User login
THE CASES
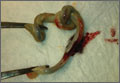
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.