User login
Delivering Complex Oncologic Care to the Veteran’s “Front Door”: A Case Report of Leveraging Nationwide VA Expertise
INTRODUCTION
Fragmentation of medical services is a significant barrier in modern patient care with contributing factors including patient and system level details. The Veterans Affairs (VA) department is the largest integrated health care organization in the US. Given the complex challenges of such a system, the VA has developed resources to lessen the impact of care fragmentation, potentially widening services and diminishing traditional barriers to care. We present a patient case as an example of how VA programs are impacting current veteran oncologic care.
CASE PRESENTATION
An 86-year-old veteran with shortness of breath and fatigue was found to have macrocytic anemia. Located nearly 200 miles from the closest VA with hematology services he was referred through the National TeleOncology (NTO) service to see hematology using clinical video telehealth (CVT) technology stationed at a VA approximately 100 miles from his home. Consultation led to lab work revealing no viral, nutritional, or rheumatologic explanation. A bone marrow biopsy was completed without clear diagnosis though molecular alterations demonstrated ASXL1, TET2 and CBL mutations. Hematopathology services were sought, and the patient’s case was presented at the NTO virtual hematologic tumor board where expert VA hematopathology, radiology and medical hematology opinions were available. A diagnosis of myelodysplastic syndrome was rendered with care recommendations including the novel agent luspatercept. Given patient age and comorbidities, transportation remained a barrier. The patient was set up to receive services through home based primary care (HBPC) with weekly lab draws and medication administration. Ultimately, the patient was able to receive the first dose of luspatercept through the NTO affiliated VA with subsequent administrations to be given by HBPC. Additional visits planned using at home VA video Connect (VVC) service and CVT visits with NTO hematology at his local community based outpatient center (CBOC) located 30 miles from his home.
DISCUSSION
Located over 3 hours from the closest in-person VA hematologist, this patient was able to receive complex care thanks to a marriage of in-person and virtual services involving specialty nurses, pharmacists, and physicians from across VA. Services such as the NTO hub-spoke model, virtual tumor boards and HBPC, reveal a care framework unique to the VA.
INTRODUCTION
Fragmentation of medical services is a significant barrier in modern patient care with contributing factors including patient and system level details. The Veterans Affairs (VA) department is the largest integrated health care organization in the US. Given the complex challenges of such a system, the VA has developed resources to lessen the impact of care fragmentation, potentially widening services and diminishing traditional barriers to care. We present a patient case as an example of how VA programs are impacting current veteran oncologic care.
CASE PRESENTATION
An 86-year-old veteran with shortness of breath and fatigue was found to have macrocytic anemia. Located nearly 200 miles from the closest VA with hematology services he was referred through the National TeleOncology (NTO) service to see hematology using clinical video telehealth (CVT) technology stationed at a VA approximately 100 miles from his home. Consultation led to lab work revealing no viral, nutritional, or rheumatologic explanation. A bone marrow biopsy was completed without clear diagnosis though molecular alterations demonstrated ASXL1, TET2 and CBL mutations. Hematopathology services were sought, and the patient’s case was presented at the NTO virtual hematologic tumor board where expert VA hematopathology, radiology and medical hematology opinions were available. A diagnosis of myelodysplastic syndrome was rendered with care recommendations including the novel agent luspatercept. Given patient age and comorbidities, transportation remained a barrier. The patient was set up to receive services through home based primary care (HBPC) with weekly lab draws and medication administration. Ultimately, the patient was able to receive the first dose of luspatercept through the NTO affiliated VA with subsequent administrations to be given by HBPC. Additional visits planned using at home VA video Connect (VVC) service and CVT visits with NTO hematology at his local community based outpatient center (CBOC) located 30 miles from his home.
DISCUSSION
Located over 3 hours from the closest in-person VA hematologist, this patient was able to receive complex care thanks to a marriage of in-person and virtual services involving specialty nurses, pharmacists, and physicians from across VA. Services such as the NTO hub-spoke model, virtual tumor boards and HBPC, reveal a care framework unique to the VA.
INTRODUCTION
Fragmentation of medical services is a significant barrier in modern patient care with contributing factors including patient and system level details. The Veterans Affairs (VA) department is the largest integrated health care organization in the US. Given the complex challenges of such a system, the VA has developed resources to lessen the impact of care fragmentation, potentially widening services and diminishing traditional barriers to care. We present a patient case as an example of how VA programs are impacting current veteran oncologic care.
CASE PRESENTATION
An 86-year-old veteran with shortness of breath and fatigue was found to have macrocytic anemia. Located nearly 200 miles from the closest VA with hematology services he was referred through the National TeleOncology (NTO) service to see hematology using clinical video telehealth (CVT) technology stationed at a VA approximately 100 miles from his home. Consultation led to lab work revealing no viral, nutritional, or rheumatologic explanation. A bone marrow biopsy was completed without clear diagnosis though molecular alterations demonstrated ASXL1, TET2 and CBL mutations. Hematopathology services were sought, and the patient’s case was presented at the NTO virtual hematologic tumor board where expert VA hematopathology, radiology and medical hematology opinions were available. A diagnosis of myelodysplastic syndrome was rendered with care recommendations including the novel agent luspatercept. Given patient age and comorbidities, transportation remained a barrier. The patient was set up to receive services through home based primary care (HBPC) with weekly lab draws and medication administration. Ultimately, the patient was able to receive the first dose of luspatercept through the NTO affiliated VA with subsequent administrations to be given by HBPC. Additional visits planned using at home VA video Connect (VVC) service and CVT visits with NTO hematology at his local community based outpatient center (CBOC) located 30 miles from his home.
