User login
What is the best initial treatment of an adult patient with healthcare-associated pneumonia?
Case
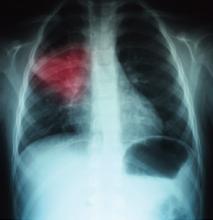
A 68-year-old man with hypertension, diabetes, and recent hip fracture with poor functional status presents from a nursing home with a productive cough, shortness of breath, and chills of two-day duration. He finished a five-day course of cephalexin for a urinary tract infection one week ago. His vital signs reveal a blood pressure of 162/80 mm/Hg, temperature of 101.9°F, respirations of 26 breaths per minute, and oxygen saturation of 88% on room air. Coarse breath sounds are noted in the right lung field and his chest X-ray reveals a right-middle-lobe infiltrate.
He is admitted to the hospital with a diagnosis of healthcare-associated pneumonia. What is the best empiric antibiotic coverage for this patient?
Overview
Modern medicine exists over a continuum of care that is delivered in a manifold of different settings. Patients routinely receive complex medical care at home, including wound care and infusion of intravenous antibiotics. Additionally, many patients are interfacing with the healthcare system on a regular basis via hemodialysis centers or sub-acute rehabilitation centers. As a result of these interactions, patients are exposed to—and colonized by—different bacterial pathogens that can result in a variety of infections.1
While patients with healthcare-associated pneumonia (HCAP) can present similarly to those with community-acquired pneumonia (CAP)—patients with CAP normally present with a lower-respiratory-tract infection—the differences in the likely etiological pathogens dictate that these patients be considered for broader-spectrum empiric antibiotics. Hospitalists will continue to be responsible for choosing the initial antibiotic regimen for these patients, and they need to be able to recognize this disease process in order to treat it appropriately.
The joint American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines released in 2005 emphasize that certain clinical HCAP risk factors center on increased interactions and encounters with healthcare facilities.2 These risk factors are evolving over time to include a patient’s functional status, recent antibiotic use, and clinical severity.
Review of the Data
Differences between HCAP and CAP
HCAP represents a diagnostic category of pneumonia created to differentiate patients with infections caused by a different microbiological subset of bacteria, including possible multi-drug-resistant (MDR) organisms, from patients with CAP. Thus far, culture data support this dichotomy.3,4
Kollef and colleagues performed a multicenter, retrospective cohort study of 4,543 patients with bacterial respiratory culture-positive pneumonia between 2002 and 2003. The study examined the bacteriological differences between CAP and HCAP. In this study, HCAP patients were defined as having: transfer from another healthcare facility; long-term hemodialysis; or prior hospitalization within 30 days in which they had non-ventilator-associated pneumonia (VAP). CAP patients were defined as having non-VAP and non-HCAP.
The study showed that the frequency of Pseudomonas aeurginosa (25% HCAP vs. 17% CAP) and Staphylococcus aureus (46% vs. 25%), which included methicillin-resistant Staphylococcus aureus (MRSA) (18% vs. 6%), was significantly higher in patients with HCAP than those with CAP. Additionally, frequency of Streptococcus pneumoniae (5% vs. 16%) and Haemophilus influenza (5% vs. 16%) infections were noted as significantly lower.3
A single-center, retrospective cohort analysis of 639 patients done by Micek et al yielded similar culture differences between CAP and HCAP patients. In this study, criteria for HCAP were defined as hospitalization in the past year, immunosuppression, nursing-home resident, or hemodialysis. The study authors found that a significantly higher percentage of HCAP patients were infected with MRSA (30% vs. 12%), Pseudomonas aeurginosa (25% vs. 4%), and other non-fermenting gram-negative rods (GNR) (10% vs. 2%). HCAP patients again were noted as having significantly fewer infections with S. pneumoniae (10% vs. 40%) and Haemophilus influenza (4% vs. 17%).
In addition to showing a difference in the bacteriology of CAP and HCAP, the Kollef study also evaluated mortality rates, length of stay, and hospital charges. Mortality rates for HCAP (19.8%) were similar to those of hospital-acquired pneumonia (HAP) (18.8%), and both of these were significantly higher than CAP (10%). Length of stay and hospital cost increased across the spectrum, from CAP to HCAP to HAP, with significant differences between each.3
ATS/IDSA Guidelines
In 2005, a joint committee of the ATS and ISDA updated its initial 1996 nosocomial pneumonia guidelines. The guideline update included the new HCAP category.2 The No. 1 goal of these guidelines was to emphasize early and appropriate antibiotics, followed by tailoring of the treatment regimen based upon culture and clinical data. To this end, HCAP risk factors were developed via extrapolation from observational data generated from HAP and VAP patients.5,6,7
The risk factors are summarized in Table 1 (see p. 19).2 Guidelines dictated that the identification of any of these risk factors in pneumonia patients at the time of admission indicates increased risk for infection with an MDR organism. These high-risk patients require placement into the diagnostic category of HCAP.
Once a patient has been diagnosed with HCAP, the guidelines recommended obtaining lower-respiratory-tract cultures and initiating broad-spectrum antibiotic therapy. Appropriate empiric antibiotic therapy was suggested to be the same as for HAP. This regimen requires coverage with two anti-pseudomonal agents, as well as an agent with activity against MRSA.
The rationale behind initial coverage with two anti-pseudomonal agents stems from the finding that pseudomonas has a high rate of resistance to many antibiotics, and that if two agents are empirically started, chances of appropriate coverage increase from the outset. This is important, as timely administration of appropriate antibiotics has been shown to decrease mortality in infections.8
Additional considerations for empiric antibiotic treatment include sensitivities of local microbiologic data, as well as any recent antibiotic regimens given to the patient. Following this broad primary antibiotic coverage, de-escalation was recommended based on results of lower respiratory cultures and clinical improvement.2
Evolution of Diagnostic Criteria and Empiric Antibiotic Coverage
Since the publication of the 2005 ATS/IDSA guidelines, the aforementioned risk factors for HCAP have been brought into question, as they have yet to be validated by prospective trials. There is a growing concern that these criteria may not be adequately specific and, therefore, might call for too many patients to be treated with a broader spectrum of antibiotic coverage, thereby increasing the likelihood of developing MDR bacteria.
In order to further analyze HCAP criteria, Poch and Ost wrote a review earlier this year examining the data behind each of the risk factors cited in the ATS/IDSA guidelines; they found considerable heterogeneity in magnitude of MDR infection risk for these criteria.9 The authors also reviewed studies looking at other risk factors for MDR infections in patients living in nursing homes or afflicted with CAP. They proposed that such additional factors as patient specific risks (including functional status and previous antibiotic exposure) and contextual risks (including nurse-to-patient ratio) be evaluated and possibly incorporated into criteria.
Of all the patients with HCAP criteria, residents in nursing homes have been studied the best. Loeb et al, while looking for a way to decrease hospitalizations for nursing-home residents, showed that patients who get pneumonia (by guideline definition HCAP) can be effectively treated as outpatients with a single antibiotic agent.10 This randomized controlled trial of 680 patients, all with HCAP, were treated with oral levofloxacin at the nursing home or admitted to the hospital. There were no significant differences between mortality (8% vs. 9%) and quality-of-life measures between the two groups. Furthermore, analysis of data from the 1980s showed that nursing-home-acquired pneumonia could be treated effectively with single agents.11,12
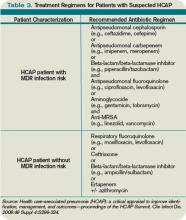
To address some of the questions regarding HCAP, national infectious-disease leaders were brought together to respond to a number of HCAP questions.13 One of the questions centered on the recommended empiric coverage for HCAP. Given the above noted studies in nursing-home patients, disagreement emerged about the need to empirically treat all HCAP patients with broad-spectrum antibiotics. Therefore, another assessment of risk factors for MDR infections was proposed (see Table 2, p. 20) and a consensus was reached, resulting in the current recommendations. The current guidelines state that once a patient has met HCAP criteria, if they have additional MDR risk factors, then broad antibiotic coverage is recommended; however, if no additional MDR risk is found, then more conservative, narrower coverage could be given (see Table 3, p. 31).13
Additional considerations
More studies are needed to refine and validate the specific diagnostic criteria for HCAP, as well as the MDR infectious risk factors. Moreover, current recommendations are for lower respiratory cultures to be obtained on all patients with pneumonia and antibiotic coverage to be titrated according to these results. This practice, however, appears to be uncommon. More data are needed to further guide treatment following initiation of empiric antibiotic coverage without the guidance of culture data, with reliance upon clinical parameters instead.
Back to the Case
This patient met initial criteria for HCAP because he was a nursing home resident, and was found to have additional MDR risk factors (poor functional status and a recent course of antibiotics). Therefore, lower respiratory cultures were obtained, supplemental oxygen was started, and piperacillin/tazobactam plus levofloxacin and vancomycin (with consideration made for local resistance patterns) was administered. He clinically improved over the next two days. His sputum cultures grew Pseudomonas aeuroginosa, which was sensitive to piperacillin/tazobactam but resistant to levofloxacin.
The vancomycin and levofloxacin were discontinued, and he was treated with a seven-day course of piperacillin/tazobactam.
