User login
Healthcare Utilization after Sepsis
Sepsis, the systemic inflammatory response to infection, is a major public health concern.[1] Worldwide, sepsis affects millions of hospitalized patients each year.[2] In the United States, it is the single most expensive cause of hospitalization.[3, 4, 5, 6] Multiple studies suggest that sepsis hospitalizations are also increasing in frequency.[3, 6, 7, 8, 9, 10]
Improved sepsis care has dramatically reduced in‐hospital mortality.[11, 12, 13] However, the result is a growing number of sepsis survivors discharged with new disability.[1, 9, 14, 15, 16] Despite being a common cause of hospitalization, little is known about how to improve postsepsis care.[15, 17, 18, 19] This contrasts with other, often less common, hospital conditions for which many studies evaluating readmission and postdischarge care are available.[20, 21, 22, 23] Identifying the factors contributing to high utilization could lend critical insight to designing interventions that improve long‐term sepsis outcomes.[24]
We conducted a retrospective study of sepsis patients discharged in 2010 at Kaiser Permanente Northern California (KPNC) to describe their posthospital trajectories. In this diverse community‐hospitalbased population, we sought to identify the patient‐level factors that impact the posthospital healthcare utilization of sepsis survivors.
METHODS
This study was approved by the KPNC institutional review board.
Setting
We conducted a retrospective study of sepsis patients aged 18 years admitted to KPNC hospitals in 2010 whose hospitalizations included an overnight stay, began in a KPNC hospital, and was not for peripartum care. We identified sepsis based on International Classification of Disease, 9th Edition principal diagnosis codes used at KPNC, which capture a similar population to that from the Angus definition (see Supporting Appendix, Table 1, in the online version of this article).[7, 25, 26] We denoted each patient's first sepsis hospitalization as the index event.
| Predicted Hospital Mortality Quartiles (n=1,586 for Each Group) | |||||
|---|---|---|---|---|---|
| Overall | 1 | 2 | 3 | 4 | |
| |||||
| Baseline | |||||
| Age, y, mean | 71.915.7 | 62.317.8 | 71.214.2 | 75.612.7 | 78.612.2 |
| By age category | |||||
| <45 years | 410 (6.5) | 290 (18.3) | 71 (4.5) | 25 (1.6) | 24 (1.5) |
| 4564 years | 1,425 (22.5) | 539 (34.0) | 407 (25.7) | 292 (18.4) | 187 (11.8) |
| 6584 years | 3,036 (47.9) | 601 (37.9) | 814 (51.3) | 832 (52.5) | 789 (49.8) |
| 85 years | 1,473 (23.2) | 156 (9.8) | 294 (18.5) | 437 (27.6) | 586 (37.0) |
| Male | 2,973 (46.9) | 686 (43.3) | 792 (49.9) | 750 (47.3) | 745 (47.0) |
| Comorbidity | |||||
| COPS2 score | 5143 | 2627 | 5441 | 6445 | 6245 |
| Charlson score | 2.01.5 | 1.31.2 | 2.11.4 | 2.41.5 | 2.41.5 |
| Hospitalization | |||||
| LAPS2 severity score | 10742 | 6621 | 9020 | 11423 | 15928 |
| Admitted via emergency department | 6,176 (97.4) | 1,522 (96.0) | 1,537 (96.9) | 1,539 (97.0) | 1,578 (99.5) |
| Direct ICU admission | 1,730 (27.3) | 169 (10.7) | 309 (19.5) | 482 (30.4) | 770 (48.6) |
| ICU transfer, at any time | 2,206 (34.8) | 279 (17.6) | 474 (29.9) | 603 (38.0) | 850 (53.6) |
| Hospital mortality | |||||
| Predicted, % | 10.513.8 | 1.00.1 | 3.40.1 | 8.32.3 | 29.415.8 |
| Observed | 865 (13.6) | 26 (1.6) | 86 (5.4) | 197 (12.4) | 556 (35.1) |
| Hospital length of stay, d | 5.86.4 | 4.43.8 | 5.45.7 | 6.68.0 | 6.66.9 |
We linked hospital episodes with existing KPNC inpatient databases to describe patient characteristics.[27, 28, 29, 30] We categorized patients by age (45, 4564, 6584, and 85 years) and used Charlson comorbidity scores and Comorbidity Point Scores 2 (COPS2) to quantify comorbid illness burden.[28, 30, 31, 32] We quantified acute severity of illness using the Laboratory Acute Physiology Scores 2 (LAPS2), which incorporates 15 laboratory values, 5 vital signs, and mental status prior to hospital admission (including emergency department data).[30] Both the COPS2 and LAPS2 are independently associated with hospital mortality.[30, 31] We also generated a summary predicted risk of hospital mortality based on a validated risk model and stratified patients by quartiles.[30] We determined whether patients were admitted to the intensive care unit (ICU).[29]
Outcomes
We used patients' health insurance administrative data to quantify postsepsis utilization. Within the KPNC integrated healthcare delivery system, uniform information systems capture all healthcare utilization of insured members including services received at non‐KPNC facilities.[28, 30] We collected utilization data from the year preceding index hospitalization (presepsis) and for the year after discharge date or until death (postsepsis). We ascertained mortality after discharge from KPNC medical records as well as state and national death record files.
We grouped services into facility‐based or outpatient categories. Facility‐based services included inpatient admission, subacute nursing facility or long‐term acute care, and emergency department visits. We grouped outpatient services as hospice, home health, outpatient surgery, clinic, or other (eg, laboratory). We excluded patients whose utilization records were not available over the full presepsis interval. Among these 1211 patients (12.5% of total), the median length of records prior to index hospitalization was 67 days, with a mean value of 117 days.
Statistical Analysis
Our primary outcomes of interest were hospital readmission and utilization in the year after sepsis. We defined a hospital readmission as any inpatient stay after the index hospitalization grouped within 1‐, 3‐, 6‐, and 12‐month intervals. We designated those within 30 days as an early readmission. We grouped readmission principal diagnoses, where available, by the 17 Healthcare Cost and Utilization Project (HCUP) Clinical Classifications Software multilevel categories with sepsis in the infectious category.[33, 34] In secondary analysis, we also designated other infectious diagnoses not included in the standard HCUP infection category (eg, pneumonia, meningitis, cellulitis) as infection (see Supporting Appendix in the online version of this article).
We quantified outpatient utilization based on the number of episodes recorded. For facility‐based utilization, we calculated patient length of stay intervals. Because patients surviving their index hospitalization might not survive the entire year after discharge, we also calculated utilization adjusted for patients' living days by dividing the total facility length of stay by the number of living days after discharge.
Continuous data are represented as mean (standard deviation [SD]) and categorical data as number (%). We compared groups with analysis of variance or 2 testing. We estimated survival with Kaplan‐Meier analysis (95% confidence interval) and compared groups with log‐rank testing. We compared pre‐ and postsepsis healthcare utilization with paired t tests.
To identify factors associated with early readmission after sepsis, we used a competing risks regression model.[35] The dependent variable was time to readmission and the competing hazard was death within 30 days without early readmission; patients without early readmission or death were censored at 30 days. The independent variables included age, gender, comorbid disease burden (COPS2), acute severity of illness (LAPS2), any use of intensive care, total index length of stay, and percentage of living days prior to sepsis hospitalization spent utilizing facility‐based care. We also used logistic regression to quantify the association between these variables and high postsepsis utilization; we defined high utilization as 15% of living days postsepsis spent in facility‐based care. For each model, we quantified the relative contribution of each predictor variable to model performance based on differences in log likelihoods.[35, 36] We conducted analyses using STATA/SE version 11.2 (StataCorp, College Station, TX) and considered a P value of <0.05 to be significant.
RESULTS
Cohort Characteristics
Our study cohort included 6344 patients with index sepsis hospitalizations in 2010 (Table 1). Mean age was 72 (SD 16) years including 1835 (28.9%) patients aged <65 years. During index hospitalizations, higher predicted mortality was associated with increased age, comorbid disease burden, and severity of illness (P<0.01 for each). ICU utilization increased across predicted mortality strata; for example, 10.7% of patients in the lowest quartile were admitted directly to the ICU compared with 48.6% in the highest quartile. In the highest quartile, observed mortality was 35.1%.
