User login
Coarctation of the Aorta
At age 27, a woman with no family history of hypertension was diagnosed with the disease, which was left untreated. Two years later—during her first pregnancy—she was still hypertensive and was prescribed methyldopa, which was switched to lisinopril in the postpartum period. Her blood pressure (BP) remained elevated, despite titration of lisinopril and the addition of a β-blocking agent.
In the same year, the woman went to the emergency department with a severe headache and near-syncope; her BP was 180/100 mm Hg. Her medications were changed, and she was discharged with a prescription for captopril 50 mg bid and the angiotensin receptor blocker (ARB) valsartan 80 mg bid. Over the following six years, her average BP remained around 140/90 mm Hg with no further medication adjustment.
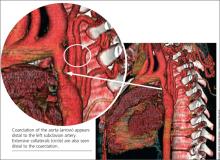
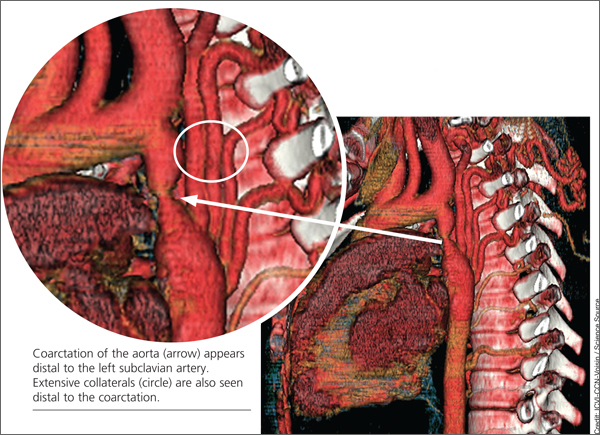
When the patient was 37, she underwent a chest x-ray (CXR), prompted by positive results on a purified protein derivative test at an employment physical; the CXR demonstrated a 3.6-cm mediastinal mass. This finding led to a chest CT exam that demonstrated a severe coarctation distal to the left subclavian artery with diffuse tubular hypoplasia, a collateral reconstitution of the descending aorta, and a true 2.7-cm aneurysm of one of the intercostal arteries.
The gradient between the ascending and descending aorta was 50 mm Hg, and the same wide pressure gradient was present between the upper and lower extremities (50 mm Hg).
Referral to pediatric cardiology was initiated. (It is not uncommon for an adult with a congenital heart lesion to be evaluated by a pediatric cardiologist in centers where adult congenital heart disease [ACHD] specialists are not available.) A cardiac MRI revealed a virtual interruption of the aortic arch in juxta-ductal position with multiple aortic collateral arteries. Subsequent cardiac catheterization demonstrated a transverse aortic arch at 1.2 cm and a narrowing to 7 mm just distal to the left subclavian artery, with a discrete coarctation of 2.5 mm.
With hypertension and a 50–mm Hg resting clinical gradient, corrective treatment was deemed necessary. Subsequently, balloon angioplasty was performed, and a drug-eluting stent was placed in the proximal distal aorta with dilation of the narrowing and a resultant decrease in BP gradient from 50 mm Hg to 7 mm Hg. Following stent placement, the aneurysm thrombosed secondary to reduced blood flow. Clinical reevaluation showed good dorsalis pedis and posterior tibial pulses with improved BP. The ARB was subsequently discontinued, and the patient continued to take captopril for mildly elevated BP (average, 130/85 mm Hg).
The patient did well until three years later, when she developed shortness of breath on exertion, claudication, and fatigue for a period of two weeks. On physical examination, her BP was noted to be elevated at 140/90 mm Hg with a clinical gradient of 20 mm between the upper and lower extremities and an increase in the gradient on echocardiogram to a peak of approximately 46 mm Hg and a mean of 21 mm Hg.
A subsequent chest CT demonstrated a narrowing of the previous stent site, and a right and left cardiac catheterization revealed neo-intimal proliferation affecting the stent with a 3–mm Hg gradient across the transverse arch and a 15–mm Hg gradient across the proximal descending aorta stent. The stent was subsequently redilated, and an additional stent was placed with no residual gradient.
The patient was discharged while taking clopidogrel 75 mg/d in addition to aspirin 325 mg/d for six months; antihypertensive medications were no longer necessary. Clinical evaluation with echocardiography was recommended every three months for the first year, and annually thereafter. At three-month and one-year follow-up, the patient was found to be symptom-free and normotensive (BP, 110/70 mm Hg).