Bottom Line
For adults who present with pneumonia from the community, special attention must be paid to certain parts of the patient’s history to determine if they have HCAP.
Patients who have HCAP can benefit from broad-spectrum empiric antibiotic coverage, which current expert consensus believes is dependent upon further MDR infection risk factors. TH
Dr. Rohde is medicine faculty hospitalist at the University of Michigan in Ann Arbor.
References
- Jernigan JA, Pullen AL, Flowers L, Bell M, Jarvis WR. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol. 2003;24(6):409-414.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(5):3854-3862.
- Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51(10):3568-3573.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
- Celis R, Torres A, Gatell JM, Almela M, Rodríguez-Roisin R, Augustí-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93(2):318-324.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18(2):362-368.
- Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31 Supple 4:S131-S138.
- Poch DS, Ost DE. What are the important risk factors for healthcare-associated pneumonia? Semin Respir Crit Care Med. 2009;30(1):26-35.
- Loeb M, Carusone SC, Goeree R, et al. Effect of clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510.
- Peterson PK, Stein D, Guay DR, et al. Prospective study of lower respiratory tract infections in an extended-care nursing home program: potential role of oral ciprofloxacin. Am J Med. 1988;85(2):164-171.
- Trenholme GM, Schmitt BA, Spear J, Gvazdinskas LC, Levin S. Randomized study of intravenous/oral ciprofloxacin versus ceftazidime in the treatment of hospital and nursing home patients with lower respiratory tract infections. Am J Med. 1989(5A);87:116S-118S.
- Kollef MH, Morrow LE, Baughman RP, et al. Healthcare-associated pneumonia (HCAP): a critical appraisal to improve identification, management and outcomes—proceedings of the HCAP summit. Clin Infect Dis. 2008;46 Suppl 4:S296-S334.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919.
- El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39(4):474-480.
If you are interested in joining our reader-involvement program, e-mail Editor Jason Carris at [email protected].
Case
A 68-year-old man with hypertension, diabetes, and recent hip fracture with poor functional status presents from a nursing home with a productive cough, shortness of breath, and chills of two-day duration. He finished a five-day course of cephalexin for a urinary tract infection one week ago. His vital signs reveal a blood pressure of 162/80 mm/Hg, temperature of 101.9°F, respirations of 26 breaths per minute, and oxygen saturation of 88% on room air. Coarse breath sounds are noted in the right lung field and his chest X-ray reveals a right-middle-lobe infiltrate.
He is admitted to the hospital with a diagnosis of healthcare-associated pneumonia. What is the best empiric antibiotic coverage for this patient?
Overview
Modern medicine exists over a continuum of care that is delivered in a manifold of different settings. Patients routinely receive complex medical care at home, including wound care and infusion of intravenous antibiotics. Additionally, many patients are interfacing with the healthcare system on a regular basis via hemodialysis centers or sub-acute rehabilitation centers. As a result of these interactions, patients are exposed to—and colonized by—different bacterial pathogens that can result in a variety of infections.1
While patients with healthcare-associated pneumonia (HCAP) can present similarly to those with community-acquired pneumonia (CAP)—patients with CAP normally present with a lower-respiratory-tract infection—the differences in the likely etiological pathogens dictate that these patients be considered for broader-spectrum empiric antibiotics. Hospitalists will continue to be responsible for choosing the initial antibiotic regimen for these patients, and they need to be able to recognize this disease process in order to treat it appropriately.
The joint American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines released in 2005 emphasize that certain clinical HCAP risk factors center on increased interactions and encounters with healthcare facilities.2 These risk factors are evolving over time to include a patient’s functional status, recent antibiotic use, and clinical severity.
Review of the Data
Differences between HCAP and CAP
HCAP represents a diagnostic category of pneumonia created to differentiate patients with infections caused by a different microbiological subset of bacteria, including possible multi-drug-resistant (MDR) organisms, from patients with CAP. Thus far, culture data support this dichotomy.3,4
Kollef and colleagues performed a multicenter, retrospective cohort study of 4,543 patients with bacterial respiratory culture-positive pneumonia between 2002 and 2003. The study examined the bacteriological differences between CAP and HCAP. In this study, HCAP patients were defined as having: transfer from another healthcare facility; long-term hemodialysis; or prior hospitalization within 30 days in which they had non-ventilator-associated pneumonia (VAP). CAP patients were defined as having non-VAP and non-HCAP.
The study showed that the frequency of Pseudomonas aeurginosa (25% HCAP vs. 17% CAP) and Staphylococcus aureus (46% vs. 25%), which included methicillin-resistant Staphylococcus aureus (MRSA) (18% vs. 6%), was significantly higher in patients with HCAP than those with CAP. Additionally, frequency of Streptococcus pneumoniae (5% vs. 16%) and Haemophilus influenza (5% vs. 16%) infections were noted as significantly lower.3
A single-center, retrospective cohort analysis of 639 patients done by Micek et al yielded similar culture differences between CAP and HCAP patients. In this study, criteria for HCAP were defined as hospitalization in the past year, immunosuppression, nursing-home resident, or hemodialysis. The study authors found that a significantly higher percentage of HCAP patients were infected with MRSA (30% vs. 12%), Pseudomonas aeurginosa (25% vs. 4%), and other non-fermenting gram-negative rods (GNR) (10% vs. 2%). HCAP patients again were noted as having significantly fewer infections with S. pneumoniae (10% vs. 40%) and Haemophilus influenza (4% vs. 17%).
In addition to showing a difference in the bacteriology of CAP and HCAP, the Kollef study also evaluated mortality rates, length of stay, and hospital charges. Mortality rates for HCAP (19.8%) were similar to those of hospital-acquired pneumonia (HAP) (18.8%), and both of these were significantly higher than CAP (10%). Length of stay and hospital cost increased across the spectrum, from CAP to HCAP to HAP, with significant differences between each.3
ATS/IDSA Guidelines
In 2005, a joint committee of the ATS and ISDA updated its initial 1996 nosocomial pneumonia guidelines. The guideline update included the new HCAP category.2 The No. 1 goal of these guidelines was to emphasize early and appropriate antibiotics, followed by tailoring of the treatment regimen based upon culture and clinical data. To this end, HCAP risk factors were developed via extrapolation from observational data generated from HAP and VAP patients.5,6,7
The risk factors are summarized in Table 1 (see p. 19).2 Guidelines dictated that the identification of any of these risk factors in pneumonia patients at the time of admission indicates increased risk for infection with an MDR organism. These high-risk patients require placement into the diagnostic category of HCAP.
Once a patient has been diagnosed with HCAP, the guidelines recommended obtaining lower-respiratory-tract cultures and initiating broad-spectrum antibiotic therapy. Appropriate empiric antibiotic therapy was suggested to be the same as for HAP. This regimen requires coverage with two anti-pseudomonal agents, as well as an agent with activity against MRSA.
The rationale behind initial coverage with two anti-pseudomonal agents stems from the finding that pseudomonas has a high rate of resistance to many antibiotics, and that if two agents are empirically started, chances of appropriate coverage increase from the outset. This is important, as timely administration of appropriate antibiotics has been shown to decrease mortality in infections.8
Additional considerations for empiric antibiotic treatment include sensitivities of local microbiologic data, as well as any recent antibiotic regimens given to the patient. Following this broad primary antibiotic coverage, de-escalation was recommended based on results of lower respiratory cultures and clinical improvement.2
Evolution of Diagnostic Criteria and Empiric Antibiotic Coverage
Since the publication of the 2005 ATS/IDSA guidelines, the aforementioned risk factors for HCAP have been brought into question, as they have yet to be validated by prospective trials. There is a growing concern that these criteria may not be adequately specific and, therefore, might call for too many patients to be treated with a broader spectrum of antibiotic coverage, thereby increasing the likelihood of developing MDR bacteria.
In order to further analyze HCAP criteria, Poch and Ost wrote a review earlier this year examining the data behind each of the risk factors cited in the ATS/IDSA guidelines; they found considerable heterogeneity in magnitude of MDR infection risk for these criteria.9 The authors also reviewed studies looking at other risk factors for MDR infections in patients living in nursing homes or afflicted with CAP. They proposed that such additional factors as patient specific risks (including functional status and previous antibiotic exposure) and contextual risks (including nurse-to-patient ratio) be evaluated and possibly incorporated into criteria.
Of all the patients with HCAP criteria, residents in nursing homes have been studied the best. Loeb et al, while looking for a way to decrease hospitalizations for nursing-home residents, showed that patients who get pneumonia (by guideline definition HCAP) can be effectively treated as outpatients with a single antibiotic agent.10 This randomized controlled trial of 680 patients, all with HCAP, were treated with oral levofloxacin at the nursing home or admitted to the hospital. There were no significant differences between mortality (8% vs. 9%) and quality-of-life measures between the two groups. Furthermore, analysis of data from the 1980s showed that nursing-home-acquired pneumonia could be treated effectively with single agents.11,12
To address some of the questions regarding HCAP, national infectious-disease leaders were brought together to respond to a number of HCAP questions.13 One of the questions centered on the recommended empiric coverage for HCAP. Given the above noted studies in nursing-home patients, disagreement emerged about the need to empirically treat all HCAP patients with broad-spectrum antibiotics. Therefore, another assessment of risk factors for MDR infections was proposed (see Table 2, p. 20) and a consensus was reached, resulting in the current recommendations. The current guidelines state that once a patient has met HCAP criteria, if they have additional MDR risk factors, then broad antibiotic coverage is recommended; however, if no additional MDR risk is found, then more conservative, narrower coverage could be given (see Table 3, p. 31).13
Additional considerations
More studies are needed to refine and validate the specific diagnostic criteria for HCAP, as well as the MDR infectious risk factors. Moreover, current recommendations are for lower respiratory cultures to be obtained on all patients with pneumonia and antibiotic coverage to be titrated according to these results. This practice, however, appears to be uncommon. More data are needed to further guide treatment following initiation of empiric antibiotic coverage without the guidance of culture data, with reliance upon clinical parameters instead.
