User login
In 2017, an estimated 22,240 women will be diagnosed with ovarian cancer, and 14,080 women will die of the disease.1 The high mortality associated with ovarian cancer is due largely to the inability to detect the disease early and the lack of effective therapeutics for women with recurrent disease. In this Update, we review important advances in the diagnosis and treatment of ovarian cancer.
Development of an effective screening tool for women at average risk has been an elusive challenge. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) examined the efficacy of transvaginal ultrasound and cancer antigen 125 (CA 125) monitoring for ovarian cancer in a large cohort of women.
For women diagnosed with ovarian cancer, treatment paradigms for the initial management of the disease have shifted dramatically. Based on data from multiple randomized controlled trials, neoadjuvant chemotherapy (NACT) is being used more frequently. The American Society of Clinical Oncology and the Society of Gynecologic Oncology developed consensus recommendations for the appropriate use of NACT and primary cytoreductive surgery for women with ovarian cancer.
Finally, all of oncology has moved toward incorporating molecularly targeted therapeutics directed toward individual genetic abnormalities in tumors, so-called precision medicine. In ovarian cancer, poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) has emerged as an important target, particularly for women with BRCA gene pathway mutations. We describe a recently published randomized controlled trial of the PARP inhibitor niraparib.
Read about ovarian cancer screening tests
Is CA 125 or ultrasound screening appropriate for the general population?
Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956.
In the United States, the overall ovarian cancer 5-year survival rate is 46.2%, resulting in more than 14,000 deaths annually.2 The poor prognosis associated with this malignancy is largely attributable to the fact that almost 75% of women have stage III or stage IV disease at the time of diagnosis.2 Ovarian cancer is usually associated with vague, nonspecific symptoms as it progresses, which contributes to delayed diagnosis and increased mortality.
Multiple studies have examined pelvic ultrasonography and tumor markers, such as CA 125, as possible screening tools to increase early detection in asymptomatic women. However, neither modality alone or in combination has sufficient sensitivity or specificity to recommend it for use in the general population.3,4 Nevertheless, the search for an appropriate screening tool continues, and the UKCTOCS trial results have reinvigorated this discussion.5
The UKCTOCS findings
The UKCTOCS was a multicenter, randomized controlled trial in the United Kingdom in which researchers allocated 202,638 women aged 50 to 74 years to 1 of 3 groups: annual multimodal screening (MMS) with serum CA 125 interpreted with the use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound screening (USS), or no screening. The median follow-up was more than 11 years.
The investigators found that equivalent rates of ovarian cancer were diagnosed in each group: 0.7% in the MMS group, 0.6% in the USS group, and 0.6% in the no-screening group. Overall, there was no significant reduction in the mortality rate from ovarian cancer in either of the 2 screening groups compared with the no-screening group.5
An important subset discovery
However, in a prespecified subset analysis excluding "prevalent cases" (women with ovarian cancer thought to be present prior to randomization and subsequent screening), ovarian cancer mortality was significantly lower in the MMS group compared with the no-screening group (P = .021). Compared with no screening, MMS was associated with a 20% reduction in mortality rate from ovarian cancer over time, with the most pronounced effects occurring at years 7 to 14 of follow-up, suggesting the possible increased effectiveness of screening over time.5
Related article:
Can CA 125 screening reduce mortality from ovarian cancer?
Concordance with other screening trials
While impressive in study magnitude and scope, the UKCTOCS results did not demonstrate a significant mortality benefit associated with MMS or USS when compared with no screening. Although the screening complications were low (<1% in both screening groups), the authors did note a false-positive surgery rate of 14 per 10,000 screens for the MMS group and 50 per 10,000 screens for the USS group. Based on the performance of screening in this trial, 641 women would need to be screened annually using MMS for 14 years to prevent 1 ovarian cancer death.
Like the UKCTOCS, the ovarian cancer-screening arm of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial in the United States was also unable to demonstrate a reduction in mortality rate with screening with CA 125 and transvaginal ultrasound. Importantly, more than one-third of women with a false-positive screen underwent surgery and 15% of them experienced a major complication.6 Based on these findings, the US Preventive Services Task Force grades screening for ovarian cancer as D, suggesting that the harms of screening may outweigh the benefits.7
While screening for ovarian cancer remains an important need, there is currently no evidence to suggest that serum tumor marker or ultrasound screening is appropriate in the general population. Studies using more specific screening tests or strategies targeted to higher-risk women are ongoing.
Read about patient selection for neoadjuvant chemotherapy
New clinical practice guideline advises neoadjuvant chemotherapy for certain women with ovarian cancer
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-3473.