DISCUSSION
Coarctation of the aorta (CoA) is a discrete narrowing of the thoracic aorta at the junction of the ductus arteriosus and the aortic arch, just distal to the subclavian artery. The specific anatomy, severity, and degree of hypoplasia proximal to the aortic coarctation are highly variable. For example, in some instances, coarctation presents as a long segment or a tubular hypoplasia.1
The defect is often associated with other congenital cardiovascular abnormalities, including bicuspid aortic valve (BAV; reported incidence, up to 85%),2,3 intracranial aneurysms (incidence, 3% to 10%),4 intrinsic abnormality in the aorta, aortic arch hypoplasia, ventricular septal defect, patent ductus arteriosus, aortic stenosis at different levels (valvular, subvalvular, or supravalvular), and mitral valve abnormalities.2,5 There is evidence of increased familial risk for CoA and increased prevalence with certain disorders, including Turner syndrome, maternal phenylketonuria syndrome, and Kabuki syndrome.6
CoA accounts for 5% to 8% of all congenital heart disease,2,3 and its incidence is 4 in 10,000 births.1 (Adults presenting with CoA represent either recoarctation or a missed diagnosis of native coarctation.) The mean life expectancy of untreated patients with aortic coarctation is 35 years; 90% die before age 50.1
Reduced life expectancy of patients with untreated CoA is due to systemic hypertension, accelerated coronary artery disease, stroke, heart failure, aortic rupture/dissection, cerebral hemorrhage, infective endarteritis/endocarditis, concomitant aortic valve disease (usually involving a BAV), and sudden cardiac death of presumed arrhythmogenic etiology.4 Even adults whose CoA has been detected early and managed with catheter-based and surgical interventions continue to face lifelong complications, including recoarctation, aneurysm formation, premature coronary artery disease, and cerebrovascular disease—mostly resulting from residual hypertension.3
Persistent hypertension has been reported in 68% of patients with repaired CoA at long-term follow-up. Hypertension may result in recurrence of CoA (incidence ranges from 5% to 50%), a residual CoA, or an idiopathic condition.7
The role of primary care providers is crucial in early detection and prompt referral to specialists in ACHD. For clinicians who manage these patients, increased morbidity and mortality from the associated cardiovascular sequelae pose an ongoing challenge.8
Presentation
Patients with hemodynamically stable coarctation in adolescence or adulthood are usually asymptomatic. Occasionally, a patient may be diagnosed with CoA based on its typical appearance on CXR or may come to medical attention because of an incidental murmur or management of hypertension.4 Symptoms vary in intensity and include headache, epistaxis, claudication, exertional fatigue, heart failure, aortic rupture, or dissection.7 Based on the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), and the 2008 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for adults with congenital heart disease, patients with hypertension and/or a history of CoA repair should be evaluated periodically for coarctation.8
Physical Examination
Physical assessment should include simultaneous palpation of the brachial and femoral pulses to assess amplitude and timing, looking for diminished arterial pulses and brachial-femoral delay. Additionally, measurement of supine bilateral arm (brachial artery) BP and right or left supine leg BP is recommended to detect differing pressures.8
The following physical findings may be suggestive of CoA or recoarctation:
• Systolic BP in the right arm higher than in the lower extremities, unless the origin of the right subclavian artery is anomalous and thus is not reliable; the left arm BP may not always be reliable because of the origin of the left subclavian artery, which may vary and may or may not be hypertensive
• Hyperdynamic carotid pulsations
• A pulse delay between the right arm and the femoral or popliteal arteries
• A murmur or bruit heard in the left interscapular position; a systolic ejection click of moderate intensity heard along the left sternal border
• In cases of BAV, an early diastolic decrescendo murmur of aortic regurgitation
Diagnostic Workup
The diagnosis of CoA is usually confirmed by echocardiography or radiographic imaging, including cardiac MRI or CT angiography.8
The initial diagnostic workup should include echocardiography, which may demonstrate left ventricular hypertrophy and secondary ST-T wave abnormalities, and a two-dimensional Doppler echocardiogram, which can establish the diagnosis and severity of the CoA, with possible associated cardiac defects.9 Also recommended is a CXR, which may occasionally reveal rib notching (caused by erosion of the inferior border of the posterior ribs by enlarged intercostal arteries), also known as the 3 sign. Finally, cardiac MRI is used to delineate the coarctation anatomy and to determine whether collateral arteries and/or associated vascular anomalies and flow abnormalities exist.9 If MRI is not possible, CT angiography can be an alternate approach. Subsequently, invasive angiography is required for better assessment of the coarctation gradient and hemodynamic measurement.9
Treatment
Management of CoA requires treatment of hypertension with β-blockers, ACE inhibitors, and/or ARBs as first-line medications. Aortic root size, the presence of aortic regurgitation, or both may influence the choice of antihypertensive agents.3
Intervention is recommended if the peak-to-peak coarctation gradient is ≥ 20 mm Hg, or the peak-to-peak coarctation gradient is < 20 mm Hg, with evidence of significant coarctation and collateral flow on radiologic imaging.8 The choice of treatment (stenting or surgery) should be decided by a team of ACHD cardiologists, interventionalists, and surgeons at an ACHD center.
Surgical intervention via a lateral left thoracotomy approach was first performed in 1944. The most common surgical repair is resection with end-to-end anastomosis, which yields a low mortality and recoarctation rate. Other techniques such as resection with replacement by a tube graft, patch aortoplasty, and bypass graft are used less frequently.10-12 Postsurgical morbidity most commonly includes recoarctation and residual hypertension.