DISCUSSION
Located over 3 hours from the closest in-person VA hematologist, this patient was able to receive complex care thanks to a marriage of in-person and virtual services involving specialty nurses, pharmacists, and physicians from across VA. Services such as the NTO hub-spoke model, virtual tumor boards and HBPC, reveal a care framework unique to the VA.
Dispensing and Monitoring Oral Anticancer Therapy
The availability and popularity of orally administered anticancer therapy has drastically increased in recent years. Currently, there are more than 40 oral anticancer medications on the market in the U.S.; and about 40% of all newly FDA-approved anticancer agents in 2013 and 2014 have been oral agents.1
The use of these agents is often driven by patients. In a review of 103 patients, an overwhelming 90% of patients who were to receive palliative chemotherapy chose oral chemotherapy over IV chemotherapy, assuming equivalent efficacy, toxicity, clinic visits, and blood work schedules. However, 70% of these patients were unwilling to sacrifice any efficacy between IV and oral chemotherapy.2 Several other factors influenced the preference of oral chemotherapy for patients, including convenience, avoidance of central venous catheter placement or need for other IV access, control of the environment in which they receive chemotherapy, and travel considerations.2 In addition to these practical benefits, patients reported a great sense of freedom with oral chemotherapy.3
Although patients may prefer oral anticancer therapies, for providers, several issues exist surrounding the shift in delivery of anticancer therapies from IV to oral therapies. The most significant concern is patient adherence, defined as “the extent to which patients take medications as prescribed by their health care providers.”4
Adherence rates in clinical trials are often excellent; however, real-life adherence rates tend to be less optimal.5 In a study of women receiving 5 years of adjuvant tamoxifen for breast cancer, the researchers determined that patients filled their prescription 87% of the time the first year of treatment. This rate of adherence dramatically decreased to only 50% by year 4.6
These results suggest that a longer duration of treatment can adversely affect adherence. Duration of treatment is of great concern for providers specifically when considering the need for indefinite duration of use of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. In 2011, Ibrahim and colleagues showed that imatinib adherence rates of 85% have been directly correlated to the loss of complete cytogenetic response (26.8% vs 1.5%, P = .0002) and lower probability of continuing imatinib (64.5% vs 90.6%, P = .006).7 Whereas several factors are known to influence adherence rates, Marin and colleagues identified the 2 main risk factors for poor adherence to imatinib: younger age and adverse effects (AEs). The median age for patients with adherence rates of 90% was 43.8 years compared with 53.8 years for patients with > 90% adherence rate. Imatinib AEs, such as asthenia, nausea, muscle cramps, and bone or joint pains, also significantly decreased imatinib adherence.8
In addition to concerns for poor therapeutic outcomes and suboptimal toxicity management, lack of adherence to oral anticancer regimens can result in significant waste of medication and increased health care costs. In most situations, IV anticancer treatment cycles are repeated every 1 to 3 weeks and allow the patient more frequent face-to-face interaction with the oncology team. Oral chemotherapy, on the other hand, is traditionally dispensed as a 28- to 30-day supply. This practice often limits the patient’s access to the oncology team for full evaluation of adherence and toxicity, which can lead to oral anticancer therapy waste.
Khandelwal and colleagues investigated the utility of a split-fill to decrease health care costs. In the splitfill process, patients were dispensed only days 1 to 16 of their oral anticancer medication at the initial fill. If the medication was tolerated and the prescribing provider deemed no changes in treatment necessary, the remaining 12 to 14 days of the cycle were then dispensed. Unfortunately, all insurance companies did not authorize the split-fill plan, thus preventing some patients in the study to participate in this cost savings strategy. However, it was determined for the patients who discontinued therapy, about 34% could have reduced wastage had they been on the split-fill plan, resulting in an average direct savings of $934.20 per patient who discontinued use.9
In 2011, the Hematology/Oncology and Pharmacy divisions at the VA Pittsburgh Healthcare System (VAPHS) examined the issues surrounding dispensing and monitoring of oral anticancer therapy. Higher utilization of oral anticancer therapy was identified and in parallel, increasing rates of patient nonadherence, toxicity, and wasted medication. Originally, most providers dispensed oral anticancer therapy as a 1-month supply. However, in efforts to increase adherence, limit toxicity, and avoid medication waste, some oncologists began only dispensing a 1- to 2-week supply of medication per visit. This shift in practice led to a pilot study evaluating the utility of limiting all oral chemotherapy to a 7- to 14-day supply during the first 3 months of treatment.