It has long been held as a central dogma that primary cytoreductive surgery (PCS) is the preferred initial treatment for women with newly diagnosed ovarian cancer.8 However, PCS is associated with substantial morbidity, and the ability to achieve optimal cytoreduction (<1 cm of residual disease), an important prognostic factor, is often compromised in women with significant tumor burden.9,10
Neoadjuvant chemotherapy, in which chemotherapy is administered prior to surgical cytoreduction, challenges the traditional treatment paradigm for advanced-stage ovarian cancer. Several randomized controlled trials have reported equivalent survival for primary surgical cytoreduction and NACT. Importantly, women who received NACT had fewer complications and were more likely to have optimal cytoreduction at the time of surgery.11,12 These studies have limitations, however, and the role of NACT remains uncertain.
To help guide clinicians, the Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an expert panel to provide recommendations and guidance on the evaluation of women for and the use of NACT in the setting of advanced ovarian cancer.13
Related article:
2015 Update on cancer
Recommendation: Clinical evaluation and patient selection
Strong clinical evidence supports that all women with suspected stage IIIC or stage IV ovarian cancer should be evaluated by a gynecologic oncologist prior to the initiation of therapy. The evaluation should include at least a computed tomography scan of the chest, abdomen, and pelvis to assess the extent of disease and resectability. A preoperative risk assessment should be performed to assess risk factors for increased morbidity and mortality.
Women who have a high perioperative risk profile or a low likelihood of achieving cytoreduction to 1 cm or less of residual tumor should receive NACT. Prior to the initiation of NACT, histologic confirmation of ovarian cancer should be obtained.13
Outcomes for neoadjuvant chemotherapy versus primary cytoreduction
Four phase 3 randomized controlled trials (EORTC 55971, CHORUS, JCOG0602, and SCORPION) suggest that NACT is noninferior to PCS with regard to progression-free survival and overall survival. NACT is associated with less perioperative and postoperative morbidity and mortality and is associated with shorter hospital stays.
To date, complete data are available only from the EORTC and CHORUS trials, which both demonstrated similar progression-free survival and overall survival for NACT and PCS. Critics have noted, however, that both trials have shorter median overall survival for the PCS groups than were previously reported in other phase 3 studies in the United States, suggesting the possibility of different patient populations or less aggressive "surgical effort." Thus, PCS remains the preferred management strategy for women with advanced-stage ovarian cancer in whom there is a high likelihood of optimal cytoreduction.13
Recommendation: Use of neoadjuvant chemotherapy
Patients who are appropriate candidates for NACT should be treated with a platinum and taxane doublet and should receive interval cytoreduction following 3 to 4 cycles of therapy if a favorable response is noted. Patients whose disease progresses despite NACT have a poor prognosis, and there is little role for surgical treatment with the exception of palliative purposes.13
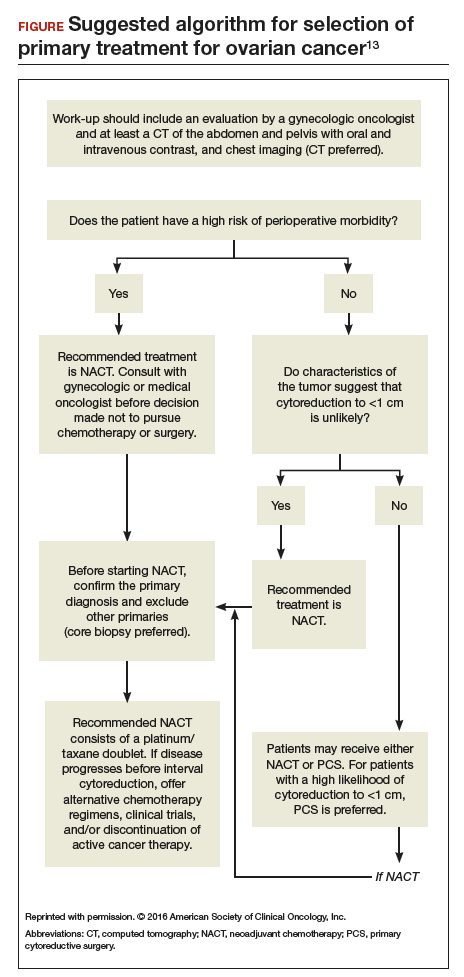
Neoadjuvant chemotherapy is a noninferior and appropriate treatment option for women who are poor surgical candidates or who have a low likelihood of optimal cytoreduction. When optimal cytoreduction is possible, however, PCS is preferred (see FIGURE). The data on the efficacy of NACT for ovarian cancer have led to increased use of this treatment in the United States.
Read about a new PARP inhibitor for maintenance therapy
Niraparib is promising as maintenance therapy in ovarian cancer
Mirza MR, Monk BJ, Herrstedt J, et al; for the ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164.
Approximately 85% of women with ovarian cancer will develop recurrent disease. Women with ovarian cancer are commonly treated with a range of antineoplastic agents over the course of their lifetime. As such, there is a great need for additional active therapeutic agents in this setting. Recently, substantial effort has been directed toward "precision" or "personalized medicine" in oncology.
