User login
Sickle Cell Disease
INTRODUCTION
Sickle cell disease is the most common inherited blood disorder in the world. It affects more than 100,000 individuals in the United States, and millions more worldwide.1 Sickle cell disease is most commonly found in individuals of African heritage, but the disease also occurs in Hispanics and people of Middle Eastern and subcontinent Indian heritage.2 The distribution of the sickle hemoglobin (hemoglobin S [HbS]) allele overlaps with the distribution of malaria; HbS carriers, or individuals with sickle cell trait, have protection against malaria,3 and are not considered to have sickle cell disease.
Sickle cell disease is a severe monogenic disorder marked by significant morbidity and mortality, affecting every organ in the body.4 The term sickle cell disease refers to all genotypes that cause sickling; the most common are the homozygous hemoglobin SS (HbSS) and compound heterozygotes hemoglobin SC (HbSC), hemoglobin S–β0-thalassemia (HbSβ0), and hemoglobin S–β+-thalassemia (HbSβ+), although HbS and several rarer hemoglobin variants such as HbSO(Arab) and HbSD(Punjab) can also cause sickle cell disease. The term sickle cell anemia refers exclusively to the most severe genotypes, HbSS and HbSβ0.5 Common sickling genotypes along with their relative clinical severity are shown in Table 1.6–11
| Table 1. Genotypes of Sickling Syndromes and Their Relative Severities | ||
Genotype | Severity | Characteristics |
HbSS | Severe | Most common form |
HbSβ0 | Severe | Clinically indistinguishable from HbSS6 |
HbSO-Arab | Severe | Relatively rare6 |
HbSD-Punjab | Severe | Mostly in northern India6 |
HbSC-Harlem | Severe | Migrates like HbSC, but rare double β-globin mutation7 |
HbCS-Antilles | Severe | Rare double β-globin mutation8 |
HbSC | Moderate | 25% of SCD9 |
HbSβ+, Mediterranean | Moderate | 5%–16% HbA6 |
HbAS-Oman | Moderate | Dominant rare double β-globin mutation10 |
HbSβ+, African | Mild | 16%–30% HbA6 |
HbSE | Mild | HbE found mostly in Southeast Asia11 |
HbS-HPFH | Very mild | Large deletions in β-globin gene complex; > 30% HbF6 |
| HbA = hemoglobin A; HbE = hemoglobin E; HbF = fetal hemoglobin; HbS-HPFH = HbS and gene deletion HPFH; HbSC = heterozygous hemoglobin SC; HbSS = homozygous hemoglobin SS; HbSβ0 = hemoglobin S-β thalassemia0; HbSβ+ = hemoglobin S-β thalassemia+; SCD = sickle cell disease. | ||
This article reviews the pathophysiology of sickle cell disease, common clinical complications, and available therapies. A complex case which illustrates diagnostic and management challenges is presented as well.
PATHOPHYSIOLOGY
HbS is the result of a substitution of valine for glutamic acid in the sixth amino acid of the β-globin chain.12 The change from a hydrophilic to a hydrophobic amino acid causes the hemoglobin molecules to stack, or polymerize, when deoxygenated. This rigid rod of hemoglobin distorts the cell, producing the characteristic crescent or sickle shape that gives the disease its name.13 Polymerization of hemoglobin within the cell is promoted by dehydration, which increases the concentration of HbS.13,14 Polymerization occurs when hemoglobin is in the deoxygenated state.13
The sickle red blood cell is abnormal; it is rigid and dense, and lacks the deformability needed to navigate the microvasculature.15 Blockages of blood flow result in painful vaso-occlusion that is the hallmark of the disease, and that also can cause damage to the spleen, kidneys, and liver.16 The sickle red cell is also fragile, with a lifespan of only 20 days compared to the 120-day lifespan of a normal red blood cell.13 Frequent hemolysis results in anemia and the release of free hemoglobin, which both scavenges nitric oxide and impairs the production of more nitric oxide, which is essential for vasodilatation.17 This contributes to vascular dysfunction and an increased risk for stroke.18 If untreated, the natural course of sickle cell anemia is mortality in early childhood in most cases.19 Common chronic and acute sickle cell disease–related complications and recommended therapies, based on 2014 National Institutes of Health guidelines, are shown in Table 2 and Table 3.20
| Table 2. Common Adult Sickle Cell Disease Chronic Complications and Recommended Therapies | ||
Chronic Complication | Recommended Therapy | Strength of Recommendation |
Chronic pain | Opioids | Consensus |
Avascular necrosis | Analgesics and physical therapy | Consensus |
Proliferative sickle retinopathy | Laser photocoagulation | Strong |
Leg ulcers | Standard wound care | Moderate |
Recurrent priapism | Consult urology | Moderate |
| Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
| Table 3. Common Adult Sickle Cell Disease Acute Complications and Recommended Therapies | ||
Acute Complication | Recommended Therapy | Strength of Recommendation |
Vaso-occlusive crisis | NSAIDs, opioids for severe pain | Moderate-consensus |
ACS | Antibiotics, oxygen | Strong |
Simple transfusiona | Weak | |
Urgent exchange transfusionb | Strong | |
Acute stroke | Exchange transfusion | Strong |
Priapism ≥ 4 hr | Aggressive hydration, pain control, and urology consult | Strong-consensus |
Gallstones, symptomatic | Cholecystectomy, laparoscopic | Strong |
Splenic sequestration | Intravenous fluids, transfuse cautiously, discuss surgical splenectomy | Strong-moderate |
Acute renal failure | Consult nephrologyc | Consensus |
ACS = acute chest syndrome; NSAIDs = nonsteroidal anti-inflammatory drugs. a For symptomatic ACS with hemoglobin > 1 g/dL below baseline but > 9.0 g/dL. b When there is progression of ACS (SpO2 < 90% despite supplemental oxygen, increasing respiratory distress, progressive pulmonary infiltrates despite simple transfusion). c For acute rise in creatinine ≥ 0.3 mg/dL; do not give transfusions unless there are other indications. Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
One of the most challenging aspects of sickle cell disease is its clinical variability. While in general, HbSS and HbSβ0 are the most severe genotypes, there are patients with HbSC and HbSb+ who have significant sickle-cell–related complications, and may have a more severe clinical course than a HbSS patient.21 A great deal of this clinical variability cannot be explained, but some can be attributed to endogenous fetal hemoglobin (HbF) levels.22–24 The importance of HbF levels in sickle cell disease was first noted by a pediatrician in the 1940s.25 She observed that sickle cell disease complications in children under the age of 1 were rare, and attributed it to the presence of HbF.25 HbF levels decline more slowly in individuals with hemoglobinopathies, reaching their nadir after the age of 5 rather than within 6 months of birth in individuals without hemoglobinopathies.26 HbF levels remain elevated lifelong in most sickle cell disease patients, especially those with the HbSS and HbSβ0 genotypes. Levels of HbF vary widely between individuals, from zero to 20% to 30%, with a median of 10%.26–28 Individuals who produce more HbF have a milder course, in general.24 An association between the 4 β-globin haplotypes and HbF levels has been reported in the past,27,29 but more sophisticated next-generation sequencing has revealed causal variants in BCL11A and HBS1L-MYB that contribute approximately 50% of the observed variability in HbF levels.30–33
Co-inheritance of α-thalassemia also modifies disease course; less available α-globin chains results in a lower hemoglobin concentration within the cell. Paradoxically, this results in a higher overall hemoglobin level, as there is a reduction in polymerization, and therefore sickling due to lower HbS concentrations in the cell. Patients therefore are less anemic, reducing the risk of stroke in childhood,34,35 but blood viscosity may be higher, resulting in more frequent pain crises and increased risk36 of avascular necrosis.34,35,37 It is often helpful to think of sickle cell patients as falling into 1 of 2 groups: high hemolysis/low hemoglobin and high viscosity/high hemoglobin. Individuals with high rates of hemolysis are at greater risk for stroke, pulmonary hypertension, and acute chest syndrome (ACS). Higher rates of hemolysis result in higher levels of free hemoglobin, which scavenges nitric oxide. This leads to the vascular damage and dysfunction that contributes to the associated clinical complications. This phenotype is most commonly seen in HbSS and HbSβ0.38 High hemoglobin/high viscosity phenotypes are most often found in HbSC patients and in sickle cell anemia with α-thalassemia coinheritance.39–42
TREATMENT OPTIONS
In high-resource countries with newborn screening, the initiation of penicillin prophylaxis has dramatically altered the natural history of the disease, allowing the majority of patients to reach adulthood.43 Penicillin prophylaxis is usually discontinued at age 5 years; however, individuals who have undergone surgical splenectomy or have had pneumococcal sepsis on penicillin prophylaxis may remain on penicillin to age 18 or beyond.20
Another advance in sickle cell care is screening for stroke risk through transcranial Doppler ultrasound (TCD).44–47 This screening tool has reduced the incidence of childhood stroke from 10% by age 11 to 1%. TCDs typically cannot be performed after the age of 16 due to changes in the skull. Individuals found to have abnormal (elevated) TCD velocities are placed on chronic transfusion therapy for primary stroke prevention. They may remain on monthly chronic transfusions, with the goal of suppressing the percentage of HbS to 30% to 50% indefinitely. A clinical trial (STOPII) designed to determine if pediatric sickle cell disease patients on chronic transfusion therapy for primary stroke prevention could be safely taken off transfusion therapy was discontinued early due to an excess of strokes and conversion to abnormal TCD velocities in the untransfused arm.44 Individuals who have experienced an ischemic stroke have a 70% risk of another stroke, and must remain on chronic transfusion therapy indefinitely. Chronic transfusion reduces their stroke risk to 13%.
The only widely used pharmacologic therapy for sickle cell disease is hydroxyurea.12,48–50 A significant portion of the benefit of hydroxyurea stems from its induction of HbF.51 HbF does not sickle, and it interrupts the polymerization of HbS in the cell, if present in high enough concentrations.50 The level of HbF needed to achieve clinical improvement is not known, but in vitro assays suggest 20% HbF is needed to prevent sickling.52,53 However, endogenous and hydroxyurea-induced HbF is not distributed evenly through the red cells, so sickling is possible regardless of the level of HbF induced.54,55 Hydroxyurea likely has other disease-modifying effects as well, including reduction of white blood cell count and reticulocyte count and reduction of red cell adhesion to the endothelium.56–58 Clinical criteria for initiation of hydroxyurea in adult sickle cell disease patients are shown in Table 4.20 Hydroxyurea is given daily and is dosed to maximum tolerated dose for the individual by following the absolute neutrophil count (ANC). The goal ANC is between 2000 and 4000/µL. At times, absolute reticulocyte count (ARC) can be dose-limiting; goal ARC is greater than 70,000/µL.59 Platelet counts may be reduced as well, especially in HbSC patients.60,61
| Table 4. Indications for Hydroxyurea in Adult Patients with Sickle Cell Disease | |
Indication | Strength of Recommendation |
SCA with ≥ 3 pain crises per year | Strong |
SCA with pain that interferes with ADL and QoL | Strong |
History of severe or recurrent ACS | Strong |
Chronic kidney disease on epoetin | Weak |
HbSβ+ and HbSC with pain that interferes with ADL and QoL; consult sickle cell disease expert | Moderate |
| ACS = acute chest syndrome; ADL = activities of daily living; QoL = quality of life; SCA = sickle cell anemia. | |
The only curative therapy for sickle cell disease is hematopoietic stem cell transplant.62 Transplant use is limited by availability of matched sibling donors,62 and even at experienced centers transplant carries a small risk for mortality, graft rejection, and graft-versus-host disease. Furthermore, consensus on disease complications for which transplant is recommended is also lacking.63–65 Clinical trials of gene therapy for sickle cell disease and thalassemia are ongoing.66
COMPLICATIONS AND DISEASE-SPECIFIC THERAPIES
CASE PRESENTATION
A 26-year-old African-American man who works as a school bus driver presents to an academic center’s emergency department complaining of pain in his left leg, similar to prior pain events. He is described as having sickle cell trait, although no hemoglobin profile is available in his chart. He describes the pain as dull and aching, 10/10 in intensity. A complete blood count (CBC) is obtained; it reveals a hemoglobin of 14.5 g/dL, white blood cell (WBC) count of 5600/µL, and platelet count of 344,000/µL. His CBC is also notable for a mean corpuscular volume (MCV) of 72 fL, a mean corpuscular hemoglobin concentration (MCHC) of 37 g/dL, and a red blood cell distribution width (RDW) of 12. Slide review of a peripheral blood smear shows 2+ target cells (Figure).
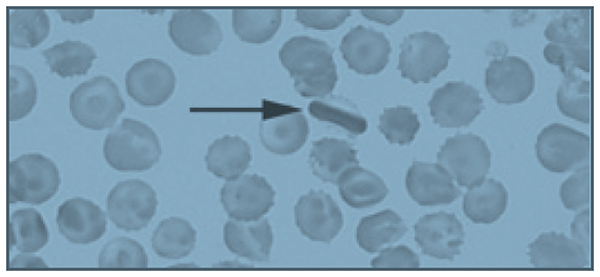
The patient is given 6 mg of morphine, which provides some relief of his pain, and is discharged with a prescription for hydrocodone bitartrate/acetaminophen 5/325 mg. The diagnosis given is musculoskeletal pain, and he is instructed to follow-up with a primary care physician. His past medical history is significant for 4 or 5 visits to the emergency department per year in the past 4 years. Prior to 4 years ago, he rarely required medical attention.
• What laboratory and clinical features might lead you to question the diagnosis of sickle cell trait in this patient?
The patient’s hemoglobin is within normal range, which is consistent with sickle cell trait; however, he is microcytic, with a normal RDW. It is possible to be mildly microcytic in the early stages of iron deficiency, prior to the development of anemia, but the RDW would typically be elevated, demonstrating the presence of newer, smaller cells produced under conditions of iron deficiency.67 It is also possible that his microcytosis with a normal RDW could represent sickle cell trait with co-inheritance of β-thalassemia. Up to 30% of African Americans have β-thalassemia,2 and 1 in 10 have sickle cell trait.68 However, a high MCHC, indicating the presence of dense cells, and target cells noted on slide review are most consistent with HbSC.9 HbSC patients, especially males, can have hemoglobin levels in the normal range.4 The biggest inconsistency with the diagnosis of sickle cell trait is his history of frequent pain events. Individuals with sickle cell trait rarely present with pain crises, except under extreme conditions of dehydration or high altitude.68 Sickle cell trait is generally regarded as a benign condition, although a study of U.S. military recruits found a 30-fold higher risk of sudden death during basic training in persons with sickle cell trait.69 Additional sickle cell trait–related complications include hematuria, risk of splenic sequestration or infarct under extreme conditions and high altitude, and a rare and usually fatal renal malignancy, renal medullary carcinoma, which is vanishingly rare in individuals without sickle cell trait.70,71 Although the patient reported having sickle cell trait, this diagnosis should have been verified with a hemoglobin panel, given his atypical presentation.20
• What is the approach to managing pain episodes in sickle cell disease?
In sickle cell disease, vaso-occlusive pain events can be common, often beginning in early childhood.17 This disease complication accounts for 95% of all adult sickle cell disease hospitalizations.72 There is a great deal of variability in pain symptoms between individuals, and within individuals at various times in their lives:73 30% have no pain events, 50% have occasional events, and 20% have monthly or more frequent events that require hospitalization.74 The frequency and severity of pain events are modulated by HbF levels, β-thalassemia status, genotypes, therapies like hydroxyurea, or in rare cases, chronic transfusion therapy.23 Personal factors, such as psychosocial stressors, also contribute to the frequency of pain events.75 Pain event triggers include exposure to cold water, windy or cold weather, temperature changes, and extreme temperatures.76–79 Patient age also contributes to pain event frequency. Many patients see an increase in pain event frequency in their late 20s, and a marked decrease in their 40s.23,73 More than 3 pain events per year is associated with reduced life expectancy.23
Acute management of pain episodes involves nonsteroidal anti-inflammatory drugs, oral opioids, and when hospitalization is required, intravenous opioids, often delivered via patient-controlled analgesia (PCA) pumps.79 As sickle cell disease patients become teenagers and young adults, some experience an increased frequency of pain episodes, with fewer pain-free days, or a failure to return to baseline before the next pain crisis occurs.80,81 This is characteristic of emerging chronic pain.82 Chronic pain is a significant problem in adult patients with sickle cell disease, with up to 85% reporting pain on most days.72,80 The development of chronic pain may be reduced by early and aggressive treatment of acute pain events, as well as use of hydroxyurea to reduce the number of pain events. Many adult sickle cell patients with chronic pain are treated with daily opioids.20 Given the significant side effects of chronic opioid use—sedation, respiratory depression, itching, nausea, and impairment of function and quality of life—non-opioid therapies are under investigation.83 Many chronic pain patients have symptoms of neuropathic pain, and may benefit from neuropathic agents like gabapentin, both to reduce opioid use and to more effectively treat chronic neuropathic pain, which is known to respond poorly to opioids.84–86
• Is the patient’s peripheral blood smear consistent with a diagnosis of sickle cell trait?