Thoracotomy was the only surgical treatment until 1982, when balloon angioplasty became available as an alternative.13 However, recoarctation, aneurysm formation, and aortic dissection are major disadvantages to balloon angioplasty.13
In the early 1990s, endovascular stents were introduced and have become an alternative approach to surgical repair.14 Aneurysms remain a significant complication in 4% to 7% of patients who undergo stent placement for CoA.14
Currently, there is insufficient evidence to indicate which is the best treatment for CoA: surgical or stent repair. Choice of treatment strategy will continue to depend on the operator’s skills or institutional preference until a prospective randomized controlled clinical trial is performed.13
Follow-Up
Based on recommendations from the ACC/AHA and the 2009 Canadian Cardiovascular Society Consensus Conference on the management of adults with congenital heart disease, lifelong follow-up is recommended for all patients with aortic coarctation (whether repaired or not), including an evaluation by a cardiologist with expertise in ACHD.4,8
A baseline cardiac MRI or CT for complete evaluation of the thoracic aorta and intracranial vessels is required for follow-up. Patients who have previously undergone surgical or interventional CoA repair should be followed annually with echocardiography to assess for potential late complications, such as aortic dilatation and aneurysm formation. Evaluation of the coarctation repair site by MRI and/or CT at intervals of five years or less is also recommended. Moreover, patients should be monitored for recurrent resting or exercise-induced hypertension, which should be treated aggressively after recoarctation is excluded.
The guidelines recommend that every patient with systemic arterial hypertension have the brachial and femoral pulses palpated simultaneously.4,8 This additional physical assessment will help detect significant aortic coarctation by assessing timing and amplitude of both pulses in search for a brachial-femoral delay. Moreover, measuring the differential pressure between bilateral arms (brachial artery) in a supine position and prone right or left supine leg (popliteal artery) BP should be performed.4,8 Initial imaging and hemodynamic evaluation by transthoracic echocardiogram is recommended in suspected aortic coarctation.
CONCLUSION
This case represents a missed CoA and provides an example of recoarctation as a late complication after repair. Unfortunately, the critical need to screen for coarctation was not recognized by the patient’s primary care providers in a timely manner. Had the guidelines for CoA screening been applied, this defect would have been detected earlier, avoiding many years of cardiovascular system stress from the sequelae of hypertension.
Measuring the BP gradient between the upper and lower extremities and searching for brachial-femoral timing delay are simple but crucial steps in the initial application of the clinical guidelines for early detection of CoA and recoarctation.
References
1. Jurcut R, Daraban AM, Lorber A, et al. Coarctation of the aorta in adults: what is the best treatment? Case report and literature review. J Med Life. 2011;4:189-195.
2. Teo LLS, Cannell T, Babu-Narayan SV, et al. Prevalence of associated cardiovascular abnormalities in 500 patients with aortic coarctation referred for cardiovascular magnetic resonance imaging to a tertiary center. Pediatr Cardiol. 2011;32:1120-1127.
3. Canniffe C. Hypertension after repair of aortic coarctation–a systematic review. Int J Cardiol. 2012;167:2456-2461.
4. Silversides CK, Kiess M, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can J Cardiol. 2010;26:e80-e97.
5. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for The Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease) Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143-e263.
6. McBride KL, Pignatelli R, Lewin M, et al. Inheritance analysis of congenital left ventricular outflow tract obstruction malformations: segregation, multiplex relative risk, and heritability. Am J Med Genet. Part A. 2005;134:
180-186.
7. Brown JW, Ruzmetov M, Hoyer MH, et al. Recurrent coarctation: is surgical repair of recurrent coarctation of the aorta safe and effective? Ann Thorac Surg. 2009;88:1923-1931.
8. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008;118:2395-2451.
9. Darabian S, Zeb I, Rezaeian P, et al. Use of noninvasive imaging in the evaluation of coarctation of aorta. J Comput Assist Tomogr. 2013;37:75-78.
10. Backer CL, Mavroudis C, Zias EA, et al. Repair of coarctation with resection and extended end-to-end anastomosis. Ann Thorac Surg. 1998;66:1365-1370.
11. Cobanoglu A, Thyagarajan GK, Dobbs JL. Surgery for coarctation of the aorta in infants younger than 3 months: end-to-end repair versus subclavian flap angioplasty: is either operation better? Eur J Cardiothorac Surg. 1998;14:19-25.
12. Walhout RJ, Lekkerkerker JC, Oron GH, et al. Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg. 2003;126:521-528.
13. Pádua LM, Garcia LC, Rubira CJ, de Oliveira Carvalho PE. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev. 2012;5:CD008204.
14. Suárez de Lezo J, Pan M, Romero M, et al. Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am J Cardiol. 1999;83:400-406.
At age 27, a woman with no family history of hypertension was diagnosed with the disease, which was left untreated. Two years later—during her first pregnancy—she was still hypertensive and was prescribed methyldopa, which was switched to lisinopril in the postpartum period. Her blood pressure (BP) remained elevated, despite titration of lisinopril and the addition of a β-blocking agent.
In the same year, the woman went to the emergency department with a severe headache and near-syncope; her BP was 180/100 mm Hg. Her medications were changed, and she was discharged with a prescription for captopril 50 mg bid and the angiotensin receptor blocker (ARB) valsartan 80 mg bid. Over the following six years, her average BP remained around 140/90 mm Hg with no further medication adjustment.
When the patient was 37, she underwent a chest x-ray (CXR), prompted by positive results on a purified protein derivative test at an employment physical; the CXR demonstrated a 3.6-cm mediastinal mass. This finding led to a chest CT exam that demonstrated a severe coarctation distal to the left subclavian artery with diffuse tubular hypoplasia, a collateral reconstitution of the descending aorta, and a true 2.7-cm aneurysm of one of the intercostal arteries.