Pilot Study
The goal of the pilot study was to increase adherence, decrease toxicity, and avoid medication waste. Patients who initiated a new oral anticancer therapy between August 15, 2011, and February 15, 2012, were enrolled in the pilot study. Each patient was to be provided only a 14-day supply of medication at each visit. Patients on concurrent chemoradiotherapy with capecitabine were dispensed only a 7-day supply (as they were at VAPHS daily for radiation) of medication. A pillbox designated for oral anticancer therapy was provided and filled by the clinical pharmacist before leaving the hematology/oncology clinic.
Patients were provided a calendar to record the time and date of their oral anticancer therapy selfadministration. Patients were also asked to record any missed doses and the reasons they missed taking the medication. In addition, patients were counseled on the importance of medication adherence, food-drug and drug-drug interaction, proper storage and administration of medications, and when/who to notify if AEs occurred.
Patients were asked to return the pillboxes to the hematology/oncology clinic for the next refill and meet with the clinical pharmacist. A pill count was performed at each visit in addition to screening for toxicity. If a toxicity was identified, the prescribing provider was contacted for further orders. If no changes were needed, the remaining 14-day supply was dispensed to the patient at that time. Adherence and toxicity were documented in the electronic medical record (EMR) at each visit.
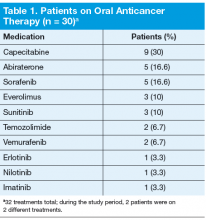
Thirty patients were started on 32 different oral anticancer therapies (Table 1) over the 6 months between August 15, 2011, and February 15, 2012. Patients already initiated on oral anticancer therapy before the start date were not included in this analysis. This number also did not include patients on lenalidomide, because this medication is mailed directly to the patient from a specialty pharmacy. All patients were male; average age was 68 (45-89) years; 83.3% of patients were white; and 83% of patients had a stage IV disease.
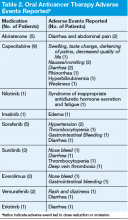
Adherence assessments using pill counts and medication calendars demonstrated that 6% (121/2,037) of doses that were dispensed were not taken. Overall adherence rate was 94%. The average patient adherence rate was 93.2%. Adverse events contributed to 62.8% of doses omitted (Table 2). Some AEs (eg, nausea, vomiting, and hypertension) were deemed preventable or modifiable with better symptom management. However, the majority of AEs that led to dose omission were not preventable.
Ten patients had their treatments discontinued midcycle, leading to 24.7% of missed doses. Adverse events led to 70% of discontinuation, whereas 20% resulted from disease progression. In both cases of disease progression, the patient was given a 30-day supply before the restaging scan, and in both cases this led to oral anticancer therapy waste. An additional 12.3% of doses were omitted due to hospitalization of patients.
Over a 6-month period, an estimated $32,314 was saved under the 14-day dosing pilot. This number was reached by subtracting the number of pills actually dispensed under pilot protocol from the number of pills that would have been dispensed under old dispensing standards (usually 28- to 30-day supply), then multiplying the difference by the cost per pill.
The results of this study were presented to the Pharmacy and Therapeutics Committee and led to the approval to continue with the 14-day dispensing protocol at VAPHS in March 2012. In addition, the pilot served as the backbone for the VHA Guidance on Oral Chemotherapy Dispensing and Monitoring.10 As part of the guidance, a monitoring guide for all the FDA-approved oral anticancer therapies is maintained and available for all VA practitioners to access on the PBM website under the Clinical Guidance subheading.
Current Practice
From the time the original pilot was conducted, the number of available oral anticancer therapies has increased along with the patient volume. Due to these factors and the lack of a dedicated outpatient oncology clinical pharmacist, oncology nurses in the outpatient clinic now direct the education, dispensing, and monitoring of patients on oral chemotherapy.
Treatment Plan
An oral anticancer treatment plan is developed by the oncology physician and entered in the EMR as a progress note titled Treatment Plan. The treatment plan includes, disease, stage, curative vs palliative intent, premedications, oral anticancer medication, dose, route and frequency, cycle length and number of cycles, baseline and continuous monitoring parameters, follow-up with provider, and staging follow-up. Once the oncology clinical pharmacist approves a treatment plan, the oncology nursing staff ensures that all the prechemotherapy laboratory tests are ordered and helps arrange any additional tests needed (echocardiogram, electrocardiogram, etc). After all the prechemotherapy testing is complete, the oncology nurse phones the patientto schedule a date for chemotherapy education and to pick up the first 14-day supply.
Initial Visit
The oncology nurse meets with each patient receiving oral anticancer therapy and provides them with an oncology clinic information packet, which includes chemotherapy education, a medication sheet, questions and answers about chemotherapy, common AEs and ways to manage them, as well as tips for meeting with the nurse and physician. The oncology nurse then reviews the oral anticancer treatment the patient is to receive, including how to administer the medication and timing, whether to take with or without food, common AEs, storage, safe handling, contact name if a toxicity arises, and importance of adherence.