Several target cells are visible, which is not typical of sickle cell trait, but may be seen in HbSC or thalassemia. The finding of an intracellular crystal is pathognomonic for HbSC or HbCC. HbC polymerizes in high oxygen conditions, opposite of HbS, which polymerizes in low oxygen conditions.9
CASE CONTINUED
The patient’s family history is significant for a sister who died at age 3 from sickle cell–related complications, and a sister with sickle cell trait who had a cholecystectomy for gallstones at age 22. His father died at age 38 due to unknown causes. The sickle cell trait status of his parents is unknown. His mother is alive, and has hypertension.
• Is the medical history of this patient’s family members consistent with sickle cell trait?
It is unlikely that sickle cell trait would result in early death in childhood, or in gallstones at age 22. Gallstones in early adulthood is a common presentation for HbSC patients not diagnosed by newborn screening.87 Any hemolytic condition can lead to the formation of hemoglobin-containing pigmented gallstones, biliary sludge, and obstruction of the gallbladder. In the presence of right-sided abdominal pain, a serum bilirubin level of more than 4 mg/dL should lead to measurement of direct bilirubin; if greater than 10% of total, imaging of the gallbladder should be obtained. In sickle cell disease, 30% of patients will have gallstones by 18 years of age. The low hemolysis/high viscosity phenotype patients are typically older at diagnosis. Co-inheritance of Gilbert syndrome and sickle cell disease is not uncommon, and can result in formation of gallstones at a young age; Gilbert syndrome alone typically results in gallstones in mid-life.88
CASE CONTINUED
Two months later, the patient presents again to the emergency department with the same complaint of leg pain, as well as abdominal pain. His hemoglobin is 12.5 g/dL, and his platelet count is 134,000/µL. His pain is not improved with 3 doses of morphine 6 mg intravenously, and he is admitted to the medicine service. A hemoglobin profile is obtained, revealing 52% HbS, 45% HbC, and 1.5% HbF, consistent with HbSC. In sickle cell trait, the hemoglobin profile is 60% HbA and 40% HbS (available α-globin prefers to pair with a normal β-globin, so the ratio of HbA to HbS is 60:40, not 50:50).
On the second hospital day, the patient’s hemoglobin drops to 7.2 g/dL and his platelet count decreases to 44,000/µL. His abdomen is distended and diffusely tender. The internist transfuses him with 2 units of packed red blood cells (PRBC), after which his hemoglobin increases to 11 g/dL, while his platelet count increases to 112,000/µL. Following the transfusion, his abdominal pain resolves, as does his anemia and thrombocytopenia.
• What caused this patient’s anemia and thrombocytopenia?
High on the differential diagnosis is a splenic sequestration. Acute splenic sequestration occurs when red cells are trapped in the splenic sinuses. Massive splenic enlargement may occur over several hours.89,90 Unrecognized splenic sequestration has a high mortality rate from severe anemia and splenic rupture.90 Splenic sequestration must be ruled out in a sickle cell patient with abdominal pain accompanied by dropping platelet and red cell counts, especially in milder subtypes that often have splenic function preserved into adolescence and adulthood. Sickle cell anemia patients usually become functionally asplenic in early childhood.89,91,92 The rise in hemoglobin, more than would be expected from 2 units of PRBC, plus the improvement in platelet count without a platelet transfusion observed in the case patient strongly supports the diagnosis of splenic sequestration.
Splenic sequestration can occur in any sickle cell patient whose spleen has not fibrosed. Splenic sequestration in adulthood is not uncommon in HbSC patients, who often have preserved splenic function into adulthood.93–95
Clinical signs of splenic sequestration include a rapid drop in hemoglobin, rise in reticulocyte count, a tender, enlarged spleen, and, in severe cases, hypovolemia.89,93 It is treated with prompt blood transfusion, but care must be taken not to overtransfuse the patient, as the spleen can trap several grams of hemoglobin, which may be released upon transfusion, potentially causing life-threatening hyperviscosity.89 Hemoglobin levels must be checked following transfusion in suspected splenic sequestration, and “mini transfusions” of 5 mL/kg are recommended in sickle cell disease patients who are hemodynamically stable.20
Hepatic sequestration may also occur, but it is much less common than splenic sequestration.96 Other conditions on the differential diagnosis include thrombotic thrombocytopenic purpura, which would be unlikely to respond to a transfusion. ACS can cause a drop in hemoglobin, and is treated with simple or exchange transfusions.97 ACS is less likely without respiratory symptoms or oxygen requirement, and usually is not associated with thrombocytopenia. Sepsis may also cause anemia and thrombocytopenia, but again would not likely respond to a simple transfusion. The patient’s response to transfusion is consistent with a sequestering event, not a destructive event as in the case of sepsis.
CASE CONTINUED
Imaging reveals a grossly enlarged spleen, which is having a mass effect on the left kidney. The patient is started on hydroxyurea therapy at 500 mg 3 times daily. Discharge instructions include following up with his primary care physician, continuing hydroxyurea therapy, and receiving yearly dilated eye exams to evaluate for proliferative sickle retinopathy.
• Are these discharge instructions complete?
Splenic sequestration has a 50% recurrence rate.98 In very young children, watchful waiting or chronic transfusion may be implemented to preserve the immunologic function of the spleen and reduce the risk of sepsis.89 Splenectomy after a single episode of sequestration in adults is a matter of debate, with experts advising both watchful waiting99 and splenectomy after recovery from the first sequestering event.100 The patient should have been informed of the risk for recurrence, and the signs and symptoms of splenic sequestration as well as the need for emergency medical attention should have been discussed. Splenic sequestration may be milder in adults than in children, but fatal sequestrations have been reported.95,101–103
Proliferative sickle cell retinopathy is a high viscosity/high hemoglobin complication that may occur more frequently in HbSC than HbSS, with an incidence of 33% in HbSC.42,104 Spontaneous regression of retinopathy occurs in approximately 32% of eyes, and laser or scatter photocoagulation is an effective intervention.105
• Would the patient need to be transfused prior to splenectomy?
Preoperative transfusion therapy is standard of care for HbSS patients undergoing general anesthesia. The TRAP study found that simple “top off” transfusion to a hemoglobin of 10 g/dL was as effective at preventing postoperative sickle cell–related complications as exchange transfusion to HbS of 30% or less, and had fewer transfusion-related complications like alloimmunization.106 There is little data regarding preoperative transfusions in HbSC disease. A retrospective study suggests that HbSC patients undergoing abdominal surgeries should be transfused.107 The higher hemoglobin level of the typical HbSC patient necessitates exchange transfusion to avoid hyperviscosity.
• Is hydroxyurea therapy indicated in this patient?
• Has it been dosed appropriately?
If the patient had the HbSS subtype, hydroxyurea would be clearly indicated, given his frequent pain events.20 HbSC patients may be placed on hydroxyurea on a case-by-case basis, but evidence for its efficacy in this sickle cell subtype is lacking.108 Large clinical trials like the Multi-Center Study of Hydroxyurea (MSH) that established the safety and efficacy of hydroxyurea in sickle cell anemia excluded HbSC and HbSβ+ patients.109 These mild to moderate subtypes produce less HbF at baseline, and typically have a minimal to modest rise in HbF on hydroxyurea.110 In sickle cell anemia, hydroxyurea is titrated to maximum tolerated dose, defined as an ANC of 2000 to 4000/µL and an ARC of 70,000/µL or higher.53 Because of their lower levels of chronic inflammation and lower reticulocyte counts due to higher hemoglobin levels, many HbSC and HbSβ+ patients have values in that range before initiating hydroxyurea therapy.9 Cytopenias, particularly of platelets in HbSC, occur at low doses of hydroxyurea.111
Of note, although the half-life of hydroxyurea would suggest that 3 times daily dosing is indicated, daily dosing has been found to have equal response and is preferred. Another concern is the monitoring of this myelosuppressive medication. This patient has repeatedly failed to obtain a primary care physician or a hematologist, and hydroxyurea requires laboratory monitoring at least every 2 months, especially in a HbSC patient with a very large spleen who is at significant risk for thrombocytopenia and neutropenia.9
CASE CONTINUED
A week after discharge from his admission for abdominal pain diagnosed as splenic sequestration, the patient presents again to the emergency department with abdominal pain which he reports is his typical sickle cell pain. Hemoglobin is 13.8 g/dL, platelet count is 388,000/µL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are both 10 times their prior value. Creatinine is 1.2 mg/dL (0.75 mg/dL on his prior admission), and total bilirubin is 3 mg/dL, with 0.3 mg/dL direct bilirubin. He undergoes an ultrasound exam of his gallbladder, which reveals sludge and a possible gallstone. There is no evidence of cholecystitis. General surgery performs a laparoscopic cholecystectomy.
• Was this cholecystectomy necessary?
In patients with sickle cell disease, symptomatic gallstones and gallbladder sludge should be observed; recurrent abdominal pain without a significant change in bilirubin may not be due to gallstones or sludge, and therefore may not be relieved by cholecystectomy.112,113 In sickle cell disease, 40% of patients with gallbladder sludge do not develop gallstones.87 The patient’s bilirubin level was at baseline, and there was no increase in the direct (conjugated) fraction. Watchful waiting would have been appropriate, with cholecystectomy being performed if he experienced recurrent symptoms associated with fatty foods accompanied by an elevation in direct bilirubin.
More concerning and deserving of investigation was his elevated liver enzymes. Patients with sickle cell disease may experience recurrent ischemia and reperfusion injuries in the liver, which is called right upper quadrant syndrome. On autopsy of 70 sickle cell patients, 91% had hepatomegaly and 34% had focal necrosis.114 AST is often elevated in sickle cell disease, as it is affected by hemolysis. In this patient, both AST and ALT are elevated, consistent with a hepatocellular disorder. His abdominal pain and ALT rise may be a sign of a hepatic crisis.115 Rapid resolution of ALT elevation in a matter of days suggests a vaso-occlusive, inflammatory event that is self- limiting. Prolonged AST elevation requires further investigation, with consideration of autoimmune hepatitis, viral hepatitis, or iron overload. Iron overload is unlikely in this patient given his lifetime history of only 1 transfusion. Hepatic iron overload typically occurs in sickle cell disease after a minimum of 10 transfusions.115
CASE CONTINUED
The patient is discharged on the day after the procedure, with instructions to continue his hydroxyurea.
• Should the patient resume hydroxyurea therapy?
Hydroxyurea is hepatically cleared and thus it should be held until his liver function tests normalize.106
CASE CONTINUED
Two months later, the patient presents to the emergency department with abdominal pain that moves to his left leg. A CBC is obtained, showing a hemoglobin of 11.8 g/dL and a platelet count of 144,000/µL. He is given 2 doses of morphine 6 mg intravenously, and reports that his leg pain is now a 4/10. He is discharged home with a prescription for hydrocodone/acetaminophen.
• Is the emergency department evaluation sufficient?
This patient remains at high risk for splenic sequestration,93 with a hemoglobin 2 g lower than it was 2 months ago and platelets less than half. This decline could be consistent with early splenic sequestration.20 Additionally, he had elevated liver function tests on a recent admission, as well as rising creatinine, without evidence of resolution. It is not appropriate to discharge him without checking a chemistry and liver panel, and abdominal imaging should be considered. The best plan would be to admit him for observation, given his risk for splenic sequestration, and consult surgery for an elective splenectomy if he has a second episode of splenic sequestration 2 months after the first.100 His abdominal pain that migrates to his left leg could be due to his massive splenomegaly compressing his left kidney, as noted on imaging during his recent admission for splenic sequestration
CASE CONTINUED
An hour after discharge from the emergency department, EMS is called to his home for intractable pain. He is found lying on the floor, and reports excruciating left leg pain. He is brought to the closest hospital, a community hospital that he has not visited previously. There, he is admitted for hydration and pain control and placed on hydromorphone 2 mg every 4 hours as needed for pain. His hemoglobin is 10.8 g/dL, and platelets are 121,000/µL. A chemistry panel is remarkable for a creatinine level of 1.5 mg/dL and a potassium level of 3.2 mEq/L. Liver function tests are not obtained. After 3 doses of hydromorphone, he falls asleep. He is not in a monitored bed, and intravenous fluids, while ordered, are not started. At 6:30 AM the day after admission, he cannot be aroused on a routine vital sign check; he has an SpO2 of 60%, a blood pressure of 80/60 mm Hg, and heart rate of 148 beats/min. A rapid response is called, and naloxone is administered along with oxygen by face mask and several fluid boluses. His systolic blood pressure increases to 100 mm Hg from a low of 70 mm Hg. His SpO2 increases to 92%, and he is arousable and alert, although he reports 10/10 leg pain. His abdomen is noted to be distended and tender.
• What may have contributed to his clinical condition?
The patient is opioid tolerant and has received equivalent doses of opioids in the past without excess sedation. He may have liver dysfunction making him unable to metabolize opioids effectively. His hemoglobin and platelets continue to decline, raising concern for splenic sequestration versus sepsis. Failure to place him on a monitor allowed his hypoxia to continue for an unknown amount of time, placing him at high risk for developing ACS. Lack of intravenous hydration while he was too sedated to drink likely exacerbated his sickling.
CASE CONTINUED
At 9:20 AM, a CBC is obtained and reveals a hemoglobin of 4.8 g/dL and a platelet count of 44,000/µL. Two units of stat O negative blood are administered, and preparations are made to administer an exchange transfusion. A liver panel is obtained 3 hours later, which reveals an AST level of 1200 U/L and an ALT level of 1050 U/L. His bilirubin is 10 mg/dL, and his lactate dehydrogenase level is 1800 U/L. His urine is dark and is positive for bilirubin and ketones. He is transferred to the intensive care unit. A chest X-ray shows pulmonary congestion. Hematology/oncology is consulted.
He receives a 7-unit red blood cell exchange, which reduces his HbS to 11%. He continues to be hypotensive, and requires norepinephrine to support his blood pressure. Antibiotic therapy is started. His creatinine concentration rises to 2.3 mg/dL, potassium is 7.8 mEq/L, and bicarbonate is 12 mEq/L. He is placed on hemodialysis.
Computed tomography of the chest and abdomen reveals lower posterior lung infiltrates and a grossly enlarged spleen. He requires intubation. He is given a diagnosis of ACS in addition to kidney failure, liver failure, and “sickle crisis.” He continues to require daily to twice daily transfusions to maintain a hemoglobin of 7 to 9 g/dL, and his abdominal distension increases. As his condition worsens, surgery is consulted to discuss a liver transplant. He is deemed to not be a surgical candidate, and he passes away 6 days after entering the hospital. The immediate cause of death is listed as vaso-occlusive crisis, with ACS and sickle crisis listed as contributors.
• Are the causes of death accurate and complete?