The gradient between the ascending and descending aorta was 50 mm Hg, and the same wide pressure gradient was present between the upper and lower extremities (50 mm Hg).
Referral to pediatric cardiology was initiated. (It is not uncommon for an adult with a congenital heart lesion to be evaluated by a pediatric cardiologist in centers where adult congenital heart disease [ACHD] specialists are not available.) A cardiac MRI revealed a virtual interruption of the aortic arch in juxta-ductal position with multiple aortic collateral arteries. Subsequent cardiac catheterization demonstrated a transverse aortic arch at 1.2 cm and a narrowing to 7 mm just distal to the left subclavian artery, with a discrete coarctation of 2.5 mm.
With hypertension and a 50–mm Hg resting clinical gradient, corrective treatment was deemed necessary. Subsequently, balloon angioplasty was performed, and a drug-eluting stent was placed in the proximal distal aorta with dilation of the narrowing and a resultant decrease in BP gradient from 50 mm Hg to 7 mm Hg. Following stent placement, the aneurysm thrombosed secondary to reduced blood flow. Clinical reevaluation showed good dorsalis pedis and posterior tibial pulses with improved BP. The ARB was subsequently discontinued, and the patient continued to take captopril for mildly elevated BP (average, 130/85 mm Hg).
The patient did well until three years later, when she developed shortness of breath on exertion, claudication, and fatigue for a period of two weeks. On physical examination, her BP was noted to be elevated at 140/90 mm Hg with a clinical gradient of 20 mm between the upper and lower extremities and an increase in the gradient on echocardiogram to a peak of approximately 46 mm Hg and a mean of 21 mm Hg.
A subsequent chest CT demonstrated a narrowing of the previous stent site, and a right and left cardiac catheterization revealed neo-intimal proliferation affecting the stent with a 3–mm Hg gradient across the transverse arch and a 15–mm Hg gradient across the proximal descending aorta stent. The stent was subsequently redilated, and an additional stent was placed with no residual gradient.
The patient was discharged while taking clopidogrel 75 mg/d in addition to aspirin 325 mg/d for six months; antihypertensive medications were no longer necessary. Clinical evaluation with echocardiography was recommended every three months for the first year, and annually thereafter. At three-month and one-year follow-up, the patient was found to be symptom-free and normotensive (BP, 110/70 mm Hg).
DISCUSSION
Coarctation of the aorta (CoA) is a discrete narrowing of the thoracic aorta at the junction of the ductus arteriosus and the aortic arch, just distal to the subclavian artery. The specific anatomy, severity, and degree of hypoplasia proximal to the aortic coarctation are highly variable. For example, in some instances, coarctation presents as a long segment or a tubular hypoplasia.1
The defect is often associated with other congenital cardiovascular abnormalities, including bicuspid aortic valve (BAV; reported incidence, up to 85%),2,3 intracranial aneurysms (incidence, 3% to 10%),4 intrinsic abnormality in the aorta, aortic arch hypoplasia, ventricular septal defect, patent ductus arteriosus, aortic stenosis at different levels (valvular, subvalvular, or supravalvular), and mitral valve abnormalities.2,5 There is evidence of increased familial risk for CoA and increased prevalence with certain disorders, including Turner syndrome, maternal phenylketonuria syndrome, and Kabuki syndrome.6
CoA accounts for 5% to 8% of all congenital heart disease,2,3 and its incidence is 4 in 10,000 births.1 (Adults presenting with CoA represent either recoarctation or a missed diagnosis of native coarctation.) The mean life expectancy of untreated patients with aortic coarctation is 35 years; 90% die before age 50.1
Reduced life expectancy of patients with untreated CoA is due to systemic hypertension, accelerated coronary artery disease, stroke, heart failure, aortic rupture/dissection, cerebral hemorrhage, infective endarteritis/endocarditis, concomitant aortic valve disease (usually involving a BAV), and sudden cardiac death of presumed arrhythmogenic etiology.4 Even adults whose CoA has been detected early and managed with catheter-based and surgical interventions continue to face lifelong complications, including recoarctation, aneurysm formation, premature coronary artery disease, and cerebrovascular disease—mostly resulting from residual hypertension.3
Persistent hypertension has been reported in 68% of patients with repaired CoA at long-term follow-up. Hypertension may result in recurrence of CoA (incidence ranges from 5% to 50%), a residual CoA, or an idiopathic condition.7
The role of primary care providers is crucial in early detection and prompt referral to specialists in ACHD. For clinicians who manage these patients, increased morbidity and mortality from the associated cardiovascular sequelae pose an ongoing challenge.8
Presentation
Patients with hemodynamically stable coarctation in adolescence or adulthood are usually asymptomatic. Occasionally, a patient may be diagnosed with CoA based on its typical appearance on CXR or may come to medical attention because of an incidental murmur or management of hypertension.4 Symptoms vary in intensity and include headache, epistaxis, claudication, exertional fatigue, heart failure, aortic rupture, or dissection.7 Based on the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), and the 2008 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for adults with congenital heart disease, patients with hypertension and/or a history of CoA repair should be evaluated periodically for coarctation.8
Physical Examination
Physical assessment should include simultaneous palpation of the brachial and femoral pulses to assess amplitude and timing, looking for diminished arterial pulses and brachial-femoral delay. Additionally, measurement of supine bilateral arm (brachial artery) BP and right or left supine leg BP is recommended to detect differing pressures.8
