User login
Takotsubo Cardiomyopathy: A Clinical Overview
Takotsubo cardiomyopathy (TTC) is characterized by transient wall motion abnormalities of the left ventricle (LV), resulting in apical ballooning. Despite sporadic reports noting right ventricular (RV) involvement, research to date has mainly focused on LV pathology. However, Elesber and colleagues, the first group to systematically evaluate RV involvement in TTC, found RV dysfunction in eight of 25 patients.1
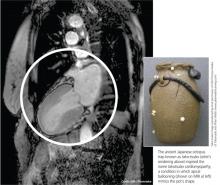
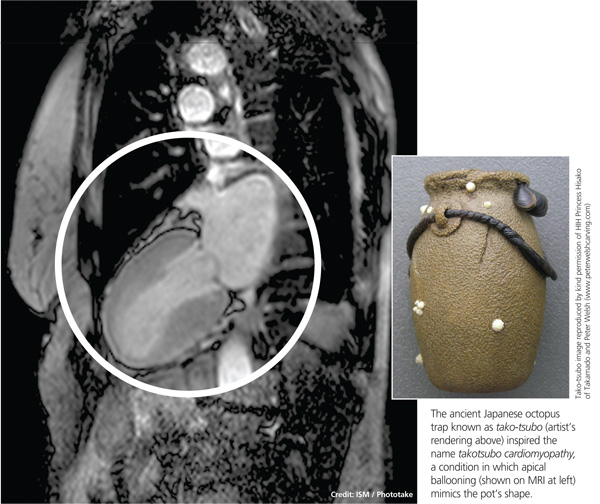
The condition is known by many different names: stress-induced cardiomyopathy, broken heart syndrome, and ampulla cardiomyopathy. The term takotsubo cardiomyopathy is derived from the appearance of the mid-ventricle and apex of the heart on echocardiography or catheterization during systole; this apical ballooning resembles a spherical bottle with a narrow neck, not unlike the ancient Japanese octopus trap called tako-tsubo (see image). First described in Japanese articles in 1991 by Dote and colleagues, TTC is not typically associated with coronary artery stenosis on angiography.2
TTC is diagnosed in approximately 1% to 2% of patients who present with signs and symptoms similar to those of acute myocardial infarction (AMI).3 The majority of affected patients are postmenopausal women, with two studies showing an 89% to 90% female predominance and mean age at presentation ranging from 58 to 77 and 58 to 75 years, respectively.4,5
The pathogenesis of TTC has been postulated to include multivessel epicardial spasm, catecholamine-mediated myocardial damage, microvascular coronary spasm or dysfunction, and neurogenic myocardial stunning.6 TTC is often not recognized on initial presentation, as it mimics AMI with ST elevation. Providers should maintain a high clinical suspicion for this transient clinical condition, which is increasingly recognized in various populations.4
Etiology and Pathophysiology
The etiology of TTC remains unclear, but a physiologic or emotional stressor usually precedes the onset of symptoms.7 It is hypothesized that a catecholamine surge in response to a stressor causes myocardial stunning through an uncertain mechanism.
Catecholamine-induced myocardial stunning due to various stressors has been documented through measurement of plasma levels of these hormones in more than 70% of patients with TTC.3 Myocardial scintillography with 123I-metaiodobenzylguanidine (MIBG) in these patients demonstrated a decreased uptake of a radiotracer in several segments of the LV, emphasizing a severe adrenalin secretion production due to stress. Considerable individual differences in MIBG uptake in patients with TTC may reflect variable responses to adrenergic stimulation, due to differences in genetic inheritance of adrenalin synthesis, functions, storage, and elimination.8
Additionally, Lyon and colleagues demonstrated a higher density of beta-adrenergic receptors in the apical region in these patients.9 They hypothesized that excessive levels of circulating catecholamines significantly alter the apical heart muscle cells, decreasing the force or energy of the muscle contraction. Conversely, Dandel and colleagues suggest that the akinetic appearance of this region can be related to high systolic apical circumferential wall stress.10
A recent literature review of 42 articles reported a possible link between TTC and drugs that overstimulate the sympathetic system.11 Consequently, clinicians should maintain a high suspicion for drug-induced TTC in patients who present with symptoms consistent with TTC but who have not experienced particularly stressful events immediately prior to onset.
The reason TTC predominately affects postmenopausal women remains unclear, but a link with reduced levels of estrogen and effects on the microvascular system has been proposed.5 One study reports a patient with TTC who carried a mutation of gene FMR1 (alleles with sizes between 40 and 55 triplet permutations); the researchers recommend further study of the role of cardiac genes in the acute phase of TTC.12 A familial apical ballooning syndrome was reported in a mother and daughter in another study, suggesting that certain women may be genetically predisposed to TTC; this may explain why only a minority of postmenopausal women are susceptible.13 However, there is no known association with single nucleotide polymorphisms for adrenergic receptors (based on a study that compared women with TTC to a control group).14
Patient Presentation
Chest pain is one of the most concerning symptoms for patients and primary care providers. The priority for a patient presenting with chest pain is to exclude catastrophic or life-threatening causes. Therefore, primary care providers are encouraged to refer patients to the nearest emergency department for appropriate diagnostic evaluation.