One‐Year Survival
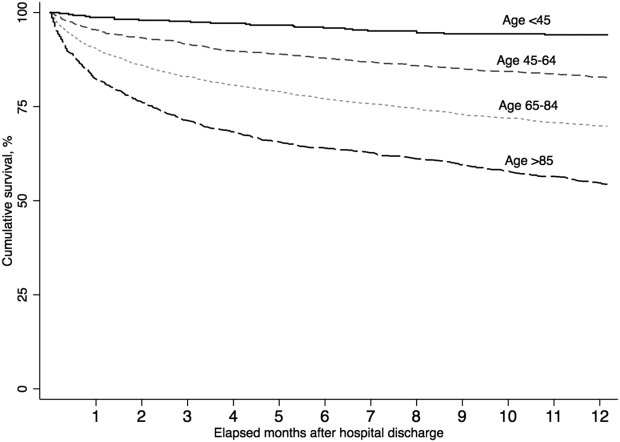
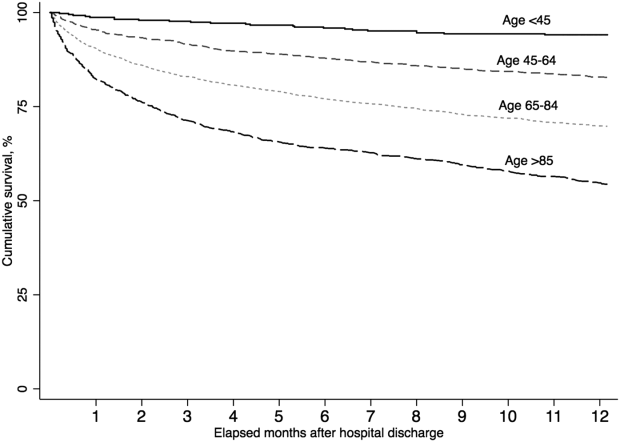
A total of 5479 (86.4%) patients survived their index sepsis hospitalization. Overall survival after living discharge was 90.5% (range, 89.6%91.2%) at 30 days and 71.3% (range, 70.1%72.5%) at 1 year. However, postsepsis survival was strongly modified by age (Figure 1). For example, 1‐year survival was 94.1% (range, 91.2%96.0%) for <45 year olds and 54.4% (range, 51.5%57.2%) for 85 year olds (P<0.01). Survival was also modified by predicted mortality, however, not by ICU admission during index hospitalization (P=0.18) (see Supporting Appendix, Figure 1, in the online version of this article).
Hospital Readmission
Overall, 978 (17.9%) patients had early readmission after index discharge (Table 2); nearly half were readmitted at least once in the year following discharge. Rehospitalization frequency was slightly lower when including patients with incomplete presepsis data (see Supporting Appendix, Table 2, in the online version of this article). The frequency of hospital readmission varied based on patient age and severity of illness. For example, 22.3% of patients in the highest predicted mortality quartile had early readmission compared with 11.6% in the lowest. The median time from discharge to early readmission was 11 days. Principal diagnoses were available for 78.6% of all readmissions (see Supporting Appendix, Table 3, in the online version of this article). Between 28.3% and 42.7% of those readmissions were for infectious diagnoses (including sepsis).
| Predicted Mortality Quartile | |||||
|---|---|---|---|---|---|
| Readmission | Overall | 1 | 2 | 3 | 4 |
| Within 30 days | 978 (17.9) | 158 (11.6) | 242 (17.7) | 274 (20.0) | 304 (22.3) |
| Within 90 days | 1,643 (30.1) | 276 (20.2) | 421 (30.8) | 463 (33.9) | 483 (35.4) |
| Within 180 days | 2,061 (37.7) | 368 (26.9) | 540 (39.5) | 584 (42.7) | 569 (41.7) |
| Within 365 days | 2,618 (47.9) | 498 (36.4) | 712 (52.1) | 723 (52.9) | 685 (50.2) |
| Variable | Hazard Ratio for Early Readmission | Odds Ratio for High Utilization | ||
|---|---|---|---|---|
| HR (95% CI) | Relative Contribution | OR (95% CI) | Relative Contribution | |
| ||||
| Age category | 1.2% | 11.1% | ||
| <45 years | 1.00 [reference] | 1.00 [reference] | ||
| 4564 years | 0.86 (0.64‐1.16) | 2.22 (1.30‐3.83)a | ||
| 6584 years | 0.92 (0.69‐1.21) | 3.66 (2.17‐6.18)a | ||
| 85 years | 0.95 (0.70‐1.28) | 4.98 (2.92‐8.50)a | ||
| Male | 0.99 (0.88‐1.13) | 0.0% | 0.86 (0.74‐1.00) | 0.1% |
| Severity of illness (LAPS2) | 1.08 (1.04‐1.12)a | 12.4% | 1.22 (1.17‐1.27)a | 11.3% |
| Comorbid illness (COPS2) | 1.16 (1.12‐1.19)a | 73.9% | 1.13 (1.09‐1.17)a | 5.9% |
| Intensive care | 1.21 (1.05‐1.40)a | 5.2% | 1.02 (0.85‐1.21) | 0.0% |
| Hospital length of stay, day | 1.01 (1.001.02)b | 6.6% | 1.04 (1.03‐1.06)a | 6.9% |
| Prior utilization, per 10% | 0.98 (0.95‐1.02) | 0.7% | 1.74 (1.61‐1.88)a | 64.2% |
Healthcare Utilization
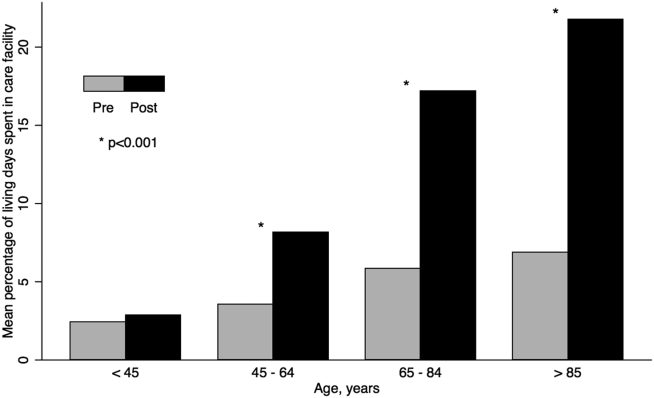
The unadjusted difference between pre‐ and postsepsis healthcare utilization among survivors was statistically significant for most categories but of modest clinical significance (see Supporting Appendix, Table 4, in the online version of this article). For example, the mean number of presepsis hospitalizations was 0.9 (1.4) compared to 1.0 (1.5) postsepsis (P<0.01). After adjusting for postsepsis living days, the difference in utilization was more pronounced (Figure 2). Overall, there was roughly a 3‐fold increase in the mean percentage of living days spent in facility‐based care between patients' pre‐ and postsepsis phases (5.3% vs 15.0%, P<0.01). Again, the difference was strongly modified by age. For patients aged <45 years, the difference was not statistically significant (2.4% vs 2.9%, P=0.32), whereas for those aged 65 years, it was highly significant (6.2% vs 18.5%, P<0.01).
Factors associated with early readmission included severity of illness, comorbid disease burden, index hospital length of stay, and intensive care (Table 3). However, the dominant factor explaining variation in the risk of early readmission was patients' prior comorbid disease burden (73.9%), followed by acute severity of illness (12.4%), total hospital length of stay (6.6%), and the need for intensive care (5.2%). Severity of illness and age were also significantly associated with higher odds of high postsepsis utilization; however, the dominant factor contributing to this risk was a history of high presepsis utilization (64.2%).
DISCUSSION
In this population‐based study in a community healthcare system, the impact of sepsis extended well beyond the initial hospitalization. One in 6 sepsis survivors was readmitted within 30 days, and roughly half were readmitted within 1 year. Fewer than half of rehospitalizations were for sepsis. Patients had a 3‐fold increase in the percentage of living days spent in hospitals or care facilities after sepsis hospitalization. Although age and acute severity of illness strongly modified healthcare utilization and mortality after sepsis, the dominant factors contributing to early readmission and high utilization ratescomorbid disease burden and presepsis healthcare utilizationwere present prior to hospitalization.
Sepsis is the single most expensive cause of US hospitalizations.[3, 4, 5] Despite its prevalence, there are little contemporary data identifying factors that impact healthcare utilization among sepsis survivors.[9, 16, 17, 19, 24, 36, 37] Recently, Prescott and others found that in Medicare beneficiaries, following severe sepsis, healthcare utilization was markedly increased.[17] More than one‐quarter of survivors were readmitted within 30 days, and 63.8% were readmitted within a year. Severe sepsis survivors also spent an average of 26% of their living days in a healthcare facility, a nearly 4‐fold increase compared to their presepsis phase. The current study included a population with a broader age and severity range; however, in a similar subgroup of patients, for those aged 65 years within the highest predicted mortality quartile, the frequency of readmission was similar. These findings are concordant with those from prior studies.[17, 19, 36, 37]
Among sepsis survivors, most readmissions were not for sepsis or infectious diagnoses, which is a novel finding with implications for designing approaches to reduce rehospitalization. The pattern in sepsis is similar to that seen in other common and costly hospital conditions.[17, 20, 23, 38, 39, 40] For example, between 18% and 25% of Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, or pneumonia were readmitted within 30 days; fewer than one‐third had the same diagnosis.[20] The timing of readmission in our sepsis cohort was also similar to that seen in other conditions.[20] For example, the median time of early readmission in this study was 11 days; it was between 10 and 12 days for patients with heart failure, pneumonia, and myocardial infarction.[20]
Krumholz and others suggest that the pattern of early rehospitalization after common acute conditions reflects a posthospital syndromean acquired, transient period of vulnerabilitythat could be the byproduct of common hospital factors.[20, 41] Such universal impairments might result from new physical and neurocognitive disability, nutritional deficiency, and sleep deprivation or delirium, among others.[41] If this construct were also true in sepsis, it could have important implications on the design of postsepsis care. However, prior studies suggest that sepsis patients may be particularly vulnerable to the sequelae of hospitalization.[2, 42, 43, 44, 45]
Among Medicare beneficiaries, Iwashyna and others reported that hospitalizations for severe sepsis resulted in significant increases in physical limitations and moderate to severe cognitive impairment.[1, 14, 46] Encephalopathy, sleep deprivation, and delirium are also frequently seen in sepsis patients.[47, 48] Furthermore, sepsis patients frequently need intensive care, which is also associated with increased patient disability and injury.[16, 46, 49, 50] We found that severity of illness and the need for intensive care were both predictive of the need for early readmission following sepsis. We also confirmed the results of prior studies suggesting that sepsis outcomes are strongly modified by age.[16, 19, 43, 51]
However, we found that the dominant factors contributing to patients' health trajectories were conditions present prior to admission. This finding is in accord with prior suggestions that acute severity of illness only partially predicts patients facing adverse posthospital sequelae.[23, 41, 52] Among sepsis patients, prior work demonstrates that inadequate consideration for presepsis level of function and utilization can result in an overestimation of the impact of sepsis on postdischarge health.[52, 53] Further, we found that the need for intensive care was not independently associated with an increased risk of high postsepsis utilization after adjusting for illness severity, a finding also seen in prior studies.[17, 23, 38, 51]
Taken together, our findings might suggest that an optimal approach to posthospital care in sepsis should focus on treatment approaches that address disease‐specific problems within the much larger context of common hospital risks. However, further study is necessary to clearly define the mechanisms by which age, severity of illness, and intensive care affect subsequent healthcare utilization. Furthermore, sepsis patients are a heterogeneous population in terms of severity of illness, site and pathogen of infection, and underlying comorbidity whose posthospital course remains incompletely characterized, limiting our ability to draw strong inferences.