The following physical findings may be suggestive of CoA or recoarctation:
• Systolic BP in the right arm higher than in the lower extremities, unless the origin of the right subclavian artery is anomalous and thus is not reliable; the left arm BP may not always be reliable because of the origin of the left subclavian artery, which may vary and may or may not be hypertensive
• Hyperdynamic carotid pulsations
• A pulse delay between the right arm and the femoral or popliteal arteries
• A murmur or bruit heard in the left interscapular position; a systolic ejection click of moderate intensity heard along the left sternal border
• In cases of BAV, an early diastolic decrescendo murmur of aortic regurgitation
Diagnostic Workup
The diagnosis of CoA is usually confirmed by echocardiography or radiographic imaging, including cardiac MRI or CT angiography.8
The initial diagnostic workup should include echocardiography, which may demonstrate left ventricular hypertrophy and secondary ST-T wave abnormalities, and a two-dimensional Doppler echocardiogram, which can establish the diagnosis and severity of the CoA, with possible associated cardiac defects.9 Also recommended is a CXR, which may occasionally reveal rib notching (caused by erosion of the inferior border of the posterior ribs by enlarged intercostal arteries), also known as the 3 sign. Finally, cardiac MRI is used to delineate the coarctation anatomy and to determine whether collateral arteries and/or associated vascular anomalies and flow abnormalities exist.9 If MRI is not possible, CT angiography can be an alternate approach. Subsequently, invasive angiography is required for better assessment of the coarctation gradient and hemodynamic measurement.9
Treatment
Management of CoA requires treatment of hypertension with β-blockers, ACE inhibitors, and/or ARBs as first-line medications. Aortic root size, the presence of aortic regurgitation, or both may influence the choice of antihypertensive agents.3
Intervention is recommended if the peak-to-peak coarctation gradient is ≥ 20 mm Hg, or the peak-to-peak coarctation gradient is < 20 mm Hg, with evidence of significant coarctation and collateral flow on radiologic imaging.8 The choice of treatment (stenting or surgery) should be decided by a team of ACHD cardiologists, interventionalists, and surgeons at an ACHD center.
Surgical intervention via a lateral left thoracotomy approach was first performed in 1944. The most common surgical repair is resection with end-to-end anastomosis, which yields a low mortality and recoarctation rate. Other techniques such as resection with replacement by a tube graft, patch aortoplasty, and bypass graft are used less frequently.10-12 Postsurgical morbidity most commonly includes recoarctation and residual hypertension.
Thoracotomy was the only surgical treatment until 1982, when balloon angioplasty became available as an alternative.13 However, recoarctation, aneurysm formation, and aortic dissection are major disadvantages to balloon angioplasty.13
In the early 1990s, endovascular stents were introduced and have become an alternative approach to surgical repair.14 Aneurysms remain a significant complication in 4% to 7% of patients who undergo stent placement for CoA.14
Currently, there is insufficient evidence to indicate which is the best treatment for CoA: surgical or stent repair. Choice of treatment strategy will continue to depend on the operator’s skills or institutional preference until a prospective randomized controlled clinical trial is performed.13
Follow-Up
Based on recommendations from the ACC/AHA and the 2009 Canadian Cardiovascular Society Consensus Conference on the management of adults with congenital heart disease, lifelong follow-up is recommended for all patients with aortic coarctation (whether repaired or not), including an evaluation by a cardiologist with expertise in ACHD.4,8
A baseline cardiac MRI or CT for complete evaluation of the thoracic aorta and intracranial vessels is required for follow-up. Patients who have previously undergone surgical or interventional CoA repair should be followed annually with echocardiography to assess for potential late complications, such as aortic dilatation and aneurysm formation. Evaluation of the coarctation repair site by MRI and/or CT at intervals of five years or less is also recommended. Moreover, patients should be monitored for recurrent resting or exercise-induced hypertension, which should be treated aggressively after recoarctation is excluded.
The guidelines recommend that every patient with systemic arterial hypertension have the brachial and femoral pulses palpated simultaneously.4,8 This additional physical assessment will help detect significant aortic coarctation by assessing timing and amplitude of both pulses in search for a brachial-femoral delay. Moreover, measuring the differential pressure between bilateral arms (brachial artery) in a supine position and prone right or left supine leg (popliteal artery) BP should be performed.4,8 Initial imaging and hemodynamic evaluation by transthoracic echocardiogram is recommended in suspected aortic coarctation.
CONCLUSION
This case represents a missed CoA and provides an example of recoarctation as a late complication after repair. Unfortunately, the critical need to screen for coarctation was not recognized by the patient’s primary care providers in a timely manner. Had the guidelines for CoA screening been applied, this defect would have been detected earlier, avoiding many years of cardiovascular system stress from the sequelae of hypertension.
Measuring the BP gradient between the upper and lower extremities and searching for brachial-femoral timing delay are simple but crucial steps in the initial application of the clinical guidelines for early detection of CoA and recoarctation.
References
1. Jurcut R, Daraban AM, Lorber A, et al. Coarctation of the aorta in adults: what is the best treatment? Case report and literature review. J Med Life. 2011;4:189-195.
