survivorship
A study of > 500 pediatric patients followed for an average 23 years reassuringly found low prevalence of adverse outcomes related to disease or treatment. Major adverse outcomes such as death due to late relapse; secondary malignancy; or development of osteoporosis, cataracts, and diminished functional status were infrequent.41 Most prevalent were growth effects (short stature or growth hormone insufficiency), likely related to certain treatment approaches.41 Guidelines for long-term follow-up of pediatric patients are available from the Children’s Oncology Group.42
A 2017 systematic review concluded that the quality of life for survivors is diminished upon treatment, and persistently over time for some patients.43 In contrast, a 2022 comparison of long-term survivors (median 20.5 years since diagnosis) of pediatric ALL with healthy controls found that survivors had better quality of life in some domains, including general health, vitality, and mental health.44 Smaller percentages of survivors rated themselves happiest about sleep quality, absence of pain, and physical abilities.44
As therapy patterns and options evolve, continued follow-up is important to ensure patients derive optimal benefit from treatment and post-treatment life.
- Puckett Y, Chan O. Acute lymphocytic leukemia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated June 27, 2022. Accessed April 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459149/
- Cancer facts & figures 2023. American Cancer Society. 2023. Accessed April 10, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia. Version 1.2022. April 4, 2022. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Childhood acute lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 16, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: management of immunotherapy-related toxicities. Version 1.2023. March 10, 2023. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- DuVall AS, Sheade J, Anderson D, et al. Updates in the management of relapsed and refractory acute lymphoplastic leukemia: an urgent plea for new treatments is being answered! JCO Oncol Pract. 2022;18(7):479-487. doi:10.1200/OP.21.00843
- Baker JM, To T, Beyene J, Zagorski B, Greenberg ML, Sung L. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk Res. 2014;38(2):204-209. doi:10.1016/j.leukres.2013.11.014
- Acute adult lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 24, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, et al. Toward a NOTCH1/FBXW7/RAS/PTEN–based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2013;31(34):4333-4342. doi:10.1200/JCO.2012.48.5292
- Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966-1973. doi:10.1200/JCO.2011.39.7661
- Gao C, Liu SG, Zhang RD, et al. NOTCH1 mutations are associated with favourable long-term prognosis in paediatric T-cell acute lymphoblastic leukaemia: a retrospective study of patients treated on BCH-2003 and CCLG-2008 protocol in China. Br J Haematol. 2014;166(2):221-228. doi:10.1111/bjh.12866
- Yang YL, Hsiao CC, Chen HY, et al. Absence of biallelic TCRγ deletion predicts induction failure and poorer outcomes in childhood T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(6):846-851. doi:10.1002/pbc.24021
- Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3816-3823. doi:10.1200/JCO.2010.28.3390
- Bandapalli OR, Zimmermann M, Kox C, et al. NOTCH1 activation clinically antagonizes the unfavorable effect of PTEN inactivation in BFM-treated children with precursor T-cell acute lymphoblastic leukemia. Haematologica. 2013;98(6):928-936. doi:10.3324/haematol.2012.073585
- Palmi C, Savino AM, Silvestri D, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7(37):59260-59272. doi:10.18632/oncotarget.10610
- Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125-134. doi:10.1016/S1470-2045(08)70339-5
- Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. doi:10.1200/JCO.2010.30.1325
- Nguyen K, Devidas M, Cheng SC, et al.; Children’s Oncology Group. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22(12):2142-2150. doi:10.1038/leu.2008.251
- Besponsa. Prescribing information. Wyeth Pharmaceuticals Inc; 2017. BESPONSA® (inotuzumab ozogamicin) Dosing & Administration |Safety Info (pfizerpro.com)
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. doi:10.1056/NEJMoa1509277
- Lv M, Liu Y, Liu W, Xing Y, Zhang S. Immunotherapy for pediatric acute lymphoblastic leukemia: recent advances and future perspectives. Front Immunol. 2022;13:921894. doi:10.3389/fimmu.2022.921894
- Blincyto. Prescribing information. Amgen; 2022. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Blincyto/blincyto_pi_hcp_english.pdf
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/NEJMoa1609783
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. doi:10.1016/S0140-6736(21)01222-8