If vaso-occlusive crisis is used to indicate a pain event, it is not an accurate cause of death. Pain is one of the most distressing complications of sickle cell disease, and frequent pain events are associated with early mortality,4,80 but they are not in themselves fatal. ACS is the number one cause of death in sickle cell disease,4 and it likely contributed to this patient’s death. Sickle crisis is a vague term that should not be used in this context. Causes of death should include splenic sequestration and multisystem organ failure. Multisystem organ failure in sickle cell disease often responds to aggressive transfusion therapy, which this patient received.116–118
CONCLUSION
Sickle cell disease is a complex chronic disease that impacts almost every organ system in the body. Clinicians may be inclined to attribute most pain in a patient with sickle cell disease to a simple vaso-occlusive crisis, treat them for this, and not investigate further. As the case presented here demonstrates, failure to identify the actual life-threatening process occurring in a patient with sickle cell disease presenting with pain can result in preventable early mortality. Clinicians must approach a sickle cell patient reporting pain in a thoughtful manner, and consider a complete differential diagnosis, including both sickle cell disease complications and those unrelated to sickle cell disease. Knowledge of the disease courses of the different sickle cell genotypes is essential, and must go beyond a superficial hierarchy of severity, but rather include an understanding of the complications each genotype is most prone to, and at what ages. Complete laboratory assessment, including a comprehensive metabolic panel, should be performed on all admitted patients, not just a complete blood count. Treating pain with high-dose opioids, while appropriate in an uncomplicated pain crisis, can lead to ACS or even respiratory failure in a patient with uninvestigated liver and kidney dysfunction. The most important lesson to remember is that even the sickle cell disease patient who has been given the unfortunate and pejorative label of “frequent flyer” by some providers has the potential for rapid deterioration into multisystem organ failure and death.
- Weatherall D, Hofman K, Rodgers G, Ruffin J, Hrynkow S. A case for developing North-South partnerships for research in sickle cell disease. Blood 2005;105:921–3.
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1998;11:1–51.
- Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg 1954;48:312–8.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–31.
- Serjeant GR, Serjeant BE. Sickle cell disease. 3rd ed. New York: Oxford University Press; 2001.
- Moo-Penn W, Bechtel K, Jue D, et al. The presence of hemoglobin S and C Harlem in an individual in the United States. Blood 1975;46:363–7.
- Monplaisir N, Merault G, Poyart C, et al. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A 1986;83:9363–7.
- Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev 2003;17:167–78.
- Nagel RL, Daar S, Romero JR, et al. HbS-oman heterozygote: a new dominant sickle syndrome. Blood 1998;92:4375–82.
- Masiello D, Heeney MM, Adewoye AH, et al. Hemoglobin SE disease: a concise review. Am J Hematol 2007;82:643–9.
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–9.
- Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985;65:183–9.
- .Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A 1979;76:670–2.
- .Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol 1989;32:104–11.
- Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol 2002;9:101–6.
- Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Med 2002;8:1383–9.
- Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013;98:464–72.
- Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med 2013;3:a011783.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48.
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol 1995;2:103–8.
- Odenheimer DJ, Sarnaik SA, Whitten CF, et al. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am J Med Genet 1987;27:525–35.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991;325:11–6.
- Falusi AG, Olatunji PO. Effects of alpha thalassaemia and haemoglobin F (HbF) level on the clinical severity of sickle-cell anaemia. Eur J Haematol 1994;52:13–5.
- Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci 1948;215:419–23.
- Marcus SJ, Ware RE. Physiologic decline in fetal hemoglobin parameters in infants with sickle cell disease: implications for pharmacological intervention. J Pediatr Hematol Oncol 1999;21:407–11.
- Schroeder WA, Powars DR, Kay LM, et al. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin 1989;13:325–53.
- Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. Am J Hematol 2003;72:121–6.
- Ngo D, Bae H, Steinberg MH, et al. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood Cells Mol Dis 2013;51:22–6.
- Galarneau G, Palmer CD, Sankaran VG, et al. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 2010;42:1049–51.
- Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A 2008;105:11869–74.
- Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839–42.
- Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A 2008;105:1620–5.
- Adams RJ, Kutlar A, McKie V, et al. Alpha thalassemia and stroke risk in sickle cell anemia. Am J Hematol 1994;45:279–82.
- Ballas SK. Effect of alpha-globin genotype on the pathophysiology of sickle cell disease. Pediatr Pathol Mol Med 2001;20:107–21.
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med 1986;314:1593–9.
- Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin 1989;13:649–55.
- .Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007;21:37–47.
- Murphy JR, Wengard M, Brereton W. Rheological studies of Hb SS blood: influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med 1976;87:475–86.
- Fabry ME, Kaul DK, Raventos-Suarez C, Chang H, Nagel RL. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest 1982;70:1315–9.
- Stuart J, Johnson CS. Rheology of the sickle cell disorders. Baillieres Clin Haematol 1987;1:747–75.
- Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136-41.
- Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr 2003;143:438–44.
- Abboud MR, Yim E, Musallam KM, Adams RJ, Investigators SIS. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood 2011;118:894–8.
- Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol 2000;15:344–9.
- Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood 2004;103:3689–94.
- Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108:847–52.
- Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 1992;79:2555–65.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22.
- Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol 1997;34:15–21.
- Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol 2010;85:403–8.
- Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 1988;318:96–9.
- Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–6.
- Maier-Redelsperger M, de Montalembert M, Flahault A, et al. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients. The French Study Group on Sickle Cell Disease. Blood 1998;91:4472–9.
- Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 2014;123:481–5.
- Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 1994;83:553–60.
- Bridges KR, Barabino GD, Brugnara C, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood 1996;88:4701–10.
- Jiang J, Jordan SJ, Barr DP, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol 1997;52:1081–6.
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
- Yates AM, Dedeken L, Smeltzer MP, et al. Hydroxyurea treatment of children with hemoglobin SC disease. Pediatr Blood Cancer 2013;60:323–5.
- Barbosa CG, Aleluia AC, Pacheco AP, et al. Genetic modulation of HbF in Brazilians with HbSC disease and sickle cell anemia. Am J Hematol 2013;88:923–4.
- Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009;361:2309–17.
- King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood 2014;123:3089–94.
- Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD007001.
- Freed J, Talano J, Small T, et al. Allogeneic cellular and autologous stem cell therapy for sickle cell disease: ‘whom, when and how’. Bone Marrow Transplant 2012;47:1489–98.
- Urbinati F, Hargrove PW, Geiger S, et al. Potentially therapeutic levels of anti-sickling globin gene expression following lentivirus-mediated gene transfer in sickle cell disease bone marrow CD34 cells. Exp Hematol 2015;43:346–51.
- Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes’s original 1934 classification to the third millennium. Curr Opin Hematol 2013;20:222–30.
- Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am Soc Hematol Educ Program 2010;2010:418–22.
- Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987;317:781–7.
- Goldsmith JC, Bonham VL, Joiner CH, et al. Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol 2012;87:340–6.
- Grant AM, Parker CS, Jordan LB, et al. Public health implications of sickle cell trait: a report of the CDC meeting. Am J Prev Med 2011;41:S435–9.
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol 2005;79:17–25.
- Serjeant GR, Ceulaer CD, Lethbridge R, et al. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol 1994;87:586–91.
- Vichinsky EP, Johnson R, Lubin BH. Multidisciplinary approach to pain management in sickle cell disease. Am J Pediatr Hematol Oncol 1982;4:328–33.
- Gil KM, Carson JW, Porter LS, et al. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol 2004;23:267–74.
- Amjad H, Bannerman RM, Judisch JM. Letter: Sickling pain and season. Br Med J 1974;2:54.
- Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Trans R Soc Trop Med Hyg 1980;74:159–61.
- Jones S, Duncan ER, Thomas N, et al. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol 2005;131:530–3.
- Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr 1991;118:407–9.
- Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol 2014;93:89–95.
- Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr 2011;160:286–90.
- Hollins M, Stonerock GL, Kisaalita NR, et al. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage 2012;43:1082–93.
- Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol 2013;88:927–9.
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer 2015 May 13. doi: 10.1002/pbc.25574. [Epub ahead of print].
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61:512–7.
- Brandow AM, Farley RA, Dasgupta M, et al. The use of neuropathic pain drugs in children with sickle cell disease is associated with older age, female sex, and longer length of hospital stay. J Pediatr Hematol Oncol 2015;37:10–5.
- Walker TM, Hambleton IR, Serjeant GR. Gallstones in sickle cell disease: observations from The Jamaican Cohort study. J Pediatr 2000;136:80–5.
- Penner E, Mayr WR, Djawan S, et al. [The genetics of Gilbert syndrome]. Schweiz Med Wochenschr 1976;106:860–2. [German]
- Powell RW, Levine GL, Yang YM, Mankad VN. Acute splenic sequestration crisis in sickle cell disease: early detection and treatment. J Pediatr Surg 1992;27:215–8.
- Al Salem AH, Qaisaruddin S, Nasserullah Z, et al. Splenectomy and acute splenic sequestration crises in sickle cell disease. Pediatr Surg Int 1996;11:26–8.
- Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med 1969;281:923–6.
- Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377:1663–72.
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 2014;166:165–76.
- Orringer EP, Fowler VG Jr, Owens CM, et al. Case report: splenic infarction and acute splenic sequestration in adults with hemoglobin SC disease. Am J Med Sci 1991;302:374–9.
- Michel JB, Hernandez JA, Buchanan GR. A fatal case of acute splenic sequestration in a 53–year-old woman with sickle-hemoglobin C disease. Am J Med 1992;92:97–100.
- Hatton CS, Bunch C, Weatherall DJ. Hepatic sequestration in sickle cell anaemia. Br Med J (Clin Res Ed) 1985;290:744–5.
- Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994;84:643–9.
- Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood 1995;86:776–83.
- Owusu-Ofori S, Hirst C. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD003425.
- 00.Al-Salem AH. Splenic complications of sickle cell anemia and the role of splenectomy. ISRN Hematol 2011;2011:864257.
- Sabarense AP, Lima GO, Silva LM, Viana MB. Characterization of mortality in children with sickle cell disease diagnosed through the Newborn Screening Program. J Pediatr (Rio J) 2015;91:242–7.
- Aslam AF, Aslam AK, Dipillo F. Fatal splenic sequestration crisis with multiorgan failure in an adult woman with sickle cell-beta+ thalassemia. Am J Med Sci 2005;329:141–3.
- Berry RA, Odumakinde EA, Lewis JP. Massive splenic infarction in doubly abnormal heterozygous sickling disorders. A new complication of acute splenic sequestration syndrome. The West J Med 1991;155:531–2.
- Bonanomi MT, Lavezzo MM. Sickle cell retinopathy: diagnosis and treatment. Arq Bras de Oftalmol 2013;76:320–7.
- Chen RW, Flynn HW Jr, Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. Am J Ophthalmol 2014;157:870–5 e1.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930–8.
- Neumayr L, Koshy M, Haberkern C, et al. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. Am J Hematol 1998;57:101–8.
- 08. Savage WJ, Buchanan GR, Yawn BP, et al. Evidence gaps in the management of sickle cell disease: A summary of needed research. Am J Hematol 2015;90:273–5.
- Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300–26.
- Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol 1997;98:838–44.
- Wang W, Brugnara C, Snyder C, et al. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. Br J Haematol 2011;152:771–6.
- Jungst C, Kullak-Ublick GA, Jungst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol 2006;20:1053–62.
- Walker TM, Serjeant GR. Biliary sludge in sickle cell disease. J Pediatr 1996;129:443–5.
- Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7.
- Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol 2010;8:483–9.
- Hiran S. Multiorgan dysfunction syndrome in sickle cell disease. J Assoc Physicians India 2005;53:19–22.
- Shao SH, Orringer EP. Case report: splenic sequestration and multiorgan failure as the presenting manifestation of hemoglobin SC disease. Am J Med Sci 1996;311:139–41.
- Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 1994;96:155–62.
INTRODUCTION
Sickle cell disease is the most common inherited blood disorder in the world. It affects more than 100,000 individuals in the United States, and millions more worldwide.1 Sickle cell disease is most commonly found in individuals of African heritage, but the disease also occurs in Hispanics and people of Middle Eastern and subcontinent Indian heritage.2 The distribution of the sickle hemoglobin (hemoglobin S [HbS]) allele overlaps with the distribution of malaria; HbS carriers, or individuals with sickle cell trait, have protection against malaria,3 and are not considered to have sickle cell disease.
Sickle cell disease is a severe monogenic disorder marked by significant morbidity and mortality, affecting every organ in the body.4 The term sickle cell disease refers to all genotypes that cause sickling; the most common are the homozygous hemoglobin SS (HbSS) and compound heterozygotes hemoglobin SC (HbSC), hemoglobin S–β0-thalassemia (HbSβ0), and hemoglobin S–β+-thalassemia (HbSβ+), although HbS and several rarer hemoglobin variants such as HbSO(Arab) and HbSD(Punjab) can also cause sickle cell disease. The term sickle cell anemia refers exclusively to the most severe genotypes, HbSS and HbSβ0.5 Common sickling genotypes along with their relative clinical severity are shown in Table 1.6–11
| Table 1. Genotypes of Sickling Syndromes and Their Relative Severities | ||
Genotype | Severity | Characteristics |
HbSS | Severe | Most common form |
HbSβ0 | Severe | Clinically indistinguishable from HbSS6 |
HbSO-Arab | Severe | Relatively rare6 |
HbSD-Punjab | Severe | Mostly in northern India6 |
HbSC-Harlem | Severe | Migrates like HbSC, but rare double β-globin mutation7 |
HbCS-Antilles | Severe | Rare double β-globin mutation8 |
HbSC | Moderate | 25% of SCD9 |
HbSβ+, Mediterranean | Moderate | 5%–16% HbA6 |
HbAS-Oman | Moderate | Dominant rare double β-globin mutation10 |
HbSβ+, African | Mild | 16%–30% HbA6 |
HbSE | Mild | HbE found mostly in Southeast Asia11 |
HbS-HPFH | Very mild | Large deletions in β-globin gene complex; > 30% HbF6 |
| HbA = hemoglobin A; HbE = hemoglobin E; HbF = fetal hemoglobin; HbS-HPFH = HbS and gene deletion HPFH; HbSC = heterozygous hemoglobin SC; HbSS = homozygous hemoglobin SS; HbSβ0 = hemoglobin S-β thalassemia0; HbSβ+ = hemoglobin S-β thalassemia+; SCD = sickle cell disease. | ||
This article reviews the pathophysiology of sickle cell disease, common clinical complications, and available therapies. A complex case which illustrates diagnostic and management challenges is presented as well.