Precision medicine, targeted therapies in oncology
Precision medicine refers to the customization of medical therapy based on the genetic characterization of the individual patient or the molecular profile of the patient's tumor. As a result of large-scale molecular profiling from projects such as the International Cancer Genome Consortium and The Cancer Genome Atlas, an abundance of molecular data has been generated through the characterization of multiple tumor types. This has led to the discovery of key cancer drivers, alterations, and specific molecular profiles that have distinct prognostic and treatment implications. These data, in combination with the commercial availability of molecular profiling tests, has made precision medicine a reality for women with ovarian cancer.
This wealth of new information has led to development of targeted therapeutics that block the growth and spread of cancer by acting on specific molecules or molecular pathways. Targeted therapies approved for cancer treatment include hormonal therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, and immunotherapies.14
How PARP inhibitors work
PARP inhibitors are a class of agents that are emerging as important therapies for ovarian cancer. These agents block the nuclear protein PARP, which functions to detect and repair single-strand DNA breaks with the resulting accumulation of double-stranded DNA breaks.15 In the setting of DNA damage, the homologous recombination repair pathway is activated for repair. However, homologous recombination deficiencies (HRD) can arise as a result of BRCA1 or BRCA2 mutations or BRCA-independent pathways, which effectively disable this DNA repair pathway. As a result, when PARP inhibitors are used in patients with HRD, the cell cannot repair double-stranded DNA breaks and this leads to "synthetic lethality."16
Understanding this molecular mechanism of PARP inhibitors as well as the frequent abnormalities in the BRCA genes and HRD pathways in ovarian cancer has provided an important potential therapeutic target in ovarian cancer. A number of PARP inhibitors are now commercially available and are undergoing testing in ovarian cancer.
Related article:
Is a minimally invasive approach to hysterectomy for Gyn cancer utilized equally in all racial and income groups?
Niraparib for ovarian cancer
In a randomized, double-blind, phase 3 trial by Mizra and colleagues, 553 women with platinum-sensitive recurrent ovarian cancer who responded to therapy were divided according to the presence or absence of a germline BRCA (gBRCA) mutation and randomly assigned to niraparib 300 mg or placebo once daily. Women in the niraparib group had a significantly longer median duration of progression-free survival than did those in the placebo group. This was most pronounced in women in the gBRCA cohort (21.0 vs 5.5 months). Importantly, niraparib was associated with improved progression-free survival in HRD-positive patients without gBRCA mutations (12.9 vs 3.8 months) as well as in the HRD-negative subgroup (6.9 vs 3.8 months).17
Overall, niraparib was well tolerated. About 15% of women discontinued the drug due to toxicity. Significant (grade 3 or 4) adverse events were seen in three-quarters of women treated with niraparib, and they most commonly consisted of hematologic toxicities. Patient-reported outcomes were similar for both groups, indicating no significant effect from niraparib on quality of life.17
This study's results suggest that niraparib has clinical activity against ovarian cancer. Importantly, niraparib was active in women with gBRCA mutations, in those with HRD without a gBRCA mutation, and potentially in women without HRD. If approved by the US Food and Drug Administration, niraparib will join olaparib and rucaparib as a newly approved therapeutic agent for ovarian cancer. This study provides important evidence that suggests niraparib maintenance therapy may be an efficacious and important addition to the treatment armamentarium for platinum-sensitive ovarian cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed January 20, 2017.
- Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306(6884):1030–1034.
- van Nagell JR Jr, Pavlik EJ. Ovarian cancer screening. Clin Obstet Gynecol. 2012;55:43–51.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305(22):2295–2303.
- Moyer VA, US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–904.
- Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3(3):111–117.
- Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):974–979; discussion 979–980.
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Goup; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257.
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460–3473.
- National Cancer Institute. Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Updated April 25, 2014. Accessed January 21, 2017.
- Drean A, Lord CJ, Ashworth A. PARP inhibitor combination therapy. Crit Rev Oncol Hematol. 2016;108:73–85.
- Ledermann JA, El-Khouly F. PARP inhibitors in ovarian cancer: clinical evidence for informed treatment decisions. Br J Cancer. 2015;113(suppl 1):S10–S16.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.
In 2017, an estimated 22,240 women will be diagnosed with ovarian cancer, and 14,080 women will die of the disease.1 The high mortality associated with ovarian cancer is due largely to the inability to detect the disease early and the lack of effective therapeutics for women with recurrent disease. In this Update, we review important advances in the diagnosis and treatment of ovarian cancer.
Development of an effective screening tool for women at average risk has been an elusive challenge. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) examined the efficacy of transvaginal ultrasound and cancer antigen 125 (CA 125) monitoring for ovarian cancer in a large cohort of women.