2. Teo LLS, Cannell T, Babu-Narayan SV, et al. Prevalence of associated cardiovascular abnormalities in 500 patients with aortic coarctation referred for cardiovascular magnetic resonance imaging to a tertiary center. Pediatr Cardiol. 2011;32:1120-1127.
3. Canniffe C. Hypertension after repair of aortic coarctation–a systematic review. Int J Cardiol. 2012;167:2456-2461.
4. Silversides CK, Kiess M, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can J Cardiol. 2010;26:e80-e97.
5. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for The Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease) Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143-e263.
6. McBride KL, Pignatelli R, Lewin M, et al. Inheritance analysis of congenital left ventricular outflow tract obstruction malformations: segregation, multiplex relative risk, and heritability. Am J Med Genet. Part A. 2005;134:
180-186.
7. Brown JW, Ruzmetov M, Hoyer MH, et al. Recurrent coarctation: is surgical repair of recurrent coarctation of the aorta safe and effective? Ann Thorac Surg. 2009;88:1923-1931.
8. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008;118:2395-2451.
9. Darabian S, Zeb I, Rezaeian P, et al. Use of noninvasive imaging in the evaluation of coarctation of aorta. J Comput Assist Tomogr. 2013;37:75-78.
10. Backer CL, Mavroudis C, Zias EA, et al. Repair of coarctation with resection and extended end-to-end anastomosis. Ann Thorac Surg. 1998;66:1365-1370.
11. Cobanoglu A, Thyagarajan GK, Dobbs JL. Surgery for coarctation of the aorta in infants younger than 3 months: end-to-end repair versus subclavian flap angioplasty: is either operation better? Eur J Cardiothorac Surg. 1998;14:19-25.
12. Walhout RJ, Lekkerkerker JC, Oron GH, et al. Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg. 2003;126:521-528.
13. Pádua LM, Garcia LC, Rubira CJ, de Oliveira Carvalho PE. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev. 2012;5:CD008204.
14. Suárez de Lezo J, Pan M, Romero M, et al. Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am J Cardiol. 1999;83:400-406.
At age 27, a woman with no family history of hypertension was diagnosed with the disease, which was left untreated. Two years later—during her first pregnancy—she was still hypertensive and was prescribed methyldopa, which was switched to lisinopril in the postpartum period. Her blood pressure (BP) remained elevated, despite titration of lisinopril and the addition of a β-blocking agent.
In the same year, the woman went to the emergency department with a severe headache and near-syncope; her BP was 180/100 mm Hg. Her medications were changed, and she was discharged with a prescription for captopril 50 mg bid and the angiotensin receptor blocker (ARB) valsartan 80 mg bid. Over the following six years, her average BP remained around 140/90 mm Hg with no further medication adjustment.
When the patient was 37, she underwent a chest x-ray (CXR), prompted by positive results on a purified protein derivative test at an employment physical; the CXR demonstrated a 3.6-cm mediastinal mass. This finding led to a chest CT exam that demonstrated a severe coarctation distal to the left subclavian artery with diffuse tubular hypoplasia, a collateral reconstitution of the descending aorta, and a true 2.7-cm aneurysm of one of the intercostal arteries.
The gradient between the ascending and descending aorta was 50 mm Hg, and the same wide pressure gradient was present between the upper and lower extremities (50 mm Hg).
Referral to pediatric cardiology was initiated. (It is not uncommon for an adult with a congenital heart lesion to be evaluated by a pediatric cardiologist in centers where adult congenital heart disease [ACHD] specialists are not available.) A cardiac MRI revealed a virtual interruption of the aortic arch in juxta-ductal position with multiple aortic collateral arteries. Subsequent cardiac catheterization demonstrated a transverse aortic arch at 1.2 cm and a narrowing to 7 mm just distal to the left subclavian artery, with a discrete coarctation of 2.5 mm.
With hypertension and a 50–mm Hg resting clinical gradient, corrective treatment was deemed necessary. Subsequently, balloon angioplasty was performed, and a drug-eluting stent was placed in the proximal distal aorta with dilation of the narrowing and a resultant decrease in BP gradient from 50 mm Hg to 7 mm Hg. Following stent placement, the aneurysm thrombosed secondary to reduced blood flow. Clinical reevaluation showed good dorsalis pedis and posterior tibial pulses with improved BP. The ARB was subsequently discontinued, and the patient continued to take captopril for mildly elevated BP (average, 130/85 mm Hg).
The patient did well until three years later, when she developed shortness of breath on exertion, claudication, and fatigue for a period of two weeks. On physical examination, her BP was noted to be elevated at 140/90 mm Hg with a clinical gradient of 20 mm between the upper and lower extremities and an increase in the gradient on echocardiogram to a peak of approximately 46 mm Hg and a mean of 21 mm Hg.
A subsequent chest CT demonstrated a narrowing of the previous stent site, and a right and left cardiac catheterization revealed neo-intimal proliferation affecting the stent with a 3–mm Hg gradient across the transverse arch and a 15–mm Hg gradient across the proximal descending aorta stent. The stent was subsequently redilated, and an additional stent was placed with no residual gradient.