PATHOPHYSIOLOGY
HbS is the result of a substitution of valine for glutamic acid in the sixth amino acid of the β-globin chain.12 The change from a hydrophilic to a hydrophobic amino acid causes the hemoglobin molecules to stack, or polymerize, when deoxygenated. This rigid rod of hemoglobin distorts the cell, producing the characteristic crescent or sickle shape that gives the disease its name.13 Polymerization of hemoglobin within the cell is promoted by dehydration, which increases the concentration of HbS.13,14 Polymerization occurs when hemoglobin is in the deoxygenated state.13
The sickle red blood cell is abnormal; it is rigid and dense, and lacks the deformability needed to navigate the microvasculature.15 Blockages of blood flow result in painful vaso-occlusion that is the hallmark of the disease, and that also can cause damage to the spleen, kidneys, and liver.16 The sickle red cell is also fragile, with a lifespan of only 20 days compared to the 120-day lifespan of a normal red blood cell.13 Frequent hemolysis results in anemia and the release of free hemoglobin, which both scavenges nitric oxide and impairs the production of more nitric oxide, which is essential for vasodilatation.17 This contributes to vascular dysfunction and an increased risk for stroke.18 If untreated, the natural course of sickle cell anemia is mortality in early childhood in most cases.19 Common chronic and acute sickle cell disease–related complications and recommended therapies, based on 2014 National Institutes of Health guidelines, are shown in Table 2 and Table 3.20
| Table 2. Common Adult Sickle Cell Disease Chronic Complications and Recommended Therapies | ||
Chronic Complication | Recommended Therapy | Strength of Recommendation |
Chronic pain | Opioids | Consensus |
Avascular necrosis | Analgesics and physical therapy | Consensus |
Proliferative sickle retinopathy | Laser photocoagulation | Strong |
Leg ulcers | Standard wound care | Moderate |
Recurrent priapism | Consult urology | Moderate |
| Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
| Table 3. Common Adult Sickle Cell Disease Acute Complications and Recommended Therapies | ||
Acute Complication | Recommended Therapy | Strength of Recommendation |
Vaso-occlusive crisis | NSAIDs, opioids for severe pain | Moderate-consensus |
ACS | Antibiotics, oxygen | Strong |
Simple transfusiona | Weak | |
Urgent exchange transfusionb | Strong | |
Acute stroke | Exchange transfusion | Strong |
Priapism ≥ 4 hr | Aggressive hydration, pain control, and urology consult | Strong-consensus |
Gallstones, symptomatic | Cholecystectomy, laparoscopic | Strong |
Splenic sequestration | Intravenous fluids, transfuse cautiously, discuss surgical splenectomy | Strong-moderate |
Acute renal failure | Consult nephrologyc | Consensus |
ACS = acute chest syndrome; NSAIDs = nonsteroidal anti-inflammatory drugs. a For symptomatic ACS with hemoglobin > 1 g/dL below baseline but > 9.0 g/dL. b When there is progression of ACS (SpO2 < 90% despite supplemental oxygen, increasing respiratory distress, progressive pulmonary infiltrates despite simple transfusion). c For acute rise in creatinine ≥ 0.3 mg/dL; do not give transfusions unless there are other indications. Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
One of the most challenging aspects of sickle cell disease is its clinical variability. While in general, HbSS and HbSβ0 are the most severe genotypes, there are patients with HbSC and HbSb+ who have significant sickle-cell–related complications, and may have a more severe clinical course than a HbSS patient.21 A great deal of this clinical variability cannot be explained, but some can be attributed to endogenous fetal hemoglobin (HbF) levels.22–24 The importance of HbF levels in sickle cell disease was first noted by a pediatrician in the 1940s.25 She observed that sickle cell disease complications in children under the age of 1 were rare, and attributed it to the presence of HbF.25 HbF levels decline more slowly in individuals with hemoglobinopathies, reaching their nadir after the age of 5 rather than within 6 months of birth in individuals without hemoglobinopathies.26 HbF levels remain elevated lifelong in most sickle cell disease patients, especially those with the HbSS and HbSβ0 genotypes. Levels of HbF vary widely between individuals, from zero to 20% to 30%, with a median of 10%.26–28 Individuals who produce more HbF have a milder course, in general.24 An association between the 4 β-globin haplotypes and HbF levels has been reported in the past,27,29 but more sophisticated next-generation sequencing has revealed causal variants in BCL11A and HBS1L-MYB that contribute approximately 50% of the observed variability in HbF levels.30–33
Co-inheritance of α-thalassemia also modifies disease course; less available α-globin chains results in a lower hemoglobin concentration within the cell. Paradoxically, this results in a higher overall hemoglobin level, as there is a reduction in polymerization, and therefore sickling due to lower HbS concentrations in the cell. Patients therefore are less anemic, reducing the risk of stroke in childhood,34,35 but blood viscosity may be higher, resulting in more frequent pain crises and increased risk36 of avascular necrosis.34,35,37 It is often helpful to think of sickle cell patients as falling into 1 of 2 groups: high hemolysis/low hemoglobin and high viscosity/high hemoglobin. Individuals with high rates of hemolysis are at greater risk for stroke, pulmonary hypertension, and acute chest syndrome (ACS). Higher rates of hemolysis result in higher levels of free hemoglobin, which scavenges nitric oxide. This leads to the vascular damage and dysfunction that contributes to the associated clinical complications. This phenotype is most commonly seen in HbSS and HbSβ0.38 High hemoglobin/high viscosity phenotypes are most often found in HbSC patients and in sickle cell anemia with α-thalassemia coinheritance.39–42
TREATMENT OPTIONS
In high-resource countries with newborn screening, the initiation of penicillin prophylaxis has dramatically altered the natural history of the disease, allowing the majority of patients to reach adulthood.43 Penicillin prophylaxis is usually discontinued at age 5 years; however, individuals who have undergone surgical splenectomy or have had pneumococcal sepsis on penicillin prophylaxis may remain on penicillin to age 18 or beyond.20
Another advance in sickle cell care is screening for stroke risk through transcranial Doppler ultrasound (TCD).44–47 This screening tool has reduced the incidence of childhood stroke from 10% by age 11 to 1%. TCDs typically cannot be performed after the age of 16 due to changes in the skull. Individuals found to have abnormal (elevated) TCD velocities are placed on chronic transfusion therapy for primary stroke prevention. They may remain on monthly chronic transfusions, with the goal of suppressing the percentage of HbS to 30% to 50% indefinitely. A clinical trial (STOPII) designed to determine if pediatric sickle cell disease patients on chronic transfusion therapy for primary stroke prevention could be safely taken off transfusion therapy was discontinued early due to an excess of strokes and conversion to abnormal TCD velocities in the untransfused arm.44 Individuals who have experienced an ischemic stroke have a 70% risk of another stroke, and must remain on chronic transfusion therapy indefinitely. Chronic transfusion reduces their stroke risk to 13%.
The only widely used pharmacologic therapy for sickle cell disease is hydroxyurea.12,48–50 A significant portion of the benefit of hydroxyurea stems from its induction of HbF.51 HbF does not sickle, and it interrupts the polymerization of HbS in the cell, if present in high enough concentrations.50 The level of HbF needed to achieve clinical improvement is not known, but in vitro assays suggest 20% HbF is needed to prevent sickling.52,53 However, endogenous and hydroxyurea-induced HbF is not distributed evenly through the red cells, so sickling is possible regardless of the level of HbF induced.54,55 Hydroxyurea likely has other disease-modifying effects as well, including reduction of white blood cell count and reticulocyte count and reduction of red cell adhesion to the endothelium.56–58 Clinical criteria for initiation of hydroxyurea in adult sickle cell disease patients are shown in Table 4.20 Hydroxyurea is given daily and is dosed to maximum tolerated dose for the individual by following the absolute neutrophil count (ANC). The goal ANC is between 2000 and 4000/µL. At times, absolute reticulocyte count (ARC) can be dose-limiting; goal ARC is greater than 70,000/µL.59 Platelet counts may be reduced as well, especially in HbSC patients.60,61
| Table 4. Indications for Hydroxyurea in Adult Patients with Sickle Cell Disease | |
Indication | Strength of Recommendation |
SCA with ≥ 3 pain crises per year | Strong |
SCA with pain that interferes with ADL and QoL | Strong |
History of severe or recurrent ACS | Strong |
Chronic kidney disease on epoetin | Weak |
HbSβ+ and HbSC with pain that interferes with ADL and QoL; consult sickle cell disease expert | Moderate |
| ACS = acute chest syndrome; ADL = activities of daily living; QoL = quality of life; SCA = sickle cell anemia. | |
The only curative therapy for sickle cell disease is hematopoietic stem cell transplant.62 Transplant use is limited by availability of matched sibling donors,62 and even at experienced centers transplant carries a small risk for mortality, graft rejection, and graft-versus-host disease. Furthermore, consensus on disease complications for which transplant is recommended is also lacking.63–65 Clinical trials of gene therapy for sickle cell disease and thalassemia are ongoing.66
COMPLICATIONS AND DISEASE-SPECIFIC THERAPIES
CASE PRESENTATION
A 26-year-old African-American man who works as a school bus driver presents to an academic center’s emergency department complaining of pain in his left leg, similar to prior pain events. He is described as having sickle cell trait, although no hemoglobin profile is available in his chart. He describes the pain as dull and aching, 10/10 in intensity. A complete blood count (CBC) is obtained; it reveals a hemoglobin of 14.5 g/dL, white blood cell (WBC) count of 5600/µL, and platelet count of 344,000/µL. His CBC is also notable for a mean corpuscular volume (MCV) of 72 fL, a mean corpuscular hemoglobin concentration (MCHC) of 37 g/dL, and a red blood cell distribution width (RDW) of 12. Slide review of a peripheral blood smear shows 2+ target cells (Figure).

The patient is given 6 mg of morphine, which provides some relief of his pain, and is discharged with a prescription for hydrocodone bitartrate/acetaminophen 5/325 mg. The diagnosis given is musculoskeletal pain, and he is instructed to follow-up with a primary care physician. His past medical history is significant for 4 or 5 visits to the emergency department per year in the past 4 years. Prior to 4 years ago, he rarely required medical attention.
• What laboratory and clinical features might lead you to question the diagnosis of sickle cell trait in this patient?
The patient’s hemoglobin is within normal range, which is consistent with sickle cell trait; however, he is microcytic, with a normal RDW. It is possible to be mildly microcytic in the early stages of iron deficiency, prior to the development of anemia, but the RDW would typically be elevated, demonstrating the presence of newer, smaller cells produced under conditions of iron deficiency.67 It is also possible that his microcytosis with a normal RDW could represent sickle cell trait with co-inheritance of β-thalassemia. Up to 30% of African Americans have β-thalassemia,2 and 1 in 10 have sickle cell trait.68 However, a high MCHC, indicating the presence of dense cells, and target cells noted on slide review are most consistent with HbSC.9 HbSC patients, especially males, can have hemoglobin levels in the normal range.4 The biggest inconsistency with the diagnosis of sickle cell trait is his history of frequent pain events. Individuals with sickle cell trait rarely present with pain crises, except under extreme conditions of dehydration or high altitude.68 Sickle cell trait is generally regarded as a benign condition, although a study of U.S. military recruits found a 30-fold higher risk of sudden death during basic training in persons with sickle cell trait.69 Additional sickle cell trait–related complications include hematuria, risk of splenic sequestration or infarct under extreme conditions and high altitude, and a rare and usually fatal renal malignancy, renal medullary carcinoma, which is vanishingly rare in individuals without sickle cell trait.70,71 Although the patient reported having sickle cell trait, this diagnosis should have been verified with a hemoglobin panel, given his atypical presentation.20
• What is the approach to managing pain episodes in sickle cell disease?
In sickle cell disease, vaso-occlusive pain events can be common, often beginning in early childhood.17 This disease complication accounts for 95% of all adult sickle cell disease hospitalizations.72 There is a great deal of variability in pain symptoms between individuals, and within individuals at various times in their lives:73 30% have no pain events, 50% have occasional events, and 20% have monthly or more frequent events that require hospitalization.74 The frequency and severity of pain events are modulated by HbF levels, β-thalassemia status, genotypes, therapies like hydroxyurea, or in rare cases, chronic transfusion therapy.23 Personal factors, such as psychosocial stressors, also contribute to the frequency of pain events.75 Pain event triggers include exposure to cold water, windy or cold weather, temperature changes, and extreme temperatures.76–79 Patient age also contributes to pain event frequency. Many patients see an increase in pain event frequency in their late 20s, and a marked decrease in their 40s.23,73 More than 3 pain events per year is associated with reduced life expectancy.23
Acute management of pain episodes involves nonsteroidal anti-inflammatory drugs, oral opioids, and when hospitalization is required, intravenous opioids, often delivered via patient-controlled analgesia (PCA) pumps.79 As sickle cell disease patients become teenagers and young adults, some experience an increased frequency of pain episodes, with fewer pain-free days, or a failure to return to baseline before the next pain crisis occurs.80,81 This is characteristic of emerging chronic pain.82 Chronic pain is a significant problem in adult patients with sickle cell disease, with up to 85% reporting pain on most days.72,80 The development of chronic pain may be reduced by early and aggressive treatment of acute pain events, as well as use of hydroxyurea to reduce the number of pain events. Many adult sickle cell patients with chronic pain are treated with daily opioids.20 Given the significant side effects of chronic opioid use—sedation, respiratory depression, itching, nausea, and impairment of function and quality of life—non-opioid therapies are under investigation.83 Many chronic pain patients have symptoms of neuropathic pain, and may benefit from neuropathic agents like gabapentin, both to reduce opioid use and to more effectively treat chronic neuropathic pain, which is known to respond poorly to opioids.84–86
• Is the patient’s peripheral blood smear consistent with a diagnosis of sickle cell trait?
Several target cells are visible, which is not typical of sickle cell trait, but may be seen in HbSC or thalassemia. The finding of an intracellular crystal is pathognomonic for HbSC or HbCC. HbC polymerizes in high oxygen conditions, opposite of HbS, which polymerizes in low oxygen conditions.9
CASE CONTINUED
The patient’s family history is significant for a sister who died at age 3 from sickle cell–related complications, and a sister with sickle cell trait who had a cholecystectomy for gallstones at age 22. His father died at age 38 due to unknown causes. The sickle cell trait status of his parents is unknown. His mother is alive, and has hypertension.
• Is the medical history of this patient’s family members consistent with sickle cell trait?
It is unlikely that sickle cell trait would result in early death in childhood, or in gallstones at age 22. Gallstones in early adulthood is a common presentation for HbSC patients not diagnosed by newborn screening.87 Any hemolytic condition can lead to the formation of hemoglobin-containing pigmented gallstones, biliary sludge, and obstruction of the gallbladder. In the presence of right-sided abdominal pain, a serum bilirubin level of more than 4 mg/dL should lead to measurement of direct bilirubin; if greater than 10% of total, imaging of the gallbladder should be obtained. In sickle cell disease, 30% of patients will have gallstones by 18 years of age. The low hemolysis/high viscosity phenotype patients are typically older at diagnosis. Co-inheritance of Gilbert syndrome and sickle cell disease is not uncommon, and can result in formation of gallstones at a young age; Gilbert syndrome alone typically results in gallstones in mid-life.88
CASE CONTINUED
Two months later, the patient presents again to the emergency department with the same complaint of leg pain, as well as abdominal pain. His hemoglobin is 12.5 g/dL, and his platelet count is 134,000/µL. His pain is not improved with 3 doses of morphine 6 mg intravenously, and he is admitted to the medicine service. A hemoglobin profile is obtained, revealing 52% HbS, 45% HbC, and 1.5% HbF, consistent with HbSC. In sickle cell trait, the hemoglobin profile is 60% HbA and 40% HbS (available α-globin prefers to pair with a normal β-globin, so the ratio of HbA to HbS is 60:40, not 50:50).
On the second hospital day, the patient’s hemoglobin drops to 7.2 g/dL and his platelet count decreases to 44,000/µL. His abdomen is distended and diffusely tender. The internist transfuses him with 2 units of packed red blood cells (PRBC), after which his hemoglobin increases to 11 g/dL, while his platelet count increases to 112,000/µL. Following the transfusion, his abdominal pain resolves, as does his anemia and thrombocytopenia.
• What caused this patient’s anemia and thrombocytopenia?
High on the differential diagnosis is a splenic sequestration. Acute splenic sequestration occurs when red cells are trapped in the splenic sinuses. Massive splenic enlargement may occur over several hours.89,90 Unrecognized splenic sequestration has a high mortality rate from severe anemia and splenic rupture.90 Splenic sequestration must be ruled out in a sickle cell patient with abdominal pain accompanied by dropping platelet and red cell counts, especially in milder subtypes that often have splenic function preserved into adolescence and adulthood. Sickle cell anemia patients usually become functionally asplenic in early childhood.89,91,92 The rise in hemoglobin, more than would be expected from 2 units of PRBC, plus the improvement in platelet count without a platelet transfusion observed in the case patient strongly supports the diagnosis of splenic sequestration.
Splenic sequestration can occur in any sickle cell patient whose spleen has not fibrosed. Splenic sequestration in adulthood is not uncommon in HbSC patients, who often have preserved splenic function into adulthood.93–95
Clinical signs of splenic sequestration include a rapid drop in hemoglobin, rise in reticulocyte count, a tender, enlarged spleen, and, in severe cases, hypovolemia.89,93 It is treated with prompt blood transfusion, but care must be taken not to overtransfuse the patient, as the spleen can trap several grams of hemoglobin, which may be released upon transfusion, potentially causing life-threatening hyperviscosity.89 Hemoglobin levels must be checked following transfusion in suspected splenic sequestration, and “mini transfusions” of 5 mL/kg are recommended in sickle cell disease patients who are hemodynamically stable.20
Hepatic sequestration may also occur, but it is much less common than splenic sequestration.96 Other conditions on the differential diagnosis include thrombotic thrombocytopenic purpura, which would be unlikely to respond to a transfusion. ACS can cause a drop in hemoglobin, and is treated with simple or exchange transfusions.97 ACS is less likely without respiratory symptoms or oxygen requirement, and usually is not associated with thrombocytopenia. Sepsis may also cause anemia and thrombocytopenia, but again would not likely respond to a simple transfusion. The patient’s response to transfusion is consistent with a sequestering event, not a destructive event as in the case of sepsis.