These results should be interpreted in light of the study's limitations. First, our cohort included patients with healthcare insurance within a community‐based healthcare system. Care within the KPNC system, which bears similarities with accountable care organizations, is enhanced through service integration and a comprehensive health information system. Although prior studies suggest that these characteristics result in improved population‐based care, it is unclear whether there is a similar impact in hospital‐based conditions such as sepsis.[54, 55] Furthermore, care within an integrated system may impact posthospital utilization patterns and could limit generalizability. However, prior studies demonstrate the similarity of KPNC members to other patients in the same region in terms of age, socioeconomics, overall health behaviors, and racial/ethnic diversity.[56] Second, our study did not characterize organ dysfunction based on diagnosis coding, a common feature of sepsis studies that lack detailed physiologic severity data.[4, 5, 6, 8, 26] Instead, we focused on using granular laboratory and vital signs data to ensure accurate risk adjustment using a validated system developed in >400,000 hospitalizations.[30] Although this method may hamper comparisons with existing studies, traditional methods of grading severity by diagnosis codes can be vulnerable to biases resulting in wide variability.[10, 23, 26, 57, 58] Nonetheless, it is likely that characterizing preexisting and acute organ dysfunction will improve risk stratification in the heterogeneous sepsis population. Third, this study did not include data regarding patients' functional status, which has been shown to strongly predict patient outcomes following hospitalization. Fourth, this study did not address the cost of care following sepsis hospitalizations.[19, 59] Finally, our study excluded patients with incomplete utilization records, a choice designed to avoid the spurious inferences that can result from such comparisons.[53]
In summary, we found that sepsis exacted a considerable toll on patients in the hospital and in the year following discharge. Sepsis patients were frequently rehospitalized within a month of discharge, and on average had a 3‐fold increase in their subsequent time spent in healthcare facilities. Although age, severity of illness, and the need for ICU care impacted postsepsis utilization, the dominant contributing factorscomorbid disease burden or presepsis utilizationwere present prior to sepsis hospitalization. Early readmission patterns in sepsis appeared similar to those seen in other important hospital conditions, suggesting a role for shared posthospital, rather than just postsepsis, care approaches.
Disclosures
The funding for this study was provided by The Permanente Medical Group, Inc. and Kaiser Foundation Hospitals. The authors have no conflict of interests to disclose relevant to this article.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–1834.
- , , , et al.; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , . Costs for hospital stays in the United States, 2010. HCUP statistical brief #16. January 2013. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb146.pdf. Accessed October 1, 2013.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250.
- , , . Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. October 2011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb122.pdf. Accessed October 1, 2013.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761.
- , , , . Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174.
- , , , et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924.
- , , , . Reducing mortality in severe sepsis: the Surviving Sepsis Campaign. Clin Chest Med. 2008;29(4):721–733, x.
- , , , et al.; Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , , . Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283.
- , , , et al.; the Scottish Critical Care Trials Group and the Scottish Intensive Care Society Audit Group. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17(2):R70.
- , , , , . Post‐Discharge Health Care Use Is Markedly Higher in Survivors of Severe Sepsis. Am J Respir Crit Care Med 2013;187:A1573.
- , , , . Long‐term survival and function after suspected gram‐negative sepsis. JAMA. 1995;274(4):338–345.
- , , , , . Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31(9):2316–2323.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , , . A systematic review and meta‐analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164(21):2315–2320.
- , . Hospitalizations for heart failure in the United States—a sign of hope. JAMA. 2011;306(15):1705–1706.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Sepsis after Scotland: enough with the averages, show us the effect modifiers. Crit Care. 2013;17(3):148.
- , , , et al. Kaiser Permanente's performance improvement system, part 3: multisite improvements in care for patients with sepsis. Jt Comm J Qual Patient Saf. 2011;37(11): 483–493.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus implementation of the International Consensus Conference Definition of Severe Sepsis [published online ahead of print September 18, 2012]. Med Care. doi: 10.1097/MLR.0b013e318268ac86. Epub ahead of print.
- . Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8 pt 2):719–724.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , , . An electronic Simplified Acute Physiology Score‐based risk adjustment score for critical illness in an integrated healthcare system. Crit Care Med. 2013;41(1):41–48.
- , , , , . Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , . The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2009;63(7):798–803.
- , , , , , . Casemix adjustment of managed care claims data using the clinical classification for health policy research method. Med Care. 1998;36(7):1108–1113.
- Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project. Clinical Classifications Software (CCS) for ICD‐9‐CM Fact Sheet. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccsfactsheet.jsp. Accessed January 20, 2013.
- , . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1997;94(446):496–509.
- , , . Severe sepsis in managed care: analysis of incidence, one‐year mortality, and associated costs of care. J Manag Care Pharm. 2004;10(6):521–530.
- , , , , , . Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med. 2004;32(4):981–985.
- , , , , et al. Readmissions among patients with severe sepsis/septic shock among inner‐city minority New Yorkers. Chest. 2012;142:286A.
- , , . Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–857.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , . The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21.
- , . Multiple systems organ failure: failure of host defense homeostasis. Crit Care Clin. 1989;5(2):199–220.
- . Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444.
- , . Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29(3):368–377.
- , , . The encephalopathy in sepsis. Crit Care Clin. 2008;24(1):67–82, viii.
- , . Sepsis‐associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–566.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509.
- , , , , . The association between sepsis and potential medical injury among hospitalized patients. Chest. 2012;142(3):606–613.
- , , , , , . Three‐year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303(9):849–856.
- , , , , , . Does acute organ dysfunction predict patient‐centered outcomes? Chest. 2002;121(6):1963–1971.
- , , , . Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–841.
- , , , , , . Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- , , , et al., Outpatient electronic health records and the clinical care and outcomes of patients with diabetes mellitus. Ann Intern Med. 2012;157(7):482–489.
- . Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2009 California Health Interview Survey. Internal Division of Research Report. Oakland, CA: Kaiser Permanente Division of Research; January 24, 2012. Available at: http://www.dor.kaiser.org/external/chis_non_kp_2009. Accessed January 20, 2013.
- , , , , . Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413.
- , . Finding pure and simple truths with administrative data. JAMA. 2012;307(13):1433–1435.
- , , , . Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46(12):1226–1233.
Sepsis, the systemic inflammatory response to infection, is a major public health concern.[1] Worldwide, sepsis affects millions of hospitalized patients each year.[2] In the United States, it is the single most expensive cause of hospitalization.[3, 4, 5, 6] Multiple studies suggest that sepsis hospitalizations are also increasing in frequency.[3, 6, 7, 8, 9, 10]
Improved sepsis care has dramatically reduced in‐hospital mortality.[11, 12, 13] However, the result is a growing number of sepsis survivors discharged with new disability.[1, 9, 14, 15, 16] Despite being a common cause of hospitalization, little is known about how to improve postsepsis care.[15, 17, 18, 19] This contrasts with other, often less common, hospital conditions for which many studies evaluating readmission and postdischarge care are available.[20, 21, 22, 23] Identifying the factors contributing to high utilization could lend critical insight to designing interventions that improve long‐term sepsis outcomes.[24]
We conducted a retrospective study of sepsis patients discharged in 2010 at Kaiser Permanente Northern California (KPNC) to describe their posthospital trajectories. In this diverse community‐hospitalbased population, we sought to identify the patient‐level factors that impact the posthospital healthcare utilization of sepsis survivors.
METHODS
This study was approved by the KPNC institutional review board.