Precipitating events are typically severe emotional or physiologic stressors, but the absence of such a stressor does not exclude the diagnosis. Examples of emotional stressors include learning of the death of a loved one, a personal financial blow, legal problems, natural disasters, and motor vehicle collisions.4 Physiologic stressors, such as a severe medical illness, worsening chronic disease, or a noncardiac surgical procedure, can also trigger an episode of TTC.15
Patients present with chest pain and dyspnea that resemble the symptoms of AMI.4 Most patients will report chest pain at rest, although some may have dyspnea as their sole presenting complaint. They may also report palpitations, nausea, and vomiting. Patients who present with TTC typically have few traditional cardiac risk factors, such as hypertension, hyperlipidemia, diabetes, smoking, or a family history of cardiovascular disease.3
Although hemodynamic stability is the norm, a small number of patients may demonstrate hypotension due to reduced stroke volume and LV outflow tract obstruction and require hemodynamic support during the acute phase. Mild-to-moderate congestive heart failure commonly accompanies the condition.16 Rarely, patients will report an episode of syncope or an out-of-hospital cardiac arrest.17 Rare complications of TTC are cardiogenic shock and formation of a thrombus at the LV apex due to apical ballooning akinesis.17,18
Diagnostic Studies
Electrocardiography (ECG) findings may demonstrate ST-segment elevation, although this occurs in only one-half of patients with TTC.17 The elevation is typically noted in the precordial leads,16 but the ECG can be normal or show nonspecific T-wave abnormalities.17 In patients with ST-segment elevation, the severity of ventricular dysfunction or prognosis does not correlate with the ECG changes noted on presentation.19 The ST-segment elevation is followed by T-wave inversions and QT prolongation.16 Torsades de pointes and QT prolongation in patients with TTC has been reported.20
Laboratory studies demonstrate mildly elevated cardiac troponin and brain natriuretic peptide levels.3,21,22 LV wall-motion abnormalities and a severely depressed ejection fraction have been noted on transthoracic echocardiography.23 Several case reports utilized cardiac magnetic resonance imaging in patients with TTC, resulting in findings of wall motion abnormalities, myocardial edema, and hyperenhancement on contrast-enhanced imaging.24,25
Diagnosis
Although universally accepted diagnostic criteria are currently unavailable,23 Bybee and colleagues have proposed criteria for the diagnosis of TTC.6 All four of the following criteria must be met:
• Transient hypokinesis, akinesis, or dyskinesis of the LV apical and midventricular segments with regional wall-motion abnormalities encompassing more than a single epicardial vascular distribution.
• Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture.
• New ECG abnormalities (either ST-segment or T-wave inversion) or modest elevation of cardiac troponin levels.
• Absence of recent significant head trauma, intracranial bleeding, pheochromocytoma, obstructive epicardial coronary artery disease, myocarditis, and hypertrophic cardiomyopathy.
The diagnosis of TTC is often made in an emergency setting because of the presenting complaint of chest pain. Typically, patients with TTC have no associated significant atherosclerotic luminal narrowing7 despite the presence of transient apical and left midventricular systolic dysfunction.
Treatment and Management
Optimal management of TTC has yet to be established, but the general approach is supportive and conservative. Because the presenting chest pain is indistinguishable from AMI, initial management should focus on preventing ischemia. Continuous ECG monitoring and administration of nitrates, morphine for pain control, aspirin, IV heparin, and beta-blockers are recommended.26
Once the diagnosis of TTC is confirmed, if there is no coexisting coronary atherosclerosis, aspirin therapy can be discontinued.17 Treatment with beta-blockers (in hemodynamically stable patients) and ACE inhibitors (in the absence of outflow tract obstruction) is usually recommended, although randomized trials have not been conducted.27
Beta-blockers, which may inhibit the release of catecholamines, could be beneficial since they are hypothesized to mediate in TTC. Additionally, beta-blockers work to reduce LV outflow tract obstruction through basal segment hypercontractility.3 For patients with associated congestive heart failure, diuretics may be effective. For significant hypotension, phenylephrine helps to increase afterload and LV cavity size; note that inotropes are contraindicated in this situation. In the rare occurrence of LV thrombus, anticoagulation is recommended.16,18 Long-term administration of beta-blockers is recommended to reduce the likelihood of TTC recurrence.17
Prognosis
In the absence of underlying comorbid conditions, the prognosis in TTC is generally good. Cardiovascular symptoms—systolic dysfunction and regional wall-motion abnormality—usually resolve completely within days to one month; an alternative diagnosis should be considered if the cardiomyopathy does not resolve after this time. Close follow-up with a cardiologist, usually with serial echocardiograms, in the weeks after diagnosis is recommended to ensure complete resolution. At six-week follow-up, the ECG usually demonstrates complete resolution, although T-wave inversion may persist.17
Inpatient mortality rates associated with TTC range from 0% to 8%.18,28 The recurrence rate has been reported as less than 10%, but additional studies are needed to track recurrence, in addition to the longitudinal effects of this condition.29 Left-sided heart failure, with or without pulmonary edema, is the most common complication associated with TTC. Others are LV mural clot, systemic or pulmonic embolic events, mitral valve regurgitation, and ventricular arrhythmias.3
Conclusion
TTC is an entity of acute heart failure that can mimic AMI. It should be considered in symptomatic postmenopausal women with a normal heart and no history of cardiovascular disease. Providers should include TTC in the differential diagnosis, especially when patients present with acute chest pain after a stressful incident. While short-term management may suffice, providers should follow these patients over time to identify the potential for long-term impact and possible causes of this condition.