CASE CONTINUED
Imaging reveals a grossly enlarged spleen, which is having a mass effect on the left kidney. The patient is started on hydroxyurea therapy at 500 mg 3 times daily. Discharge instructions include following up with his primary care physician, continuing hydroxyurea therapy, and receiving yearly dilated eye exams to evaluate for proliferative sickle retinopathy.
• Are these discharge instructions complete?
Splenic sequestration has a 50% recurrence rate.98 In very young children, watchful waiting or chronic transfusion may be implemented to preserve the immunologic function of the spleen and reduce the risk of sepsis.89 Splenectomy after a single episode of sequestration in adults is a matter of debate, with experts advising both watchful waiting99 and splenectomy after recovery from the first sequestering event.100 The patient should have been informed of the risk for recurrence, and the signs and symptoms of splenic sequestration as well as the need for emergency medical attention should have been discussed. Splenic sequestration may be milder in adults than in children, but fatal sequestrations have been reported.95,101–103
Proliferative sickle cell retinopathy is a high viscosity/high hemoglobin complication that may occur more frequently in HbSC than HbSS, with an incidence of 33% in HbSC.42,104 Spontaneous regression of retinopathy occurs in approximately 32% of eyes, and laser or scatter photocoagulation is an effective intervention.105
• Would the patient need to be transfused prior to splenectomy?
Preoperative transfusion therapy is standard of care for HbSS patients undergoing general anesthesia. The TRAP study found that simple “top off” transfusion to a hemoglobin of 10 g/dL was as effective at preventing postoperative sickle cell–related complications as exchange transfusion to HbS of 30% or less, and had fewer transfusion-related complications like alloimmunization.106 There is little data regarding preoperative transfusions in HbSC disease. A retrospective study suggests that HbSC patients undergoing abdominal surgeries should be transfused.107 The higher hemoglobin level of the typical HbSC patient necessitates exchange transfusion to avoid hyperviscosity.
• Is hydroxyurea therapy indicated in this patient?
• Has it been dosed appropriately?
If the patient had the HbSS subtype, hydroxyurea would be clearly indicated, given his frequent pain events.20 HbSC patients may be placed on hydroxyurea on a case-by-case basis, but evidence for its efficacy in this sickle cell subtype is lacking.108 Large clinical trials like the Multi-Center Study of Hydroxyurea (MSH) that established the safety and efficacy of hydroxyurea in sickle cell anemia excluded HbSC and HbSβ+ patients.109 These mild to moderate subtypes produce less HbF at baseline, and typically have a minimal to modest rise in HbF on hydroxyurea.110 In sickle cell anemia, hydroxyurea is titrated to maximum tolerated dose, defined as an ANC of 2000 to 4000/µL and an ARC of 70,000/µL or higher.53 Because of their lower levels of chronic inflammation and lower reticulocyte counts due to higher hemoglobin levels, many HbSC and HbSβ+ patients have values in that range before initiating hydroxyurea therapy.9 Cytopenias, particularly of platelets in HbSC, occur at low doses of hydroxyurea.111
Of note, although the half-life of hydroxyurea would suggest that 3 times daily dosing is indicated, daily dosing has been found to have equal response and is preferred. Another concern is the monitoring of this myelosuppressive medication. This patient has repeatedly failed to obtain a primary care physician or a hematologist, and hydroxyurea requires laboratory monitoring at least every 2 months, especially in a HbSC patient with a very large spleen who is at significant risk for thrombocytopenia and neutropenia.9
CASE CONTINUED
A week after discharge from his admission for abdominal pain diagnosed as splenic sequestration, the patient presents again to the emergency department with abdominal pain which he reports is his typical sickle cell pain. Hemoglobin is 13.8 g/dL, platelet count is 388,000/µL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are both 10 times their prior value. Creatinine is 1.2 mg/dL (0.75 mg/dL on his prior admission), and total bilirubin is 3 mg/dL, with 0.3 mg/dL direct bilirubin. He undergoes an ultrasound exam of his gallbladder, which reveals sludge and a possible gallstone. There is no evidence of cholecystitis. General surgery performs a laparoscopic cholecystectomy.
• Was this cholecystectomy necessary?
In patients with sickle cell disease, symptomatic gallstones and gallbladder sludge should be observed; recurrent abdominal pain without a significant change in bilirubin may not be due to gallstones or sludge, and therefore may not be relieved by cholecystectomy.112,113 In sickle cell disease, 40% of patients with gallbladder sludge do not develop gallstones.87 The patient’s bilirubin level was at baseline, and there was no increase in the direct (conjugated) fraction. Watchful waiting would have been appropriate, with cholecystectomy being performed if he experienced recurrent symptoms associated with fatty foods accompanied by an elevation in direct bilirubin.
More concerning and deserving of investigation was his elevated liver enzymes. Patients with sickle cell disease may experience recurrent ischemia and reperfusion injuries in the liver, which is called right upper quadrant syndrome. On autopsy of 70 sickle cell patients, 91% had hepatomegaly and 34% had focal necrosis.114 AST is often elevated in sickle cell disease, as it is affected by hemolysis. In this patient, both AST and ALT are elevated, consistent with a hepatocellular disorder. His abdominal pain and ALT rise may be a sign of a hepatic crisis.115 Rapid resolution of ALT elevation in a matter of days suggests a vaso-occlusive, inflammatory event that is self- limiting. Prolonged AST elevation requires further investigation, with consideration of autoimmune hepatitis, viral hepatitis, or iron overload. Iron overload is unlikely in this patient given his lifetime history of only 1 transfusion. Hepatic iron overload typically occurs in sickle cell disease after a minimum of 10 transfusions.115
CASE CONTINUED
The patient is discharged on the day after the procedure, with instructions to continue his hydroxyurea.
• Should the patient resume hydroxyurea therapy?
Hydroxyurea is hepatically cleared and thus it should be held until his liver function tests normalize.106
CASE CONTINUED
Two months later, the patient presents to the emergency department with abdominal pain that moves to his left leg. A CBC is obtained, showing a hemoglobin of 11.8 g/dL and a platelet count of 144,000/µL. He is given 2 doses of morphine 6 mg intravenously, and reports that his leg pain is now a 4/10. He is discharged home with a prescription for hydrocodone/acetaminophen.
• Is the emergency department evaluation sufficient?
This patient remains at high risk for splenic sequestration,93 with a hemoglobin 2 g lower than it was 2 months ago and platelets less than half. This decline could be consistent with early splenic sequestration.20 Additionally, he had elevated liver function tests on a recent admission, as well as rising creatinine, without evidence of resolution. It is not appropriate to discharge him without checking a chemistry and liver panel, and abdominal imaging should be considered. The best plan would be to admit him for observation, given his risk for splenic sequestration, and consult surgery for an elective splenectomy if he has a second episode of splenic sequestration 2 months after the first.100 His abdominal pain that migrates to his left leg could be due to his massive splenomegaly compressing his left kidney, as noted on imaging during his recent admission for splenic sequestration
CASE CONTINUED
An hour after discharge from the emergency department, EMS is called to his home for intractable pain. He is found lying on the floor, and reports excruciating left leg pain. He is brought to the closest hospital, a community hospital that he has not visited previously. There, he is admitted for hydration and pain control and placed on hydromorphone 2 mg every 4 hours as needed for pain. His hemoglobin is 10.8 g/dL, and platelets are 121,000/µL. A chemistry panel is remarkable for a creatinine level of 1.5 mg/dL and a potassium level of 3.2 mEq/L. Liver function tests are not obtained. After 3 doses of hydromorphone, he falls asleep. He is not in a monitored bed, and intravenous fluids, while ordered, are not started. At 6:30 AM the day after admission, he cannot be aroused on a routine vital sign check; he has an SpO2 of 60%, a blood pressure of 80/60 mm Hg, and heart rate of 148 beats/min. A rapid response is called, and naloxone is administered along with oxygen by face mask and several fluid boluses. His systolic blood pressure increases to 100 mm Hg from a low of 70 mm Hg. His SpO2 increases to 92%, and he is arousable and alert, although he reports 10/10 leg pain. His abdomen is noted to be distended and tender.
• What may have contributed to his clinical condition?
The patient is opioid tolerant and has received equivalent doses of opioids in the past without excess sedation. He may have liver dysfunction making him unable to metabolize opioids effectively. His hemoglobin and platelets continue to decline, raising concern for splenic sequestration versus sepsis. Failure to place him on a monitor allowed his hypoxia to continue for an unknown amount of time, placing him at high risk for developing ACS. Lack of intravenous hydration while he was too sedated to drink likely exacerbated his sickling.
CASE CONTINUED
At 9:20 AM, a CBC is obtained and reveals a hemoglobin of 4.8 g/dL and a platelet count of 44,000/µL. Two units of stat O negative blood are administered, and preparations are made to administer an exchange transfusion. A liver panel is obtained 3 hours later, which reveals an AST level of 1200 U/L and an ALT level of 1050 U/L. His bilirubin is 10 mg/dL, and his lactate dehydrogenase level is 1800 U/L. His urine is dark and is positive for bilirubin and ketones. He is transferred to the intensive care unit. A chest X-ray shows pulmonary congestion. Hematology/oncology is consulted.
He receives a 7-unit red blood cell exchange, which reduces his HbS to 11%. He continues to be hypotensive, and requires norepinephrine to support his blood pressure. Antibiotic therapy is started. His creatinine concentration rises to 2.3 mg/dL, potassium is 7.8 mEq/L, and bicarbonate is 12 mEq/L. He is placed on hemodialysis.
Computed tomography of the chest and abdomen reveals lower posterior lung infiltrates and a grossly enlarged spleen. He requires intubation. He is given a diagnosis of ACS in addition to kidney failure, liver failure, and “sickle crisis.” He continues to require daily to twice daily transfusions to maintain a hemoglobin of 7 to 9 g/dL, and his abdominal distension increases. As his condition worsens, surgery is consulted to discuss a liver transplant. He is deemed to not be a surgical candidate, and he passes away 6 days after entering the hospital. The immediate cause of death is listed as vaso-occlusive crisis, with ACS and sickle crisis listed as contributors.
• Are the causes of death accurate and complete?
If vaso-occlusive crisis is used to indicate a pain event, it is not an accurate cause of death. Pain is one of the most distressing complications of sickle cell disease, and frequent pain events are associated with early mortality,4,80 but they are not in themselves fatal. ACS is the number one cause of death in sickle cell disease,4 and it likely contributed to this patient’s death. Sickle crisis is a vague term that should not be used in this context. Causes of death should include splenic sequestration and multisystem organ failure. Multisystem organ failure in sickle cell disease often responds to aggressive transfusion therapy, which this patient received.116–118
CONCLUSION
Sickle cell disease is a complex chronic disease that impacts almost every organ system in the body. Clinicians may be inclined to attribute most pain in a patient with sickle cell disease to a simple vaso-occlusive crisis, treat them for this, and not investigate further. As the case presented here demonstrates, failure to identify the actual life-threatening process occurring in a patient with sickle cell disease presenting with pain can result in preventable early mortality. Clinicians must approach a sickle cell patient reporting pain in a thoughtful manner, and consider a complete differential diagnosis, including both sickle cell disease complications and those unrelated to sickle cell disease. Knowledge of the disease courses of the different sickle cell genotypes is essential, and must go beyond a superficial hierarchy of severity, but rather include an understanding of the complications each genotype is most prone to, and at what ages. Complete laboratory assessment, including a comprehensive metabolic panel, should be performed on all admitted patients, not just a complete blood count. Treating pain with high-dose opioids, while appropriate in an uncomplicated pain crisis, can lead to ACS or even respiratory failure in a patient with uninvestigated liver and kidney dysfunction. The most important lesson to remember is that even the sickle cell disease patient who has been given the unfortunate and pejorative label of “frequent flyer” by some providers has the potential for rapid deterioration into multisystem organ failure and death.
INTRODUCTION
Sickle cell disease is the most common inherited blood disorder in the world. It affects more than 100,000 individuals in the United States, and millions more worldwide.1 Sickle cell disease is most commonly found in individuals of African heritage, but the disease also occurs in Hispanics and people of Middle Eastern and subcontinent Indian heritage.2 The distribution of the sickle hemoglobin (hemoglobin S [HbS]) allele overlaps with the distribution of malaria; HbS carriers, or individuals with sickle cell trait, have protection against malaria,3 and are not considered to have sickle cell disease.
Sickle cell disease is a severe monogenic disorder marked by significant morbidity and mortality, affecting every organ in the body.4 The term sickle cell disease refers to all genotypes that cause sickling; the most common are the homozygous hemoglobin SS (HbSS) and compound heterozygotes hemoglobin SC (HbSC), hemoglobin S–β0-thalassemia (HbSβ0), and hemoglobin S–β+-thalassemia (HbSβ+), although HbS and several rarer hemoglobin variants such as HbSO(Arab) and HbSD(Punjab) can also cause sickle cell disease. The term sickle cell anemia refers exclusively to the most severe genotypes, HbSS and HbSβ0.5 Common sickling genotypes along with their relative clinical severity are shown in Table 1.6–11
| Table 1. Genotypes of Sickling Syndromes and Their Relative Severities | ||
Genotype | Severity | Characteristics |
HbSS | Severe | Most common form |
HbSβ0 | Severe | Clinically indistinguishable from HbSS6 |
HbSO-Arab | Severe | Relatively rare6 |
HbSD-Punjab | Severe | Mostly in northern India6 |
HbSC-Harlem | Severe | Migrates like HbSC, but rare double β-globin mutation7 |
HbCS-Antilles | Severe | Rare double β-globin mutation8 |
HbSC | Moderate | 25% of SCD9 |
HbSβ+, Mediterranean | Moderate | 5%–16% HbA6 |
HbAS-Oman | Moderate | Dominant rare double β-globin mutation10 |
HbSβ+, African | Mild | 16%–30% HbA6 |
HbSE | Mild | HbE found mostly in Southeast Asia11 |
HbS-HPFH | Very mild | Large deletions in β-globin gene complex; > 30% HbF6 |
| HbA = hemoglobin A; HbE = hemoglobin E; HbF = fetal hemoglobin; HbS-HPFH = HbS and gene deletion HPFH; HbSC = heterozygous hemoglobin SC; HbSS = homozygous hemoglobin SS; HbSβ0 = hemoglobin S-β thalassemia0; HbSβ+ = hemoglobin S-β thalassemia+; SCD = sickle cell disease. | ||
This article reviews the pathophysiology of sickle cell disease, common clinical complications, and available therapies. A complex case which illustrates diagnostic and management challenges is presented as well.