Back to the Case
This patient met initial criteria for HCAP because he was a nursing home resident, and was found to have additional MDR risk factors (poor functional status and a recent course of antibiotics). Therefore, lower respiratory cultures were obtained, supplemental oxygen was started, and piperacillin/tazobactam plus levofloxacin and vancomycin (with consideration made for local resistance patterns) was administered. He clinically improved over the next two days. His sputum cultures grew Pseudomonas aeuroginosa, which was sensitive to piperacillin/tazobactam but resistant to levofloxacin.
The vancomycin and levofloxacin were discontinued, and he was treated with a seven-day course of piperacillin/tazobactam.
Bottom Line
For adults who present with pneumonia from the community, special attention must be paid to certain parts of the patient’s history to determine if they have HCAP.
Patients who have HCAP can benefit from broad-spectrum empiric antibiotic coverage, which current expert consensus believes is dependent upon further MDR infection risk factors. TH
Dr. Rohde is medicine faculty hospitalist at the University of Michigan in Ann Arbor.
References
- Jernigan JA, Pullen AL, Flowers L, Bell M, Jarvis WR. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol. 2003;24(6):409-414.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(5):3854-3862.
- Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51(10):3568-3573.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
- Celis R, Torres A, Gatell JM, Almela M, Rodríguez-Roisin R, Augustí-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93(2):318-324.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18(2):362-368.
- Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31 Supple 4:S131-S138.
- Poch DS, Ost DE. What are the important risk factors for healthcare-associated pneumonia? Semin Respir Crit Care Med. 2009;30(1):26-35.
- Loeb M, Carusone SC, Goeree R, et al. Effect of clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510.
- Peterson PK, Stein D, Guay DR, et al. Prospective study of lower respiratory tract infections in an extended-care nursing home program: potential role of oral ciprofloxacin. Am J Med. 1988;85(2):164-171.
- Trenholme GM, Schmitt BA, Spear J, Gvazdinskas LC, Levin S. Randomized study of intravenous/oral ciprofloxacin versus ceftazidime in the treatment of hospital and nursing home patients with lower respiratory tract infections. Am J Med. 1989(5A);87:116S-118S.
- Kollef MH, Morrow LE, Baughman RP, et al. Healthcare-associated pneumonia (HCAP): a critical appraisal to improve identification, management and outcomes—proceedings of the HCAP summit. Clin Infect Dis. 2008;46 Suppl 4:S296-S334.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919.
- El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39(4):474-480.
If you are interested in joining our reader-involvement program, e-mail Editor Jason Carris at [email protected].
Case
A 68-year-old man with hypertension, diabetes, and recent hip fracture with poor functional status presents from a nursing home with a productive cough, shortness of breath, and chills of two-day duration. He finished a five-day course of cephalexin for a urinary tract infection one week ago. His vital signs reveal a blood pressure of 162/80 mm/Hg, temperature of 101.9°F, respirations of 26 breaths per minute, and oxygen saturation of 88% on room air. Coarse breath sounds are noted in the right lung field and his chest X-ray reveals a right-middle-lobe infiltrate.
He is admitted to the hospital with a diagnosis of healthcare-associated pneumonia. What is the best empiric antibiotic coverage for this patient?
Overview
Modern medicine exists over a continuum of care that is delivered in a manifold of different settings. Patients routinely receive complex medical care at home, including wound care and infusion of intravenous antibiotics. Additionally, many patients are interfacing with the healthcare system on a regular basis via hemodialysis centers or sub-acute rehabilitation centers. As a result of these interactions, patients are exposed to—and colonized by—different bacterial pathogens that can result in a variety of infections.1
While patients with healthcare-associated pneumonia (HCAP) can present similarly to those with community-acquired pneumonia (CAP)—patients with CAP normally present with a lower-respiratory-tract infection—the differences in the likely etiological pathogens dictate that these patients be considered for broader-spectrum empiric antibiotics. Hospitalists will continue to be responsible for choosing the initial antibiotic regimen for these patients, and they need to be able to recognize this disease process in order to treat it appropriately.
The joint American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines released in 2005 emphasize that certain clinical HCAP risk factors center on increased interactions and encounters with healthcare facilities.2 These risk factors are evolving over time to include a patient’s functional status, recent antibiotic use, and clinical severity.
Review of the Data
Differences between HCAP and CAP
HCAP represents a diagnostic category of pneumonia created to differentiate patients with infections caused by a different microbiological subset of bacteria, including possible multi-drug-resistant (MDR) organisms, from patients with CAP. Thus far, culture data support this dichotomy.3,4
Kollef and colleagues performed a multicenter, retrospective cohort study of 4,543 patients with bacterial respiratory culture-positive pneumonia between 2002 and 2003. The study examined the bacteriological differences between CAP and HCAP. In this study, HCAP patients were defined as having: transfer from another healthcare facility; long-term hemodialysis; or prior hospitalization within 30 days in which they had non-ventilator-associated pneumonia (VAP). CAP patients were defined as having non-VAP and non-HCAP.
The study showed that the frequency of Pseudomonas aeurginosa (25% HCAP vs. 17% CAP) and Staphylococcus aureus (46% vs. 25%), which included methicillin-resistant Staphylococcus aureus (MRSA) (18% vs. 6%), was significantly higher in patients with HCAP than those with CAP. Additionally, frequency of Streptococcus pneumoniae (5% vs. 16%) and Haemophilus influenza (5% vs. 16%) infections were noted as significantly lower.3
A single-center, retrospective cohort analysis of 639 patients done by Micek et al yielded similar culture differences between CAP and HCAP patients. In this study, criteria for HCAP were defined as hospitalization in the past year, immunosuppression, nursing-home resident, or hemodialysis. The study authors found that a significantly higher percentage of HCAP patients were infected with MRSA (30% vs. 12%), Pseudomonas aeurginosa (25% vs. 4%), and other non-fermenting gram-negative rods (GNR) (10% vs. 2%). HCAP patients again were noted as having significantly fewer infections with S. pneumoniae (10% vs. 40%) and Haemophilus influenza (4% vs. 17%).
In addition to showing a difference in the bacteriology of CAP and HCAP, the Kollef study also evaluated mortality rates, length of stay, and hospital charges. Mortality rates for HCAP (19.8%) were similar to those of hospital-acquired pneumonia (HAP) (18.8%), and both of these were significantly higher than CAP (10%). Length of stay and hospital cost increased across the spectrum, from CAP to HCAP to HAP, with significant differences between each.3
ATS/IDSA Guidelines
In 2005, a joint committee of the ATS and ISDA updated its initial 1996 nosocomial pneumonia guidelines. The guideline update included the new HCAP category.2 The No. 1 goal of these guidelines was to emphasize early and appropriate antibiotics, followed by tailoring of the treatment regimen based upon culture and clinical data. To this end, HCAP risk factors were developed via extrapolation from observational data generated from HAP and VAP patients.5,6,7
The risk factors are summarized in Table 1 (see p. 19).2 Guidelines dictated that the identification of any of these risk factors in pneumonia patients at the time of admission indicates increased risk for infection with an MDR organism. These high-risk patients require placement into the diagnostic category of HCAP.
Once a patient has been diagnosed with HCAP, the guidelines recommended obtaining lower-respiratory-tract cultures and initiating broad-spectrum antibiotic therapy. Appropriate empiric antibiotic therapy was suggested to be the same as for HAP. This regimen requires coverage with two anti-pseudomonal agents, as well as an agent with activity against MRSA.
The rationale behind initial coverage with two anti-pseudomonal agents stems from the finding that pseudomonas has a high rate of resistance to many antibiotics, and that if two agents are empirically started, chances of appropriate coverage increase from the outset. This is important, as timely administration of appropriate antibiotics has been shown to decrease mortality in infections.8
Additional considerations for empiric antibiotic treatment include sensitivities of local microbiologic data, as well as any recent antibiotic regimens given to the patient. Following this broad primary antibiotic coverage, de-escalation was recommended based on results of lower respiratory cultures and clinical improvement.2
Evolution of Diagnostic Criteria and Empiric Antibiotic Coverage
Since the publication of the 2005 ATS/IDSA guidelines, the aforementioned risk factors for HCAP have been brought into question, as they have yet to be validated by prospective trials. There is a growing concern that these criteria may not be adequately specific and, therefore, might call for too many patients to be treated with a broader spectrum of antibiotic coverage, thereby increasing the likelihood of developing MDR bacteria.