The patient is provided with a pillbox and encouraged to track any missed doses. The oncology nurse then reschedules the patient for the next appointment at the clinic no more than 14 days later. Some treatments require more frequent monitoring and therefore are only dispensed 7 days at a time.
First Follow-up Visit (7-14 days)
At the first follow-up visit, the oncology nurse reviews adherence and toxicity with the patients. If any toxicity is identified, the oncology nurse contacts the oncology physician for additional assessment and orders. If the patient demonstrates adherence and tolerability, an additional 7- to 14-day supply is dispensed and the next appointment is scheduled 7 to 14 days later.
Subsequent Follow-up Visits
The patient continues to follow up at least every 28 days after cycle 1. The oncology nurse practices veterancentered care when trying to determine the appropriate follow-up for each patient. Continuous monitoring of toxicity and adherence occurs at each visit. If toxicity develops, monitoring may be increased at the discretion of the oncology nurse or physician.
Conclusions
Patients at VAPHS have been very receptive to the oral anticancer therapy protocol. Few patients have refused the initial biweekly visits, and many patients appreciate the special attention being focused on their treatment. The facility hopes to be able to expand its oral anticancer monitoring protocol to a telehealth clinic to help reduce the travel time of many patients. Additionally, as the program continues to expand, it is hoped it will be able to support a full-time outpatient oncology clinical pharmacist with a scope of practice to help manage toxicity and continue to improve adherence rates.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Center Watch. FDA approved drugs. Center Watch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/year/2014. Accessed October 24, 2014.
2. Liu G, Franssen E, Fitch Mi, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110-115.
3. Catania C, Didier F, Leon ME, et al. Perception that oral anticancer treatments are less efficacious: Development of a questionnaire to assess the possible prejudices of patients with cancer. Breast Cancer Res Treat. 2005;92(3):265-272.
4. Kelly A, Agius CR. Improving adherence to endocrine therapies: The role of advanced practice nurses. Oncology (Williston Park). 2006;20(10 Nurse Ed):50-54.
5. Prasad V, Massey PR, Fojo T. Oral anticancer drugs: How limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol. 2014;32(15):1620-1629.
6. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606.
7. Ibrahim A, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on longterm therapy. Blood. 2011;117(14):3733-3736.
8. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
9. Khandelwal N, Duncan I, Ahmed T, Rubinstein E, Pegus C. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admission. J Natl Compr Canc Netw. 2012;10(5):618-625.
10. Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. VHA guidance on oral anticancer drugs dispensing and monitoring. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; September 2012.
The availability and popularity of orally administered anticancer therapy has drastically increased in recent years. Currently, there are more than 40 oral anticancer medications on the market in the U.S.; and about 40% of all newly FDA-approved anticancer agents in 2013 and 2014 have been oral agents.1
The use of these agents is often driven by patients. In a review of 103 patients, an overwhelming 90% of patients who were to receive palliative chemotherapy chose oral chemotherapy over IV chemotherapy, assuming equivalent efficacy, toxicity, clinic visits, and blood work schedules. However, 70% of these patients were unwilling to sacrifice any efficacy between IV and oral chemotherapy.2 Several other factors influenced the preference of oral chemotherapy for patients, including convenience, avoidance of central venous catheter placement or need for other IV access, control of the environment in which they receive chemotherapy, and travel considerations.2 In addition to these practical benefits, patients reported a great sense of freedom with oral chemotherapy.3
Although patients may prefer oral anticancer therapies, for providers, several issues exist surrounding the shift in delivery of anticancer therapies from IV to oral therapies. The most significant concern is patient adherence, defined as “the extent to which patients take medications as prescribed by their health care providers.”4
Adherence rates in clinical trials are often excellent; however, real-life adherence rates tend to be less optimal.5 In a study of women receiving 5 years of adjuvant tamoxifen for breast cancer, the researchers determined that patients filled their prescription 87% of the time the first year of treatment. This rate of adherence dramatically decreased to only 50% by year 4.6
These results suggest that a longer duration of treatment can adversely affect adherence. Duration of treatment is of great concern for providers specifically when considering the need for indefinite duration of use of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. In 2011, Ibrahim and colleagues showed that imatinib adherence rates of 85% have been directly correlated to the loss of complete cytogenetic response (26.8% vs 1.5%, P = .0002) and lower probability of continuing imatinib (64.5% vs 90.6%, P = .006).7 Whereas several factors are known to influence adherence rates, Marin and colleagues identified the 2 main risk factors for poor adherence to imatinib: younger age and adverse effects (AEs). The median age for patients with adherence rates of 90% was 43.8 years compared with 53.8 years for patients with > 90% adherence rate. Imatinib AEs, such as asthenia, nausea, muscle cramps, and bone or joint pains, also significantly decreased imatinib adherence.8
In addition to concerns for poor therapeutic outcomes and suboptimal toxicity management, lack of adherence to oral anticancer regimens can result in significant waste of medication and increased health care costs. In most situations, IV anticancer treatment cycles are repeated every 1 to 3 weeks and allow the patient more frequent face-to-face interaction with the oncology team. Oral chemotherapy, on the other hand, is traditionally dispensed as a 28- to 30-day supply. This practice often limits the patient’s access to the oncology team for full evaluation of adherence and toxicity, which can lead to oral anticancer therapy waste.