- Bhaskar ST, Dholaria BR, Singsayadeth S, Savani BN, Oluwole OO. Role of bridging therapy during chimeric antigen receptor T cell therapy. EJHaem. 2021;3(suppl 1):39-45. doi:10.1002/jha2.335
- Granroth G, Rosenthal A, McCallen M, et al. Supportive care for patients with lymphoma
undergoing CAR-T-cell therapy: the advanced practice provider’s perspective. Curr Oncol Rep. 2022;24(12):1863-1872. doi:10.1007/s11912-022-01330-z - Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphocytic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664-1669. doi:10.1200/JCO.22.00642
- Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. doi:10.1186/s13045-022-01379-0
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi:10.1136/jitc-2020-001511
- Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39(35):3978-3992. doi:10.1200/JCO.21.01992
- Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transpl. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
- LeBar K, Murawski S, Umayam S, Quinn V. The role of advanced practice providers and telemedicine in reinventing care: the transition of a CAR T-cell transplantation program to the outpatient setting. J Adv Pract Oncol. 2020;11(7):757-763. doi:10.6004/jadpro.2020.11.7.8
- Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy for aggressive B-cell lymphomas and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):e002056. doi:10.1136/jitc-2020-002056
- Kymriah. Prescribing information. Novartis Pharmaceuticals Corporation; 2022. https://www.fda.gov/media/107296/download
- Tecartus. Prescribing information. Kite Pharma, Inc; 2021. https://www.fda.gov/media/140409/download
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 2022;18(12):800-807. doi:10.1200/OP.22.00315
- Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135(3):159-166. doi:10.1182/blood.2019001557
- Summers RJ, Teachey DT. SOHO state of the art updates and next questions: novel approaches to pediatric T-cell ALL and T-lymphoblastic lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22(10):718-725. doi:10.1016/j.clml.2022.07.010
- Hogan LE, Bhatla T, Teachey DT, et al. Efficacy and safety of daratumumab (DARA) in pediatric and young adult patients (pts) with relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL): results from the phase 2 DELPHINUS study. J Clin Oncol. 2022;40(16 suppl):Abstract 10001. doi:10.1200/JCO.2022.40.16_suppl.10001
- Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study. Lancet Oncol. 2014;15(8):841-851. doi:10.1016/S1470-2045(14)70265-7
- Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Children’s Oncology Group. October 2018. Accessed April 10, 2023. http://www.survivorshipguidelines.org
- Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9). doi:10.1002/pbc.26489
- Chantziara S, Musoro J, Rowsell AC, et al; European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QLG) and Children’s Leukemia Group (CLG). Quality of life of long-term childhood acute lymphoblastic leukemia survivors: comparison with healthy controls. Psychooncology. 2022;31(12):2159-2168. doi:10.1002/pon.6060
- Puckett Y, Chan O. Acute lymphocytic leukemia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated June 27, 2022. Accessed April 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459149/
- Cancer facts & figures 2023. American Cancer Society. 2023. Accessed April 10, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia. Version 1.2022. April 4, 2022. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Childhood acute lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 16, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: management of immunotherapy-related toxicities. Version 1.2023. March 10, 2023. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- DuVall AS, Sheade J, Anderson D, et al. Updates in the management of relapsed and refractory acute lymphoplastic leukemia: an urgent plea for new treatments is being answered! JCO Oncol Pract. 2022;18(7):479-487. doi:10.1200/OP.21.00843
- Baker JM, To T, Beyene J, Zagorski B, Greenberg ML, Sung L. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk Res. 2014;38(2):204-209. doi:10.1016/j.leukres.2013.11.014
- Acute adult lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 24, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, et al. Toward a NOTCH1/FBXW7/RAS/PTEN–based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2013;31(34):4333-4342. doi:10.1200/JCO.2012.48.5292
- Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966-1973. doi:10.1200/JCO.2011.39.7661
- Gao C, Liu SG, Zhang RD, et al. NOTCH1 mutations are associated with favourable long-term prognosis in paediatric T-cell acute lymphoblastic leukaemia: a retrospective study of patients treated on BCH-2003 and CCLG-2008 protocol in China. Br J Haematol. 2014;166(2):221-228. doi:10.1111/bjh.12866