In order to further analyze HCAP criteria, Poch and Ost wrote a review earlier this year examining the data behind each of the risk factors cited in the ATS/IDSA guidelines; they found considerable heterogeneity in magnitude of MDR infection risk for these criteria.9 The authors also reviewed studies looking at other risk factors for MDR infections in patients living in nursing homes or afflicted with CAP. They proposed that such additional factors as patient specific risks (including functional status and previous antibiotic exposure) and contextual risks (including nurse-to-patient ratio) be evaluated and possibly incorporated into criteria.
Of all the patients with HCAP criteria, residents in nursing homes have been studied the best. Loeb et al, while looking for a way to decrease hospitalizations for nursing-home residents, showed that patients who get pneumonia (by guideline definition HCAP) can be effectively treated as outpatients with a single antibiotic agent.10 This randomized controlled trial of 680 patients, all with HCAP, were treated with oral levofloxacin at the nursing home or admitted to the hospital. There were no significant differences between mortality (8% vs. 9%) and quality-of-life measures between the two groups. Furthermore, analysis of data from the 1980s showed that nursing-home-acquired pneumonia could be treated effectively with single agents.11,12
To address some of the questions regarding HCAP, national infectious-disease leaders were brought together to respond to a number of HCAP questions.13 One of the questions centered on the recommended empiric coverage for HCAP. Given the above noted studies in nursing-home patients, disagreement emerged about the need to empirically treat all HCAP patients with broad-spectrum antibiotics. Therefore, another assessment of risk factors for MDR infections was proposed (see Table 2, p. 20) and a consensus was reached, resulting in the current recommendations. The current guidelines state that once a patient has met HCAP criteria, if they have additional MDR risk factors, then broad antibiotic coverage is recommended; however, if no additional MDR risk is found, then more conservative, narrower coverage could be given (see Table 3, p. 31).13
Additional considerations
More studies are needed to refine and validate the specific diagnostic criteria for HCAP, as well as the MDR infectious risk factors. Moreover, current recommendations are for lower respiratory cultures to be obtained on all patients with pneumonia and antibiotic coverage to be titrated according to these results. This practice, however, appears to be uncommon. More data are needed to further guide treatment following initiation of empiric antibiotic coverage without the guidance of culture data, with reliance upon clinical parameters instead.
Back to the Case
This patient met initial criteria for HCAP because he was a nursing home resident, and was found to have additional MDR risk factors (poor functional status and a recent course of antibiotics). Therefore, lower respiratory cultures were obtained, supplemental oxygen was started, and piperacillin/tazobactam plus levofloxacin and vancomycin (with consideration made for local resistance patterns) was administered. He clinically improved over the next two days. His sputum cultures grew Pseudomonas aeuroginosa, which was sensitive to piperacillin/tazobactam but resistant to levofloxacin.
The vancomycin and levofloxacin were discontinued, and he was treated with a seven-day course of piperacillin/tazobactam.
Bottom Line
For adults who present with pneumonia from the community, special attention must be paid to certain parts of the patient’s history to determine if they have HCAP.
Patients who have HCAP can benefit from broad-spectrum empiric antibiotic coverage, which current expert consensus believes is dependent upon further MDR infection risk factors. TH
Dr. Rohde is medicine faculty hospitalist at the University of Michigan in Ann Arbor.
References
- Jernigan JA, Pullen AL, Flowers L, Bell M, Jarvis WR. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol. 2003;24(6):409-414.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(5):3854-3862.
- Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51(10):3568-3573.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
- Celis R, Torres A, Gatell JM, Almela M, Rodríguez-Roisin R, Augustí-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93(2):318-324.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18(2):362-368.
- Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31 Supple 4:S131-S138.
- Poch DS, Ost DE. What are the important risk factors for healthcare-associated pneumonia? Semin Respir Crit Care Med. 2009;30(1):26-35.
- Loeb M, Carusone SC, Goeree R, et al. Effect of clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510.
- Peterson PK, Stein D, Guay DR, et al. Prospective study of lower respiratory tract infections in an extended-care nursing home program: potential role of oral ciprofloxacin. Am J Med. 1988;85(2):164-171.
- Trenholme GM, Schmitt BA, Spear J, Gvazdinskas LC, Levin S. Randomized study of intravenous/oral ciprofloxacin versus ceftazidime in the treatment of hospital and nursing home patients with lower respiratory tract infections. Am J Med. 1989(5A);87:116S-118S.
- Kollef MH, Morrow LE, Baughman RP, et al. Healthcare-associated pneumonia (HCAP): a critical appraisal to improve identification, management and outcomes—proceedings of the HCAP summit. Clin Infect Dis. 2008;46 Suppl 4:S296-S334.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919.
- El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39(4):474-480.
If you are interested in joining our reader-involvement program, e-mail Editor Jason Carris at [email protected].
Acute Bacterial Meningitis in Adults
Background Acute bacterial meningitis is an inflammation of the meninges, which results from bacterially mediated recruitment and activation of inflammatory cells in the cerebrospinal fluid (CSF). Bacterial meningitis was an almost invariably fatal disease at the start of the 20th century. With the development of and advancements in antimicrobial therapy, however, there has been a significant reduction in the mortality rate, although this has remained stable during the past 20 years (1). One large study of adults with community-acquired bacterial meningitis reported an overall mortality rate of 21%, including a 30% mortality rate associated with Streptococcus pneumoniae meningitis and a 7% mortality rate for Neisseria meningitidis (2). In adults, the most commonly identified organisms are S. pneumoniae (40–50%), Neisseria meningitidis (14–37%), and Listeria monocytogenes (4–10%) (2-4).
Clinical Presentation
Bacterial meningitis is a serious illness that often progresses rapidly. The classic clinical presentation consists of fever, nuchal rigidity, and mental status change (3). One large review of 10 critically appraised studies showed that almost all (99–100%) of the patients with bacterial meningitis presented with at least one of these clinical findings; and 95% of the patients had at least 2 of the clinical findings (5). In contrast, less than half of the patients presented with all 3 findings. Thus, in the absence of all 3 of these classic findings, the diagnosis of meningitis can virtually be dismissed, and further evaluation for meningitis need not be pursued. Individually, fever was the most common presenting finding, with a sensitivity of 85%. Nuchal rigidity had a sensitivity of 70%, and mental status change was 67%. While these physical examination findings may be of value in determining the diagnosis of bacterial meningitis, the accuracy of the clinical history including features such as headache, nausea and vomiting, and neck pain was too low to be of use clinically.
Signs of meningeal irritation may be of benefit in the clinical diagnosis of bacterial meningitis. Kernig’s and Brudzinski’s signs were first described nearly a century ago and have been used by most clinicians in the clinical realm; however, their diagnostic utility has been evaluated only in a limited number of studies. Kernig’s sign is positive when a patient in the supine position with his/her hips flexed at 90 degrees develops pain in the lower back or posterior thigh during an attempt to extend the knee. Brudzinski’s sign is positive when a patient in the supine position whose neck is passively flexed responds with flexion of his/her knees and hips. Recently, a bedside maneuver called jolt accentuation of headache was found to be potentially useful. In this maneuver, the patient is asked to turn his/her head horizontally 2–3 times per second, and a worsening headache is considered a positive sign. A small study showed that this maneuver had 97% sensitivity and 60% specificity for patients with CSF pleocytosis (6).
Other clinical manifestations in patients with bacterial meningitis include photophobia, seizure, rash, focal neurologic deficits, and signs of increased intracranial pressure. While these various findings may be present in many patients with bacterial meningitis, their sensitivities have been found to be low. Thus, their clinical utility in ruling out the diagnosis of bacterial meningitis is limited (5).
Laboratory Findings
Any patient who presents with a reasonable likelihood of having bacterial meningitis should undergo a lumbar puncture (LP) to evaluate the CSF as soon as possible. The initial CSF study should measure the opening pressure. One study demonstrated that 39% of patients with bacterial meningitis had opening pressures greater than 300 mg H20 (3). Other CSF laboratory studies should be sent for analysis in 4 sterile tubes filled with approximately 1 mL of CSF each. The first tube is typically reserved for gram stain and culture. The gram stain is positive in about 70% of patients with bacterial meningitis, and the culture will be positive in about 80% of cases. The second tube is sent for protein and glucose levels. Patients who have markedly elevated CSF protein counts (>500 mg/dL) and low glucose levels (<45 mg/dL, or ratio of serum: CSF glucose levels <0.4) are likely to have bacterial meningitis. The third tube is sent for cell count and differential. Patients with bacterial meningitis are likely to have >10 WBC/μL that are predominantly polymorphonucleocytes and have few or no red blood cells in the absence of a traumatic LP. We recommend the fourth tube be used for any viral, fungal, or other miscellaneous studies. In addition to the CSF studies, other diagnostic evaluations should include blood cultures, complete blood count with platelets and differential (CBCPD), and basic chemistry labs.