Khandelwal and colleagues investigated the utility of a split-fill to decrease health care costs. In the splitfill process, patients were dispensed only days 1 to 16 of their oral anticancer medication at the initial fill. If the medication was tolerated and the prescribing provider deemed no changes in treatment necessary, the remaining 12 to 14 days of the cycle were then dispensed. Unfortunately, all insurance companies did not authorize the split-fill plan, thus preventing some patients in the study to participate in this cost savings strategy. However, it was determined for the patients who discontinued therapy, about 34% could have reduced wastage had they been on the split-fill plan, resulting in an average direct savings of $934.20 per patient who discontinued use.9
In 2011, the Hematology/Oncology and Pharmacy divisions at the VA Pittsburgh Healthcare System (VAPHS) examined the issues surrounding dispensing and monitoring of oral anticancer therapy. Higher utilization of oral anticancer therapy was identified and in parallel, increasing rates of patient nonadherence, toxicity, and wasted medication. Originally, most providers dispensed oral anticancer therapy as a 1-month supply. However, in efforts to increase adherence, limit toxicity, and avoid medication waste, some oncologists began only dispensing a 1- to 2-week supply of medication per visit. This shift in practice led to a pilot study evaluating the utility of limiting all oral chemotherapy to a 7- to 14-day supply during the first 3 months of treatment.
Pilot Study
The goal of the pilot study was to increase adherence, decrease toxicity, and avoid medication waste. Patients who initiated a new oral anticancer therapy between August 15, 2011, and February 15, 2012, were enrolled in the pilot study. Each patient was to be provided only a 14-day supply of medication at each visit. Patients on concurrent chemoradiotherapy with capecitabine were dispensed only a 7-day supply (as they were at VAPHS daily for radiation) of medication. A pillbox designated for oral anticancer therapy was provided and filled by the clinical pharmacist before leaving the hematology/oncology clinic.
Patients were provided a calendar to record the time and date of their oral anticancer therapy selfadministration. Patients were also asked to record any missed doses and the reasons they missed taking the medication. In addition, patients were counseled on the importance of medication adherence, food-drug and drug-drug interaction, proper storage and administration of medications, and when/who to notify if AEs occurred.
Patients were asked to return the pillboxes to the hematology/oncology clinic for the next refill and meet with the clinical pharmacist. A pill count was performed at each visit in addition to screening for toxicity. If a toxicity was identified, the prescribing provider was contacted for further orders. If no changes were needed, the remaining 14-day supply was dispensed to the patient at that time. Adherence and toxicity were documented in the electronic medical record (EMR) at each visit.
Thirty patients were started on 32 different oral anticancer therapies (Table 1) over the 6 months between August 15, 2011, and February 15, 2012. Patients already initiated on oral anticancer therapy before the start date were not included in this analysis. This number also did not include patients on lenalidomide, because this medication is mailed directly to the patient from a specialty pharmacy. All patients were male; average age was 68 (45-89) years; 83.3% of patients were white; and 83% of patients had a stage IV disease.
Adherence assessments using pill counts and medication calendars demonstrated that 6% (121/2,037) of doses that were dispensed were not taken. Overall adherence rate was 94%. The average patient adherence rate was 93.2%. Adverse events contributed to 62.8% of doses omitted (Table 2). Some AEs (eg, nausea, vomiting, and hypertension) were deemed preventable or modifiable with better symptom management. However, the majority of AEs that led to dose omission were not preventable.
Ten patients had their treatments discontinued midcycle, leading to 24.7% of missed doses. Adverse events led to 70% of discontinuation, whereas 20% resulted from disease progression. In both cases of disease progression, the patient was given a 30-day supply before the restaging scan, and in both cases this led to oral anticancer therapy waste. An additional 12.3% of doses were omitted due to hospitalization of patients.
Over a 6-month period, an estimated $32,314 was saved under the 14-day dosing pilot. This number was reached by subtracting the number of pills actually dispensed under pilot protocol from the number of pills that would have been dispensed under old dispensing standards (usually 28- to 30-day supply), then multiplying the difference by the cost per pill.
The results of this study were presented to the Pharmacy and Therapeutics Committee and led to the approval to continue with the 14-day dispensing protocol at VAPHS in March 2012. In addition, the pilot served as the backbone for the VHA Guidance on Oral Chemotherapy Dispensing and Monitoring.10 As part of the guidance, a monitoring guide for all the FDA-approved oral anticancer therapies is maintained and available for all VA practitioners to access on the PBM website under the Clinical Guidance subheading.
Current Practice
From the time the original pilot was conducted, the number of available oral anticancer therapies has increased along with the patient volume. Due to these factors and the lack of a dedicated outpatient oncology clinical pharmacist, oncology nurses in the outpatient clinic now direct the education, dispensing, and monitoring of patients on oral chemotherapy.