Setting
We conducted a retrospective study of sepsis patients aged 18 years admitted to KPNC hospitals in 2010 whose hospitalizations included an overnight stay, began in a KPNC hospital, and was not for peripartum care. We identified sepsis based on International Classification of Disease, 9th Edition principal diagnosis codes used at KPNC, which capture a similar population to that from the Angus definition (see Supporting Appendix, Table 1, in the online version of this article).[7, 25, 26] We denoted each patient's first sepsis hospitalization as the index event.
| Predicted Hospital Mortality Quartiles (n=1,586 for Each Group) | |||||
|---|---|---|---|---|---|
| Overall | 1 | 2 | 3 | 4 | |
| |||||
| Baseline | |||||
| Age, y, mean | 71.915.7 | 62.317.8 | 71.214.2 | 75.612.7 | 78.612.2 |
| By age category | |||||
| <45 years | 410 (6.5) | 290 (18.3) | 71 (4.5) | 25 (1.6) | 24 (1.5) |
| 4564 years | 1,425 (22.5) | 539 (34.0) | 407 (25.7) | 292 (18.4) | 187 (11.8) |
| 6584 years | 3,036 (47.9) | 601 (37.9) | 814 (51.3) | 832 (52.5) | 789 (49.8) |
| 85 years | 1,473 (23.2) | 156 (9.8) | 294 (18.5) | 437 (27.6) | 586 (37.0) |
| Male | 2,973 (46.9) | 686 (43.3) | 792 (49.9) | 750 (47.3) | 745 (47.0) |
| Comorbidity | |||||
| COPS2 score | 5143 | 2627 | 5441 | 6445 | 6245 |
| Charlson score | 2.01.5 | 1.31.2 | 2.11.4 | 2.41.5 | 2.41.5 |
| Hospitalization | |||||
| LAPS2 severity score | 10742 | 6621 | 9020 | 11423 | 15928 |
| Admitted via emergency department | 6,176 (97.4) | 1,522 (96.0) | 1,537 (96.9) | 1,539 (97.0) | 1,578 (99.5) |
| Direct ICU admission | 1,730 (27.3) | 169 (10.7) | 309 (19.5) | 482 (30.4) | 770 (48.6) |
| ICU transfer, at any time | 2,206 (34.8) | 279 (17.6) | 474 (29.9) | 603 (38.0) | 850 (53.6) |
| Hospital mortality | |||||
| Predicted, % | 10.513.8 | 1.00.1 | 3.40.1 | 8.32.3 | 29.415.8 |
| Observed | 865 (13.6) | 26 (1.6) | 86 (5.4) | 197 (12.4) | 556 (35.1) |
| Hospital length of stay, d | 5.86.4 | 4.43.8 | 5.45.7 | 6.68.0 | 6.66.9 |
We linked hospital episodes with existing KPNC inpatient databases to describe patient characteristics.[27, 28, 29, 30] We categorized patients by age (45, 4564, 6584, and 85 years) and used Charlson comorbidity scores and Comorbidity Point Scores 2 (COPS2) to quantify comorbid illness burden.[28, 30, 31, 32] We quantified acute severity of illness using the Laboratory Acute Physiology Scores 2 (LAPS2), which incorporates 15 laboratory values, 5 vital signs, and mental status prior to hospital admission (including emergency department data).[30] Both the COPS2 and LAPS2 are independently associated with hospital mortality.[30, 31] We also generated a summary predicted risk of hospital mortality based on a validated risk model and stratified patients by quartiles.[30] We determined whether patients were admitted to the intensive care unit (ICU).[29]
Outcomes
We used patients' health insurance administrative data to quantify postsepsis utilization. Within the KPNC integrated healthcare delivery system, uniform information systems capture all healthcare utilization of insured members including services received at non‐KPNC facilities.[28, 30] We collected utilization data from the year preceding index hospitalization (presepsis) and for the year after discharge date or until death (postsepsis). We ascertained mortality after discharge from KPNC medical records as well as state and national death record files.
We grouped services into facility‐based or outpatient categories. Facility‐based services included inpatient admission, subacute nursing facility or long‐term acute care, and emergency department visits. We grouped outpatient services as hospice, home health, outpatient surgery, clinic, or other (eg, laboratory). We excluded patients whose utilization records were not available over the full presepsis interval. Among these 1211 patients (12.5% of total), the median length of records prior to index hospitalization was 67 days, with a mean value of 117 days.
Statistical Analysis
Our primary outcomes of interest were hospital readmission and utilization in the year after sepsis. We defined a hospital readmission as any inpatient stay after the index hospitalization grouped within 1‐, 3‐, 6‐, and 12‐month intervals. We designated those within 30 days as an early readmission. We grouped readmission principal diagnoses, where available, by the 17 Healthcare Cost and Utilization Project (HCUP) Clinical Classifications Software multilevel categories with sepsis in the infectious category.[33, 34] In secondary analysis, we also designated other infectious diagnoses not included in the standard HCUP infection category (eg, pneumonia, meningitis, cellulitis) as infection (see Supporting Appendix in the online version of this article).
We quantified outpatient utilization based on the number of episodes recorded. For facility‐based utilization, we calculated patient length of stay intervals. Because patients surviving their index hospitalization might not survive the entire year after discharge, we also calculated utilization adjusted for patients' living days by dividing the total facility length of stay by the number of living days after discharge.
Continuous data are represented as mean (standard deviation [SD]) and categorical data as number (%). We compared groups with analysis of variance or 2 testing. We estimated survival with Kaplan‐Meier analysis (95% confidence interval) and compared groups with log‐rank testing. We compared pre‐ and postsepsis healthcare utilization with paired t tests.
To identify factors associated with early readmission after sepsis, we used a competing risks regression model.[35] The dependent variable was time to readmission and the competing hazard was death within 30 days without early readmission; patients without early readmission or death were censored at 30 days. The independent variables included age, gender, comorbid disease burden (COPS2), acute severity of illness (LAPS2), any use of intensive care, total index length of stay, and percentage of living days prior to sepsis hospitalization spent utilizing facility‐based care. We also used logistic regression to quantify the association between these variables and high postsepsis utilization; we defined high utilization as 15% of living days postsepsis spent in facility‐based care. For each model, we quantified the relative contribution of each predictor variable to model performance based on differences in log likelihoods.[35, 36] We conducted analyses using STATA/SE version 11.2 (StataCorp, College Station, TX) and considered a P value of <0.05 to be significant.
RESULTS
Cohort Characteristics
Our study cohort included 6344 patients with index sepsis hospitalizations in 2010 (Table 1). Mean age was 72 (SD 16) years including 1835 (28.9%) patients aged <65 years. During index hospitalizations, higher predicted mortality was associated with increased age, comorbid disease burden, and severity of illness (P<0.01 for each). ICU utilization increased across predicted mortality strata; for example, 10.7% of patients in the lowest quartile were admitted directly to the ICU compared with 48.6% in the highest quartile. In the highest quartile, observed mortality was 35.1%.
One‐Year Survival
A total of 5479 (86.4%) patients survived their index sepsis hospitalization. Overall survival after living discharge was 90.5% (range, 89.6%91.2%) at 30 days and 71.3% (range, 70.1%72.5%) at 1 year. However, postsepsis survival was strongly modified by age (Figure 1). For example, 1‐year survival was 94.1% (range, 91.2%96.0%) for <45 year olds and 54.4% (range, 51.5%57.2%) for 85 year olds (P<0.01). Survival was also modified by predicted mortality, however, not by ICU admission during index hospitalization (P=0.18) (see Supporting Appendix, Figure 1, in the online version of this article).

Hospital Readmission
Overall, 978 (17.9%) patients had early readmission after index discharge (Table 2); nearly half were readmitted at least once in the year following discharge. Rehospitalization frequency was slightly lower when including patients with incomplete presepsis data (see Supporting Appendix, Table 2, in the online version of this article). The frequency of hospital readmission varied based on patient age and severity of illness. For example, 22.3% of patients in the highest predicted mortality quartile had early readmission compared with 11.6% in the lowest. The median time from discharge to early readmission was 11 days. Principal diagnoses were available for 78.6% of all readmissions (see Supporting Appendix, Table 3, in the online version of this article). Between 28.3% and 42.7% of those readmissions were for infectious diagnoses (including sepsis).