References
1. Elesber AA, Prasad A, Bybee KA, et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006;47:1082-1083.
2. Dote K, Sato H, Tateishi H, et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a view of 5 cases. J Cardiol. 1991;24:471-476.
3. Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol. 2008;124:283-292.
4. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy; a systematic review. Eur Heart J. 2006;27: 1523-1529.
5. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417.
6. Bybee KA, Prasad A, Barsness GW. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004; 94:343-346.
7. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472-479.
8. Soares-Filho GL, Felix RC, Azevedo JC, et al. Broken heart or takotsubo syndrome: support for the neurohumoral hypothesis of stress cardiomyopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:
247-249.
9. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22-29.
10. Dandel M, Lehmkuhl H, Knosalla C, Hetzer R. Left ventricular wall motion abnormality and myocardial dysfunction in stress cardiomyopathy: new pathophysiological aspects suggested by echocardiography. Int J Cardiol. 2009;135:e40-43.
11. Amariles P. A comprehensive literature search: drugs as possible triggers of Takotsubo cardiomyopathy. Curr Clin Pharmacol. 2011;6:1-11.
12. Kleinfeldt T, Schneider H, Akin I, et al. Detection of FMR1-gene in Takotsubo cardiomyopathy: a new piece of the puzzle. Int J Cardiol. 2009;137:e81-83.
13. Kumar G, Holmes DR, Prasad A. “Familial” apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2010;144:444-445.
14. Sharkey SW, Maron BJ, Nelson P, et al. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J Cardiol. 2009;53:53-57.
15. Le Ven F, Pennec PY, Timsit S, Blanc JJ. Takotsubo syndrome associated with seizures: an underestimated cause of sudden death in epilepsy? Int J Cardiol. 2011;146:475-479.
16. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858-865.
17. Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation, 2007;111:e56-e59.
18. Lee PH, Song JK, Sun BJ, et al. Outcomes of patients with stress-induced cardiomyopathy diagnosed by echocardiography in a tertiary referral hospital. J Am Soc Echocardiogr. 2010;23:766-771.
19. Dib C, Asirvatham S, Elesber A, et al. Clinical correlates and prognostic significance of electrocardiographic abnormalities in apical ballooning syndrome (Takotsubo/stress-induced cardiomyopathy). Am Heart J. 2009;157:933-938.
20. Denney SD, Lakkireddy DR, Khan IA. Long QT syndrome and torsade de pointes in transient left ventricular apical ballooning syndrome. Int J Cardio. 2005;100:499-501.
21. Song BG, Park SJ, Noh HJ, et al. Clinical characteristics, and laboratory and echocardiographic findings in takotsubo cardiomyopathy presenting as cardiogenic shock. J Crit Care. 2010;25:329-335.
22. Primetshofer D, Agladze R, Kratzer H, et al. Tako-Tsubo syndrome: an important differential diagnosis in patients with acute chest pain. Wien Klin Wochenschr. 2010;122:37-44.
23. Ando G, Trio O, Gregorio C. Transient left ventricular apical ballooning syndrome and cardiac dysfunction after subarachnoid hemorrhage: similar clinical entities? Open Emerg Med J. 2009;2:8-10.
24. Caudron J, Rey N, Dacher JN. Midventricular Takotsubo cardiomyopathy associated with ventricular fibrillation during general anaesthesia in 34-year-old woman: insight from cardiac computed tomography and magnetic resonance imaging. Arch Cardiovasc Dis. 2012;105:329-331.
25. Matsumoto H, Matsuda T, Miyamoto K. Early enhancement on contrast-enhanced cardiovascular magnetic resonance imaging in takotsubo cardiomyopathy: two cases. Int J Cardiol. 2012;155:e54-e56.
26. Van de Werf F, Bas J, Betriu A, et al; ESC Committee for Practice Guidelines. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909-2945.
27. Tomich EB, Luerssen E, Kang CS. Takotsubo cardiomyopathy. http://emedicine.medscape.com/article/1513631-overview. Accessed August 14, 2013.
28. Silva C, Goncalves A, Almedia R, et al. Transient left ventricular ballooning syndrome. Eur J Intern Med. 2009;20:454-456.
29. Elesber AA, Prasad A, Lennon RJ, et al. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448-452.
Takotsubo cardiomyopathy (TTC) is characterized by transient wall motion abnormalities of the left ventricle (LV), resulting in apical ballooning. Despite sporadic reports noting right ventricular (RV) involvement, research to date has mainly focused on LV pathology. However, Elesber and colleagues, the first group to systematically evaluate RV involvement in TTC, found RV dysfunction in eight of 25 patients.1
The condition is known by many different names: stress-induced cardiomyopathy, broken heart syndrome, and ampulla cardiomyopathy. The term takotsubo cardiomyopathy is derived from the appearance of the mid-ventricle and apex of the heart on echocardiography or catheterization during systole; this apical ballooning resembles a spherical bottle with a narrow neck, not unlike the ancient Japanese octopus trap called tako-tsubo (see image). First described in Japanese articles in 1991 by Dote and colleagues, TTC is not typically associated with coronary artery stenosis on angiography.2
TTC is diagnosed in approximately 1% to 2% of patients who present with signs and symptoms similar to those of acute myocardial infarction (AMI).3 The majority of affected patients are postmenopausal women, with two studies showing an 89% to 90% female predominance and mean age at presentation ranging from 58 to 77 and 58 to 75 years, respectively.4,5
The pathogenesis of TTC has been postulated to include multivessel epicardial spasm, catecholamine-mediated myocardial damage, microvascular coronary spasm or dysfunction, and neurogenic myocardial stunning.6 TTC is often not recognized on initial presentation, as it mimics AMI with ST elevation. Providers should maintain a high clinical suspicion for this transient clinical condition, which is increasingly recognized in various populations.4
Etiology and Pathophysiology
The etiology of TTC remains unclear, but a physiologic or emotional stressor usually precedes the onset of symptoms.7 It is hypothesized that a catecholamine surge in response to a stressor causes myocardial stunning through an uncertain mechanism.