Treatment Plan
An oral anticancer treatment plan is developed by the oncology physician and entered in the EMR as a progress note titled Treatment Plan. The treatment plan includes, disease, stage, curative vs palliative intent, premedications, oral anticancer medication, dose, route and frequency, cycle length and number of cycles, baseline and continuous monitoring parameters, follow-up with provider, and staging follow-up. Once the oncology clinical pharmacist approves a treatment plan, the oncology nursing staff ensures that all the prechemotherapy laboratory tests are ordered and helps arrange any additional tests needed (echocardiogram, electrocardiogram, etc). After all the prechemotherapy testing is complete, the oncology nurse phones the patientto schedule a date for chemotherapy education and to pick up the first 14-day supply.
Initial Visit
The oncology nurse meets with each patient receiving oral anticancer therapy and provides them with an oncology clinic information packet, which includes chemotherapy education, a medication sheet, questions and answers about chemotherapy, common AEs and ways to manage them, as well as tips for meeting with the nurse and physician. The oncology nurse then reviews the oral anticancer treatment the patient is to receive, including how to administer the medication and timing, whether to take with or without food, common AEs, storage, safe handling, contact name if a toxicity arises, and importance of adherence.
The patient is provided with a pillbox and encouraged to track any missed doses. The oncology nurse then reschedules the patient for the next appointment at the clinic no more than 14 days later. Some treatments require more frequent monitoring and therefore are only dispensed 7 days at a time.
First Follow-up Visit (7-14 days)
At the first follow-up visit, the oncology nurse reviews adherence and toxicity with the patients. If any toxicity is identified, the oncology nurse contacts the oncology physician for additional assessment and orders. If the patient demonstrates adherence and tolerability, an additional 7- to 14-day supply is dispensed and the next appointment is scheduled 7 to 14 days later.
Subsequent Follow-up Visits
The patient continues to follow up at least every 28 days after cycle 1. The oncology nurse practices veterancentered care when trying to determine the appropriate follow-up for each patient. Continuous monitoring of toxicity and adherence occurs at each visit. If toxicity develops, monitoring may be increased at the discretion of the oncology nurse or physician.
Conclusions
Patients at VAPHS have been very receptive to the oral anticancer therapy protocol. Few patients have refused the initial biweekly visits, and many patients appreciate the special attention being focused on their treatment. The facility hopes to be able to expand its oral anticancer monitoring protocol to a telehealth clinic to help reduce the travel time of many patients. Additionally, as the program continues to expand, it is hoped it will be able to support a full-time outpatient oncology clinical pharmacist with a scope of practice to help manage toxicity and continue to improve adherence rates.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The availability and popularity of orally administered anticancer therapy has drastically increased in recent years. Currently, there are more than 40 oral anticancer medications on the market in the U.S.; and about 40% of all newly FDA-approved anticancer agents in 2013 and 2014 have been oral agents.1
The use of these agents is often driven by patients. In a review of 103 patients, an overwhelming 90% of patients who were to receive palliative chemotherapy chose oral chemotherapy over IV chemotherapy, assuming equivalent efficacy, toxicity, clinic visits, and blood work schedules. However, 70% of these patients were unwilling to sacrifice any efficacy between IV and oral chemotherapy.2 Several other factors influenced the preference of oral chemotherapy for patients, including convenience, avoidance of central venous catheter placement or need for other IV access, control of the environment in which they receive chemotherapy, and travel considerations.2 In addition to these practical benefits, patients reported a great sense of freedom with oral chemotherapy.3
Although patients may prefer oral anticancer therapies, for providers, several issues exist surrounding the shift in delivery of anticancer therapies from IV to oral therapies. The most significant concern is patient adherence, defined as “the extent to which patients take medications as prescribed by their health care providers.”4
Adherence rates in clinical trials are often excellent; however, real-life adherence rates tend to be less optimal.5 In a study of women receiving 5 years of adjuvant tamoxifen for breast cancer, the researchers determined that patients filled their prescription 87% of the time the first year of treatment. This rate of adherence dramatically decreased to only 50% by year 4.6
These results suggest that a longer duration of treatment can adversely affect adherence. Duration of treatment is of great concern for providers specifically when considering the need for indefinite duration of use of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. In 2011, Ibrahim and colleagues showed that imatinib adherence rates of 85% have been directly correlated to the loss of complete cytogenetic response (26.8% vs 1.5%, P = .0002) and lower probability of continuing imatinib (64.5% vs 90.6%, P = .006).7 Whereas several factors are known to influence adherence rates, Marin and colleagues identified the 2 main risk factors for poor adherence to imatinib: younger age and adverse effects (AEs). The median age for patients with adherence rates of 90% was 43.8 years compared with 53.8 years for patients with > 90% adherence rate. Imatinib AEs, such as asthenia, nausea, muscle cramps, and bone or joint pains, also significantly decreased imatinib adherence.8
In addition to concerns for poor therapeutic outcomes and suboptimal toxicity management, lack of adherence to oral anticancer regimens can result in significant waste of medication and increased health care costs. In most situations, IV anticancer treatment cycles are repeated every 1 to 3 weeks and allow the patient more frequent face-to-face interaction with the oncology team. Oral chemotherapy, on the other hand, is traditionally dispensed as a 28- to 30-day supply. This practice often limits the patient’s access to the oncology team for full evaluation of adherence and toxicity, which can lead to oral anticancer therapy waste.