PATHOPHYSIOLOGY
HbS is the result of a substitution of valine for glutamic acid in the sixth amino acid of the β-globin chain.12 The change from a hydrophilic to a hydrophobic amino acid causes the hemoglobin molecules to stack, or polymerize, when deoxygenated. This rigid rod of hemoglobin distorts the cell, producing the characteristic crescent or sickle shape that gives the disease its name.13 Polymerization of hemoglobin within the cell is promoted by dehydration, which increases the concentration of HbS.13,14 Polymerization occurs when hemoglobin is in the deoxygenated state.13
The sickle red blood cell is abnormal; it is rigid and dense, and lacks the deformability needed to navigate the microvasculature.15 Blockages of blood flow result in painful vaso-occlusion that is the hallmark of the disease, and that also can cause damage to the spleen, kidneys, and liver.16 The sickle red cell is also fragile, with a lifespan of only 20 days compared to the 120-day lifespan of a normal red blood cell.13 Frequent hemolysis results in anemia and the release of free hemoglobin, which both scavenges nitric oxide and impairs the production of more nitric oxide, which is essential for vasodilatation.17 This contributes to vascular dysfunction and an increased risk for stroke.18 If untreated, the natural course of sickle cell anemia is mortality in early childhood in most cases.19 Common chronic and acute sickle cell disease–related complications and recommended therapies, based on 2014 National Institutes of Health guidelines, are shown in Table 2 and Table 3.20
| Table 2. Common Adult Sickle Cell Disease Chronic Complications and Recommended Therapies | ||
Chronic Complication | Recommended Therapy | Strength of Recommendation |
Chronic pain | Opioids | Consensus |
Avascular necrosis | Analgesics and physical therapy | Consensus |
Proliferative sickle retinopathy | Laser photocoagulation | Strong |
Leg ulcers | Standard wound care | Moderate |
Recurrent priapism | Consult urology | Moderate |
| Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
| Table 3. Common Adult Sickle Cell Disease Acute Complications and Recommended Therapies | ||
Acute Complication | Recommended Therapy | Strength of Recommendation |
Vaso-occlusive crisis | NSAIDs, opioids for severe pain | Moderate-consensus |
ACS | Antibiotics, oxygen | Strong |
Simple transfusiona | Weak | |
Urgent exchange transfusionb | Strong | |
Acute stroke | Exchange transfusion | Strong |
Priapism ≥ 4 hr | Aggressive hydration, pain control, and urology consult | Strong-consensus |
Gallstones, symptomatic | Cholecystectomy, laparoscopic | Strong |
Splenic sequestration | Intravenous fluids, transfuse cautiously, discuss surgical splenectomy | Strong-moderate |
Acute renal failure | Consult nephrologyc | Consensus |
ACS = acute chest syndrome; NSAIDs = nonsteroidal anti-inflammatory drugs. a For symptomatic ACS with hemoglobin > 1 g/dL below baseline but > 9.0 g/dL. b When there is progression of ACS (SpO2 < 90% despite supplemental oxygen, increasing respiratory distress, progressive pulmonary infiltrates despite simple transfusion). c For acute rise in creatinine ≥ 0.3 mg/dL; do not give transfusions unless there are other indications. Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
One of the most challenging aspects of sickle cell disease is its clinical variability. While in general, HbSS and HbSβ0 are the most severe genotypes, there are patients with HbSC and HbSb+ who have significant sickle-cell–related complications, and may have a more severe clinical course than a HbSS patient.21 A great deal of this clinical variability cannot be explained, but some can be attributed to endogenous fetal hemoglobin (HbF) levels.22–24 The importance of HbF levels in sickle cell disease was first noted by a pediatrician in the 1940s.25 She observed that sickle cell disease complications in children under the age of 1 were rare, and attributed it to the presence of HbF.25 HbF levels decline more slowly in individuals with hemoglobinopathies, reaching their nadir after the age of 5 rather than within 6 months of birth in individuals without hemoglobinopathies.26 HbF levels remain elevated lifelong in most sickle cell disease patients, especially those with the HbSS and HbSβ0 genotypes. Levels of HbF vary widely between individuals, from zero to 20% to 30%, with a median of 10%.26–28 Individuals who produce more HbF have a milder course, in general.24 An association between the 4 β-globin haplotypes and HbF levels has been reported in the past,27,29 but more sophisticated next-generation sequencing has revealed causal variants in BCL11A and HBS1L-MYB that contribute approximately 50% of the observed variability in HbF levels.30–33
Co-inheritance of α-thalassemia also modifies disease course; less available α-globin chains results in a lower hemoglobin concentration within the cell. Paradoxically, this results in a higher overall hemoglobin level, as there is a reduction in polymerization, and therefore sickling due to lower HbS concentrations in the cell. Patients therefore are less anemic, reducing the risk of stroke in childhood,34,35 but blood viscosity may be higher, resulting in more frequent pain crises and increased risk36 of avascular necrosis.34,35,37 It is often helpful to think of sickle cell patients as falling into 1 of 2 groups: high hemolysis/low hemoglobin and high viscosity/high hemoglobin. Individuals with high rates of hemolysis are at greater risk for stroke, pulmonary hypertension, and acute chest syndrome (ACS). Higher rates of hemolysis result in higher levels of free hemoglobin, which scavenges nitric oxide. This leads to the vascular damage and dysfunction that contributes to the associated clinical complications. This phenotype is most commonly seen in HbSS and HbSβ0.38 High hemoglobin/high viscosity phenotypes are most often found in HbSC patients and in sickle cell anemia with α-thalassemia coinheritance.39–42
TREATMENT OPTIONS
In high-resource countries with newborn screening, the initiation of penicillin prophylaxis has dramatically altered the natural history of the disease, allowing the majority of patients to reach adulthood.43 Penicillin prophylaxis is usually discontinued at age 5 years; however, individuals who have undergone surgical splenectomy or have had pneumococcal sepsis on penicillin prophylaxis may remain on penicillin to age 18 or beyond.20
Another advance in sickle cell care is screening for stroke risk through transcranial Doppler ultrasound (TCD).44–47 This screening tool has reduced the incidence of childhood stroke from 10% by age 11 to 1%. TCDs typically cannot be performed after the age of 16 due to changes in the skull. Individuals found to have abnormal (elevated) TCD velocities are placed on chronic transfusion therapy for primary stroke prevention. They may remain on monthly chronic transfusions, with the goal of suppressing the percentage of HbS to 30% to 50% indefinitely. A clinical trial (STOPII) designed to determine if pediatric sickle cell disease patients on chronic transfusion therapy for primary stroke prevention could be safely taken off transfusion therapy was discontinued early due to an excess of strokes and conversion to abnormal TCD velocities in the untransfused arm.44 Individuals who have experienced an ischemic stroke have a 70% risk of another stroke, and must remain on chronic transfusion therapy indefinitely. Chronic transfusion reduces their stroke risk to 13%.
The only widely used pharmacologic therapy for sickle cell disease is hydroxyurea.12,48–50 A significant portion of the benefit of hydroxyurea stems from its induction of HbF.51 HbF does not sickle, and it interrupts the polymerization of HbS in the cell, if present in high enough concentrations.50 The level of HbF needed to achieve clinical improvement is not known, but in vitro assays suggest 20% HbF is needed to prevent sickling.52,53 However, endogenous and hydroxyurea-induced HbF is not distributed evenly through the red cells, so sickling is possible regardless of the level of HbF induced.54,55 Hydroxyurea likely has other disease-modifying effects as well, including reduction of white blood cell count and reticulocyte count and reduction of red cell adhesion to the endothelium.56–58 Clinical criteria for initiation of hydroxyurea in adult sickle cell disease patients are shown in Table 4.20 Hydroxyurea is given daily and is dosed to maximum tolerated dose for the individual by following the absolute neutrophil count (ANC). The goal ANC is between 2000 and 4000/µL. At times, absolute reticulocyte count (ARC) can be dose-limiting; goal ARC is greater than 70,000/µL.59 Platelet counts may be reduced as well, especially in HbSC patients.60,61
| Table 4. Indications for Hydroxyurea in Adult Patients with Sickle Cell Disease | |
Indication | Strength of Recommendation |
SCA with ≥ 3 pain crises per year | Strong |
SCA with pain that interferes with ADL and QoL | Strong |
History of severe or recurrent ACS | Strong |
Chronic kidney disease on epoetin | Weak |
HbSβ+ and HbSC with pain that interferes with ADL and QoL; consult sickle cell disease expert | Moderate |
| ACS = acute chest syndrome; ADL = activities of daily living; QoL = quality of life; SCA = sickle cell anemia. | |
The only curative therapy for sickle cell disease is hematopoietic stem cell transplant.62 Transplant use is limited by availability of matched sibling donors,62 and even at experienced centers transplant carries a small risk for mortality, graft rejection, and graft-versus-host disease. Furthermore, consensus on disease complications for which transplant is recommended is also lacking.63–65 Clinical trials of gene therapy for sickle cell disease and thalassemia are ongoing.66
COMPLICATIONS AND DISEASE-SPECIFIC THERAPIES
CASE PRESENTATION
A 26-year-old African-American man who works as a school bus driver presents to an academic center’s emergency department complaining of pain in his left leg, similar to prior pain events. He is described as having sickle cell trait, although no hemoglobin profile is available in his chart. He describes the pain as dull and aching, 10/10 in intensity. A complete blood count (CBC) is obtained; it reveals a hemoglobin of 14.5 g/dL, white blood cell (WBC) count of 5600/µL, and platelet count of 344,000/µL. His CBC is also notable for a mean corpuscular volume (MCV) of 72 fL, a mean corpuscular hemoglobin concentration (MCHC) of 37 g/dL, and a red blood cell distribution width (RDW) of 12. Slide review of a peripheral blood smear shows 2+ target cells (Figure).

The patient is given 6 mg of morphine, which provides some relief of his pain, and is discharged with a prescription for hydrocodone bitartrate/acetaminophen 5/325 mg. The diagnosis given is musculoskeletal pain, and he is instructed to follow-up with a primary care physician. His past medical history is significant for 4 or 5 visits to the emergency department per year in the past 4 years. Prior to 4 years ago, he rarely required medical attention.
• What laboratory and clinical features might lead you to question the diagnosis of sickle cell trait in this patient?
The patient’s hemoglobin is within normal range, which is consistent with sickle cell trait; however, he is microcytic, with a normal RDW. It is possible to be mildly microcytic in the early stages of iron deficiency, prior to the development of anemia, but the RDW would typically be elevated, demonstrating the presence of newer, smaller cells produced under conditions of iron deficiency.67 It is also possible that his microcytosis with a normal RDW could represent sickle cell trait with co-inheritance of β-thalassemia. Up to 30% of African Americans have β-thalassemia,2 and 1 in 10 have sickle cell trait.68 However, a high MCHC, indicating the presence of dense cells, and target cells noted on slide review are most consistent with HbSC.9 HbSC patients, especially males, can have hemoglobin levels in the normal range.4 The biggest inconsistency with the diagnosis of sickle cell trait is his history of frequent pain events. Individuals with sickle cell trait rarely present with pain crises, except under extreme conditions of dehydration or high altitude.68 Sickle cell trait is generally regarded as a benign condition, although a study of U.S. military recruits found a 30-fold higher risk of sudden death during basic training in persons with sickle cell trait.69 Additional sickle cell trait–related complications include hematuria, risk of splenic sequestration or infarct under extreme conditions and high altitude, and a rare and usually fatal renal malignancy, renal medullary carcinoma, which is vanishingly rare in individuals without sickle cell trait.70,71 Although the patient reported having sickle cell trait, this diagnosis should have been verified with a hemoglobin panel, given his atypical presentation.20
• What is the approach to managing pain episodes in sickle cell disease?
In sickle cell disease, vaso-occlusive pain events can be common, often beginning in early childhood.17 This disease complication accounts for 95% of all adult sickle cell disease hospitalizations.72 There is a great deal of variability in pain symptoms between individuals, and within individuals at various times in their lives:73 30% have no pain events, 50% have occasional events, and 20% have monthly or more frequent events that require hospitalization.74 The frequency and severity of pain events are modulated by HbF levels, β-thalassemia status, genotypes, therapies like hydroxyurea, or in rare cases, chronic transfusion therapy.23 Personal factors, such as psychosocial stressors, also contribute to the frequency of pain events.75 Pain event triggers include exposure to cold water, windy or cold weather, temperature changes, and extreme temperatures.76–79 Patient age also contributes to pain event frequency. Many patients see an increase in pain event frequency in their late 20s, and a marked decrease in their 40s.23,73 More than 3 pain events per year is associated with reduced life expectancy.23
Acute management of pain episodes involves nonsteroidal anti-inflammatory drugs, oral opioids, and when hospitalization is required, intravenous opioids, often delivered via patient-controlled analgesia (PCA) pumps.79 As sickle cell disease patients become teenagers and young adults, some experience an increased frequency of pain episodes, with fewer pain-free days, or a failure to return to baseline before the next pain crisis occurs.80,81 This is characteristic of emerging chronic pain.82 Chronic pain is a significant problem in adult patients with sickle cell disease, with up to 85% reporting pain on most days.72,80 The development of chronic pain may be reduced by early and aggressive treatment of acute pain events, as well as use of hydroxyurea to reduce the number of pain events. Many adult sickle cell patients with chronic pain are treated with daily opioids.20 Given the significant side effects of chronic opioid use—sedation, respiratory depression, itching, nausea, and impairment of function and quality of life—non-opioid therapies are under investigation.83 Many chronic pain patients have symptoms of neuropathic pain, and may benefit from neuropathic agents like gabapentin, both to reduce opioid use and to more effectively treat chronic neuropathic pain, which is known to respond poorly to opioids.84–86
• Is the patient’s peripheral blood smear consistent with a diagnosis of sickle cell trait?
Several target cells are visible, which is not typical of sickle cell trait, but may be seen in HbSC or thalassemia. The finding of an intracellular crystal is pathognomonic for HbSC or HbCC. HbC polymerizes in high oxygen conditions, opposite of HbS, which polymerizes in low oxygen conditions.9
CASE CONTINUED
The patient’s family history is significant for a sister who died at age 3 from sickle cell–related complications, and a sister with sickle cell trait who had a cholecystectomy for gallstones at age 22. His father died at age 38 due to unknown causes. The sickle cell trait status of his parents is unknown. His mother is alive, and has hypertension.
• Is the medical history of this patient’s family members consistent with sickle cell trait?
It is unlikely that sickle cell trait would result in early death in childhood, or in gallstones at age 22. Gallstones in early adulthood is a common presentation for HbSC patients not diagnosed by newborn screening.87 Any hemolytic condition can lead to the formation of hemoglobin-containing pigmented gallstones, biliary sludge, and obstruction of the gallbladder. In the presence of right-sided abdominal pain, a serum bilirubin level of more than 4 mg/dL should lead to measurement of direct bilirubin; if greater than 10% of total, imaging of the gallbladder should be obtained. In sickle cell disease, 30% of patients will have gallstones by 18 years of age. The low hemolysis/high viscosity phenotype patients are typically older at diagnosis. Co-inheritance of Gilbert syndrome and sickle cell disease is not uncommon, and can result in formation of gallstones at a young age; Gilbert syndrome alone typically results in gallstones in mid-life.88
CASE CONTINUED
Two months later, the patient presents again to the emergency department with the same complaint of leg pain, as well as abdominal pain. His hemoglobin is 12.5 g/dL, and his platelet count is 134,000/µL. His pain is not improved with 3 doses of morphine 6 mg intravenously, and he is admitted to the medicine service. A hemoglobin profile is obtained, revealing 52% HbS, 45% HbC, and 1.5% HbF, consistent with HbSC. In sickle cell trait, the hemoglobin profile is 60% HbA and 40% HbS (available α-globin prefers to pair with a normal β-globin, so the ratio of HbA to HbS is 60:40, not 50:50).
On the second hospital day, the patient’s hemoglobin drops to 7.2 g/dL and his platelet count decreases to 44,000/µL. His abdomen is distended and diffusely tender. The internist transfuses him with 2 units of packed red blood cells (PRBC), after which his hemoglobin increases to 11 g/dL, while his platelet count increases to 112,000/µL. Following the transfusion, his abdominal pain resolves, as does his anemia and thrombocytopenia.
• What caused this patient’s anemia and thrombocytopenia?
High on the differential diagnosis is a splenic sequestration. Acute splenic sequestration occurs when red cells are trapped in the splenic sinuses. Massive splenic enlargement may occur over several hours.89,90 Unrecognized splenic sequestration has a high mortality rate from severe anemia and splenic rupture.90 Splenic sequestration must be ruled out in a sickle cell patient with abdominal pain accompanied by dropping platelet and red cell counts, especially in milder subtypes that often have splenic function preserved into adolescence and adulthood. Sickle cell anemia patients usually become functionally asplenic in early childhood.89,91,92 The rise in hemoglobin, more than would be expected from 2 units of PRBC, plus the improvement in platelet count without a platelet transfusion observed in the case patient strongly supports the diagnosis of splenic sequestration.
Splenic sequestration can occur in any sickle cell patient whose spleen has not fibrosed. Splenic sequestration in adulthood is not uncommon in HbSC patients, who often have preserved splenic function into adulthood.93–95
Clinical signs of splenic sequestration include a rapid drop in hemoglobin, rise in reticulocyte count, a tender, enlarged spleen, and, in severe cases, hypovolemia.89,93 It is treated with prompt blood transfusion, but care must be taken not to overtransfuse the patient, as the spleen can trap several grams of hemoglobin, which may be released upon transfusion, potentially causing life-threatening hyperviscosity.89 Hemoglobin levels must be checked following transfusion in suspected splenic sequestration, and “mini transfusions” of 5 mL/kg are recommended in sickle cell disease patients who are hemodynamically stable.20
Hepatic sequestration may also occur, but it is much less common than splenic sequestration.96 Other conditions on the differential diagnosis include thrombotic thrombocytopenic purpura, which would be unlikely to respond to a transfusion. ACS can cause a drop in hemoglobin, and is treated with simple or exchange transfusions.97 ACS is less likely without respiratory symptoms or oxygen requirement, and usually is not associated with thrombocytopenia. Sepsis may also cause anemia and thrombocytopenia, but again would not likely respond to a simple transfusion. The patient’s response to transfusion is consistent with a sequestering event, not a destructive event as in the case of sepsis.