Catecholamine-induced myocardial stunning due to various stressors has been documented through measurement of plasma levels of these hormones in more than 70% of patients with TTC.3 Myocardial scintillography with 123I-metaiodobenzylguanidine (MIBG) in these patients demonstrated a decreased uptake of a radiotracer in several segments of the LV, emphasizing a severe adrenalin secretion production due to stress. Considerable individual differences in MIBG uptake in patients with TTC may reflect variable responses to adrenergic stimulation, due to differences in genetic inheritance of adrenalin synthesis, functions, storage, and elimination.8
Additionally, Lyon and colleagues demonstrated a higher density of beta-adrenergic receptors in the apical region in these patients.9 They hypothesized that excessive levels of circulating catecholamines significantly alter the apical heart muscle cells, decreasing the force or energy of the muscle contraction. Conversely, Dandel and colleagues suggest that the akinetic appearance of this region can be related to high systolic apical circumferential wall stress.10
A recent literature review of 42 articles reported a possible link between TTC and drugs that overstimulate the sympathetic system.11 Consequently, clinicians should maintain a high suspicion for drug-induced TTC in patients who present with symptoms consistent with TTC but who have not experienced particularly stressful events immediately prior to onset.
The reason TTC predominately affects postmenopausal women remains unclear, but a link with reduced levels of estrogen and effects on the microvascular system has been proposed.5 One study reports a patient with TTC who carried a mutation of gene FMR1 (alleles with sizes between 40 and 55 triplet permutations); the researchers recommend further study of the role of cardiac genes in the acute phase of TTC.12 A familial apical ballooning syndrome was reported in a mother and daughter in another study, suggesting that certain women may be genetically predisposed to TTC; this may explain why only a minority of postmenopausal women are susceptible.13 However, there is no known association with single nucleotide polymorphisms for adrenergic receptors (based on a study that compared women with TTC to a control group).14
Patient Presentation
Chest pain is one of the most concerning symptoms for patients and primary care providers. The priority for a patient presenting with chest pain is to exclude catastrophic or life-threatening causes. Therefore, primary care providers are encouraged to refer patients to the nearest emergency department for appropriate diagnostic evaluation.
Precipitating events are typically severe emotional or physiologic stressors, but the absence of such a stressor does not exclude the diagnosis. Examples of emotional stressors include learning of the death of a loved one, a personal financial blow, legal problems, natural disasters, and motor vehicle collisions.4 Physiologic stressors, such as a severe medical illness, worsening chronic disease, or a noncardiac surgical procedure, can also trigger an episode of TTC.15
Patients present with chest pain and dyspnea that resemble the symptoms of AMI.4 Most patients will report chest pain at rest, although some may have dyspnea as their sole presenting complaint. They may also report palpitations, nausea, and vomiting. Patients who present with TTC typically have few traditional cardiac risk factors, such as hypertension, hyperlipidemia, diabetes, smoking, or a family history of cardiovascular disease.3
Although hemodynamic stability is the norm, a small number of patients may demonstrate hypotension due to reduced stroke volume and LV outflow tract obstruction and require hemodynamic support during the acute phase. Mild-to-moderate congestive heart failure commonly accompanies the condition.16 Rarely, patients will report an episode of syncope or an out-of-hospital cardiac arrest.17 Rare complications of TTC are cardiogenic shock and formation of a thrombus at the LV apex due to apical ballooning akinesis.17,18
Diagnostic Studies
Electrocardiography (ECG) findings may demonstrate ST-segment elevation, although this occurs in only one-half of patients with TTC.17 The elevation is typically noted in the precordial leads,16 but the ECG can be normal or show nonspecific T-wave abnormalities.17 In patients with ST-segment elevation, the severity of ventricular dysfunction or prognosis does not correlate with the ECG changes noted on presentation.19 The ST-segment elevation is followed by T-wave inversions and QT prolongation.16 Torsades de pointes and QT prolongation in patients with TTC has been reported.20
Laboratory studies demonstrate mildly elevated cardiac troponin and brain natriuretic peptide levels.3,21,22 LV wall-motion abnormalities and a severely depressed ejection fraction have been noted on transthoracic echocardiography.23 Several case reports utilized cardiac magnetic resonance imaging in patients with TTC, resulting in findings of wall motion abnormalities, myocardial edema, and hyperenhancement on contrast-enhanced imaging.24,25
Diagnosis
Although universally accepted diagnostic criteria are currently unavailable,23 Bybee and colleagues have proposed criteria for the diagnosis of TTC.6 All four of the following criteria must be met:
• Transient hypokinesis, akinesis, or dyskinesis of the LV apical and midventricular segments with regional wall-motion abnormalities encompassing more than a single epicardial vascular distribution.
• Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture.
• New ECG abnormalities (either ST-segment or T-wave inversion) or modest elevation of cardiac troponin levels.
• Absence of recent significant head trauma, intracranial bleeding, pheochromocytoma, obstructive epicardial coronary artery disease, myocarditis, and hypertrophic cardiomyopathy.
The diagnosis of TTC is often made in an emergency setting because of the presenting complaint of chest pain. Typically, patients with TTC have no associated significant atherosclerotic luminal narrowing7 despite the presence of transient apical and left midventricular systolic dysfunction.
Treatment and Management
Optimal management of TTC has yet to be established, but the general approach is supportive and conservative. Because the presenting chest pain is indistinguishable from AMI, initial management should focus on preventing ischemia. Continuous ECG monitoring and administration of nitrates, morphine for pain control, aspirin, IV heparin, and beta-blockers are recommended.26
Once the diagnosis of TTC is confirmed, if there is no coexisting coronary atherosclerosis, aspirin therapy can be discontinued.17 Treatment with beta-blockers (in hemodynamically stable patients) and ACE inhibitors (in the absence of outflow tract obstruction) is usually recommended, although randomized trials have not been conducted.27
Beta-blockers, which may inhibit the release of catecholamines, could be beneficial since they are hypothesized to mediate in TTC. Additionally, beta-blockers work to reduce LV outflow tract obstruction through basal segment hypercontractility.3 For patients with associated congestive heart failure, diuretics may be effective. For significant hypotension, phenylephrine helps to increase afterload and LV cavity size; note that inotropes are contraindicated in this situation. In the rare occurrence of LV thrombus, anticoagulation is recommended.16,18 Long-term administration of beta-blockers is recommended to reduce the likelihood of TTC recurrence.17
Prognosis
In the absence of underlying comorbid conditions, the prognosis in TTC is generally good. Cardiovascular symptoms—systolic dysfunction and regional wall-motion abnormality—usually resolve completely within days to one month; an alternative diagnosis should be considered if the cardiomyopathy does not resolve after this time. Close follow-up with a cardiologist, usually with serial echocardiograms, in the weeks after diagnosis is recommended to ensure complete resolution. At six-week follow-up, the ECG usually demonstrates complete resolution, although T-wave inversion may persist.17
Inpatient mortality rates associated with TTC range from 0% to 8%.18,28 The recurrence rate has been reported as less than 10%, but additional studies are needed to track recurrence, in addition to the longitudinal effects of this condition.29 Left-sided heart failure, with or without pulmonary edema, is the most common complication associated with TTC. Others are LV mural clot, systemic or pulmonic embolic events, mitral valve regurgitation, and ventricular arrhythmias.3
Conclusion
TTC is an entity of acute heart failure that can mimic AMI. It should be considered in symptomatic postmenopausal women with a normal heart and no history of cardiovascular disease. Providers should include TTC in the differential diagnosis, especially when patients present with acute chest pain after a stressful incident. While short-term management may suffice, providers should follow these patients over time to identify the potential for long-term impact and possible causes of this condition.
References
1. Elesber AA, Prasad A, Bybee KA, et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006;47:1082-1083.
2. Dote K, Sato H, Tateishi H, et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a view of 5 cases. J Cardiol. 1991;24:471-476.
3. Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol. 2008;124:283-292.
4. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy; a systematic review. Eur Heart J. 2006;27: 1523-1529.
5. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417.
6. Bybee KA, Prasad A, Barsness GW. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004; 94:343-346.
7. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472-479.
8. Soares-Filho GL, Felix RC, Azevedo JC, et al. Broken heart or takotsubo syndrome: support for the neurohumoral hypothesis of stress cardiomyopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:
247-249.
9. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22-29.
10. Dandel M, Lehmkuhl H, Knosalla C, Hetzer R. Left ventricular wall motion abnormality and myocardial dysfunction in stress cardiomyopathy: new pathophysiological aspects suggested by echocardiography. Int J Cardiol. 2009;135:e40-43.
11. Amariles P. A comprehensive literature search: drugs as possible triggers of Takotsubo cardiomyopathy. Curr Clin Pharmacol. 2011;6:1-11.
12. Kleinfeldt T, Schneider H, Akin I, et al. Detection of FMR1-gene in Takotsubo cardiomyopathy: a new piece of the puzzle. Int J Cardiol. 2009;137:e81-83.
13. Kumar G, Holmes DR, Prasad A. “Familial” apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2010;144:444-445.
14. Sharkey SW, Maron BJ, Nelson P, et al. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J Cardiol. 2009;53:53-57.
15. Le Ven F, Pennec PY, Timsit S, Blanc JJ. Takotsubo syndrome associated with seizures: an underestimated cause of sudden death in epilepsy? Int J Cardiol. 2011;146:475-479.
16. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858-865.
17. Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation, 2007;111:e56-e59.
18. Lee PH, Song JK, Sun BJ, et al. Outcomes of patients with stress-induced cardiomyopathy diagnosed by echocardiography in a tertiary referral hospital. J Am Soc Echocardiogr. 2010;23:766-771.