For women diagnosed with ovarian cancer, treatment paradigms for the initial management of the disease have shifted dramatically. Based on data from multiple randomized controlled trials, neoadjuvant chemotherapy (NACT) is being used more frequently. The American Society of Clinical Oncology and the Society of Gynecologic Oncology developed consensus recommendations for the appropriate use of NACT and primary cytoreductive surgery for women with ovarian cancer.
Finally, all of oncology has moved toward incorporating molecularly targeted therapeutics directed toward individual genetic abnormalities in tumors, so-called precision medicine. In ovarian cancer, poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) has emerged as an important target, particularly for women with BRCA gene pathway mutations. We describe a recently published randomized controlled trial of the PARP inhibitor niraparib.
Read about ovarian cancer screening tests
Is CA 125 or ultrasound screening appropriate for the general population?
Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956.
In the United States, the overall ovarian cancer 5-year survival rate is 46.2%, resulting in more than 14,000 deaths annually.2 The poor prognosis associated with this malignancy is largely attributable to the fact that almost 75% of women have stage III or stage IV disease at the time of diagnosis.2 Ovarian cancer is usually associated with vague, nonspecific symptoms as it progresses, which contributes to delayed diagnosis and increased mortality.
Multiple studies have examined pelvic ultrasonography and tumor markers, such as CA 125, as possible screening tools to increase early detection in asymptomatic women. However, neither modality alone or in combination has sufficient sensitivity or specificity to recommend it for use in the general population.3,4 Nevertheless, the search for an appropriate screening tool continues, and the UKCTOCS trial results have reinvigorated this discussion.5

The UKCTOCS findings
The UKCTOCS was a multicenter, randomized controlled trial in the United Kingdom in which researchers allocated 202,638 women aged 50 to 74 years to 1 of 3 groups: annual multimodal screening (MMS) with serum CA 125 interpreted with the use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound screening (USS), or no screening. The median follow-up was more than 11 years.
The investigators found that equivalent rates of ovarian cancer were diagnosed in each group: 0.7% in the MMS group, 0.6% in the USS group, and 0.6% in the no-screening group. Overall, there was no significant reduction in the mortality rate from ovarian cancer in either of the 2 screening groups compared with the no-screening group.5
An important subset discovery
However, in a prespecified subset analysis excluding "prevalent cases" (women with ovarian cancer thought to be present prior to randomization and subsequent screening), ovarian cancer mortality was significantly lower in the MMS group compared with the no-screening group (P = .021). Compared with no screening, MMS was associated with a 20% reduction in mortality rate from ovarian cancer over time, with the most pronounced effects occurring at years 7 to 14 of follow-up, suggesting the possible increased effectiveness of screening over time.5
Related article:
Can CA 125 screening reduce mortality from ovarian cancer?
Concordance with other screening trials
While impressive in study magnitude and scope, the UKCTOCS results did not demonstrate a significant mortality benefit associated with MMS or USS when compared with no screening. Although the screening complications were low (<1% in both screening groups), the authors did note a false-positive surgery rate of 14 per 10,000 screens for the MMS group and 50 per 10,000 screens for the USS group. Based on the performance of screening in this trial, 641 women would need to be screened annually using MMS for 14 years to prevent 1 ovarian cancer death.
Like the UKCTOCS, the ovarian cancer-screening arm of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial in the United States was also unable to demonstrate a reduction in mortality rate with screening with CA 125 and transvaginal ultrasound. Importantly, more than one-third of women with a false-positive screen underwent surgery and 15% of them experienced a major complication.6 Based on these findings, the US Preventive Services Task Force grades screening for ovarian cancer as D, suggesting that the harms of screening may outweigh the benefits.7
While screening for ovarian cancer remains an important need, there is currently no evidence to suggest that serum tumor marker or ultrasound screening is appropriate in the general population. Studies using more specific screening tests or strategies targeted to higher-risk women are ongoing.
Read about patient selection for neoadjuvant chemotherapy
New clinical practice guideline advises neoadjuvant chemotherapy for certain women with ovarian cancer
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-3473.
It has long been held as a central dogma that primary cytoreductive surgery (PCS) is the preferred initial treatment for women with newly diagnosed ovarian cancer.8 However, PCS is associated with substantial morbidity, and the ability to achieve optimal cytoreduction (<1 cm of residual disease), an important prognostic factor, is often compromised in women with significant tumor burden.9,10
Neoadjuvant chemotherapy, in which chemotherapy is administered prior to surgical cytoreduction, challenges the traditional treatment paradigm for advanced-stage ovarian cancer. Several randomized controlled trials have reported equivalent survival for primary surgical cytoreduction and NACT. Importantly, women who received NACT had fewer complications and were more likely to have optimal cytoreduction at the time of surgery.11,12 These studies have limitations, however, and the role of NACT remains uncertain.