| Predicted Mortality Quartile | |||||
|---|---|---|---|---|---|
| Readmission | Overall | 1 | 2 | 3 | 4 |
| Within 30 days | 978 (17.9) | 158 (11.6) | 242 (17.7) | 274 (20.0) | 304 (22.3) |
| Within 90 days | 1,643 (30.1) | 276 (20.2) | 421 (30.8) | 463 (33.9) | 483 (35.4) |
| Within 180 days | 2,061 (37.7) | 368 (26.9) | 540 (39.5) | 584 (42.7) | 569 (41.7) |
| Within 365 days | 2,618 (47.9) | 498 (36.4) | 712 (52.1) | 723 (52.9) | 685 (50.2) |
| Variable | Hazard Ratio for Early Readmission | Odds Ratio for High Utilization | ||
|---|---|---|---|---|
| HR (95% CI) | Relative Contribution | OR (95% CI) | Relative Contribution | |
| ||||
| Age category | 1.2% | 11.1% | ||
| <45 years | 1.00 [reference] | 1.00 [reference] | ||
| 4564 years | 0.86 (0.64‐1.16) | 2.22 (1.30‐3.83)a | ||
| 6584 years | 0.92 (0.69‐1.21) | 3.66 (2.17‐6.18)a | ||
| 85 years | 0.95 (0.70‐1.28) | 4.98 (2.92‐8.50)a | ||
| Male | 0.99 (0.88‐1.13) | 0.0% | 0.86 (0.74‐1.00) | 0.1% |
| Severity of illness (LAPS2) | 1.08 (1.04‐1.12)a | 12.4% | 1.22 (1.17‐1.27)a | 11.3% |
| Comorbid illness (COPS2) | 1.16 (1.12‐1.19)a | 73.9% | 1.13 (1.09‐1.17)a | 5.9% |
| Intensive care | 1.21 (1.05‐1.40)a | 5.2% | 1.02 (0.85‐1.21) | 0.0% |
| Hospital length of stay, day | 1.01 (1.001.02)b | 6.6% | 1.04 (1.03‐1.06)a | 6.9% |
| Prior utilization, per 10% | 0.98 (0.95‐1.02) | 0.7% | 1.74 (1.61‐1.88)a | 64.2% |
Healthcare Utilization
The unadjusted difference between pre‐ and postsepsis healthcare utilization among survivors was statistically significant for most categories but of modest clinical significance (see Supporting Appendix, Table 4, in the online version of this article). For example, the mean number of presepsis hospitalizations was 0.9 (1.4) compared to 1.0 (1.5) postsepsis (P<0.01). After adjusting for postsepsis living days, the difference in utilization was more pronounced (Figure 2). Overall, there was roughly a 3‐fold increase in the mean percentage of living days spent in facility‐based care between patients' pre‐ and postsepsis phases (5.3% vs 15.0%, P<0.01). Again, the difference was strongly modified by age. For patients aged <45 years, the difference was not statistically significant (2.4% vs 2.9%, P=0.32), whereas for those aged 65 years, it was highly significant (6.2% vs 18.5%, P<0.01).

Factors associated with early readmission included severity of illness, comorbid disease burden, index hospital length of stay, and intensive care (Table 3). However, the dominant factor explaining variation in the risk of early readmission was patients' prior comorbid disease burden (73.9%), followed by acute severity of illness (12.4%), total hospital length of stay (6.6%), and the need for intensive care (5.2%). Severity of illness and age were also significantly associated with higher odds of high postsepsis utilization; however, the dominant factor contributing to this risk was a history of high presepsis utilization (64.2%).
DISCUSSION
In this population‐based study in a community healthcare system, the impact of sepsis extended well beyond the initial hospitalization. One in 6 sepsis survivors was readmitted within 30 days, and roughly half were readmitted within 1 year. Fewer than half of rehospitalizations were for sepsis. Patients had a 3‐fold increase in the percentage of living days spent in hospitals or care facilities after sepsis hospitalization. Although age and acute severity of illness strongly modified healthcare utilization and mortality after sepsis, the dominant factors contributing to early readmission and high utilization ratescomorbid disease burden and presepsis healthcare utilizationwere present prior to hospitalization.
Sepsis is the single most expensive cause of US hospitalizations.[3, 4, 5] Despite its prevalence, there are little contemporary data identifying factors that impact healthcare utilization among sepsis survivors.[9, 16, 17, 19, 24, 36, 37] Recently, Prescott and others found that in Medicare beneficiaries, following severe sepsis, healthcare utilization was markedly increased.[17] More than one‐quarter of survivors were readmitted within 30 days, and 63.8% were readmitted within a year. Severe sepsis survivors also spent an average of 26% of their living days in a healthcare facility, a nearly 4‐fold increase compared to their presepsis phase. The current study included a population with a broader age and severity range; however, in a similar subgroup of patients, for those aged 65 years within the highest predicted mortality quartile, the frequency of readmission was similar. These findings are concordant with those from prior studies.[17, 19, 36, 37]
Among sepsis survivors, most readmissions were not for sepsis or infectious diagnoses, which is a novel finding with implications for designing approaches to reduce rehospitalization. The pattern in sepsis is similar to that seen in other common and costly hospital conditions.[17, 20, 23, 38, 39, 40] For example, between 18% and 25% of Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, or pneumonia were readmitted within 30 days; fewer than one‐third had the same diagnosis.[20] The timing of readmission in our sepsis cohort was also similar to that seen in other conditions.[20] For example, the median time of early readmission in this study was 11 days; it was between 10 and 12 days for patients with heart failure, pneumonia, and myocardial infarction.[20]
Krumholz and others suggest that the pattern of early rehospitalization after common acute conditions reflects a posthospital syndromean acquired, transient period of vulnerabilitythat could be the byproduct of common hospital factors.[20, 41] Such universal impairments might result from new physical and neurocognitive disability, nutritional deficiency, and sleep deprivation or delirium, among others.[41] If this construct were also true in sepsis, it could have important implications on the design of postsepsis care. However, prior studies suggest that sepsis patients may be particularly vulnerable to the sequelae of hospitalization.[2, 42, 43, 44, 45]
Among Medicare beneficiaries, Iwashyna and others reported that hospitalizations for severe sepsis resulted in significant increases in physical limitations and moderate to severe cognitive impairment.[1, 14, 46] Encephalopathy, sleep deprivation, and delirium are also frequently seen in sepsis patients.[47, 48] Furthermore, sepsis patients frequently need intensive care, which is also associated with increased patient disability and injury.[16, 46, 49, 50] We found that severity of illness and the need for intensive care were both predictive of the need for early readmission following sepsis. We also confirmed the results of prior studies suggesting that sepsis outcomes are strongly modified by age.[16, 19, 43, 51]
However, we found that the dominant factors contributing to patients' health trajectories were conditions present prior to admission. This finding is in accord with prior suggestions that acute severity of illness only partially predicts patients facing adverse posthospital sequelae.[23, 41, 52] Among sepsis patients, prior work demonstrates that inadequate consideration for presepsis level of function and utilization can result in an overestimation of the impact of sepsis on postdischarge health.[52, 53] Further, we found that the need for intensive care was not independently associated with an increased risk of high postsepsis utilization after adjusting for illness severity, a finding also seen in prior studies.[17, 23, 38, 51]
Taken together, our findings might suggest that an optimal approach to posthospital care in sepsis should focus on treatment approaches that address disease‐specific problems within the much larger context of common hospital risks. However, further study is necessary to clearly define the mechanisms by which age, severity of illness, and intensive care affect subsequent healthcare utilization. Furthermore, sepsis patients are a heterogeneous population in terms of severity of illness, site and pathogen of infection, and underlying comorbidity whose posthospital course remains incompletely characterized, limiting our ability to draw strong inferences.
These results should be interpreted in light of the study's limitations. First, our cohort included patients with healthcare insurance within a community‐based healthcare system. Care within the KPNC system, which bears similarities with accountable care organizations, is enhanced through service integration and a comprehensive health information system. Although prior studies suggest that these characteristics result in improved population‐based care, it is unclear whether there is a similar impact in hospital‐based conditions such as sepsis.[54, 55] Furthermore, care within an integrated system may impact posthospital utilization patterns and could limit generalizability. However, prior studies demonstrate the similarity of KPNC members to other patients in the same region in terms of age, socioeconomics, overall health behaviors, and racial/ethnic diversity.[56] Second, our study did not characterize organ dysfunction based on diagnosis coding, a common feature of sepsis studies that lack detailed physiologic severity data.[4, 5, 6, 8, 26] Instead, we focused on using granular laboratory and vital signs data to ensure accurate risk adjustment using a validated system developed in >400,000 hospitalizations.[30] Although this method may hamper comparisons with existing studies, traditional methods of grading severity by diagnosis codes can be vulnerable to biases resulting in wide variability.[10, 23, 26, 57, 58] Nonetheless, it is likely that characterizing preexisting and acute organ dysfunction will improve risk stratification in the heterogeneous sepsis population. Third, this study did not include data regarding patients' functional status, which has been shown to strongly predict patient outcomes following hospitalization. Fourth, this study did not address the cost of care following sepsis hospitalizations.[19, 59] Finally, our study excluded patients with incomplete utilization records, a choice designed to avoid the spurious inferences that can result from such comparisons.[53]
In summary, we found that sepsis exacted a considerable toll on patients in the hospital and in the year following discharge. Sepsis patients were frequently rehospitalized within a month of discharge, and on average had a 3‐fold increase in their subsequent time spent in healthcare facilities. Although age, severity of illness, and the need for ICU care impacted postsepsis utilization, the dominant contributing factorscomorbid disease burden or presepsis utilizationwere present prior to sepsis hospitalization. Early readmission patterns in sepsis appeared similar to those seen in other important hospital conditions, suggesting a role for shared posthospital, rather than just postsepsis, care approaches.
Disclosures
The funding for this study was provided by The Permanente Medical Group, Inc. and Kaiser Foundation Hospitals. The authors have no conflict of interests to disclose relevant to this article.
