User login
Navigating challenges in COVID-19 care
Early strategies for adapting to a moving target
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
Early strategies for adapting to a moving target
Early strategies for adapting to a moving target
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
The state of inpatient COVID-19 care
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.
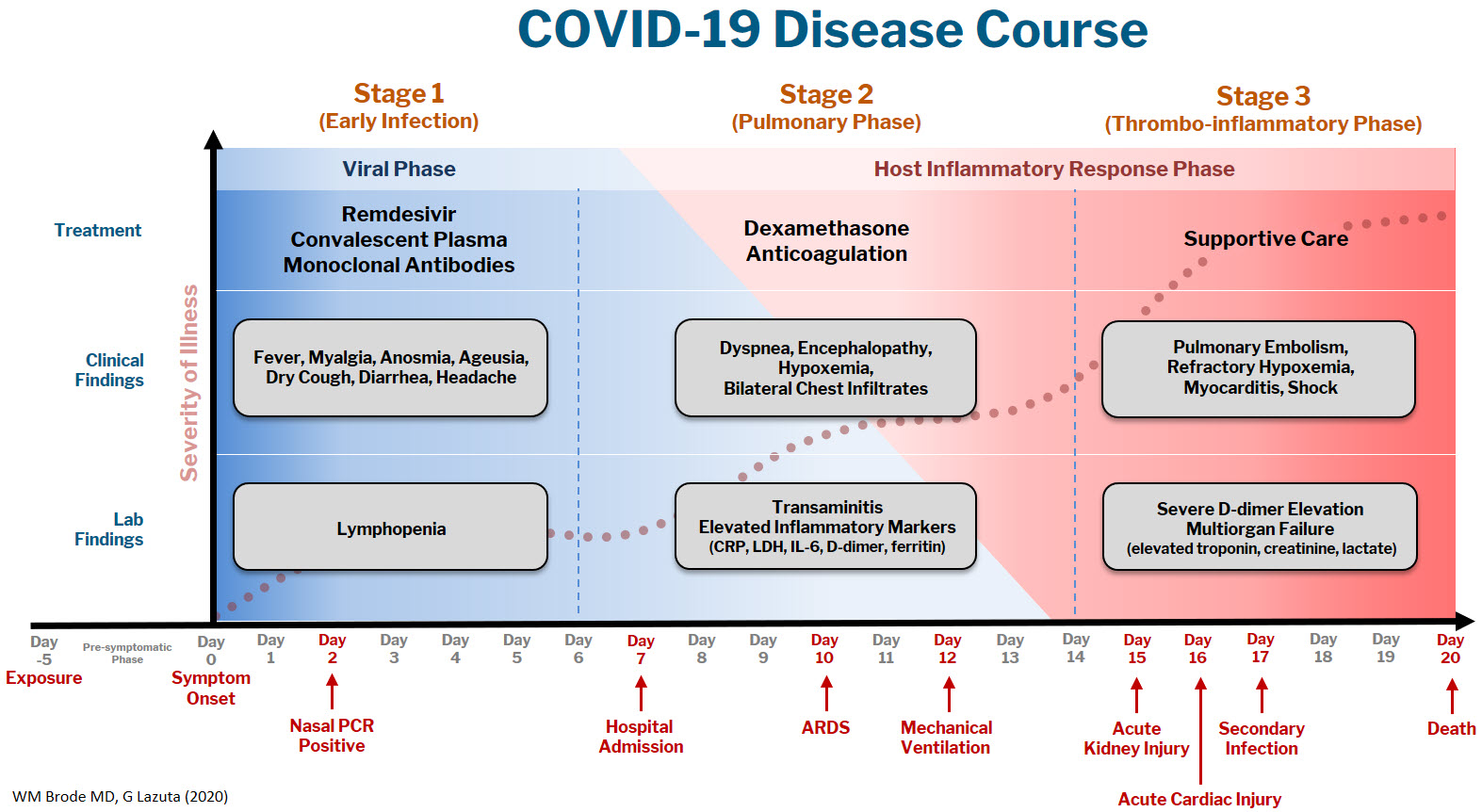
At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
A brief evidence-based review of everything we have learned
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

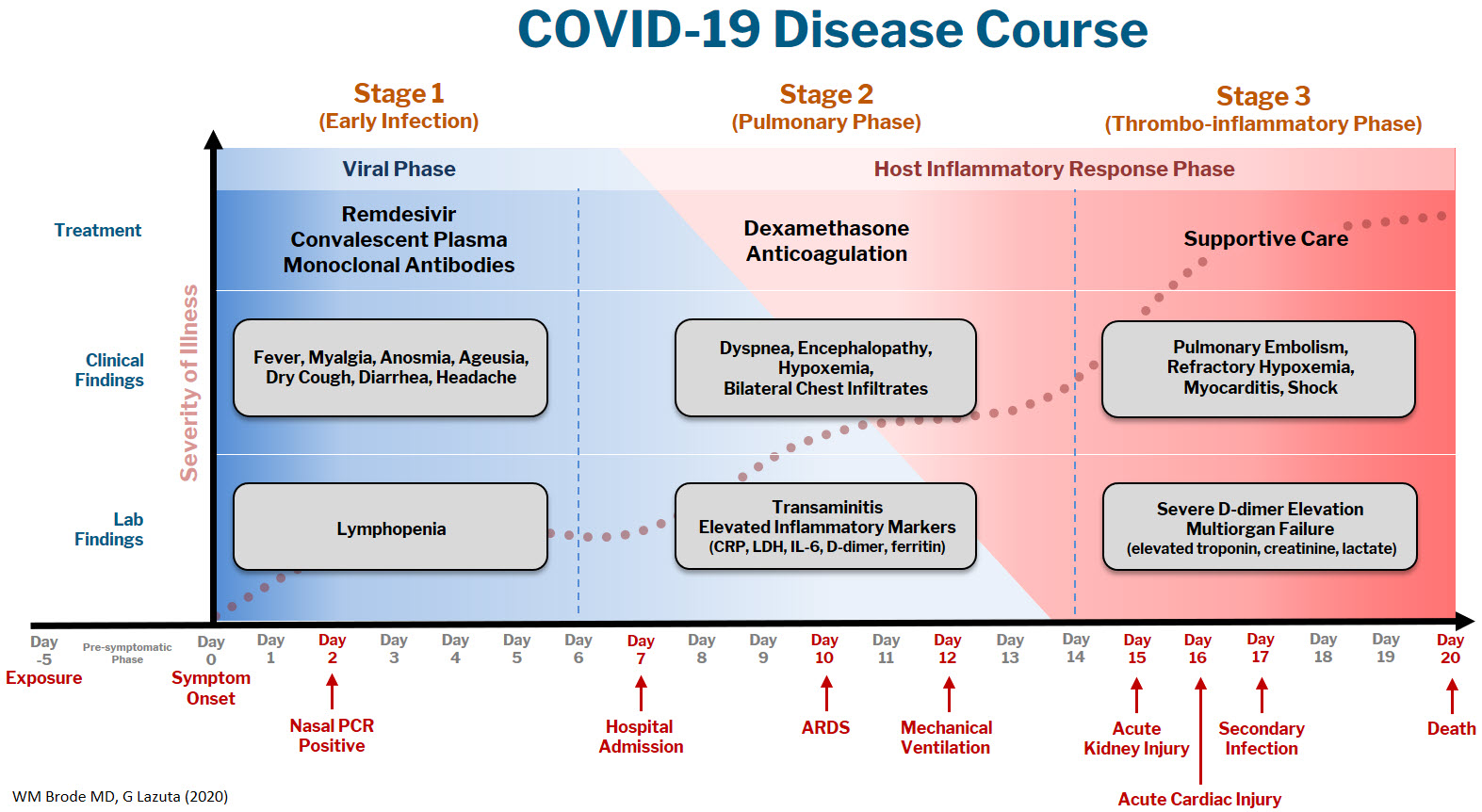
At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.