The patient was discharged while taking clopidogrel 75 mg/d in addition to aspirin 325 mg/d for six months; antihypertensive medications were no longer necessary. Clinical evaluation with echocardiography was recommended every three months for the first year, and annually thereafter. At three-month and one-year follow-up, the patient was found to be symptom-free and normotensive (BP, 110/70 mm Hg).
DISCUSSION
Coarctation of the aorta (CoA) is a discrete narrowing of the thoracic aorta at the junction of the ductus arteriosus and the aortic arch, just distal to the subclavian artery. The specific anatomy, severity, and degree of hypoplasia proximal to the aortic coarctation are highly variable. For example, in some instances, coarctation presents as a long segment or a tubular hypoplasia.1
The defect is often associated with other congenital cardiovascular abnormalities, including bicuspid aortic valve (BAV; reported incidence, up to 85%),2,3 intracranial aneurysms (incidence, 3% to 10%),4 intrinsic abnormality in the aorta, aortic arch hypoplasia, ventricular septal defect, patent ductus arteriosus, aortic stenosis at different levels (valvular, subvalvular, or supravalvular), and mitral valve abnormalities.2,5 There is evidence of increased familial risk for CoA and increased prevalence with certain disorders, including Turner syndrome, maternal phenylketonuria syndrome, and Kabuki syndrome.6
CoA accounts for 5% to 8% of all congenital heart disease,2,3 and its incidence is 4 in 10,000 births.1 (Adults presenting with CoA represent either recoarctation or a missed diagnosis of native coarctation.) The mean life expectancy of untreated patients with aortic coarctation is 35 years; 90% die before age 50.1
Reduced life expectancy of patients with untreated CoA is due to systemic hypertension, accelerated coronary artery disease, stroke, heart failure, aortic rupture/dissection, cerebral hemorrhage, infective endarteritis/endocarditis, concomitant aortic valve disease (usually involving a BAV), and sudden cardiac death of presumed arrhythmogenic etiology.4 Even adults whose CoA has been detected early and managed with catheter-based and surgical interventions continue to face lifelong complications, including recoarctation, aneurysm formation, premature coronary artery disease, and cerebrovascular disease—mostly resulting from residual hypertension.3
Persistent hypertension has been reported in 68% of patients with repaired CoA at long-term follow-up. Hypertension may result in recurrence of CoA (incidence ranges from 5% to 50%), a residual CoA, or an idiopathic condition.7
The role of primary care providers is crucial in early detection and prompt referral to specialists in ACHD. For clinicians who manage these patients, increased morbidity and mortality from the associated cardiovascular sequelae pose an ongoing challenge.8
Presentation
Patients with hemodynamically stable coarctation in adolescence or adulthood are usually asymptomatic. Occasionally, a patient may be diagnosed with CoA based on its typical appearance on CXR or may come to medical attention because of an incidental murmur or management of hypertension.4 Symptoms vary in intensity and include headache, epistaxis, claudication, exertional fatigue, heart failure, aortic rupture, or dissection.7 Based on the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), and the 2008 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for adults with congenital heart disease, patients with hypertension and/or a history of CoA repair should be evaluated periodically for coarctation.8
Physical Examination
Physical assessment should include simultaneous palpation of the brachial and femoral pulses to assess amplitude and timing, looking for diminished arterial pulses and brachial-femoral delay. Additionally, measurement of supine bilateral arm (brachial artery) BP and right or left supine leg BP is recommended to detect differing pressures.8
The following physical findings may be suggestive of CoA or recoarctation:
• Systolic BP in the right arm higher than in the lower extremities, unless the origin of the right subclavian artery is anomalous and thus is not reliable; the left arm BP may not always be reliable because of the origin of the left subclavian artery, which may vary and may or may not be hypertensive
• Hyperdynamic carotid pulsations
• A pulse delay between the right arm and the femoral or popliteal arteries
• A murmur or bruit heard in the left interscapular position; a systolic ejection click of moderate intensity heard along the left sternal border
• In cases of BAV, an early diastolic decrescendo murmur of aortic regurgitation
Diagnostic Workup
The diagnosis of CoA is usually confirmed by echocardiography or radiographic imaging, including cardiac MRI or CT angiography.8
The initial diagnostic workup should include echocardiography, which may demonstrate left ventricular hypertrophy and secondary ST-T wave abnormalities, and a two-dimensional Doppler echocardiogram, which can establish the diagnosis and severity of the CoA, with possible associated cardiac defects.9 Also recommended is a CXR, which may occasionally reveal rib notching (caused by erosion of the inferior border of the posterior ribs by enlarged intercostal arteries), also known as the 3 sign. Finally, cardiac MRI is used to delineate the coarctation anatomy and to determine whether collateral arteries and/or associated vascular anomalies and flow abnormalities exist.9 If MRI is not possible, CT angiography can be an alternate approach. Subsequently, invasive angiography is required for better assessment of the coarctation gradient and hemodynamic measurement.9
Treatment
Management of CoA requires treatment of hypertension with β-blockers, ACE inhibitors, and/or ARBs as first-line medications. Aortic root size, the presence of aortic regurgitation, or both may influence the choice of antihypertensive agents.3
Intervention is recommended if the peak-to-peak coarctation gradient is ≥ 20 mm Hg, or the peak-to-peak coarctation gradient is < 20 mm Hg, with evidence of significant coarctation and collateral flow on radiologic imaging.8 The choice of treatment (stenting or surgery) should be decided by a team of ACHD cardiologists, interventionalists, and surgeons at an ACHD center.