Sepsis, the systemic inflammatory response to infection, is a major public health concern.[1] Worldwide, sepsis affects millions of hospitalized patients each year.[2] In the United States, it is the single most expensive cause of hospitalization.[3, 4, 5, 6] Multiple studies suggest that sepsis hospitalizations are also increasing in frequency.[3, 6, 7, 8, 9, 10]
Improved sepsis care has dramatically reduced in‐hospital mortality.[11, 12, 13] However, the result is a growing number of sepsis survivors discharged with new disability.[1, 9, 14, 15, 16] Despite being a common cause of hospitalization, little is known about how to improve postsepsis care.[15, 17, 18, 19] This contrasts with other, often less common, hospital conditions for which many studies evaluating readmission and postdischarge care are available.[20, 21, 22, 23] Identifying the factors contributing to high utilization could lend critical insight to designing interventions that improve long‐term sepsis outcomes.[24]
We conducted a retrospective study of sepsis patients discharged in 2010 at Kaiser Permanente Northern California (KPNC) to describe their posthospital trajectories. In this diverse community‐hospitalbased population, we sought to identify the patient‐level factors that impact the posthospital healthcare utilization of sepsis survivors.
METHODS
This study was approved by the KPNC institutional review board.
Setting
We conducted a retrospective study of sepsis patients aged 18 years admitted to KPNC hospitals in 2010 whose hospitalizations included an overnight stay, began in a KPNC hospital, and was not for peripartum care. We identified sepsis based on International Classification of Disease, 9th Edition principal diagnosis codes used at KPNC, which capture a similar population to that from the Angus definition (see Supporting Appendix, Table 1, in the online version of this article).[7, 25, 26] We denoted each patient's first sepsis hospitalization as the index event.
| Predicted Hospital Mortality Quartiles (n=1,586 for Each Group) | |||||
|---|---|---|---|---|---|
| Overall | 1 | 2 | 3 | 4 | |
| |||||
| Baseline | |||||
| Age, y, mean | 71.915.7 | 62.317.8 | 71.214.2 | 75.612.7 | 78.612.2 |
| By age category | |||||
| <45 years | 410 (6.5) | 290 (18.3) | 71 (4.5) | 25 (1.6) | 24 (1.5) |
| 4564 years | 1,425 (22.5) | 539 (34.0) | 407 (25.7) | 292 (18.4) | 187 (11.8) |
| 6584 years | 3,036 (47.9) | 601 (37.9) | 814 (51.3) | 832 (52.5) | 789 (49.8) |
| 85 years | 1,473 (23.2) | 156 (9.8) | 294 (18.5) | 437 (27.6) | 586 (37.0) |
| Male | 2,973 (46.9) | 686 (43.3) | 792 (49.9) | 750 (47.3) | 745 (47.0) |
| Comorbidity | |||||
| COPS2 score | 5143 | 2627 | 5441 | 6445 | 6245 |
| Charlson score | 2.01.5 | 1.31.2 | 2.11.4 | 2.41.5 | 2.41.5 |
| Hospitalization | |||||
| LAPS2 severity score | 10742 | 6621 | 9020 | 11423 | 15928 |
| Admitted via emergency department | 6,176 (97.4) | 1,522 (96.0) | 1,537 (96.9) | 1,539 (97.0) | 1,578 (99.5) |
| Direct ICU admission | 1,730 (27.3) | 169 (10.7) | 309 (19.5) | 482 (30.4) | 770 (48.6) |
| ICU transfer, at any time | 2,206 (34.8) | 279 (17.6) | 474 (29.9) | 603 (38.0) | 850 (53.6) |
| Hospital mortality | |||||
| Predicted, % | 10.513.8 | 1.00.1 | 3.40.1 | 8.32.3 | 29.415.8 |
| Observed | 865 (13.6) | 26 (1.6) | 86 (5.4) | 197 (12.4) | 556 (35.1) |
| Hospital length of stay, d | 5.86.4 | 4.43.8 | 5.45.7 | 6.68.0 | 6.66.9 |
We linked hospital episodes with existing KPNC inpatient databases to describe patient characteristics.[27, 28, 29, 30] We categorized patients by age (45, 4564, 6584, and 85 years) and used Charlson comorbidity scores and Comorbidity Point Scores 2 (COPS2) to quantify comorbid illness burden.[28, 30, 31, 32] We quantified acute severity of illness using the Laboratory Acute Physiology Scores 2 (LAPS2), which incorporates 15 laboratory values, 5 vital signs, and mental status prior to hospital admission (including emergency department data).[30] Both the COPS2 and LAPS2 are independently associated with hospital mortality.[30, 31] We also generated a summary predicted risk of hospital mortality based on a validated risk model and stratified patients by quartiles.[30] We determined whether patients were admitted to the intensive care unit (ICU).[29]
Outcomes
We used patients' health insurance administrative data to quantify postsepsis utilization. Within the KPNC integrated healthcare delivery system, uniform information systems capture all healthcare utilization of insured members including services received at non‐KPNC facilities.[28, 30] We collected utilization data from the year preceding index hospitalization (presepsis) and for the year after discharge date or until death (postsepsis). We ascertained mortality after discharge from KPNC medical records as well as state and national death record files.
We grouped services into facility‐based or outpatient categories. Facility‐based services included inpatient admission, subacute nursing facility or long‐term acute care, and emergency department visits. We grouped outpatient services as hospice, home health, outpatient surgery, clinic, or other (eg, laboratory). We excluded patients whose utilization records were not available over the full presepsis interval. Among these 1211 patients (12.5% of total), the median length of records prior to index hospitalization was 67 days, with a mean value of 117 days.
Statistical Analysis
Our primary outcomes of interest were hospital readmission and utilization in the year after sepsis. We defined a hospital readmission as any inpatient stay after the index hospitalization grouped within 1‐, 3‐, 6‐, and 12‐month intervals. We designated those within 30 days as an early readmission. We grouped readmission principal diagnoses, where available, by the 17 Healthcare Cost and Utilization Project (HCUP) Clinical Classifications Software multilevel categories with sepsis in the infectious category.[33, 34] In secondary analysis, we also designated other infectious diagnoses not included in the standard HCUP infection category (eg, pneumonia, meningitis, cellulitis) as infection (see Supporting Appendix in the online version of this article).
We quantified outpatient utilization based on the number of episodes recorded. For facility‐based utilization, we calculated patient length of stay intervals. Because patients surviving their index hospitalization might not survive the entire year after discharge, we also calculated utilization adjusted for patients' living days by dividing the total facility length of stay by the number of living days after discharge.
Continuous data are represented as mean (standard deviation [SD]) and categorical data as number (%). We compared groups with analysis of variance or 2 testing. We estimated survival with Kaplan‐Meier analysis (95% confidence interval) and compared groups with log‐rank testing. We compared pre‐ and postsepsis healthcare utilization with paired t tests.
To identify factors associated with early readmission after sepsis, we used a competing risks regression model.[35] The dependent variable was time to readmission and the competing hazard was death within 30 days without early readmission; patients without early readmission or death were censored at 30 days. The independent variables included age, gender, comorbid disease burden (COPS2), acute severity of illness (LAPS2), any use of intensive care, total index length of stay, and percentage of living days prior to sepsis hospitalization spent utilizing facility‐based care. We also used logistic regression to quantify the association between these variables and high postsepsis utilization; we defined high utilization as 15% of living days postsepsis spent in facility‐based care. For each model, we quantified the relative contribution of each predictor variable to model performance based on differences in log likelihoods.[35, 36] We conducted analyses using STATA/SE version 11.2 (StataCorp, College Station, TX) and considered a P value of <0.05 to be significant.
RESULTS
Cohort Characteristics
Our study cohort included 6344 patients with index sepsis hospitalizations in 2010 (Table 1). Mean age was 72 (SD 16) years including 1835 (28.9%) patients aged <65 years. During index hospitalizations, higher predicted mortality was associated with increased age, comorbid disease burden, and severity of illness (P<0.01 for each). ICU utilization increased across predicted mortality strata; for example, 10.7% of patients in the lowest quartile were admitted directly to the ICU compared with 48.6% in the highest quartile. In the highest quartile, observed mortality was 35.1%.
One‐Year Survival
A total of 5479 (86.4%) patients survived their index sepsis hospitalization. Overall survival after living discharge was 90.5% (range, 89.6%91.2%) at 30 days and 71.3% (range, 70.1%72.5%) at 1 year. However, postsepsis survival was strongly modified by age (Figure 1). For example, 1‐year survival was 94.1% (range, 91.2%96.0%) for <45 year olds and 54.4% (range, 51.5%57.2%) for 85 year olds (P<0.01). Survival was also modified by predicted mortality, however, not by ICU admission during index hospitalization (P=0.18) (see Supporting Appendix, Figure 1, in the online version of this article).