To help guide clinicians, the Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an expert panel to provide recommendations and guidance on the evaluation of women for and the use of NACT in the setting of advanced ovarian cancer.13
Related article:
2015 Update on cancer
Recommendation: Clinical evaluation and patient selection
Strong clinical evidence supports that all women with suspected stage IIIC or stage IV ovarian cancer should be evaluated by a gynecologic oncologist prior to the initiation of therapy. The evaluation should include at least a computed tomography scan of the chest, abdomen, and pelvis to assess the extent of disease and resectability. A preoperative risk assessment should be performed to assess risk factors for increased morbidity and mortality.
Women who have a high perioperative risk profile or a low likelihood of achieving cytoreduction to 1 cm or less of residual tumor should receive NACT. Prior to the initiation of NACT, histologic confirmation of ovarian cancer should be obtained.13
Outcomes for neoadjuvant chemotherapy versus primary cytoreduction
Four phase 3 randomized controlled trials (EORTC 55971, CHORUS, JCOG0602, and SCORPION) suggest that NACT is noninferior to PCS with regard to progression-free survival and overall survival. NACT is associated with less perioperative and postoperative morbidity and mortality and is associated with shorter hospital stays.
To date, complete data are available only from the EORTC and CHORUS trials, which both demonstrated similar progression-free survival and overall survival for NACT and PCS. Critics have noted, however, that both trials have shorter median overall survival for the PCS groups than were previously reported in other phase 3 studies in the United States, suggesting the possibility of different patient populations or less aggressive "surgical effort." Thus, PCS remains the preferred management strategy for women with advanced-stage ovarian cancer in whom there is a high likelihood of optimal cytoreduction.13
Recommendation: Use of neoadjuvant chemotherapy
Patients who are appropriate candidates for NACT should be treated with a platinum and taxane doublet and should receive interval cytoreduction following 3 to 4 cycles of therapy if a favorable response is noted. Patients whose disease progresses despite NACT have a poor prognosis, and there is little role for surgical treatment with the exception of palliative purposes.13
Neoadjuvant chemotherapy is a noninferior and appropriate treatment option for women who are poor surgical candidates or who have a low likelihood of optimal cytoreduction. When optimal cytoreduction is possible, however, PCS is preferred (see FIGURE). The data on the efficacy of NACT for ovarian cancer have led to increased use of this treatment in the United States.

Read about a new PARP inhibitor for maintenance therapy
Niraparib is promising as maintenance therapy in ovarian cancer
Mirza MR, Monk BJ, Herrstedt J, et al; for the ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164.
Approximately 85% of women with ovarian cancer will develop recurrent disease. Women with ovarian cancer are commonly treated with a range of antineoplastic agents over the course of their lifetime. As such, there is a great need for additional active therapeutic agents in this setting. Recently, substantial effort has been directed toward "precision" or "personalized medicine" in oncology.
Precision medicine, targeted therapies in oncology
Precision medicine refers to the customization of medical therapy based on the genetic characterization of the individual patient or the molecular profile of the patient's tumor. As a result of large-scale molecular profiling from projects such as the International Cancer Genome Consortium and The Cancer Genome Atlas, an abundance of molecular data has been generated through the characterization of multiple tumor types. This has led to the discovery of key cancer drivers, alterations, and specific molecular profiles that have distinct prognostic and treatment implications. These data, in combination with the commercial availability of molecular profiling tests, has made precision medicine a reality for women with ovarian cancer.
This wealth of new information has led to development of targeted therapeutics that block the growth and spread of cancer by acting on specific molecules or molecular pathways. Targeted therapies approved for cancer treatment include hormonal therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, and immunotherapies.14
How PARP inhibitors work
PARP inhibitors are a class of agents that are emerging as important therapies for ovarian cancer. These agents block the nuclear protein PARP, which functions to detect and repair single-strand DNA breaks with the resulting accumulation of double-stranded DNA breaks.15 In the setting of DNA damage, the homologous recombination repair pathway is activated for repair. However, homologous recombination deficiencies (HRD) can arise as a result of BRCA1 or BRCA2 mutations or BRCA-independent pathways, which effectively disable this DNA repair pathway. As a result, when PARP inhibitors are used in patients with HRD, the cell cannot repair double-stranded DNA breaks and this leads to "synthetic lethality."16
Understanding this molecular mechanism of PARP inhibitors as well as the frequent abnormalities in the BRCA genes and HRD pathways in ovarian cancer has provided an important potential therapeutic target in ovarian cancer. A number of PARP inhibitors are now commercially available and are undergoing testing in ovarian cancer.
Related article:
Is a minimally invasive approach to hysterectomy for Gyn cancer utilized equally in all racial and income groups?