19. Dib C, Asirvatham S, Elesber A, et al. Clinical correlates and prognostic significance of electrocardiographic abnormalities in apical ballooning syndrome (Takotsubo/stress-induced cardiomyopathy). Am Heart J. 2009;157:933-938.
20. Denney SD, Lakkireddy DR, Khan IA. Long QT syndrome and torsade de pointes in transient left ventricular apical ballooning syndrome. Int J Cardio. 2005;100:499-501.
21. Song BG, Park SJ, Noh HJ, et al. Clinical characteristics, and laboratory and echocardiographic findings in takotsubo cardiomyopathy presenting as cardiogenic shock. J Crit Care. 2010;25:329-335.
22. Primetshofer D, Agladze R, Kratzer H, et al. Tako-Tsubo syndrome: an important differential diagnosis in patients with acute chest pain. Wien Klin Wochenschr. 2010;122:37-44.
23. Ando G, Trio O, Gregorio C. Transient left ventricular apical ballooning syndrome and cardiac dysfunction after subarachnoid hemorrhage: similar clinical entities? Open Emerg Med J. 2009;2:8-10.
24. Caudron J, Rey N, Dacher JN. Midventricular Takotsubo cardiomyopathy associated with ventricular fibrillation during general anaesthesia in 34-year-old woman: insight from cardiac computed tomography and magnetic resonance imaging. Arch Cardiovasc Dis. 2012;105:329-331.
25. Matsumoto H, Matsuda T, Miyamoto K. Early enhancement on contrast-enhanced cardiovascular magnetic resonance imaging in takotsubo cardiomyopathy: two cases. Int J Cardiol. 2012;155:e54-e56.
26. Van de Werf F, Bas J, Betriu A, et al; ESC Committee for Practice Guidelines. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909-2945.
27. Tomich EB, Luerssen E, Kang CS. Takotsubo cardiomyopathy. http://emedicine.medscape.com/article/1513631-overview. Accessed August 14, 2013.
28. Silva C, Goncalves A, Almedia R, et al. Transient left ventricular ballooning syndrome. Eur J Intern Med. 2009;20:454-456.
29. Elesber AA, Prasad A, Lennon RJ, et al. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448-452.
Takotsubo cardiomyopathy (TTC) is characterized by transient wall motion abnormalities of the left ventricle (LV), resulting in apical ballooning. Despite sporadic reports noting right ventricular (RV) involvement, research to date has mainly focused on LV pathology. However, Elesber and colleagues, the first group to systematically evaluate RV involvement in TTC, found RV dysfunction in eight of 25 patients.1
The condition is known by many different names: stress-induced cardiomyopathy, broken heart syndrome, and ampulla cardiomyopathy. The term takotsubo cardiomyopathy is derived from the appearance of the mid-ventricle and apex of the heart on echocardiography or catheterization during systole; this apical ballooning resembles a spherical bottle with a narrow neck, not unlike the ancient Japanese octopus trap called tako-tsubo (see image). First described in Japanese articles in 1991 by Dote and colleagues, TTC is not typically associated with coronary artery stenosis on angiography.2
TTC is diagnosed in approximately 1% to 2% of patients who present with signs and symptoms similar to those of acute myocardial infarction (AMI).3 The majority of affected patients are postmenopausal women, with two studies showing an 89% to 90% female predominance and mean age at presentation ranging from 58 to 77 and 58 to 75 years, respectively.4,5
The pathogenesis of TTC has been postulated to include multivessel epicardial spasm, catecholamine-mediated myocardial damage, microvascular coronary spasm or dysfunction, and neurogenic myocardial stunning.6 TTC is often not recognized on initial presentation, as it mimics AMI with ST elevation. Providers should maintain a high clinical suspicion for this transient clinical condition, which is increasingly recognized in various populations.4
Etiology and Pathophysiology
The etiology of TTC remains unclear, but a physiologic or emotional stressor usually precedes the onset of symptoms.7 It is hypothesized that a catecholamine surge in response to a stressor causes myocardial stunning through an uncertain mechanism.
Catecholamine-induced myocardial stunning due to various stressors has been documented through measurement of plasma levels of these hormones in more than 70% of patients with TTC.3 Myocardial scintillography with 123I-metaiodobenzylguanidine (MIBG) in these patients demonstrated a decreased uptake of a radiotracer in several segments of the LV, emphasizing a severe adrenalin secretion production due to stress. Considerable individual differences in MIBG uptake in patients with TTC may reflect variable responses to adrenergic stimulation, due to differences in genetic inheritance of adrenalin synthesis, functions, storage, and elimination.8
Additionally, Lyon and colleagues demonstrated a higher density of beta-adrenergic receptors in the apical region in these patients.9 They hypothesized that excessive levels of circulating catecholamines significantly alter the apical heart muscle cells, decreasing the force or energy of the muscle contraction. Conversely, Dandel and colleagues suggest that the akinetic appearance of this region can be related to high systolic apical circumferential wall stress.10
A recent literature review of 42 articles reported a possible link between TTC and drugs that overstimulate the sympathetic system.11 Consequently, clinicians should maintain a high suspicion for drug-induced TTC in patients who present with symptoms consistent with TTC but who have not experienced particularly stressful events immediately prior to onset.
