User login
Caring for women with HIV: Unique needs and challenges
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
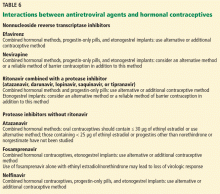
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
KEY POINTS
- The number of women living with HIV has increased over the past 30 years, and African American women bear a disproportionate burden of disease.
- Women of all ages are at risk of acquiring HIV; therefore, HIV testing should be part of routine care.
- Preconception counseling is an essential component of both primary and preventive care and should be the standard of care for all women of reproductive age with HIV.
- Women with HIV have the same gynecologic problems as all women but may be more vulnerable to certain conditions, such as human papillomavirus infection.