Niraparib for ovarian cancer
In a randomized, double-blind, phase 3 trial by Mizra and colleagues, 553 women with platinum-sensitive recurrent ovarian cancer who responded to therapy were divided according to the presence or absence of a germline BRCA (gBRCA) mutation and randomly assigned to niraparib 300 mg or placebo once daily. Women in the niraparib group had a significantly longer median duration of progression-free survival than did those in the placebo group. This was most pronounced in women in the gBRCA cohort (21.0 vs 5.5 months). Importantly, niraparib was associated with improved progression-free survival in HRD-positive patients without gBRCA mutations (12.9 vs 3.8 months) as well as in the HRD-negative subgroup (6.9 vs 3.8 months).17
Overall, niraparib was well tolerated. About 15% of women discontinued the drug due to toxicity. Significant (grade 3 or 4) adverse events were seen in three-quarters of women treated with niraparib, and they most commonly consisted of hematologic toxicities. Patient-reported outcomes were similar for both groups, indicating no significant effect from niraparib on quality of life.17
This study's results suggest that niraparib has clinical activity against ovarian cancer. Importantly, niraparib was active in women with gBRCA mutations, in those with HRD without a gBRCA mutation, and potentially in women without HRD. If approved by the US Food and Drug Administration, niraparib will join olaparib and rucaparib as a newly approved therapeutic agent for ovarian cancer. This study provides important evidence that suggests niraparib maintenance therapy may be an efficacious and important addition to the treatment armamentarium for platinum-sensitive ovarian cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In 2017, an estimated 22,240 women will be diagnosed with ovarian cancer, and 14,080 women will die of the disease.1 The high mortality associated with ovarian cancer is due largely to the inability to detect the disease early and the lack of effective therapeutics for women with recurrent disease. In this Update, we review important advances in the diagnosis and treatment of ovarian cancer.
Development of an effective screening tool for women at average risk has been an elusive challenge. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) examined the efficacy of transvaginal ultrasound and cancer antigen 125 (CA 125) monitoring for ovarian cancer in a large cohort of women.
For women diagnosed with ovarian cancer, treatment paradigms for the initial management of the disease have shifted dramatically. Based on data from multiple randomized controlled trials, neoadjuvant chemotherapy (NACT) is being used more frequently. The American Society of Clinical Oncology and the Society of Gynecologic Oncology developed consensus recommendations for the appropriate use of NACT and primary cytoreductive surgery for women with ovarian cancer.
Finally, all of oncology has moved toward incorporating molecularly targeted therapeutics directed toward individual genetic abnormalities in tumors, so-called precision medicine. In ovarian cancer, poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) has emerged as an important target, particularly for women with BRCA gene pathway mutations. We describe a recently published randomized controlled trial of the PARP inhibitor niraparib.
Read about ovarian cancer screening tests
Is CA 125 or ultrasound screening appropriate for the general population?
Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956.
In the United States, the overall ovarian cancer 5-year survival rate is 46.2%, resulting in more than 14,000 deaths annually.2 The poor prognosis associated with this malignancy is largely attributable to the fact that almost 75% of women have stage III or stage IV disease at the time of diagnosis.2 Ovarian cancer is usually associated with vague, nonspecific symptoms as it progresses, which contributes to delayed diagnosis and increased mortality.
Multiple studies have examined pelvic ultrasonography and tumor markers, such as CA 125, as possible screening tools to increase early detection in asymptomatic women. However, neither modality alone or in combination has sufficient sensitivity or specificity to recommend it for use in the general population.3,4 Nevertheless, the search for an appropriate screening tool continues, and the UKCTOCS trial results have reinvigorated this discussion.5

The UKCTOCS findings
The UKCTOCS was a multicenter, randomized controlled trial in the United Kingdom in which researchers allocated 202,638 women aged 50 to 74 years to 1 of 3 groups: annual multimodal screening (MMS) with serum CA 125 interpreted with the use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound screening (USS), or no screening. The median follow-up was more than 11 years.
The investigators found that equivalent rates of ovarian cancer were diagnosed in each group: 0.7% in the MMS group, 0.6% in the USS group, and 0.6% in the no-screening group. Overall, there was no significant reduction in the mortality rate from ovarian cancer in either of the 2 screening groups compared with the no-screening group.5
An important subset discovery
However, in a prespecified subset analysis excluding "prevalent cases" (women with ovarian cancer thought to be present prior to randomization and subsequent screening), ovarian cancer mortality was significantly lower in the MMS group compared with the no-screening group (P = .021). Compared with no screening, MMS was associated with a 20% reduction in mortality rate from ovarian cancer over time, with the most pronounced effects occurring at years 7 to 14 of follow-up, suggesting the possible increased effectiveness of screening over time.5
Related article:
Can CA 125 screening reduce mortality from ovarian cancer?