The reason TTC predominately affects postmenopausal women remains unclear, but a link with reduced levels of estrogen and effects on the microvascular system has been proposed.5 One study reports a patient with TTC who carried a mutation of gene FMR1 (alleles with sizes between 40 and 55 triplet permutations); the researchers recommend further study of the role of cardiac genes in the acute phase of TTC.12 A familial apical ballooning syndrome was reported in a mother and daughter in another study, suggesting that certain women may be genetically predisposed to TTC; this may explain why only a minority of postmenopausal women are susceptible.13 However, there is no known association with single nucleotide polymorphisms for adrenergic receptors (based on a study that compared women with TTC to a control group).14
Patient Presentation
Chest pain is one of the most concerning symptoms for patients and primary care providers. The priority for a patient presenting with chest pain is to exclude catastrophic or life-threatening causes. Therefore, primary care providers are encouraged to refer patients to the nearest emergency department for appropriate diagnostic evaluation.
Precipitating events are typically severe emotional or physiologic stressors, but the absence of such a stressor does not exclude the diagnosis. Examples of emotional stressors include learning of the death of a loved one, a personal financial blow, legal problems, natural disasters, and motor vehicle collisions.4 Physiologic stressors, such as a severe medical illness, worsening chronic disease, or a noncardiac surgical procedure, can also trigger an episode of TTC.15
Patients present with chest pain and dyspnea that resemble the symptoms of AMI.4 Most patients will report chest pain at rest, although some may have dyspnea as their sole presenting complaint. They may also report palpitations, nausea, and vomiting. Patients who present with TTC typically have few traditional cardiac risk factors, such as hypertension, hyperlipidemia, diabetes, smoking, or a family history of cardiovascular disease.3
Although hemodynamic stability is the norm, a small number of patients may demonstrate hypotension due to reduced stroke volume and LV outflow tract obstruction and require hemodynamic support during the acute phase. Mild-to-moderate congestive heart failure commonly accompanies the condition.16 Rarely, patients will report an episode of syncope or an out-of-hospital cardiac arrest.17 Rare complications of TTC are cardiogenic shock and formation of a thrombus at the LV apex due to apical ballooning akinesis.17,18
Diagnostic Studies
Electrocardiography (ECG) findings may demonstrate ST-segment elevation, although this occurs in only one-half of patients with TTC.17 The elevation is typically noted in the precordial leads,16 but the ECG can be normal or show nonspecific T-wave abnormalities.17 In patients with ST-segment elevation, the severity of ventricular dysfunction or prognosis does not correlate with the ECG changes noted on presentation.19 The ST-segment elevation is followed by T-wave inversions and QT prolongation.16 Torsades de pointes and QT prolongation in patients with TTC has been reported.20
Laboratory studies demonstrate mildly elevated cardiac troponin and brain natriuretic peptide levels.3,21,22 LV wall-motion abnormalities and a severely depressed ejection fraction have been noted on transthoracic echocardiography.23 Several case reports utilized cardiac magnetic resonance imaging in patients with TTC, resulting in findings of wall motion abnormalities, myocardial edema, and hyperenhancement on contrast-enhanced imaging.24,25
Diagnosis
Although universally accepted diagnostic criteria are currently unavailable,23 Bybee and colleagues have proposed criteria for the diagnosis of TTC.6 All four of the following criteria must be met:
• Transient hypokinesis, akinesis, or dyskinesis of the LV apical and midventricular segments with regional wall-motion abnormalities encompassing more than a single epicardial vascular distribution.
• Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture.
• New ECG abnormalities (either ST-segment or T-wave inversion) or modest elevation of cardiac troponin levels.
• Absence of recent significant head trauma, intracranial bleeding, pheochromocytoma, obstructive epicardial coronary artery disease, myocarditis, and hypertrophic cardiomyopathy.
The diagnosis of TTC is often made in an emergency setting because of the presenting complaint of chest pain. Typically, patients with TTC have no associated significant atherosclerotic luminal narrowing7 despite the presence of transient apical and left midventricular systolic dysfunction.
Treatment and Management
Optimal management of TTC has yet to be established, but the general approach is supportive and conservative. Because the presenting chest pain is indistinguishable from AMI, initial management should focus on preventing ischemia. Continuous ECG monitoring and administration of nitrates, morphine for pain control, aspirin, IV heparin, and beta-blockers are recommended.26
Once the diagnosis of TTC is confirmed, if there is no coexisting coronary atherosclerosis, aspirin therapy can be discontinued.17 Treatment with beta-blockers (in hemodynamically stable patients) and ACE inhibitors (in the absence of outflow tract obstruction) is usually recommended, although randomized trials have not been conducted.27
Beta-blockers, which may inhibit the release of catecholamines, could be beneficial since they are hypothesized to mediate in TTC. Additionally, beta-blockers work to reduce LV outflow tract obstruction through basal segment hypercontractility.3 For patients with associated congestive heart failure, diuretics may be effective. For significant hypotension, phenylephrine helps to increase afterload and LV cavity size; note that inotropes are contraindicated in this situation. In the rare occurrence of LV thrombus, anticoagulation is recommended.16,18 Long-term administration of beta-blockers is recommended to reduce the likelihood of TTC recurrence.17
Prognosis
In the absence of underlying comorbid conditions, the prognosis in TTC is generally good. Cardiovascular symptoms—systolic dysfunction and regional wall-motion abnormality—usually resolve completely within days to one month; an alternative diagnosis should be considered if the cardiomyopathy does not resolve after this time. Close follow-up with a cardiologist, usually with serial echocardiograms, in the weeks after diagnosis is recommended to ensure complete resolution. At six-week follow-up, the ECG usually demonstrates complete resolution, although T-wave inversion may persist.17
Inpatient mortality rates associated with TTC range from 0% to 8%.18,28 The recurrence rate has been reported as less than 10%, but additional studies are needed to track recurrence, in addition to the longitudinal effects of this condition.29 Left-sided heart failure, with or without pulmonary edema, is the most common complication associated with TTC. Others are LV mural clot, systemic or pulmonic embolic events, mitral valve regurgitation, and ventricular arrhythmias.3
Conclusion
TTC is an entity of acute heart failure that can mimic AMI. It should be considered in symptomatic postmenopausal women with a normal heart and no history of cardiovascular disease. Providers should include TTC in the differential diagnosis, especially when patients present with acute chest pain after a stressful incident. While short-term management may suffice, providers should follow these patients over time to identify the potential for long-term impact and possible causes of this condition.