Khandelwal and colleagues investigated the utility of a split-fill to decrease health care costs. In the splitfill process, patients were dispensed only days 1 to 16 of their oral anticancer medication at the initial fill. If the medication was tolerated and the prescribing provider deemed no changes in treatment necessary, the remaining 12 to 14 days of the cycle were then dispensed. Unfortunately, all insurance companies did not authorize the split-fill plan, thus preventing some patients in the study to participate in this cost savings strategy. However, it was determined for the patients who discontinued therapy, about 34% could have reduced wastage had they been on the split-fill plan, resulting in an average direct savings of $934.20 per patient who discontinued use.9
In 2011, the Hematology/Oncology and Pharmacy divisions at the VA Pittsburgh Healthcare System (VAPHS) examined the issues surrounding dispensing and monitoring of oral anticancer therapy. Higher utilization of oral anticancer therapy was identified and in parallel, increasing rates of patient nonadherence, toxicity, and wasted medication. Originally, most providers dispensed oral anticancer therapy as a 1-month supply. However, in efforts to increase adherence, limit toxicity, and avoid medication waste, some oncologists began only dispensing a 1- to 2-week supply of medication per visit. This shift in practice led to a pilot study evaluating the utility of limiting all oral chemotherapy to a 7- to 14-day supply during the first 3 months of treatment.
Pilot Study
The goal of the pilot study was to increase adherence, decrease toxicity, and avoid medication waste. Patients who initiated a new oral anticancer therapy between August 15, 2011, and February 15, 2012, were enrolled in the pilot study. Each patient was to be provided only a 14-day supply of medication at each visit. Patients on concurrent chemoradiotherapy with capecitabine were dispensed only a 7-day supply (as they were at VAPHS daily for radiation) of medication. A pillbox designated for oral anticancer therapy was provided and filled by the clinical pharmacist before leaving the hematology/oncology clinic.
Patients were provided a calendar to record the time and date of their oral anticancer therapy selfadministration. Patients were also asked to record any missed doses and the reasons they missed taking the medication. In addition, patients were counseled on the importance of medication adherence, food-drug and drug-drug interaction, proper storage and administration of medications, and when/who to notify if AEs occurred.
Patients were asked to return the pillboxes to the hematology/oncology clinic for the next refill and meet with the clinical pharmacist. A pill count was performed at each visit in addition to screening for toxicity. If a toxicity was identified, the prescribing provider was contacted for further orders. If no changes were needed, the remaining 14-day supply was dispensed to the patient at that time. Adherence and toxicity were documented in the electronic medical record (EMR) at each visit.
Thirty patients were started on 32 different oral anticancer therapies (Table 1) over the 6 months between August 15, 2011, and February 15, 2012. Patients already initiated on oral anticancer therapy before the start date were not included in this analysis. This number also did not include patients on lenalidomide, because this medication is mailed directly to the patient from a specialty pharmacy. All patients were male; average age was 68 (45-89) years; 83.3% of patients were white; and 83% of patients had a stage IV disease.
Adherence assessments using pill counts and medication calendars demonstrated that 6% (121/2,037) of doses that were dispensed were not taken. Overall adherence rate was 94%. The average patient adherence rate was 93.2%. Adverse events contributed to 62.8% of doses omitted (Table 2). Some AEs (eg, nausea, vomiting, and hypertension) were deemed preventable or modifiable with better symptom management. However, the majority of AEs that led to dose omission were not preventable.
Ten patients had their treatments discontinued midcycle, leading to 24.7% of missed doses. Adverse events led to 70% of discontinuation, whereas 20% resulted from disease progression. In both cases of disease progression, the patient was given a 30-day supply before the restaging scan, and in both cases this led to oral anticancer therapy waste. An additional 12.3% of doses were omitted due to hospitalization of patients.
Over a 6-month period, an estimated $32,314 was saved under the 14-day dosing pilot. This number was reached by subtracting the number of pills actually dispensed under pilot protocol from the number of pills that would have been dispensed under old dispensing standards (usually 28- to 30-day supply), then multiplying the difference by the cost per pill.
The results of this study were presented to the Pharmacy and Therapeutics Committee and led to the approval to continue with the 14-day dispensing protocol at VAPHS in March 2012. In addition, the pilot served as the backbone for the VHA Guidance on Oral Chemotherapy Dispensing and Monitoring.10 As part of the guidance, a monitoring guide for all the FDA-approved oral anticancer therapies is maintained and available for all VA practitioners to access on the PBM website under the Clinical Guidance subheading.