Hospital Readmission
Overall, 978 (17.9%) patients had early readmission after index discharge (Table 2); nearly half were readmitted at least once in the year following discharge. Rehospitalization frequency was slightly lower when including patients with incomplete presepsis data (see Supporting Appendix, Table 2, in the online version of this article). The frequency of hospital readmission varied based on patient age and severity of illness. For example, 22.3% of patients in the highest predicted mortality quartile had early readmission compared with 11.6% in the lowest. The median time from discharge to early readmission was 11 days. Principal diagnoses were available for 78.6% of all readmissions (see Supporting Appendix, Table 3, in the online version of this article). Between 28.3% and 42.7% of those readmissions were for infectious diagnoses (including sepsis).
| Predicted Mortality Quartile | |||||
|---|---|---|---|---|---|
| Readmission | Overall | 1 | 2 | 3 | 4 |
| Within 30 days | 978 (17.9) | 158 (11.6) | 242 (17.7) | 274 (20.0) | 304 (22.3) |
| Within 90 days | 1,643 (30.1) | 276 (20.2) | 421 (30.8) | 463 (33.9) | 483 (35.4) |
| Within 180 days | 2,061 (37.7) | 368 (26.9) | 540 (39.5) | 584 (42.7) | 569 (41.7) |
| Within 365 days | 2,618 (47.9) | 498 (36.4) | 712 (52.1) | 723 (52.9) | 685 (50.2) |
| Variable | Hazard Ratio for Early Readmission | Odds Ratio for High Utilization | ||
|---|---|---|---|---|
| HR (95% CI) | Relative Contribution | OR (95% CI) | Relative Contribution | |
| ||||
| Age category | 1.2% | 11.1% | ||
| <45 years | 1.00 [reference] | 1.00 [reference] | ||
| 4564 years | 0.86 (0.64‐1.16) | 2.22 (1.30‐3.83)a | ||
| 6584 years | 0.92 (0.69‐1.21) | 3.66 (2.17‐6.18)a | ||
| 85 years | 0.95 (0.70‐1.28) | 4.98 (2.92‐8.50)a | ||
| Male | 0.99 (0.88‐1.13) | 0.0% | 0.86 (0.74‐1.00) | 0.1% |
| Severity of illness (LAPS2) | 1.08 (1.04‐1.12)a | 12.4% | 1.22 (1.17‐1.27)a | 11.3% |
| Comorbid illness (COPS2) | 1.16 (1.12‐1.19)a | 73.9% | 1.13 (1.09‐1.17)a | 5.9% |
| Intensive care | 1.21 (1.05‐1.40)a | 5.2% | 1.02 (0.85‐1.21) | 0.0% |
| Hospital length of stay, day | 1.01 (1.001.02)b | 6.6% | 1.04 (1.03‐1.06)a | 6.9% |
| Prior utilization, per 10% | 0.98 (0.95‐1.02) | 0.7% | 1.74 (1.61‐1.88)a | 64.2% |
Healthcare Utilization
The unadjusted difference between pre‐ and postsepsis healthcare utilization among survivors was statistically significant for most categories but of modest clinical significance (see Supporting Appendix, Table 4, in the online version of this article). For example, the mean number of presepsis hospitalizations was 0.9 (1.4) compared to 1.0 (1.5) postsepsis (P<0.01). After adjusting for postsepsis living days, the difference in utilization was more pronounced (Figure 2). Overall, there was roughly a 3‐fold increase in the mean percentage of living days spent in facility‐based care between patients' pre‐ and postsepsis phases (5.3% vs 15.0%, P<0.01). Again, the difference was strongly modified by age. For patients aged <45 years, the difference was not statistically significant (2.4% vs 2.9%, P=0.32), whereas for those aged 65 years, it was highly significant (6.2% vs 18.5%, P<0.01).

Factors associated with early readmission included severity of illness, comorbid disease burden, index hospital length of stay, and intensive care (Table 3). However, the dominant factor explaining variation in the risk of early readmission was patients' prior comorbid disease burden (73.9%), followed by acute severity of illness (12.4%), total hospital length of stay (6.6%), and the need for intensive care (5.2%). Severity of illness and age were also significantly associated with higher odds of high postsepsis utilization; however, the dominant factor contributing to this risk was a history of high presepsis utilization (64.2%).
DISCUSSION
In this population‐based study in a community healthcare system, the impact of sepsis extended well beyond the initial hospitalization. One in 6 sepsis survivors was readmitted within 30 days, and roughly half were readmitted within 1 year. Fewer than half of rehospitalizations were for sepsis. Patients had a 3‐fold increase in the percentage of living days spent in hospitals or care facilities after sepsis hospitalization. Although age and acute severity of illness strongly modified healthcare utilization and mortality after sepsis, the dominant factors contributing to early readmission and high utilization ratescomorbid disease burden and presepsis healthcare utilizationwere present prior to hospitalization.
Sepsis is the single most expensive cause of US hospitalizations.[3, 4, 5] Despite its prevalence, there are little contemporary data identifying factors that impact healthcare utilization among sepsis survivors.[9, 16, 17, 19, 24, 36, 37] Recently, Prescott and others found that in Medicare beneficiaries, following severe sepsis, healthcare utilization was markedly increased.[17] More than one‐quarter of survivors were readmitted within 30 days, and 63.8% were readmitted within a year. Severe sepsis survivors also spent an average of 26% of their living days in a healthcare facility, a nearly 4‐fold increase compared to their presepsis phase. The current study included a population with a broader age and severity range; however, in a similar subgroup of patients, for those aged 65 years within the highest predicted mortality quartile, the frequency of readmission was similar. These findings are concordant with those from prior studies.[17, 19, 36, 37]
Among sepsis survivors, most readmissions were not for sepsis or infectious diagnoses, which is a novel finding with implications for designing approaches to reduce rehospitalization. The pattern in sepsis is similar to that seen in other common and costly hospital conditions.[17, 20, 23, 38, 39, 40] For example, between 18% and 25% of Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, or pneumonia were readmitted within 30 days; fewer than one‐third had the same diagnosis.[20] The timing of readmission in our sepsis cohort was also similar to that seen in other conditions.[20] For example, the median time of early readmission in this study was 11 days; it was between 10 and 12 days for patients with heart failure, pneumonia, and myocardial infarction.[20]
Krumholz and others suggest that the pattern of early rehospitalization after common acute conditions reflects a posthospital syndromean acquired, transient period of vulnerabilitythat could be the byproduct of common hospital factors.[20, 41] Such universal impairments might result from new physical and neurocognitive disability, nutritional deficiency, and sleep deprivation or delirium, among others.[41] If this construct were also true in sepsis, it could have important implications on the design of postsepsis care. However, prior studies suggest that sepsis patients may be particularly vulnerable to the sequelae of hospitalization.[2, 42, 43, 44, 45]
Among Medicare beneficiaries, Iwashyna and others reported that hospitalizations for severe sepsis resulted in significant increases in physical limitations and moderate to severe cognitive impairment.[1, 14, 46] Encephalopathy, sleep deprivation, and delirium are also frequently seen in sepsis patients.[47, 48] Furthermore, sepsis patients frequently need intensive care, which is also associated with increased patient disability and injury.[16, 46, 49, 50] We found that severity of illness and the need for intensive care were both predictive of the need for early readmission following sepsis. We also confirmed the results of prior studies suggesting that sepsis outcomes are strongly modified by age.[16, 19, 43, 51]
However, we found that the dominant factors contributing to patients' health trajectories were conditions present prior to admission. This finding is in accord with prior suggestions that acute severity of illness only partially predicts patients facing adverse posthospital sequelae.[23, 41, 52] Among sepsis patients, prior work demonstrates that inadequate consideration for presepsis level of function and utilization can result in an overestimation of the impact of sepsis on postdischarge health.[52, 53] Further, we found that the need for intensive care was not independently associated with an increased risk of high postsepsis utilization after adjusting for illness severity, a finding also seen in prior studies.[17, 23, 38, 51]
Taken together, our findings might suggest that an optimal approach to posthospital care in sepsis should focus on treatment approaches that address disease‐specific problems within the much larger context of common hospital risks. However, further study is necessary to clearly define the mechanisms by which age, severity of illness, and intensive care affect subsequent healthcare utilization. Furthermore, sepsis patients are a heterogeneous population in terms of severity of illness, site and pathogen of infection, and underlying comorbidity whose posthospital course remains incompletely characterized, limiting our ability to draw strong inferences.