References
1. Elesber AA, Prasad A, Bybee KA, et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006;47:1082-1083.
2. Dote K, Sato H, Tateishi H, et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a view of 5 cases. J Cardiol. 1991;24:471-476.
3. Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol. 2008;124:283-292.
4. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy; a systematic review. Eur Heart J. 2006;27: 1523-1529.
5. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417.
6. Bybee KA, Prasad A, Barsness GW. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004; 94:343-346.
7. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472-479.
8. Soares-Filho GL, Felix RC, Azevedo JC, et al. Broken heart or takotsubo syndrome: support for the neurohumoral hypothesis of stress cardiomyopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:
247-249.
9. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22-29.
10. Dandel M, Lehmkuhl H, Knosalla C, Hetzer R. Left ventricular wall motion abnormality and myocardial dysfunction in stress cardiomyopathy: new pathophysiological aspects suggested by echocardiography. Int J Cardiol. 2009;135:e40-43.
11. Amariles P. A comprehensive literature search: drugs as possible triggers of Takotsubo cardiomyopathy. Curr Clin Pharmacol. 2011;6:1-11.
12. Kleinfeldt T, Schneider H, Akin I, et al. Detection of FMR1-gene in Takotsubo cardiomyopathy: a new piece of the puzzle. Int J Cardiol. 2009;137:e81-83.
13. Kumar G, Holmes DR, Prasad A. “Familial” apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2010;144:444-445.
14. Sharkey SW, Maron BJ, Nelson P, et al. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J Cardiol. 2009;53:53-57.
15. Le Ven F, Pennec PY, Timsit S, Blanc JJ. Takotsubo syndrome associated with seizures: an underestimated cause of sudden death in epilepsy? Int J Cardiol. 2011;146:475-479.
16. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858-865.
17. Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation, 2007;111:e56-e59.
18. Lee PH, Song JK, Sun BJ, et al. Outcomes of patients with stress-induced cardiomyopathy diagnosed by echocardiography in a tertiary referral hospital. J Am Soc Echocardiogr. 2010;23:766-771.
19. Dib C, Asirvatham S, Elesber A, et al. Clinical correlates and prognostic significance of electrocardiographic abnormalities in apical ballooning syndrome (Takotsubo/stress-induced cardiomyopathy). Am Heart J. 2009;157:933-938.
20. Denney SD, Lakkireddy DR, Khan IA. Long QT syndrome and torsade de pointes in transient left ventricular apical ballooning syndrome. Int J Cardio. 2005;100:499-501.
21. Song BG, Park SJ, Noh HJ, et al. Clinical characteristics, and laboratory and echocardiographic findings in takotsubo cardiomyopathy presenting as cardiogenic shock. J Crit Care. 2010;25:329-335.
22. Primetshofer D, Agladze R, Kratzer H, et al. Tako-Tsubo syndrome: an important differential diagnosis in patients with acute chest pain. Wien Klin Wochenschr. 2010;122:37-44.
23. Ando G, Trio O, Gregorio C. Transient left ventricular apical ballooning syndrome and cardiac dysfunction after subarachnoid hemorrhage: similar clinical entities? Open Emerg Med J. 2009;2:8-10.
24. Caudron J, Rey N, Dacher JN. Midventricular Takotsubo cardiomyopathy associated with ventricular fibrillation during general anaesthesia in 34-year-old woman: insight from cardiac computed tomography and magnetic resonance imaging. Arch Cardiovasc Dis. 2012;105:329-331.
25. Matsumoto H, Matsuda T, Miyamoto K. Early enhancement on contrast-enhanced cardiovascular magnetic resonance imaging in takotsubo cardiomyopathy: two cases. Int J Cardiol. 2012;155:e54-e56.
26. Van de Werf F, Bas J, Betriu A, et al; ESC Committee for Practice Guidelines. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909-2945.
27. Tomich EB, Luerssen E, Kang CS. Takotsubo cardiomyopathy. http://emedicine.medscape.com/article/1513631-overview. Accessed August 14, 2013.
28. Silva C, Goncalves A, Almedia R, et al. Transient left ventricular ballooning syndrome. Eur J Intern Med. 2009;20:454-456.
29. Elesber AA, Prasad A, Lennon RJ, et al. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448-452.