The CSF studies described above are the primary tools in diagnosing bacterial meningitis; however, there are other studies that may be helpful in certain clinical settings. Latex agglutination tests for bacterial antigens may be used in cases in which bacterial meningitis remains a possible diagnosis despite negative CSF studies. This test is available for S. pneumoniae, N. meningitidis, H. influenzae type B, group B Streptococcus, and E. coli. The polymerase chain reaction (PCR) test of the CSF has been developed for some bacterial pathogens including S. pneumoniae, N. meningitidis, H. influenzae type B, and Mycobacterium tuberculosis. The limulus amebocyte lysate assay is a very sensitive test for gram-negative endotoxins, which may aid in identifying gram-negative organisms as potential pathogens in the CSF. While these alternative CSF diagnostic tests are available, many laboratories do not perform the tests on site and require send-out to a specialty laboratory. The time required for this may negate the clinical utility of these tests.
Role of Brain Imaging
The decision to obtain a brain imaging study prior to performing an LP has been a controversial issue for both patient safety and medical-legal reasons. Two large studies have been published in an attempt to derive a clinically useful decision analysis tool (7,8). In summary, the studies found that 5 clinical features were associated with an abnormal head cranial tomography (CT) scan. These were:
- Age >60 years
- Immunocompromised state
- Any history of central nervous system (CNS) disease
- A history of seizure within 1 week prior to presentation
- Presence of a focal neurologic abnormality, including altered level of consciousness, inability to answer or follow 2 consecutive requests, gaze palsy, abnormal visual fields, facial palsy, arm or leg drift, and abnormal language.
In patients with none of these findings, there was a 97% negative predictive value of having an abnormal CT scan, with the few patients with positive scans nonetheless tolerating LP without adverse effects. Thus, in patients with none of these findings, it appears that an LP can safely be performed without obtaining a CT scan. One study also demonstrated that patients who underwent a CT scan prior to their LP waited, on average, 2 hours longer to get an LP; with antibiotic administration delayed by an average of 1 hour (8). Antibiotic administration should not be delayed in any patient suspected of having bacterial meningitis, whether brain imaging is performed or not.
Differential Diagnosis
Given the severe nature of this disease, the diagnosis of bacterial meningitis must be differentiated from other conditions that may present in similar ways. Infectious causes that may present similarly to bacterial meningitis include other types of meningitis (viral, tuberculous, Lyme disease, syphilitic), viral encephalitis, Rocky Mountain spotted fever, fungal meningitis, parasitic causes, brain abscess, and epidural and subdural empyema. Other infectious etiologies not originating from the CNS may be mistaken for bacterial meningitis when these patients present with concomitant mental-status changes. This is especially common in elderly patients with pneumonia and urinary tract infections. Other noninfectious considerations include a CNS bleed such as a subarachnoid hemorrhage, drug-induced aseptic meningitis, and CNS vasculitis.
Treatment
When the patient’s presentation is suggestive of bacterial meningitis, empiric antibiotics should be administered without delay, while awaiting diagnostic evaluation. The initial dose of antibiotics should not alter the results of the diagnostic studies significantly. The choice of antibiotics is based upon the most likely offending organism from epidemiologic data and underlying predisposing conditions. S. pneumoniae and N. meningitidis are the 2 most common causes of bacterial meningitis in adults.
The development of antibiotic resistance by S. pneumoniae to penicillin and cephalosporins has been one of the major developments in the past 20 years. Due to this resistance, the recommended empiric therapy is a combination of a third-generation cephalosporin (ceftriaxone or cefotaxime) and vancomycin. For special cases, additional or alternative therapy should be given. Ampicillin should be added for patients at risk for Listeria monocytogenes; and postsurgical or post-trauma patients should have expanded coverage to include staphylococcal and gram-negative infections. Table 1 lists the recommended antibiotic therapy for patients with possible bacterial meningitis, along with the most commonly associated organisms.
Once the offending organism has been identified, antibiotic therapy should be narrowed to target the bacteria based on laboratory minimal inhibitory concentrations (MIC). The antibiotic should also have excellent CSF penetration and bactericidal activity. For S. pneumoniae that are susceptible to penicillin, penicillin G and ampicillin remain the therapy of choice (9). The increasing trend toward antibiotic resistance by S. pneumoniae has increased the use of vancomycin as therapy. In patients with resistant strains of S. pneumoniae, however, vancomycin should not be used alone. Vancomycin should be used in combination with a third-generation cephalosporin while keeping the serum vancomycin levels in the range of 15–20 μg/mL (10). It is imperative that the treatment course outlined be completed through its full duration. Table 2 lists specific antibiotic therapy with dosages and recommended duration of therapy based on isolated organisms.
Adjunctive Therapy
The release and production of inflammatory cytokines in bacterial meningitis is thought to be a major cause of adverse outcomes. To counteract this inflammatory process, use of adjunctive steroids in patients with bacterial meningitis has been evaluated. Initial data from children with bacterial meningitis, mostly due to H. influenzae and S. pneumoniae, demonstrated improved neurologic outcomes, with significant reductions in deafness, in patients treated with dexamethasone as an adjunctive therapy to antibiotics (11). In adults with bacterial meningitis, a recent major trial demonstrated that treatment with adjunctive steroids, along with antibiotics, led to significant improvement in mortality and morbidity in patients with meningitis due to S. pneumoniae (12). Among patients with meningococcal meningitis, there was a trend toward improved outcomes. Patients with suspected pneumococcal meningitis should receive their first dose of dexamethasone 20–30 minutes prior to or at the same time as the initial antibiotic administration. The recommended dose and duration is 0.15 mg/kg every 6 hours for 2 to 4 days. The use of dexamethasone appears to have no benefit if administered after antibiotics have already been given, and data are lacking for patients with meningitis due to organisms other than S. pneumoniae. Most experts recommend against the use of adjunctive corticosteroids in these cases (10-13). Several questions, however, remain unanswered with regard to adjunctive corticosteroid use. These include the optimal duration of treatment, whether the penetration of vancomycin into the CSF is significantly decreased by dexamethasone, and whether they should be administered to immunocompromised patients (14).
Prevention
Currently, prevention of some types of bacterial meningitis can be accomplished by appropriate use of vaccines, or through antibiotic chemoprophylaxis in certain situations. For adults, vaccines are available against the 2 most common causes of bacterial meningitis. The 23 polyvalent pneumococcal vaccine is recommended for all adults >65 years of age and for anyone age >2 with a compromised immune status. The meningococcal vaccine is available as a quadravalent vaccine (serotypes A, C, Y, and W-135) and should be administered to anyone with functional asplenia, terminal complement deficiencies, those traveling to endemic areas of meningococcal meningitis, and any college freshman requesting the vaccine who will be living in college dormitories (15).
Antibiotic chemoprophylaxis can be administered to individuals who have had close contact with an index patient with meningococcal meningitis. Antibiotics should be administered as soon as exposure has been determined. There are several options available for meningococcal meningitis exposure. Ciprofloxacin is probably the simplest regimen due to its 1-time 500-mg oral dose. Other options include rifampin 600 mg every 12 hours ×4 doses and ceftriaxone 250 mg IM as a 1-time dose. Pregnant women should avoid ciprofloxacin and rifampin due to their potential teratogenic effects.
Prognosis and Follow-up
Prognosis of bacterial meningitis is closely linked to the causative organism, the severity of disease at the time of presentation, and the speed at which the disease progresses. One large retrospective study demonstrated in-hospital mortality rates of 25% for S. pneumoniae, 10% for N. meningitidis, and 21% for L. monocytogenes. Conditions associated with an increased risk of mortality included age >60, state of obtundation on admission, and development of seizure within 24 hours of admission. This study also showed that 21% of patients developed some type of neurologic deficits, and, overall, 9% had persistence of these deficits at time of discharge (3). Another study showed that baseline features of hypotension, mental status changes, and seizures were associated with increased mortality and neurologic morbidity (16). A more recent large study evaluating the efficacy of adjunctive corticosteroids reported a mortality rate of 15% in the control arm, with mortality of 34% in patients infected with S. pneumoniae (12). Another study suggested that if patients had a rapid progression of their disease, this seemed to correlate with worse outcomes. These investigators found an uncertain correlation between antibiotic timing and unfavorable outcomes (16).
Patients discharged from the hospital should have close follow-up with their primary care physician or infectious disease specialist. Evaluation in the short-term should focus on any complications that may have developed as a result of the bacterial meningitis; such as mental status change, seizure, focal neurologic deficits, and hearing loss. Long-term evaluations should also address cognitive functioning and the neuropsychiatric well-being of the patient, in addition to those issues addressed during short-term follow-up (11, 12).
Dr. Kim may be reached at [email protected].
References
- Swartz, Morton N. Bacterial Meningitis—A View of the Past 90 Years. N Engl J Med. 2004;351:1826-8.
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-59.
- Durand ML, Calderwood SB, Weber DJ, et al. Acute Bacterial Meningitis in Adults. A review of 493 episodes. N Engl J Med. 1993;328:21-8.
- Schuchat A, Robinson K, Wenger J, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970-6.
- Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175-181.
- Uchihara T, Tsukagoshi H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31:167-71.
- Gopal AK, Whitehouse JD, Simel DL, Corey RG. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. 1999;159:2681-5.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before LP in adults with suspected meningitis. N Engl J Med. 2001;345:1727-33.
- Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708-16.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis. 2004;39:1267-84.
- McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:928-31.
- de Gans J, van de Beek D Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-56.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004;4: 139-43.
- Pile JC, Longworth DL. Should adults with suspected acute bacterial meningitis get adjunctive corticosteroids? Cleve Clin J Med. 2005;72:67-70.
- Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;4613-21.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862-9.
Background Acute bacterial meningitis is an inflammation of the meninges, which results from bacterially mediated recruitment and activation of inflammatory cells in the cerebrospinal fluid (CSF). Bacterial meningitis was an almost invariably fatal disease at the start of the 20th century. With the development of and advancements in antimicrobial therapy, however, there has been a significant reduction in the mortality rate, although this has remained stable during the past 20 years (1). One large study of adults with community-acquired bacterial meningitis reported an overall mortality rate of 21%, including a 30% mortality rate associated with Streptococcus pneumoniae meningitis and a 7% mortality rate for Neisseria meningitidis (2). In adults, the most commonly identified organisms are S. pneumoniae (40–50%), Neisseria meningitidis (14–37%), and Listeria monocytogenes (4–10%) (2-4).
Clinical Presentation
Bacterial meningitis is a serious illness that often progresses rapidly. The classic clinical presentation consists of fever, nuchal rigidity, and mental status change (3). One large review of 10 critically appraised studies showed that almost all (99–100%) of the patients with bacterial meningitis presented with at least one of these clinical findings; and 95% of the patients had at least 2 of the clinical findings (5). In contrast, less than half of the patients presented with all 3 findings. Thus, in the absence of all 3 of these classic findings, the diagnosis of meningitis can virtually be dismissed, and further evaluation for meningitis need not be pursued. Individually, fever was the most common presenting finding, with a sensitivity of 85%. Nuchal rigidity had a sensitivity of 70%, and mental status change was 67%. While these physical examination findings may be of value in determining the diagnosis of bacterial meningitis, the accuracy of the clinical history including features such as headache, nausea and vomiting, and neck pain was too low to be of use clinically.
Signs of meningeal irritation may be of benefit in the clinical diagnosis of bacterial meningitis. Kernig’s and Brudzinski’s signs were first described nearly a century ago and have been used by most clinicians in the clinical realm; however, their diagnostic utility has been evaluated only in a limited number of studies. Kernig’s sign is positive when a patient in the supine position with his/her hips flexed at 90 degrees develops pain in the lower back or posterior thigh during an attempt to extend the knee. Brudzinski’s sign is positive when a patient in the supine position whose neck is passively flexed responds with flexion of his/her knees and hips. Recently, a bedside maneuver called jolt accentuation of headache was found to be potentially useful. In this maneuver, the patient is asked to turn his/her head horizontally 2–3 times per second, and a worsening headache is considered a positive sign. A small study showed that this maneuver had 97% sensitivity and 60% specificity for patients with CSF pleocytosis (6).
Other clinical manifestations in patients with bacterial meningitis include photophobia, seizure, rash, focal neurologic deficits, and signs of increased intracranial pressure. While these various findings may be present in many patients with bacterial meningitis, their sensitivities have been found to be low. Thus, their clinical utility in ruling out the diagnosis of bacterial meningitis is limited (5).
Laboratory Findings
Any patient who presents with a reasonable likelihood of having bacterial meningitis should undergo a lumbar puncture (LP) to evaluate the CSF as soon as possible. The initial CSF study should measure the opening pressure. One study demonstrated that 39% of patients with bacterial meningitis had opening pressures greater than 300 mg H20 (3). Other CSF laboratory studies should be sent for analysis in 4 sterile tubes filled with approximately 1 mL of CSF each. The first tube is typically reserved for gram stain and culture. The gram stain is positive in about 70% of patients with bacterial meningitis, and the culture will be positive in about 80% of cases. The second tube is sent for protein and glucose levels. Patients who have markedly elevated CSF protein counts (>500 mg/dL) and low glucose levels (<45 mg/dL, or ratio of serum: CSF glucose levels <0.4) are likely to have bacterial meningitis. The third tube is sent for cell count and differential. Patients with bacterial meningitis are likely to have >10 WBC/μL that are predominantly polymorphonucleocytes and have few or no red blood cells in the absence of a traumatic LP. We recommend the fourth tube be used for any viral, fungal, or other miscellaneous studies. In addition to the CSF studies, other diagnostic evaluations should include blood cultures, complete blood count with platelets and differential (CBCPD), and basic chemistry labs.
The CSF studies described above are the primary tools in diagnosing bacterial meningitis; however, there are other studies that may be helpful in certain clinical settings. Latex agglutination tests for bacterial antigens may be used in cases in which bacterial meningitis remains a possible diagnosis despite negative CSF studies. This test is available for S. pneumoniae, N. meningitidis, H. influenzae type B, group B Streptococcus, and E. coli. The polymerase chain reaction (PCR) test of the CSF has been developed for some bacterial pathogens including S. pneumoniae, N. meningitidis, H. influenzae type B, and Mycobacterium tuberculosis. The limulus amebocyte lysate assay is a very sensitive test for gram-negative endotoxins, which may aid in identifying gram-negative organisms as potential pathogens in the CSF. While these alternative CSF diagnostic tests are available, many laboratories do not perform the tests on site and require send-out to a specialty laboratory. The time required for this may negate the clinical utility of these tests.
Role of Brain Imaging
The decision to obtain a brain imaging study prior to performing an LP has been a controversial issue for both patient safety and medical-legal reasons. Two large studies have been published in an attempt to derive a clinically useful decision analysis tool (7,8). In summary, the studies found that 5 clinical features were associated with an abnormal head cranial tomography (CT) scan. These were:
- Age >60 years
- Immunocompromised state
- Any history of central nervous system (CNS) disease
- A history of seizure within 1 week prior to presentation
- Presence of a focal neurologic abnormality, including altered level of consciousness, inability to answer or follow 2 consecutive requests, gaze palsy, abnormal visual fields, facial palsy, arm or leg drift, and abnormal language.
In patients with none of these findings, there was a 97% negative predictive value of having an abnormal CT scan, with the few patients with positive scans nonetheless tolerating LP without adverse effects. Thus, in patients with none of these findings, it appears that an LP can safely be performed without obtaining a CT scan. One study also demonstrated that patients who underwent a CT scan prior to their LP waited, on average, 2 hours longer to get an LP; with antibiotic administration delayed by an average of 1 hour (8). Antibiotic administration should not be delayed in any patient suspected of having bacterial meningitis, whether brain imaging is performed or not.
Differential Diagnosis
Given the severe nature of this disease, the diagnosis of bacterial meningitis must be differentiated from other conditions that may present in similar ways. Infectious causes that may present similarly to bacterial meningitis include other types of meningitis (viral, tuberculous, Lyme disease, syphilitic), viral encephalitis, Rocky Mountain spotted fever, fungal meningitis, parasitic causes, brain abscess, and epidural and subdural empyema. Other infectious etiologies not originating from the CNS may be mistaken for bacterial meningitis when these patients present with concomitant mental-status changes. This is especially common in elderly patients with pneumonia and urinary tract infections. Other noninfectious considerations include a CNS bleed such as a subarachnoid hemorrhage, drug-induced aseptic meningitis, and CNS vasculitis.
Treatment
When the patient’s presentation is suggestive of bacterial meningitis, empiric antibiotics should be administered without delay, while awaiting diagnostic evaluation. The initial dose of antibiotics should not alter the results of the diagnostic studies significantly. The choice of antibiotics is based upon the most likely offending organism from epidemiologic data and underlying predisposing conditions. S. pneumoniae and N. meningitidis are the 2 most common causes of bacterial meningitis in adults.
The development of antibiotic resistance by S. pneumoniae to penicillin and cephalosporins has been one of the major developments in the past 20 years. Due to this resistance, the recommended empiric therapy is a combination of a third-generation cephalosporin (ceftriaxone or cefotaxime) and vancomycin. For special cases, additional or alternative therapy should be given. Ampicillin should be added for patients at risk for Listeria monocytogenes; and postsurgical or post-trauma patients should have expanded coverage to include staphylococcal and gram-negative infections. Table 1 lists the recommended antibiotic therapy for patients with possible bacterial meningitis, along with the most commonly associated organisms.
Once the offending organism has been identified, antibiotic therapy should be narrowed to target the bacteria based on laboratory minimal inhibitory concentrations (MIC). The antibiotic should also have excellent CSF penetration and bactericidal activity. For S. pneumoniae that are susceptible to penicillin, penicillin G and ampicillin remain the therapy of choice (9). The increasing trend toward antibiotic resistance by S. pneumoniae has increased the use of vancomycin as therapy. In patients with resistant strains of S. pneumoniae, however, vancomycin should not be used alone. Vancomycin should be used in combination with a third-generation cephalosporin while keeping the serum vancomycin levels in the range of 15–20 μg/mL (10). It is imperative that the treatment course outlined be completed through its full duration. Table 2 lists specific antibiotic therapy with dosages and recommended duration of therapy based on isolated organisms.