CASE CONTINUED
Imaging reveals a grossly enlarged spleen, which is having a mass effect on the left kidney. The patient is started on hydroxyurea therapy at 500 mg 3 times daily. Discharge instructions include following up with his primary care physician, continuing hydroxyurea therapy, and receiving yearly dilated eye exams to evaluate for proliferative sickle retinopathy.
• Are these discharge instructions complete?
Splenic sequestration has a 50% recurrence rate.98 In very young children, watchful waiting or chronic transfusion may be implemented to preserve the immunologic function of the spleen and reduce the risk of sepsis.89 Splenectomy after a single episode of sequestration in adults is a matter of debate, with experts advising both watchful waiting99 and splenectomy after recovery from the first sequestering event.100 The patient should have been informed of the risk for recurrence, and the signs and symptoms of splenic sequestration as well as the need for emergency medical attention should have been discussed. Splenic sequestration may be milder in adults than in children, but fatal sequestrations have been reported.95,101–103
Proliferative sickle cell retinopathy is a high viscosity/high hemoglobin complication that may occur more frequently in HbSC than HbSS, with an incidence of 33% in HbSC.42,104 Spontaneous regression of retinopathy occurs in approximately 32% of eyes, and laser or scatter photocoagulation is an effective intervention.105
• Would the patient need to be transfused prior to splenectomy?
Preoperative transfusion therapy is standard of care for HbSS patients undergoing general anesthesia. The TRAP study found that simple “top off” transfusion to a hemoglobin of 10 g/dL was as effective at preventing postoperative sickle cell–related complications as exchange transfusion to HbS of 30% or less, and had fewer transfusion-related complications like alloimmunization.106 There is little data regarding preoperative transfusions in HbSC disease. A retrospective study suggests that HbSC patients undergoing abdominal surgeries should be transfused.107 The higher hemoglobin level of the typical HbSC patient necessitates exchange transfusion to avoid hyperviscosity.
• Is hydroxyurea therapy indicated in this patient?
• Has it been dosed appropriately?
If the patient had the HbSS subtype, hydroxyurea would be clearly indicated, given his frequent pain events.20 HbSC patients may be placed on hydroxyurea on a case-by-case basis, but evidence for its efficacy in this sickle cell subtype is lacking.108 Large clinical trials like the Multi-Center Study of Hydroxyurea (MSH) that established the safety and efficacy of hydroxyurea in sickle cell anemia excluded HbSC and HbSβ+ patients.109 These mild to moderate subtypes produce less HbF at baseline, and typically have a minimal to modest rise in HbF on hydroxyurea.110 In sickle cell anemia, hydroxyurea is titrated to maximum tolerated dose, defined as an ANC of 2000 to 4000/µL and an ARC of 70,000/µL or higher.53 Because of their lower levels of chronic inflammation and lower reticulocyte counts due to higher hemoglobin levels, many HbSC and HbSβ+ patients have values in that range before initiating hydroxyurea therapy.9 Cytopenias, particularly of platelets in HbSC, occur at low doses of hydroxyurea.111
Of note, although the half-life of hydroxyurea would suggest that 3 times daily dosing is indicated, daily dosing has been found to have equal response and is preferred. Another concern is the monitoring of this myelosuppressive medication. This patient has repeatedly failed to obtain a primary care physician or a hematologist, and hydroxyurea requires laboratory monitoring at least every 2 months, especially in a HbSC patient with a very large spleen who is at significant risk for thrombocytopenia and neutropenia.9
CASE CONTINUED
A week after discharge from his admission for abdominal pain diagnosed as splenic sequestration, the patient presents again to the emergency department with abdominal pain which he reports is his typical sickle cell pain. Hemoglobin is 13.8 g/dL, platelet count is 388,000/µL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are both 10 times their prior value. Creatinine is 1.2 mg/dL (0.75 mg/dL on his prior admission), and total bilirubin is 3 mg/dL, with 0.3 mg/dL direct bilirubin. He undergoes an ultrasound exam of his gallbladder, which reveals sludge and a possible gallstone. There is no evidence of cholecystitis. General surgery performs a laparoscopic cholecystectomy.
• Was this cholecystectomy necessary?
In patients with sickle cell disease, symptomatic gallstones and gallbladder sludge should be observed; recurrent abdominal pain without a significant change in bilirubin may not be due to gallstones or sludge, and therefore may not be relieved by cholecystectomy.112,113 In sickle cell disease, 40% of patients with gallbladder sludge do not develop gallstones.87 The patient’s bilirubin level was at baseline, and there was no increase in the direct (conjugated) fraction. Watchful waiting would have been appropriate, with cholecystectomy being performed if he experienced recurrent symptoms associated with fatty foods accompanied by an elevation in direct bilirubin.
More concerning and deserving of investigation was his elevated liver enzymes. Patients with sickle cell disease may experience recurrent ischemia and reperfusion injuries in the liver, which is called right upper quadrant syndrome. On autopsy of 70 sickle cell patients, 91% had hepatomegaly and 34% had focal necrosis.114 AST is often elevated in sickle cell disease, as it is affected by hemolysis. In this patient, both AST and ALT are elevated, consistent with a hepatocellular disorder. His abdominal pain and ALT rise may be a sign of a hepatic crisis.115 Rapid resolution of ALT elevation in a matter of days suggests a vaso-occlusive, inflammatory event that is self- limiting. Prolonged AST elevation requires further investigation, with consideration of autoimmune hepatitis, viral hepatitis, or iron overload. Iron overload is unlikely in this patient given his lifetime history of only 1 transfusion. Hepatic iron overload typically occurs in sickle cell disease after a minimum of 10 transfusions.115
CASE CONTINUED
The patient is discharged on the day after the procedure, with instructions to continue his hydroxyurea.
• Should the patient resume hydroxyurea therapy?
Hydroxyurea is hepatically cleared and thus it should be held until his liver function tests normalize.106
CASE CONTINUED
Two months later, the patient presents to the emergency department with abdominal pain that moves to his left leg. A CBC is obtained, showing a hemoglobin of 11.8 g/dL and a platelet count of 144,000/µL. He is given 2 doses of morphine 6 mg intravenously, and reports that his leg pain is now a 4/10. He is discharged home with a prescription for hydrocodone/acetaminophen.
• Is the emergency department evaluation sufficient?
This patient remains at high risk for splenic sequestration,93 with a hemoglobin 2 g lower than it was 2 months ago and platelets less than half. This decline could be consistent with early splenic sequestration.20 Additionally, he had elevated liver function tests on a recent admission, as well as rising creatinine, without evidence of resolution. It is not appropriate to discharge him without checking a chemistry and liver panel, and abdominal imaging should be considered. The best plan would be to admit him for observation, given his risk for splenic sequestration, and consult surgery for an elective splenectomy if he has a second episode of splenic sequestration 2 months after the first.100 His abdominal pain that migrates to his left leg could be due to his massive splenomegaly compressing his left kidney, as noted on imaging during his recent admission for splenic sequestration
CASE CONTINUED
An hour after discharge from the emergency department, EMS is called to his home for intractable pain. He is found lying on the floor, and reports excruciating left leg pain. He is brought to the closest hospital, a community hospital that he has not visited previously. There, he is admitted for hydration and pain control and placed on hydromorphone 2 mg every 4 hours as needed for pain. His hemoglobin is 10.8 g/dL, and platelets are 121,000/µL. A chemistry panel is remarkable for a creatinine level of 1.5 mg/dL and a potassium level of 3.2 mEq/L. Liver function tests are not obtained. After 3 doses of hydromorphone, he falls asleep. He is not in a monitored bed, and intravenous fluids, while ordered, are not started. At 6:30 AM the day after admission, he cannot be aroused on a routine vital sign check; he has an SpO2 of 60%, a blood pressure of 80/60 mm Hg, and heart rate of 148 beats/min. A rapid response is called, and naloxone is administered along with oxygen by face mask and several fluid boluses. His systolic blood pressure increases to 100 mm Hg from a low of 70 mm Hg. His SpO2 increases to 92%, and he is arousable and alert, although he reports 10/10 leg pain. His abdomen is noted to be distended and tender.
• What may have contributed to his clinical condition?
The patient is opioid tolerant and has received equivalent doses of opioids in the past without excess sedation. He may have liver dysfunction making him unable to metabolize opioids effectively. His hemoglobin and platelets continue to decline, raising concern for splenic sequestration versus sepsis. Failure to place him on a monitor allowed his hypoxia to continue for an unknown amount of time, placing him at high risk for developing ACS. Lack of intravenous hydration while he was too sedated to drink likely exacerbated his sickling.
CASE CONTINUED
At 9:20 AM, a CBC is obtained and reveals a hemoglobin of 4.8 g/dL and a platelet count of 44,000/µL. Two units of stat O negative blood are administered, and preparations are made to administer an exchange transfusion. A liver panel is obtained 3 hours later, which reveals an AST level of 1200 U/L and an ALT level of 1050 U/L. His bilirubin is 10 mg/dL, and his lactate dehydrogenase level is 1800 U/L. His urine is dark and is positive for bilirubin and ketones. He is transferred to the intensive care unit. A chest X-ray shows pulmonary congestion. Hematology/oncology is consulted.
He receives a 7-unit red blood cell exchange, which reduces his HbS to 11%. He continues to be hypotensive, and requires norepinephrine to support his blood pressure. Antibiotic therapy is started. His creatinine concentration rises to 2.3 mg/dL, potassium is 7.8 mEq/L, and bicarbonate is 12 mEq/L. He is placed on hemodialysis.
Computed tomography of the chest and abdomen reveals lower posterior lung infiltrates and a grossly enlarged spleen. He requires intubation. He is given a diagnosis of ACS in addition to kidney failure, liver failure, and “sickle crisis.” He continues to require daily to twice daily transfusions to maintain a hemoglobin of 7 to 9 g/dL, and his abdominal distension increases. As his condition worsens, surgery is consulted to discuss a liver transplant. He is deemed to not be a surgical candidate, and he passes away 6 days after entering the hospital. The immediate cause of death is listed as vaso-occlusive crisis, with ACS and sickle crisis listed as contributors.
• Are the causes of death accurate and complete?
If vaso-occlusive crisis is used to indicate a pain event, it is not an accurate cause of death. Pain is one of the most distressing complications of sickle cell disease, and frequent pain events are associated with early mortality,4,80 but they are not in themselves fatal. ACS is the number one cause of death in sickle cell disease,4 and it likely contributed to this patient’s death. Sickle crisis is a vague term that should not be used in this context. Causes of death should include splenic sequestration and multisystem organ failure. Multisystem organ failure in sickle cell disease often responds to aggressive transfusion therapy, which this patient received.116–118
CONCLUSION
Sickle cell disease is a complex chronic disease that impacts almost every organ system in the body. Clinicians may be inclined to attribute most pain in a patient with sickle cell disease to a simple vaso-occlusive crisis, treat them for this, and not investigate further. As the case presented here demonstrates, failure to identify the actual life-threatening process occurring in a patient with sickle cell disease presenting with pain can result in preventable early mortality. Clinicians must approach a sickle cell patient reporting pain in a thoughtful manner, and consider a complete differential diagnosis, including both sickle cell disease complications and those unrelated to sickle cell disease. Knowledge of the disease courses of the different sickle cell genotypes is essential, and must go beyond a superficial hierarchy of severity, but rather include an understanding of the complications each genotype is most prone to, and at what ages. Complete laboratory assessment, including a comprehensive metabolic panel, should be performed on all admitted patients, not just a complete blood count. Treating pain with high-dose opioids, while appropriate in an uncomplicated pain crisis, can lead to ACS or even respiratory failure in a patient with uninvestigated liver and kidney dysfunction. The most important lesson to remember is that even the sickle cell disease patient who has been given the unfortunate and pejorative label of “frequent flyer” by some providers has the potential for rapid deterioration into multisystem organ failure and death.
- Weatherall D, Hofman K, Rodgers G, Ruffin J, Hrynkow S. A case for developing North-South partnerships for research in sickle cell disease. Blood 2005;105:921–3.
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1998;11:1–51.
- Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg 1954;48:312–8.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–31.
- Serjeant GR, Serjeant BE. Sickle cell disease. 3rd ed. New York: Oxford University Press; 2001.
- Moo-Penn W, Bechtel K, Jue D, et al. The presence of hemoglobin S and C Harlem in an individual in the United States. Blood 1975;46:363–7.
- Monplaisir N, Merault G, Poyart C, et al. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A 1986;83:9363–7.
- Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev 2003;17:167–78.
- Nagel RL, Daar S, Romero JR, et al. HbS-oman heterozygote: a new dominant sickle syndrome. Blood 1998;92:4375–82.
- Masiello D, Heeney MM, Adewoye AH, et al. Hemoglobin SE disease: a concise review. Am J Hematol 2007;82:643–9.
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–9.
- Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985;65:183–9.
- .Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A 1979;76:670–2.
- .Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol 1989;32:104–11.
- Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol 2002;9:101–6.
- Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Med 2002;8:1383–9.
- Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013;98:464–72.
- Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med 2013;3:a011783.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48.
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol 1995;2:103–8.
- Odenheimer DJ, Sarnaik SA, Whitten CF, et al. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am J Med Genet 1987;27:525–35.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991;325:11–6.
- Falusi AG, Olatunji PO. Effects of alpha thalassaemia and haemoglobin F (HbF) level on the clinical severity of sickle-cell anaemia. Eur J Haematol 1994;52:13–5.
- Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci 1948;215:419–23.
- Marcus SJ, Ware RE. Physiologic decline in fetal hemoglobin parameters in infants with sickle cell disease: implications for pharmacological intervention. J Pediatr Hematol Oncol 1999;21:407–11.
- Schroeder WA, Powars DR, Kay LM, et al. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin 1989;13:325–53.
- Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. Am J Hematol 2003;72:121–6.
- Ngo D, Bae H, Steinberg MH, et al. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood Cells Mol Dis 2013;51:22–6.
- Galarneau G, Palmer CD, Sankaran VG, et al. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 2010;42:1049–51.
- Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A 2008;105:11869–74.
- Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839–42.
- Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A 2008;105:1620–5.
- Adams RJ, Kutlar A, McKie V, et al. Alpha thalassemia and stroke risk in sickle cell anemia. Am J Hematol 1994;45:279–82.
- Ballas SK. Effect of alpha-globin genotype on the pathophysiology of sickle cell disease. Pediatr Pathol Mol Med 2001;20:107–21.
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med 1986;314:1593–9.
- Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin 1989;13:649–55.
- .Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007;21:37–47.
- Murphy JR, Wengard M, Brereton W. Rheological studies of Hb SS blood: influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med 1976;87:475–86.
- Fabry ME, Kaul DK, Raventos-Suarez C, Chang H, Nagel RL. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest 1982;70:1315–9.
- Stuart J, Johnson CS. Rheology of the sickle cell disorders. Baillieres Clin Haematol 1987;1:747–75.
- Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136-41.
- Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr 2003;143:438–44.
- Abboud MR, Yim E, Musallam KM, Adams RJ, Investigators SIS. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood 2011;118:894–8.
- Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol 2000;15:344–9.
- Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood 2004;103:3689–94.
- Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108:847–52.
- Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 1992;79:2555–65.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22.
- Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol 1997;34:15–21.
- Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol 2010;85:403–8.
- Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 1988;318:96–9.
- Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–6.
- Maier-Redelsperger M, de Montalembert M, Flahault A, et al. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients. The French Study Group on Sickle Cell Disease. Blood 1998;91:4472–9.
- Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 2014;123:481–5.
- Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 1994;83:553–60.
- Bridges KR, Barabino GD, Brugnara C, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood 1996;88:4701–10.
- Jiang J, Jordan SJ, Barr DP, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol 1997;52:1081–6.
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
- Yates AM, Dedeken L, Smeltzer MP, et al. Hydroxyurea treatment of children with hemoglobin SC disease. Pediatr Blood Cancer 2013;60:323–5.