Concordance with other screening trials
While impressive in study magnitude and scope, the UKCTOCS results did not demonstrate a significant mortality benefit associated with MMS or USS when compared with no screening. Although the screening complications were low (<1% in both screening groups), the authors did note a false-positive surgery rate of 14 per 10,000 screens for the MMS group and 50 per 10,000 screens for the USS group. Based on the performance of screening in this trial, 641 women would need to be screened annually using MMS for 14 years to prevent 1 ovarian cancer death.
Like the UKCTOCS, the ovarian cancer-screening arm of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial in the United States was also unable to demonstrate a reduction in mortality rate with screening with CA 125 and transvaginal ultrasound. Importantly, more than one-third of women with a false-positive screen underwent surgery and 15% of them experienced a major complication.6 Based on these findings, the US Preventive Services Task Force grades screening for ovarian cancer as D, suggesting that the harms of screening may outweigh the benefits.7
While screening for ovarian cancer remains an important need, there is currently no evidence to suggest that serum tumor marker or ultrasound screening is appropriate in the general population. Studies using more specific screening tests or strategies targeted to higher-risk women are ongoing.
Read about patient selection for neoadjuvant chemotherapy
New clinical practice guideline advises neoadjuvant chemotherapy for certain women with ovarian cancer
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-3473.
It has long been held as a central dogma that primary cytoreductive surgery (PCS) is the preferred initial treatment for women with newly diagnosed ovarian cancer.8 However, PCS is associated with substantial morbidity, and the ability to achieve optimal cytoreduction (<1 cm of residual disease), an important prognostic factor, is often compromised in women with significant tumor burden.9,10
Neoadjuvant chemotherapy, in which chemotherapy is administered prior to surgical cytoreduction, challenges the traditional treatment paradigm for advanced-stage ovarian cancer. Several randomized controlled trials have reported equivalent survival for primary surgical cytoreduction and NACT. Importantly, women who received NACT had fewer complications and were more likely to have optimal cytoreduction at the time of surgery.11,12 These studies have limitations, however, and the role of NACT remains uncertain.
To help guide clinicians, the Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an expert panel to provide recommendations and guidance on the evaluation of women for and the use of NACT in the setting of advanced ovarian cancer.13
Related article:
2015 Update on cancer
Recommendation: Clinical evaluation and patient selection
Strong clinical evidence supports that all women with suspected stage IIIC or stage IV ovarian cancer should be evaluated by a gynecologic oncologist prior to the initiation of therapy. The evaluation should include at least a computed tomography scan of the chest, abdomen, and pelvis to assess the extent of disease and resectability. A preoperative risk assessment should be performed to assess risk factors for increased morbidity and mortality.
Women who have a high perioperative risk profile or a low likelihood of achieving cytoreduction to 1 cm or less of residual tumor should receive NACT. Prior to the initiation of NACT, histologic confirmation of ovarian cancer should be obtained.13
Outcomes for neoadjuvant chemotherapy versus primary cytoreduction
Four phase 3 randomized controlled trials (EORTC 55971, CHORUS, JCOG0602, and SCORPION) suggest that NACT is noninferior to PCS with regard to progression-free survival and overall survival. NACT is associated with less perioperative and postoperative morbidity and mortality and is associated with shorter hospital stays.
To date, complete data are available only from the EORTC and CHORUS trials, which both demonstrated similar progression-free survival and overall survival for NACT and PCS. Critics have noted, however, that both trials have shorter median overall survival for the PCS groups than were previously reported in other phase 3 studies in the United States, suggesting the possibility of different patient populations or less aggressive "surgical effort." Thus, PCS remains the preferred management strategy for women with advanced-stage ovarian cancer in whom there is a high likelihood of optimal cytoreduction.13
Recommendation: Use of neoadjuvant chemotherapy
Patients who are appropriate candidates for NACT should be treated with a platinum and taxane doublet and should receive interval cytoreduction following 3 to 4 cycles of therapy if a favorable response is noted. Patients whose disease progresses despite NACT have a poor prognosis, and there is little role for surgical treatment with the exception of palliative purposes.13
Neoadjuvant chemotherapy is a noninferior and appropriate treatment option for women who are poor surgical candidates or who have a low likelihood of optimal cytoreduction. When optimal cytoreduction is possible, however, PCS is preferred (see FIGURE). The data on the efficacy of NACT for ovarian cancer have led to increased use of this treatment in the United States.

Read about a new PARP inhibitor for maintenance therapy
Niraparib is promising as maintenance therapy in ovarian cancer
Mirza MR, Monk BJ, Herrstedt J, et al; for the ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164.
Approximately 85% of women with ovarian cancer will develop recurrent disease. Women with ovarian cancer are commonly treated with a range of antineoplastic agents over the course of their lifetime. As such, there is a great need for additional active therapeutic agents in this setting. Recently, substantial effort has been directed toward "precision" or "personalized medicine" in oncology.