Adjunctive Therapy
The release and production of inflammatory cytokines in bacterial meningitis is thought to be a major cause of adverse outcomes. To counteract this inflammatory process, use of adjunctive steroids in patients with bacterial meningitis has been evaluated. Initial data from children with bacterial meningitis, mostly due to H. influenzae and S. pneumoniae, demonstrated improved neurologic outcomes, with significant reductions in deafness, in patients treated with dexamethasone as an adjunctive therapy to antibiotics (11). In adults with bacterial meningitis, a recent major trial demonstrated that treatment with adjunctive steroids, along with antibiotics, led to significant improvement in mortality and morbidity in patients with meningitis due to S. pneumoniae (12). Among patients with meningococcal meningitis, there was a trend toward improved outcomes. Patients with suspected pneumococcal meningitis should receive their first dose of dexamethasone 20–30 minutes prior to or at the same time as the initial antibiotic administration. The recommended dose and duration is 0.15 mg/kg every 6 hours for 2 to 4 days. The use of dexamethasone appears to have no benefit if administered after antibiotics have already been given, and data are lacking for patients with meningitis due to organisms other than S. pneumoniae. Most experts recommend against the use of adjunctive corticosteroids in these cases (10-13). Several questions, however, remain unanswered with regard to adjunctive corticosteroid use. These include the optimal duration of treatment, whether the penetration of vancomycin into the CSF is significantly decreased by dexamethasone, and whether they should be administered to immunocompromised patients (14).
Prevention
Currently, prevention of some types of bacterial meningitis can be accomplished by appropriate use of vaccines, or through antibiotic chemoprophylaxis in certain situations. For adults, vaccines are available against the 2 most common causes of bacterial meningitis. The 23 polyvalent pneumococcal vaccine is recommended for all adults >65 years of age and for anyone age >2 with a compromised immune status. The meningococcal vaccine is available as a quadravalent vaccine (serotypes A, C, Y, and W-135) and should be administered to anyone with functional asplenia, terminal complement deficiencies, those traveling to endemic areas of meningococcal meningitis, and any college freshman requesting the vaccine who will be living in college dormitories (15).
Antibiotic chemoprophylaxis can be administered to individuals who have had close contact with an index patient with meningococcal meningitis. Antibiotics should be administered as soon as exposure has been determined. There are several options available for meningococcal meningitis exposure. Ciprofloxacin is probably the simplest regimen due to its 1-time 500-mg oral dose. Other options include rifampin 600 mg every 12 hours ×4 doses and ceftriaxone 250 mg IM as a 1-time dose. Pregnant women should avoid ciprofloxacin and rifampin due to their potential teratogenic effects.
Prognosis and Follow-up
Prognosis of bacterial meningitis is closely linked to the causative organism, the severity of disease at the time of presentation, and the speed at which the disease progresses. One large retrospective study demonstrated in-hospital mortality rates of 25% for S. pneumoniae, 10% for N. meningitidis, and 21% for L. monocytogenes. Conditions associated with an increased risk of mortality included age >60, state of obtundation on admission, and development of seizure within 24 hours of admission. This study also showed that 21% of patients developed some type of neurologic deficits, and, overall, 9% had persistence of these deficits at time of discharge (3). Another study showed that baseline features of hypotension, mental status changes, and seizures were associated with increased mortality and neurologic morbidity (16). A more recent large study evaluating the efficacy of adjunctive corticosteroids reported a mortality rate of 15% in the control arm, with mortality of 34% in patients infected with S. pneumoniae (12). Another study suggested that if patients had a rapid progression of their disease, this seemed to correlate with worse outcomes. These investigators found an uncertain correlation between antibiotic timing and unfavorable outcomes (16).
Patients discharged from the hospital should have close follow-up with their primary care physician or infectious disease specialist. Evaluation in the short-term should focus on any complications that may have developed as a result of the bacterial meningitis; such as mental status change, seizure, focal neurologic deficits, and hearing loss. Long-term evaluations should also address cognitive functioning and the neuropsychiatric well-being of the patient, in addition to those issues addressed during short-term follow-up (11, 12).
Dr. Kim may be reached at [email protected].
References
- Swartz, Morton N. Bacterial Meningitis—A View of the Past 90 Years. N Engl J Med. 2004;351:1826-8.
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-59.
- Durand ML, Calderwood SB, Weber DJ, et al. Acute Bacterial Meningitis in Adults. A review of 493 episodes. N Engl J Med. 1993;328:21-8.
- Schuchat A, Robinson K, Wenger J, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970-6.
- Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175-181.
- Uchihara T, Tsukagoshi H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31:167-71.
- Gopal AK, Whitehouse JD, Simel DL, Corey RG. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. 1999;159:2681-5.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before LP in adults with suspected meningitis. N Engl J Med. 2001;345:1727-33.
- Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708-16.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis. 2004;39:1267-84.
- McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:928-31.
- de Gans J, van de Beek D Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-56.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004;4: 139-43.
- Pile JC, Longworth DL. Should adults with suspected acute bacterial meningitis get adjunctive corticosteroids? Cleve Clin J Med. 2005;72:67-70.
- Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;4613-21.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862-9.
Background Acute bacterial meningitis is an inflammation of the meninges, which results from bacterially mediated recruitment and activation of inflammatory cells in the cerebrospinal fluid (CSF). Bacterial meningitis was an almost invariably fatal disease at the start of the 20th century. With the development of and advancements in antimicrobial therapy, however, there has been a significant reduction in the mortality rate, although this has remained stable during the past 20 years (1). One large study of adults with community-acquired bacterial meningitis reported an overall mortality rate of 21%, including a 30% mortality rate associated with Streptococcus pneumoniae meningitis and a 7% mortality rate for Neisseria meningitidis (2). In adults, the most commonly identified organisms are S. pneumoniae (40–50%), Neisseria meningitidis (14–37%), and Listeria monocytogenes (4–10%) (2-4).
Clinical Presentation
Bacterial meningitis is a serious illness that often progresses rapidly. The classic clinical presentation consists of fever, nuchal rigidity, and mental status change (3). One large review of 10 critically appraised studies showed that almost all (99–100%) of the patients with bacterial meningitis presented with at least one of these clinical findings; and 95% of the patients had at least 2 of the clinical findings (5). In contrast, less than half of the patients presented with all 3 findings. Thus, in the absence of all 3 of these classic findings, the diagnosis of meningitis can virtually be dismissed, and further evaluation for meningitis need not be pursued. Individually, fever was the most common presenting finding, with a sensitivity of 85%. Nuchal rigidity had a sensitivity of 70%, and mental status change was 67%. While these physical examination findings may be of value in determining the diagnosis of bacterial meningitis, the accuracy of the clinical history including features such as headache, nausea and vomiting, and neck pain was too low to be of use clinically.
Signs of meningeal irritation may be of benefit in the clinical diagnosis of bacterial meningitis. Kernig’s and Brudzinski’s signs were first described nearly a century ago and have been used by most clinicians in the clinical realm; however, their diagnostic utility has been evaluated only in a limited number of studies. Kernig’s sign is positive when a patient in the supine position with his/her hips flexed at 90 degrees develops pain in the lower back or posterior thigh during an attempt to extend the knee. Brudzinski’s sign is positive when a patient in the supine position whose neck is passively flexed responds with flexion of his/her knees and hips. Recently, a bedside maneuver called jolt accentuation of headache was found to be potentially useful. In this maneuver, the patient is asked to turn his/her head horizontally 2–3 times per second, and a worsening headache is considered a positive sign. A small study showed that this maneuver had 97% sensitivity and 60% specificity for patients with CSF pleocytosis (6).
Other clinical manifestations in patients with bacterial meningitis include photophobia, seizure, rash, focal neurologic deficits, and signs of increased intracranial pressure. While these various findings may be present in many patients with bacterial meningitis, their sensitivities have been found to be low. Thus, their clinical utility in ruling out the diagnosis of bacterial meningitis is limited (5).
Laboratory Findings
Any patient who presents with a reasonable likelihood of having bacterial meningitis should undergo a lumbar puncture (LP) to evaluate the CSF as soon as possible. The initial CSF study should measure the opening pressure. One study demonstrated that 39% of patients with bacterial meningitis had opening pressures greater than 300 mg H20 (3). Other CSF laboratory studies should be sent for analysis in 4 sterile tubes filled with approximately 1 mL of CSF each. The first tube is typically reserved for gram stain and culture. The gram stain is positive in about 70% of patients with bacterial meningitis, and the culture will be positive in about 80% of cases. The second tube is sent for protein and glucose levels. Patients who have markedly elevated CSF protein counts (>500 mg/dL) and low glucose levels (<45 mg/dL, or ratio of serum: CSF glucose levels <0.4) are likely to have bacterial meningitis. The third tube is sent for cell count and differential. Patients with bacterial meningitis are likely to have >10 WBC/μL that are predominantly polymorphonucleocytes and have few or no red blood cells in the absence of a traumatic LP. We recommend the fourth tube be used for any viral, fungal, or other miscellaneous studies. In addition to the CSF studies, other diagnostic evaluations should include blood cultures, complete blood count with platelets and differential (CBCPD), and basic chemistry labs.
The CSF studies described above are the primary tools in diagnosing bacterial meningitis; however, there are other studies that may be helpful in certain clinical settings. Latex agglutination tests for bacterial antigens may be used in cases in which bacterial meningitis remains a possible diagnosis despite negative CSF studies. This test is available for S. pneumoniae, N. meningitidis, H. influenzae type B, group B Streptococcus, and E. coli. The polymerase chain reaction (PCR) test of the CSF has been developed for some bacterial pathogens including S. pneumoniae, N. meningitidis, H. influenzae type B, and Mycobacterium tuberculosis. The limulus amebocyte lysate assay is a very sensitive test for gram-negative endotoxins, which may aid in identifying gram-negative organisms as potential pathogens in the CSF. While these alternative CSF diagnostic tests are available, many laboratories do not perform the tests on site and require send-out to a specialty laboratory. The time required for this may negate the clinical utility of these tests.