Surgical intervention via a lateral left thoracotomy approach was first performed in 1944. The most common surgical repair is resection with end-to-end anastomosis, which yields a low mortality and recoarctation rate. Other techniques such as resection with replacement by a tube graft, patch aortoplasty, and bypass graft are used less frequently.10-12 Postsurgical morbidity most commonly includes recoarctation and residual hypertension.
Thoracotomy was the only surgical treatment until 1982, when balloon angioplasty became available as an alternative.13 However, recoarctation, aneurysm formation, and aortic dissection are major disadvantages to balloon angioplasty.13
In the early 1990s, endovascular stents were introduced and have become an alternative approach to surgical repair.14 Aneurysms remain a significant complication in 4% to 7% of patients who undergo stent placement for CoA.14
Currently, there is insufficient evidence to indicate which is the best treatment for CoA: surgical or stent repair. Choice of treatment strategy will continue to depend on the operator’s skills or institutional preference until a prospective randomized controlled clinical trial is performed.13
Follow-Up
Based on recommendations from the ACC/AHA and the 2009 Canadian Cardiovascular Society Consensus Conference on the management of adults with congenital heart disease, lifelong follow-up is recommended for all patients with aortic coarctation (whether repaired or not), including an evaluation by a cardiologist with expertise in ACHD.4,8
A baseline cardiac MRI or CT for complete evaluation of the thoracic aorta and intracranial vessels is required for follow-up. Patients who have previously undergone surgical or interventional CoA repair should be followed annually with echocardiography to assess for potential late complications, such as aortic dilatation and aneurysm formation. Evaluation of the coarctation repair site by MRI and/or CT at intervals of five years or less is also recommended. Moreover, patients should be monitored for recurrent resting or exercise-induced hypertension, which should be treated aggressively after recoarctation is excluded.
The guidelines recommend that every patient with systemic arterial hypertension have the brachial and femoral pulses palpated simultaneously.4,8 This additional physical assessment will help detect significant aortic coarctation by assessing timing and amplitude of both pulses in search for a brachial-femoral delay. Moreover, measuring the differential pressure between bilateral arms (brachial artery) in a supine position and prone right or left supine leg (popliteal artery) BP should be performed.4,8 Initial imaging and hemodynamic evaluation by transthoracic echocardiogram is recommended in suspected aortic coarctation.
CONCLUSION
This case represents a missed CoA and provides an example of recoarctation as a late complication after repair. Unfortunately, the critical need to screen for coarctation was not recognized by the patient’s primary care providers in a timely manner. Had the guidelines for CoA screening been applied, this defect would have been detected earlier, avoiding many years of cardiovascular system stress from the sequelae of hypertension.
Measuring the BP gradient between the upper and lower extremities and searching for brachial-femoral timing delay are simple but crucial steps in the initial application of the clinical guidelines for early detection of CoA and recoarctation.
References
1. Jurcut R, Daraban AM, Lorber A, et al. Coarctation of the aorta in adults: what is the best treatment? Case report and literature review. J Med Life. 2011;4:189-195.
2. Teo LLS, Cannell T, Babu-Narayan SV, et al. Prevalence of associated cardiovascular abnormalities in 500 patients with aortic coarctation referred for cardiovascular magnetic resonance imaging to a tertiary center. Pediatr Cardiol. 2011;32:1120-1127.
3. Canniffe C. Hypertension after repair of aortic coarctation–a systematic review. Int J Cardiol. 2012;167:2456-2461.
4. Silversides CK, Kiess M, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can J Cardiol. 2010;26:e80-e97.
5. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for The Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease) Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143-e263.
6. McBride KL, Pignatelli R, Lewin M, et al. Inheritance analysis of congenital left ventricular outflow tract obstruction malformations: segregation, multiplex relative risk, and heritability. Am J Med Genet. Part A. 2005;134:
180-186.
7. Brown JW, Ruzmetov M, Hoyer MH, et al. Recurrent coarctation: is surgical repair of recurrent coarctation of the aorta safe and effective? Ann Thorac Surg. 2009;88:1923-1931.
8. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008;118:2395-2451.
9. Darabian S, Zeb I, Rezaeian P, et al. Use of noninvasive imaging in the evaluation of coarctation of aorta. J Comput Assist Tomogr. 2013;37:75-78.
10. Backer CL, Mavroudis C, Zias EA, et al. Repair of coarctation with resection and extended end-to-end anastomosis. Ann Thorac Surg. 1998;66:1365-1370.
11. Cobanoglu A, Thyagarajan GK, Dobbs JL. Surgery for coarctation of the aorta in infants younger than 3 months: end-to-end repair versus subclavian flap angioplasty: is either operation better? Eur J Cardiothorac Surg. 1998;14:19-25.
12. Walhout RJ, Lekkerkerker JC, Oron GH, et al. Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg. 2003;126:521-528.
13. Pádua LM, Garcia LC, Rubira CJ, de Oliveira Carvalho PE. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev. 2012;5:CD008204.
14. Suárez de Lezo J, Pan M, Romero M, et al. Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am J Cardiol. 1999;83:400-406.