Current Practice
From the time the original pilot was conducted, the number of available oral anticancer therapies has increased along with the patient volume. Due to these factors and the lack of a dedicated outpatient oncology clinical pharmacist, oncology nurses in the outpatient clinic now direct the education, dispensing, and monitoring of patients on oral chemotherapy.
Treatment Plan
An oral anticancer treatment plan is developed by the oncology physician and entered in the EMR as a progress note titled Treatment Plan. The treatment plan includes, disease, stage, curative vs palliative intent, premedications, oral anticancer medication, dose, route and frequency, cycle length and number of cycles, baseline and continuous monitoring parameters, follow-up with provider, and staging follow-up. Once the oncology clinical pharmacist approves a treatment plan, the oncology nursing staff ensures that all the prechemotherapy laboratory tests are ordered and helps arrange any additional tests needed (echocardiogram, electrocardiogram, etc). After all the prechemotherapy testing is complete, the oncology nurse phones the patientto schedule a date for chemotherapy education and to pick up the first 14-day supply.
Initial Visit
The oncology nurse meets with each patient receiving oral anticancer therapy and provides them with an oncology clinic information packet, which includes chemotherapy education, a medication sheet, questions and answers about chemotherapy, common AEs and ways to manage them, as well as tips for meeting with the nurse and physician. The oncology nurse then reviews the oral anticancer treatment the patient is to receive, including how to administer the medication and timing, whether to take with or without food, common AEs, storage, safe handling, contact name if a toxicity arises, and importance of adherence.
The patient is provided with a pillbox and encouraged to track any missed doses. The oncology nurse then reschedules the patient for the next appointment at the clinic no more than 14 days later. Some treatments require more frequent monitoring and therefore are only dispensed 7 days at a time.
First Follow-up Visit (7-14 days)
At the first follow-up visit, the oncology nurse reviews adherence and toxicity with the patients. If any toxicity is identified, the oncology nurse contacts the oncology physician for additional assessment and orders. If the patient demonstrates adherence and tolerability, an additional 7- to 14-day supply is dispensed and the next appointment is scheduled 7 to 14 days later.
Subsequent Follow-up Visits
The patient continues to follow up at least every 28 days after cycle 1. The oncology nurse practices veterancentered care when trying to determine the appropriate follow-up for each patient. Continuous monitoring of toxicity and adherence occurs at each visit. If toxicity develops, monitoring may be increased at the discretion of the oncology nurse or physician.
Conclusions
Patients at VAPHS have been very receptive to the oral anticancer therapy protocol. Few patients have refused the initial biweekly visits, and many patients appreciate the special attention being focused on their treatment. The facility hopes to be able to expand its oral anticancer monitoring protocol to a telehealth clinic to help reduce the travel time of many patients. Additionally, as the program continues to expand, it is hoped it will be able to support a full-time outpatient oncology clinical pharmacist with a scope of practice to help manage toxicity and continue to improve adherence rates.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Center Watch. FDA approved drugs. Center Watch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/year/2014. Accessed October 24, 2014.
2. Liu G, Franssen E, Fitch Mi, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110-115.
3. Catania C, Didier F, Leon ME, et al. Perception that oral anticancer treatments are less efficacious: Development of a questionnaire to assess the possible prejudices of patients with cancer. Breast Cancer Res Treat. 2005;92(3):265-272.
4. Kelly A, Agius CR. Improving adherence to endocrine therapies: The role of advanced practice nurses. Oncology (Williston Park). 2006;20(10 Nurse Ed):50-54.
5. Prasad V, Massey PR, Fojo T. Oral anticancer drugs: How limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol. 2014;32(15):1620-1629.
6. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606.
7. Ibrahim A, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on longterm therapy. Blood. 2011;117(14):3733-3736.
8. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
9. Khandelwal N, Duncan I, Ahmed T, Rubinstein E, Pegus C. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admission. J Natl Compr Canc Netw. 2012;10(5):618-625.
10. Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. VHA guidance on oral anticancer drugs dispensing and monitoring. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; September 2012.
1. Center Watch. FDA approved drugs. Center Watch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/year/2014. Accessed October 24, 2014.
2. Liu G, Franssen E, Fitch Mi, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110-115.
3. Catania C, Didier F, Leon ME, et al. Perception that oral anticancer treatments are less efficacious: Development of a questionnaire to assess the possible prejudices of patients with cancer. Breast Cancer Res Treat. 2005;92(3):265-272.
4. Kelly A, Agius CR. Improving adherence to endocrine therapies: The role of advanced practice nurses. Oncology (Williston Park). 2006;20(10 Nurse Ed):50-54.
5. Prasad V, Massey PR, Fojo T. Oral anticancer drugs: How limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol. 2014;32(15):1620-1629.
6. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606.
7. Ibrahim A, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on longterm therapy. Blood. 2011;117(14):3733-3736.
8. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
9. Khandelwal N, Duncan I, Ahmed T, Rubinstein E, Pegus C. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admission. J Natl Compr Canc Netw. 2012;10(5):618-625.
10. Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. VHA guidance on oral anticancer drugs dispensing and monitoring. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; September 2012.