- Barbosa CG, Aleluia AC, Pacheco AP, et al. Genetic modulation of HbF in Brazilians with HbSC disease and sickle cell anemia. Am J Hematol 2013;88:923–4.
- Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009;361:2309–17.
- King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood 2014;123:3089–94.
- Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD007001.
- Freed J, Talano J, Small T, et al. Allogeneic cellular and autologous stem cell therapy for sickle cell disease: ‘whom, when and how’. Bone Marrow Transplant 2012;47:1489–98.
- Urbinati F, Hargrove PW, Geiger S, et al. Potentially therapeutic levels of anti-sickling globin gene expression following lentivirus-mediated gene transfer in sickle cell disease bone marrow CD34 cells. Exp Hematol 2015;43:346–51.
- Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes’s original 1934 classification to the third millennium. Curr Opin Hematol 2013;20:222–30.
- Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am Soc Hematol Educ Program 2010;2010:418–22.
- Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987;317:781–7.
- Goldsmith JC, Bonham VL, Joiner CH, et al. Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol 2012;87:340–6.
- Grant AM, Parker CS, Jordan LB, et al. Public health implications of sickle cell trait: a report of the CDC meeting. Am J Prev Med 2011;41:S435–9.
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol 2005;79:17–25.
- Serjeant GR, Ceulaer CD, Lethbridge R, et al. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol 1994;87:586–91.
- Vichinsky EP, Johnson R, Lubin BH. Multidisciplinary approach to pain management in sickle cell disease. Am J Pediatr Hematol Oncol 1982;4:328–33.
- Gil KM, Carson JW, Porter LS, et al. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol 2004;23:267–74.
- Amjad H, Bannerman RM, Judisch JM. Letter: Sickling pain and season. Br Med J 1974;2:54.
- Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Trans R Soc Trop Med Hyg 1980;74:159–61.
- Jones S, Duncan ER, Thomas N, et al. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol 2005;131:530–3.
- Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr 1991;118:407–9.
- Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol 2014;93:89–95.
- Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr 2011;160:286–90.
- Hollins M, Stonerock GL, Kisaalita NR, et al. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage 2012;43:1082–93.
- Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol 2013;88:927–9.
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer 2015 May 13. doi: 10.1002/pbc.25574. [Epub ahead of print].
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61:512–7.
- Brandow AM, Farley RA, Dasgupta M, et al. The use of neuropathic pain drugs in children with sickle cell disease is associated with older age, female sex, and longer length of hospital stay. J Pediatr Hematol Oncol 2015;37:10–5.
- Walker TM, Hambleton IR, Serjeant GR. Gallstones in sickle cell disease: observations from The Jamaican Cohort study. J Pediatr 2000;136:80–5.
- Penner E, Mayr WR, Djawan S, et al. [The genetics of Gilbert syndrome]. Schweiz Med Wochenschr 1976;106:860–2. [German]
- Powell RW, Levine GL, Yang YM, Mankad VN. Acute splenic sequestration crisis in sickle cell disease: early detection and treatment. J Pediatr Surg 1992;27:215–8.
- Al Salem AH, Qaisaruddin S, Nasserullah Z, et al. Splenectomy and acute splenic sequestration crises in sickle cell disease. Pediatr Surg Int 1996;11:26–8.
- Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med 1969;281:923–6.
- Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377:1663–72.
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 2014;166:165–76.
- Orringer EP, Fowler VG Jr, Owens CM, et al. Case report: splenic infarction and acute splenic sequestration in adults with hemoglobin SC disease. Am J Med Sci 1991;302:374–9.
- Michel JB, Hernandez JA, Buchanan GR. A fatal case of acute splenic sequestration in a 53–year-old woman with sickle-hemoglobin C disease. Am J Med 1992;92:97–100.
- Hatton CS, Bunch C, Weatherall DJ. Hepatic sequestration in sickle cell anaemia. Br Med J (Clin Res Ed) 1985;290:744–5.
- Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994;84:643–9.
- Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood 1995;86:776–83.
- Owusu-Ofori S, Hirst C. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD003425.
- 00.Al-Salem AH. Splenic complications of sickle cell anemia and the role of splenectomy. ISRN Hematol 2011;2011:864257.
- Sabarense AP, Lima GO, Silva LM, Viana MB. Characterization of mortality in children with sickle cell disease diagnosed through the Newborn Screening Program. J Pediatr (Rio J) 2015;91:242–7.
- Aslam AF, Aslam AK, Dipillo F. Fatal splenic sequestration crisis with multiorgan failure in an adult woman with sickle cell-beta+ thalassemia. Am J Med Sci 2005;329:141–3.
- Berry RA, Odumakinde EA, Lewis JP. Massive splenic infarction in doubly abnormal heterozygous sickling disorders. A new complication of acute splenic sequestration syndrome. The West J Med 1991;155:531–2.
- Bonanomi MT, Lavezzo MM. Sickle cell retinopathy: diagnosis and treatment. Arq Bras de Oftalmol 2013;76:320–7.
- Chen RW, Flynn HW Jr, Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. Am J Ophthalmol 2014;157:870–5 e1.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930–8.
- Neumayr L, Koshy M, Haberkern C, et al. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. Am J Hematol 1998;57:101–8.
- 08. Savage WJ, Buchanan GR, Yawn BP, et al. Evidence gaps in the management of sickle cell disease: A summary of needed research. Am J Hematol 2015;90:273–5.
- Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300–26.
- Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol 1997;98:838–44.
- Wang W, Brugnara C, Snyder C, et al. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. Br J Haematol 2011;152:771–6.
- Jungst C, Kullak-Ublick GA, Jungst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol 2006;20:1053–62.
- Walker TM, Serjeant GR. Biliary sludge in sickle cell disease. J Pediatr 1996;129:443–5.
- Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7.
- Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol 2010;8:483–9.
- Hiran S. Multiorgan dysfunction syndrome in sickle cell disease. J Assoc Physicians India 2005;53:19–22.
- Shao SH, Orringer EP. Case report: splenic sequestration and multiorgan failure as the presenting manifestation of hemoglobin SC disease. Am J Med Sci 1996;311:139–41.
- Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 1994;96:155–62.
- Weatherall D, Hofman K, Rodgers G, Ruffin J, Hrynkow S. A case for developing North-South partnerships for research in sickle cell disease. Blood 2005;105:921–3.
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1998;11:1–51.
- Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg 1954;48:312–8.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–31.
- Serjeant GR, Serjeant BE. Sickle cell disease. 3rd ed. New York: Oxford University Press; 2001.
- Moo-Penn W, Bechtel K, Jue D, et al. The presence of hemoglobin S and C Harlem in an individual in the United States. Blood 1975;46:363–7.
- Monplaisir N, Merault G, Poyart C, et al. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A 1986;83:9363–7.
- Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev 2003;17:167–78.
- Nagel RL, Daar S, Romero JR, et al. HbS-oman heterozygote: a new dominant sickle syndrome. Blood 1998;92:4375–82.
- Masiello D, Heeney MM, Adewoye AH, et al. Hemoglobin SE disease: a concise review. Am J Hematol 2007;82:643–9.
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–9.
- Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985;65:183–9.
- .Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A 1979;76:670–2.
- .Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol 1989;32:104–11.
- Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol 2002;9:101–6.
- Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Med 2002;8:1383–9.
- Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013;98:464–72.
- Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med 2013;3:a011783.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48.
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol 1995;2:103–8.
- Odenheimer DJ, Sarnaik SA, Whitten CF, et al. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am J Med Genet 1987;27:525–35.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991;325:11–6.
- Falusi AG, Olatunji PO. Effects of alpha thalassaemia and haemoglobin F (HbF) level on the clinical severity of sickle-cell anaemia. Eur J Haematol 1994;52:13–5.
- Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci 1948;215:419–23.
- Marcus SJ, Ware RE. Physiologic decline in fetal hemoglobin parameters in infants with sickle cell disease: implications for pharmacological intervention. J Pediatr Hematol Oncol 1999;21:407–11.
- Schroeder WA, Powars DR, Kay LM, et al. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin 1989;13:325–53.
- Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. Am J Hematol 2003;72:121–6.
- Ngo D, Bae H, Steinberg MH, et al. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood Cells Mol Dis 2013;51:22–6.
- Galarneau G, Palmer CD, Sankaran VG, et al. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 2010;42:1049–51.
- Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A 2008;105:11869–74.
- Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839–42.
- Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A 2008;105:1620–5.
- Adams RJ, Kutlar A, McKie V, et al. Alpha thalassemia and stroke risk in sickle cell anemia. Am J Hematol 1994;45:279–82.
- Ballas SK. Effect of alpha-globin genotype on the pathophysiology of sickle cell disease. Pediatr Pathol Mol Med 2001;20:107–21.
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med 1986;314:1593–9.
- Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin 1989;13:649–55.
- .Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007;21:37–47.
- Murphy JR, Wengard M, Brereton W. Rheological studies of Hb SS blood: influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med 1976;87:475–86.
- Fabry ME, Kaul DK, Raventos-Suarez C, Chang H, Nagel RL. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest 1982;70:1315–9.
- Stuart J, Johnson CS. Rheology of the sickle cell disorders. Baillieres Clin Haematol 1987;1:747–75.
- Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136-41.
- Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr 2003;143:438–44.
- Abboud MR, Yim E, Musallam KM, Adams RJ, Investigators SIS. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood 2011;118:894–8.
- Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol 2000;15:344–9.
- Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood 2004;103:3689–94.
- Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108:847–52.
- Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 1992;79:2555–65.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22.
- Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol 1997;34:15–21.
- Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol 2010;85:403–8.
- Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 1988;318:96–9.
- Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–6.
- Maier-Redelsperger M, de Montalembert M, Flahault A, et al. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients. The French Study Group on Sickle Cell Disease. Blood 1998;91:4472–9.
- Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 2014;123:481–5.
- Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 1994;83:553–60.
- Bridges KR, Barabino GD, Brugnara C, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood 1996;88:4701–10.
- Jiang J, Jordan SJ, Barr DP, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol 1997;52:1081–6.
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
- Yates AM, Dedeken L, Smeltzer MP, et al. Hydroxyurea treatment of children with hemoglobin SC disease. Pediatr Blood Cancer 2013;60:323–5.
- Barbosa CG, Aleluia AC, Pacheco AP, et al. Genetic modulation of HbF in Brazilians with HbSC disease and sickle cell anemia. Am J Hematol 2013;88:923–4.
- Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009;361:2309–17.
- King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood 2014;123:3089–94.
- Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD007001.
- Freed J, Talano J, Small T, et al. Allogeneic cellular and autologous stem cell therapy for sickle cell disease: ‘whom, when and how’. Bone Marrow Transplant 2012;47:1489–98.
- Urbinati F, Hargrove PW, Geiger S, et al. Potentially therapeutic levels of anti-sickling globin gene expression following lentivirus-mediated gene transfer in sickle cell disease bone marrow CD34 cells. Exp Hematol 2015;43:346–51.
- Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes’s original 1934 classification to the third millennium. Curr Opin Hematol 2013;20:222–30.
- Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am Soc Hematol Educ Program 2010;2010:418–22.
- Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987;317:781–7.
- Goldsmith JC, Bonham VL, Joiner CH, et al. Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol 2012;87:340–6.
- Grant AM, Parker CS, Jordan LB, et al. Public health implications of sickle cell trait: a report of the CDC meeting. Am J Prev Med 2011;41:S435–9.
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol 2005;79:17–25.
- Serjeant GR, Ceulaer CD, Lethbridge R, et al. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol 1994;87:586–91.
- Vichinsky EP, Johnson R, Lubin BH. Multidisciplinary approach to pain management in sickle cell disease. Am J Pediatr Hematol Oncol 1982;4:328–33.
- Gil KM, Carson JW, Porter LS, et al. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol 2004;23:267–74.
- Amjad H, Bannerman RM, Judisch JM. Letter: Sickling pain and season. Br Med J 1974;2:54.
- Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Trans R Soc Trop Med Hyg 1980;74:159–61.
- Jones S, Duncan ER, Thomas N, et al. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol 2005;131:530–3.
- Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr 1991;118:407–9.
- Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol 2014;93:89–95.
- Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr 2011;160:286–90.
- Hollins M, Stonerock GL, Kisaalita NR, et al. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage 2012;43:1082–93.
- Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol 2013;88:927–9.
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer 2015 May 13. doi: 10.1002/pbc.25574. [Epub ahead of print].
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61:512–7.
- Brandow AM, Farley RA, Dasgupta M, et al. The use of neuropathic pain drugs in children with sickle cell disease is associated with older age, female sex, and longer length of hospital stay. J Pediatr Hematol Oncol 2015;37:10–5.
- Walker TM, Hambleton IR, Serjeant GR. Gallstones in sickle cell disease: observations from The Jamaican Cohort study. J Pediatr 2000;136:80–5.
- Penner E, Mayr WR, Djawan S, et al. [The genetics of Gilbert syndrome]. Schweiz Med Wochenschr 1976;106:860–2. [German]
- Powell RW, Levine GL, Yang YM, Mankad VN. Acute splenic sequestration crisis in sickle cell disease: early detection and treatment. J Pediatr Surg 1992;27:215–8.
- Al Salem AH, Qaisaruddin S, Nasserullah Z, et al. Splenectomy and acute splenic sequestration crises in sickle cell disease. Pediatr Surg Int 1996;11:26–8.
- Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med 1969;281:923–6.
- Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377:1663–72.
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 2014;166:165–76.
- Orringer EP, Fowler VG Jr, Owens CM, et al. Case report: splenic infarction and acute splenic sequestration in adults with hemoglobin SC disease. Am J Med Sci 1991;302:374–9.
- Michel JB, Hernandez JA, Buchanan GR. A fatal case of acute splenic sequestration in a 53–year-old woman with sickle-hemoglobin C disease. Am J Med 1992;92:97–100.
- Hatton CS, Bunch C, Weatherall DJ. Hepatic sequestration in sickle cell anaemia. Br Med J (Clin Res Ed) 1985;290:744–5.
- Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994;84:643–9.
- Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood 1995;86:776–83.
- Owusu-Ofori S, Hirst C. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD003425.
- 00.Al-Salem AH. Splenic complications of sickle cell anemia and the role of splenectomy. ISRN Hematol 2011;2011:864257.
- Sabarense AP, Lima GO, Silva LM, Viana MB. Characterization of mortality in children with sickle cell disease diagnosed through the Newborn Screening Program. J Pediatr (Rio J) 2015;91:242–7.
- Aslam AF, Aslam AK, Dipillo F. Fatal splenic sequestration crisis with multiorgan failure in an adult woman with sickle cell-beta+ thalassemia. Am J Med Sci 2005;329:141–3.
- Berry RA, Odumakinde EA, Lewis JP. Massive splenic infarction in doubly abnormal heterozygous sickling disorders. A new complication of acute splenic sequestration syndrome. The West J Med 1991;155:531–2.
- Bonanomi MT, Lavezzo MM. Sickle cell retinopathy: diagnosis and treatment. Arq Bras de Oftalmol 2013;76:320–7.
- Chen RW, Flynn HW Jr, Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. Am J Ophthalmol 2014;157:870–5 e1.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930–8.
- Neumayr L, Koshy M, Haberkern C, et al. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. Am J Hematol 1998;57:101–8.
- 08. Savage WJ, Buchanan GR, Yawn BP, et al. Evidence gaps in the management of sickle cell disease: A summary of needed research. Am J Hematol 2015;90:273–5.
- Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300–26.
- Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol 1997;98:838–44.
- Wang W, Brugnara C, Snyder C, et al. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. Br J Haematol 2011;152:771–6.
- Jungst C, Kullak-Ublick GA, Jungst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol 2006;20:1053–62.
- Walker TM, Serjeant GR. Biliary sludge in sickle cell disease. J Pediatr 1996;129:443–5.
- Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7.
- Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol 2010;8:483–9.
- Hiran S. Multiorgan dysfunction syndrome in sickle cell disease. J Assoc Physicians India 2005;53:19–22.
- Shao SH, Orringer EP. Case report: splenic sequestration and multiorgan failure as the presenting manifestation of hemoglobin SC disease. Am J Med Sci 1996;311:139–41.
- Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 1994;96:155–62.