Role of Brain Imaging
The decision to obtain a brain imaging study prior to performing an LP has been a controversial issue for both patient safety and medical-legal reasons. Two large studies have been published in an attempt to derive a clinically useful decision analysis tool (7,8). In summary, the studies found that 5 clinical features were associated with an abnormal head cranial tomography (CT) scan. These were:
- Age >60 years
- Immunocompromised state
- Any history of central nervous system (CNS) disease
- A history of seizure within 1 week prior to presentation
- Presence of a focal neurologic abnormality, including altered level of consciousness, inability to answer or follow 2 consecutive requests, gaze palsy, abnormal visual fields, facial palsy, arm or leg drift, and abnormal language.
In patients with none of these findings, there was a 97% negative predictive value of having an abnormal CT scan, with the few patients with positive scans nonetheless tolerating LP without adverse effects. Thus, in patients with none of these findings, it appears that an LP can safely be performed without obtaining a CT scan. One study also demonstrated that patients who underwent a CT scan prior to their LP waited, on average, 2 hours longer to get an LP; with antibiotic administration delayed by an average of 1 hour (8). Antibiotic administration should not be delayed in any patient suspected of having bacterial meningitis, whether brain imaging is performed or not.
Differential Diagnosis
Given the severe nature of this disease, the diagnosis of bacterial meningitis must be differentiated from other conditions that may present in similar ways. Infectious causes that may present similarly to bacterial meningitis include other types of meningitis (viral, tuberculous, Lyme disease, syphilitic), viral encephalitis, Rocky Mountain spotted fever, fungal meningitis, parasitic causes, brain abscess, and epidural and subdural empyema. Other infectious etiologies not originating from the CNS may be mistaken for bacterial meningitis when these patients present with concomitant mental-status changes. This is especially common in elderly patients with pneumonia and urinary tract infections. Other noninfectious considerations include a CNS bleed such as a subarachnoid hemorrhage, drug-induced aseptic meningitis, and CNS vasculitis.
Treatment
When the patient’s presentation is suggestive of bacterial meningitis, empiric antibiotics should be administered without delay, while awaiting diagnostic evaluation. The initial dose of antibiotics should not alter the results of the diagnostic studies significantly. The choice of antibiotics is based upon the most likely offending organism from epidemiologic data and underlying predisposing conditions. S. pneumoniae and N. meningitidis are the 2 most common causes of bacterial meningitis in adults.
The development of antibiotic resistance by S. pneumoniae to penicillin and cephalosporins has been one of the major developments in the past 20 years. Due to this resistance, the recommended empiric therapy is a combination of a third-generation cephalosporin (ceftriaxone or cefotaxime) and vancomycin. For special cases, additional or alternative therapy should be given. Ampicillin should be added for patients at risk for Listeria monocytogenes; and postsurgical or post-trauma patients should have expanded coverage to include staphylococcal and gram-negative infections. Table 1 lists the recommended antibiotic therapy for patients with possible bacterial meningitis, along with the most commonly associated organisms.
Once the offending organism has been identified, antibiotic therapy should be narrowed to target the bacteria based on laboratory minimal inhibitory concentrations (MIC). The antibiotic should also have excellent CSF penetration and bactericidal activity. For S. pneumoniae that are susceptible to penicillin, penicillin G and ampicillin remain the therapy of choice (9). The increasing trend toward antibiotic resistance by S. pneumoniae has increased the use of vancomycin as therapy. In patients with resistant strains of S. pneumoniae, however, vancomycin should not be used alone. Vancomycin should be used in combination with a third-generation cephalosporin while keeping the serum vancomycin levels in the range of 15–20 μg/mL (10). It is imperative that the treatment course outlined be completed through its full duration. Table 2 lists specific antibiotic therapy with dosages and recommended duration of therapy based on isolated organisms.
Adjunctive Therapy
The release and production of inflammatory cytokines in bacterial meningitis is thought to be a major cause of adverse outcomes. To counteract this inflammatory process, use of adjunctive steroids in patients with bacterial meningitis has been evaluated. Initial data from children with bacterial meningitis, mostly due to H. influenzae and S. pneumoniae, demonstrated improved neurologic outcomes, with significant reductions in deafness, in patients treated with dexamethasone as an adjunctive therapy to antibiotics (11). In adults with bacterial meningitis, a recent major trial demonstrated that treatment with adjunctive steroids, along with antibiotics, led to significant improvement in mortality and morbidity in patients with meningitis due to S. pneumoniae (12). Among patients with meningococcal meningitis, there was a trend toward improved outcomes. Patients with suspected pneumococcal meningitis should receive their first dose of dexamethasone 20–30 minutes prior to or at the same time as the initial antibiotic administration. The recommended dose and duration is 0.15 mg/kg every 6 hours for 2 to 4 days. The use of dexamethasone appears to have no benefit if administered after antibiotics have already been given, and data are lacking for patients with meningitis due to organisms other than S. pneumoniae. Most experts recommend against the use of adjunctive corticosteroids in these cases (10-13). Several questions, however, remain unanswered with regard to adjunctive corticosteroid use. These include the optimal duration of treatment, whether the penetration of vancomycin into the CSF is significantly decreased by dexamethasone, and whether they should be administered to immunocompromised patients (14).
Prevention
Currently, prevention of some types of bacterial meningitis can be accomplished by appropriate use of vaccines, or through antibiotic chemoprophylaxis in certain situations. For adults, vaccines are available against the 2 most common causes of bacterial meningitis. The 23 polyvalent pneumococcal vaccine is recommended for all adults >65 years of age and for anyone age >2 with a compromised immune status. The meningococcal vaccine is available as a quadravalent vaccine (serotypes A, C, Y, and W-135) and should be administered to anyone with functional asplenia, terminal complement deficiencies, those traveling to endemic areas of meningococcal meningitis, and any college freshman requesting the vaccine who will be living in college dormitories (15).
Antibiotic chemoprophylaxis can be administered to individuals who have had close contact with an index patient with meningococcal meningitis. Antibiotics should be administered as soon as exposure has been determined. There are several options available for meningococcal meningitis exposure. Ciprofloxacin is probably the simplest regimen due to its 1-time 500-mg oral dose. Other options include rifampin 600 mg every 12 hours ×4 doses and ceftriaxone 250 mg IM as a 1-time dose. Pregnant women should avoid ciprofloxacin and rifampin due to their potential teratogenic effects.
Prognosis and Follow-up
Prognosis of bacterial meningitis is closely linked to the causative organism, the severity of disease at the time of presentation, and the speed at which the disease progresses. One large retrospective study demonstrated in-hospital mortality rates of 25% for S. pneumoniae, 10% for N. meningitidis, and 21% for L. monocytogenes. Conditions associated with an increased risk of mortality included age >60, state of obtundation on admission, and development of seizure within 24 hours of admission. This study also showed that 21% of patients developed some type of neurologic deficits, and, overall, 9% had persistence of these deficits at time of discharge (3). Another study showed that baseline features of hypotension, mental status changes, and seizures were associated with increased mortality and neurologic morbidity (16). A more recent large study evaluating the efficacy of adjunctive corticosteroids reported a mortality rate of 15% in the control arm, with mortality of 34% in patients infected with S. pneumoniae (12). Another study suggested that if patients had a rapid progression of their disease, this seemed to correlate with worse outcomes. These investigators found an uncertain correlation between antibiotic timing and unfavorable outcomes (16).
Patients discharged from the hospital should have close follow-up with their primary care physician or infectious disease specialist. Evaluation in the short-term should focus on any complications that may have developed as a result of the bacterial meningitis; such as mental status change, seizure, focal neurologic deficits, and hearing loss. Long-term evaluations should also address cognitive functioning and the neuropsychiatric well-being of the patient, in addition to those issues addressed during short-term follow-up (11, 12).
Dr. Kim may be reached at [email protected].
References
- Swartz, Morton N. Bacterial Meningitis—A View of the Past 90 Years. N Engl J Med. 2004;351:1826-8.
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-59.
- Durand ML, Calderwood SB, Weber DJ, et al. Acute Bacterial Meningitis in Adults. A review of 493 episodes. N Engl J Med. 1993;328:21-8.
- Schuchat A, Robinson K, Wenger J, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970-6.
- Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175-181.
- Uchihara T, Tsukagoshi H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31:167-71.
- Gopal AK, Whitehouse JD, Simel DL, Corey RG. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. 1999;159:2681-5.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before LP in adults with suspected meningitis. N Engl J Med. 2001;345:1727-33.
- Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708-16.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis. 2004;39:1267-84.
- McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:928-31.
- de Gans J, van de Beek D Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-56.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004;4: 139-43.
- Pile JC, Longworth DL. Should adults with suspected acute bacterial meningitis get adjunctive corticosteroids? Cleve Clin J Med. 2005;72:67-70.
- Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;4613-21.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862-9.