These results should be interpreted in light of the study's limitations. First, our cohort included patients with healthcare insurance within a community‐based healthcare system. Care within the KPNC system, which bears similarities with accountable care organizations, is enhanced through service integration and a comprehensive health information system. Although prior studies suggest that these characteristics result in improved population‐based care, it is unclear whether there is a similar impact in hospital‐based conditions such as sepsis.[54, 55] Furthermore, care within an integrated system may impact posthospital utilization patterns and could limit generalizability. However, prior studies demonstrate the similarity of KPNC members to other patients in the same region in terms of age, socioeconomics, overall health behaviors, and racial/ethnic diversity.[56] Second, our study did not characterize organ dysfunction based on diagnosis coding, a common feature of sepsis studies that lack detailed physiologic severity data.[4, 5, 6, 8, 26] Instead, we focused on using granular laboratory and vital signs data to ensure accurate risk adjustment using a validated system developed in >400,000 hospitalizations.[30] Although this method may hamper comparisons with existing studies, traditional methods of grading severity by diagnosis codes can be vulnerable to biases resulting in wide variability.[10, 23, 26, 57, 58] Nonetheless, it is likely that characterizing preexisting and acute organ dysfunction will improve risk stratification in the heterogeneous sepsis population. Third, this study did not include data regarding patients' functional status, which has been shown to strongly predict patient outcomes following hospitalization. Fourth, this study did not address the cost of care following sepsis hospitalizations.[19, 59] Finally, our study excluded patients with incomplete utilization records, a choice designed to avoid the spurious inferences that can result from such comparisons.[53]
In summary, we found that sepsis exacted a considerable toll on patients in the hospital and in the year following discharge. Sepsis patients were frequently rehospitalized within a month of discharge, and on average had a 3‐fold increase in their subsequent time spent in healthcare facilities. Although age, severity of illness, and the need for ICU care impacted postsepsis utilization, the dominant contributing factorscomorbid disease burden or presepsis utilizationwere present prior to sepsis hospitalization. Early readmission patterns in sepsis appeared similar to those seen in other important hospital conditions, suggesting a role for shared posthospital, rather than just postsepsis, care approaches.
Disclosures
The funding for this study was provided by The Permanente Medical Group, Inc. and Kaiser Foundation Hospitals. The authors have no conflict of interests to disclose relevant to this article.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–1834.
- , , , et al.; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , . Costs for hospital stays in the United States, 2010. HCUP statistical brief #16. January 2013. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb146.pdf. Accessed October 1, 2013.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250.
- , , . Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. October 2011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb122.pdf. Accessed October 1, 2013.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761.
- , , , . Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174.
- , , , et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924.
- , , , . Reducing mortality in severe sepsis: the Surviving Sepsis Campaign. Clin Chest Med. 2008;29(4):721–733, x.
- , , , et al.; Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , , . Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283.
- , , , et al.; the Scottish Critical Care Trials Group and the Scottish Intensive Care Society Audit Group. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17(2):R70.
- , , , , . Post‐Discharge Health Care Use Is Markedly Higher in Survivors of Severe Sepsis. Am J Respir Crit Care Med 2013;187:A1573.
- , , , . Long‐term survival and function after suspected gram‐negative sepsis. JAMA. 1995;274(4):338–345.
- , , , , . Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31(9):2316–2323.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , , . A systematic review and meta‐analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164(21):2315–2320.
- , . Hospitalizations for heart failure in the United States—a sign of hope. JAMA. 2011;306(15):1705–1706.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Sepsis after Scotland: enough with the averages, show us the effect modifiers. Crit Care. 2013;17(3):148.
- , , , et al. Kaiser Permanente's performance improvement system, part 3: multisite improvements in care for patients with sepsis. Jt Comm J Qual Patient Saf. 2011;37(11): 483–493.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus implementation of the International Consensus Conference Definition of Severe Sepsis [published online ahead of print September 18, 2012]. Med Care. doi: 10.1097/MLR.0b013e318268ac86. Epub ahead of print.
- . Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8 pt 2):719–724.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , , . An electronic Simplified Acute Physiology Score‐based risk adjustment score for critical illness in an integrated healthcare system. Crit Care Med. 2013;41(1):41–48.
- , , , , . Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , . The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2009;63(7):798–803.
- , , , , , . Casemix adjustment of managed care claims data using the clinical classification for health policy research method. Med Care. 1998;36(7):1108–1113.
- Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project. Clinical Classifications Software (CCS) for ICD‐9‐CM Fact Sheet. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccsfactsheet.jsp. Accessed January 20, 2013.
- , . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1997;94(446):496–509.
- , , . Severe sepsis in managed care: analysis of incidence, one‐year mortality, and associated costs of care. J Manag Care Pharm. 2004;10(6):521–530.
- , , , , , . Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med. 2004;32(4):981–985.
- , , , , et al. Readmissions among patients with severe sepsis/septic shock among inner‐city minority New Yorkers. Chest. 2012;142:286A.
- , , . Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–857.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , . The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21.
- , . Multiple systems organ failure: failure of host defense homeostasis. Crit Care Clin. 1989;5(2):199–220.
- . Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444.
- , . Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29(3):368–377.
- , , . The encephalopathy in sepsis. Crit Care Clin. 2008;24(1):67–82, viii.
- , . Sepsis‐associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–566.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509.
- , , , , . The association between sepsis and potential medical injury among hospitalized patients. Chest. 2012;142(3):606–613.
- , , , , , . Three‐year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303(9):849–856.
- , , , , , . Does acute organ dysfunction predict patient‐centered outcomes? Chest. 2002;121(6):1963–1971.
- , , , . Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–841.
- , , , , , . Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- , , , et al., Outpatient electronic health records and the clinical care and outcomes of patients with diabetes mellitus. Ann Intern Med. 2012;157(7):482–489.
- . Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2009 California Health Interview Survey. Internal Division of Research Report. Oakland, CA: Kaiser Permanente Division of Research; January 24, 2012. Available at: http://www.dor.kaiser.org/external/chis_non_kp_2009. Accessed January 20, 2013.
- , , , , . Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413.
- , . Finding pure and simple truths with administrative data. JAMA. 2012;307(13):1433–1435.
- , , , . Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46(12):1226–1233.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–1834.
- , , , et al.; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , . Costs for hospital stays in the United States, 2010. HCUP statistical brief #16. January 2013. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb146.pdf. Accessed October 1, 2013.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250.
- , , . Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. October 2011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb122.pdf. Accessed October 1, 2013.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761.
- , , , . Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174.
- , , , et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924.
- , , , . Reducing mortality in severe sepsis: the Surviving Sepsis Campaign. Clin Chest Med. 2008;29(4):721–733, x.
- , , , et al.; Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , , . Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283.
- , , , et al.; the Scottish Critical Care Trials Group and the Scottish Intensive Care Society Audit Group. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17(2):R70.
- , , , , . Post‐Discharge Health Care Use Is Markedly Higher in Survivors of Severe Sepsis. Am J Respir Crit Care Med 2013;187:A1573.
- , , , . Long‐term survival and function after suspected gram‐negative sepsis. JAMA. 1995;274(4):338–345.
- , , , , . Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31(9):2316–2323.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , , . A systematic review and meta‐analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164(21):2315–2320.
- , . Hospitalizations for heart failure in the United States—a sign of hope. JAMA. 2011;306(15):1705–1706.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Sepsis after Scotland: enough with the averages, show us the effect modifiers. Crit Care. 2013;17(3):148.
- , , , et al. Kaiser Permanente's performance improvement system, part 3: multisite improvements in care for patients with sepsis. Jt Comm J Qual Patient Saf. 2011;37(11): 483–493.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus implementation of the International Consensus Conference Definition of Severe Sepsis [published online ahead of print September 18, 2012]. Med Care. doi: 10.1097/MLR.0b013e318268ac86. Epub ahead of print.
- . Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8 pt 2):719–724.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , , . An electronic Simplified Acute Physiology Score‐based risk adjustment score for critical illness in an integrated healthcare system. Crit Care Med. 2013;41(1):41–48.
- , , , , . Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , . The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2009;63(7):798–803.
- , , , , , . Casemix adjustment of managed care claims data using the clinical classification for health policy research method. Med Care. 1998;36(7):1108–1113.
- Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project. Clinical Classifications Software (CCS) for ICD‐9‐CM Fact Sheet. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccsfactsheet.jsp. Accessed January 20, 2013.
- , . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1997;94(446):496–509.
- , , . Severe sepsis in managed care: analysis of incidence, one‐year mortality, and associated costs of care. J Manag Care Pharm. 2004;10(6):521–530.
- , , , , , . Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med. 2004;32(4):981–985.
- , , , , et al. Readmissions among patients with severe sepsis/septic shock among inner‐city minority New Yorkers. Chest. 2012;142:286A.
- , , . Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–857.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , . The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21.
- , . Multiple systems organ failure: failure of host defense homeostasis. Crit Care Clin. 1989;5(2):199–220.
- . Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444.
- , . Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29(3):368–377.
- , , . The encephalopathy in sepsis. Crit Care Clin. 2008;24(1):67–82, viii.
- , . Sepsis‐associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–566.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509.
- , , , , . The association between sepsis and potential medical injury among hospitalized patients. Chest. 2012;142(3):606–613.
- , , , , , . Three‐year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303(9):849–856.
- , , , , , . Does acute organ dysfunction predict patient‐centered outcomes? Chest. 2002;121(6):1963–1971.
- , , , . Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–841.
- , , , , , . Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- , , , et al., Outpatient electronic health records and the clinical care and outcomes of patients with diabetes mellitus. Ann Intern Med. 2012;157(7):482–489.
- . Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2009 California Health Interview Survey. Internal Division of Research Report. Oakland, CA: Kaiser Permanente Division of Research; January 24, 2012. Available at: http://www.dor.kaiser.org/external/chis_non_kp_2009. Accessed January 20, 2013.
- , , , , . Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413.
- , . Finding pure and simple truths with administrative data. JAMA. 2012;307(13):1433–1435.
- , , , . Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46(12):1226–1233.
© 2014 Society of Hospital Medicine