Precision medicine, targeted therapies in oncology
Precision medicine refers to the customization of medical therapy based on the genetic characterization of the individual patient or the molecular profile of the patient's tumor. As a result of large-scale molecular profiling from projects such as the International Cancer Genome Consortium and The Cancer Genome Atlas, an abundance of molecular data has been generated through the characterization of multiple tumor types. This has led to the discovery of key cancer drivers, alterations, and specific molecular profiles that have distinct prognostic and treatment implications. These data, in combination with the commercial availability of molecular profiling tests, has made precision medicine a reality for women with ovarian cancer.
This wealth of new information has led to development of targeted therapeutics that block the growth and spread of cancer by acting on specific molecules or molecular pathways. Targeted therapies approved for cancer treatment include hormonal therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, and immunotherapies.14
How PARP inhibitors work
PARP inhibitors are a class of agents that are emerging as important therapies for ovarian cancer. These agents block the nuclear protein PARP, which functions to detect and repair single-strand DNA breaks with the resulting accumulation of double-stranded DNA breaks.15 In the setting of DNA damage, the homologous recombination repair pathway is activated for repair. However, homologous recombination deficiencies (HRD) can arise as a result of BRCA1 or BRCA2 mutations or BRCA-independent pathways, which effectively disable this DNA repair pathway. As a result, when PARP inhibitors are used in patients with HRD, the cell cannot repair double-stranded DNA breaks and this leads to "synthetic lethality."16
Understanding this molecular mechanism of PARP inhibitors as well as the frequent abnormalities in the BRCA genes and HRD pathways in ovarian cancer has provided an important potential therapeutic target in ovarian cancer. A number of PARP inhibitors are now commercially available and are undergoing testing in ovarian cancer.
Related article:
Is a minimally invasive approach to hysterectomy for Gyn cancer utilized equally in all racial and income groups?
Niraparib for ovarian cancer
In a randomized, double-blind, phase 3 trial by Mizra and colleagues, 553 women with platinum-sensitive recurrent ovarian cancer who responded to therapy were divided according to the presence or absence of a germline BRCA (gBRCA) mutation and randomly assigned to niraparib 300 mg or placebo once daily. Women in the niraparib group had a significantly longer median duration of progression-free survival than did those in the placebo group. This was most pronounced in women in the gBRCA cohort (21.0 vs 5.5 months). Importantly, niraparib was associated with improved progression-free survival in HRD-positive patients without gBRCA mutations (12.9 vs 3.8 months) as well as in the HRD-negative subgroup (6.9 vs 3.8 months).17
Overall, niraparib was well tolerated. About 15% of women discontinued the drug due to toxicity. Significant (grade 3 or 4) adverse events were seen in three-quarters of women treated with niraparib, and they most commonly consisted of hematologic toxicities. Patient-reported outcomes were similar for both groups, indicating no significant effect from niraparib on quality of life.17
This study's results suggest that niraparib has clinical activity against ovarian cancer. Importantly, niraparib was active in women with gBRCA mutations, in those with HRD without a gBRCA mutation, and potentially in women without HRD. If approved by the US Food and Drug Administration, niraparib will join olaparib and rucaparib as a newly approved therapeutic agent for ovarian cancer. This study provides important evidence that suggests niraparib maintenance therapy may be an efficacious and important addition to the treatment armamentarium for platinum-sensitive ovarian cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed January 20, 2017.
- Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306(6884):1030–1034.
- van Nagell JR Jr, Pavlik EJ. Ovarian cancer screening. Clin Obstet Gynecol. 2012;55:43–51.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305(22):2295–2303.
- Moyer VA, US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–904.
- Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3(3):111–117.
- Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):974–979; discussion 979–980.
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Goup; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257.
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460–3473.
- National Cancer Institute. Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Updated April 25, 2014. Accessed January 21, 2017.
- Drean A, Lord CJ, Ashworth A. PARP inhibitor combination therapy. Crit Rev Oncol Hematol. 2016;108:73–85.
- Ledermann JA, El-Khouly F. PARP inhibitors in ovarian cancer: clinical evidence for informed treatment decisions. Br J Cancer. 2015;113(suppl 1):S10–S16.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed January 20, 2017.
- Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306(6884):1030–1034.
- van Nagell JR Jr, Pavlik EJ. Ovarian cancer screening. Clin Obstet Gynecol. 2012;55:43–51.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305(22):2295–2303.
- Moyer VA, US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–904.
- Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3(3):111–117.
- Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):974–979; discussion 979–980.
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Goup; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257.
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460–3473.
- National Cancer Institute. Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Updated April 25, 2014. Accessed January 21, 2017.
- Drean A, Lord CJ, Ashworth A. PARP inhibitor combination therapy. Crit Rev Oncol Hematol. 2016;108:73–85.
- Ledermann JA, El-Khouly F. PARP inhibitors in ovarian cancer: clinical evidence for informed treatment decisions. Br J Cancer. 2015;113(suppl 1):S10–S16.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.