- Yang YL, Hsiao CC, Chen HY, et al. Absence of biallelic TCRγ deletion predicts induction failure and poorer outcomes in childhood T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(6):846-851. doi:10.1002/pbc.24021
- Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3816-3823. doi:10.1200/JCO.2010.28.3390
- Bandapalli OR, Zimmermann M, Kox C, et al. NOTCH1 activation clinically antagonizes the unfavorable effect of PTEN inactivation in BFM-treated children with precursor T-cell acute lymphoblastic leukemia. Haematologica. 2013;98(6):928-936. doi:10.3324/haematol.2012.073585
- Palmi C, Savino AM, Silvestri D, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7(37):59260-59272. doi:10.18632/oncotarget.10610
- Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125-134. doi:10.1016/S1470-2045(08)70339-5
- Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. doi:10.1200/JCO.2010.30.1325
- Nguyen K, Devidas M, Cheng SC, et al.; Children’s Oncology Group. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22(12):2142-2150. doi:10.1038/leu.2008.251
- Besponsa. Prescribing information. Wyeth Pharmaceuticals Inc; 2017. BESPONSA® (inotuzumab ozogamicin) Dosing & Administration |Safety Info (pfizerpro.com)
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. doi:10.1056/NEJMoa1509277
- Lv M, Liu Y, Liu W, Xing Y, Zhang S. Immunotherapy for pediatric acute lymphoblastic leukemia: recent advances and future perspectives. Front Immunol. 2022;13:921894. doi:10.3389/fimmu.2022.921894
- Blincyto. Prescribing information. Amgen; 2022. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Blincyto/blincyto_pi_hcp_english.pdf
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/NEJMoa1609783
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. doi:10.1016/S0140-6736(21)01222-8
- Bhaskar ST, Dholaria BR, Singsayadeth S, Savani BN, Oluwole OO. Role of bridging therapy during chimeric antigen receptor T cell therapy. EJHaem. 2021;3(suppl 1):39-45. doi:10.1002/jha2.335
- Granroth G, Rosenthal A, McCallen M, et al. Supportive care for patients with lymphoma
undergoing CAR-T-cell therapy: the advanced practice provider’s perspective. Curr Oncol Rep. 2022;24(12):1863-1872. doi:10.1007/s11912-022-01330-z - Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphocytic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664-1669. doi:10.1200/JCO.22.00642
- Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. doi:10.1186/s13045-022-01379-0
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi:10.1136/jitc-2020-001511
- Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39(35):3978-3992. doi:10.1200/JCO.21.01992
- Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transpl. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
- LeBar K, Murawski S, Umayam S, Quinn V. The role of advanced practice providers and telemedicine in reinventing care: the transition of a CAR T-cell transplantation program to the outpatient setting. J Adv Pract Oncol. 2020;11(7):757-763. doi:10.6004/jadpro.2020.11.7.8
- Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy for aggressive B-cell lymphomas and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):e002056. doi:10.1136/jitc-2020-002056
- Kymriah. Prescribing information. Novartis Pharmaceuticals Corporation; 2022. https://www.fda.gov/media/107296/download
- Tecartus. Prescribing information. Kite Pharma, Inc; 2021. https://www.fda.gov/media/140409/download
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 2022;18(12):800-807. doi:10.1200/OP.22.00315
- Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135(3):159-166. doi:10.1182/blood.2019001557
- Summers RJ, Teachey DT. SOHO state of the art updates and next questions: novel approaches to pediatric T-cell ALL and T-lymphoblastic lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22(10):718-725. doi:10.1016/j.clml.2022.07.010
- Hogan LE, Bhatla T, Teachey DT, et al. Efficacy and safety of daratumumab (DARA) in pediatric and young adult patients (pts) with relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL): results from the phase 2 DELPHINUS study. J Clin Oncol. 2022;40(16 suppl):Abstract 10001. doi:10.1200/JCO.2022.40.16_suppl.10001
- Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study. Lancet Oncol. 2014;15(8):841-851. doi:10.1016/S1470-2045(14)70265-7
- Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Children’s Oncology Group. October 2018. Accessed April 10, 2023. http://www.survivorshipguidelines.org
- Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9). doi:10.1002/pbc.26489
- Chantziara S, Musoro J, Rowsell AC, et al; European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QLG) and Children’s Leukemia Group (CLG). Quality of life of long-term childhood acute lymphoblastic leukemia survivors: comparison with healthy controls. Psychooncology. 2022;31(12):2159-2168. doi:10.1002/pon.6060