User login
In many ways, vascular disease in the leg is similar to that in the heart. The risk factors, underlying conditions, and pathogenetic processes are the same, and in many cases, patients have both conditions. And just as cardiologists and emergency physicians have learned that in acute myocardial infarction “time is muscle,” we are coming to appreciate that in many cases of limb ischemia, “time is limb.”
Most physicians well understand the clinical spectrum of coronary artery disease, which ranges from stable angina to ST-elevation myocardial infarction. In the leg, the same situation exists: at the more benign end of the spectrum, patients experience no symptoms, but often that is because they lead a sedentary lifestyle, modifying their activity level to avoid pain. As the disease worsens, they can develop claudication and critical leg ischemia, comparable to non-ST-elevation myocardial infarction. The most severe condition is acute limb ischemia, analagous to ST-elevation myocardial infarction.
Distinguishing acute from critical limb ischemia is essential in patients who present with leg problems, whether it be leg pain or ulcers. The farther along the clinical spectrum the patient’s condition is, the more important it is to be aggressive in diagnosis and treatment. The history and physical examination are the most important first steps, focusing on the onset of symptoms, history, risk factors, and past interventions.
Peripheral artery disease is increasingly becoming a worldwide problem that is now being emphasized by the World Health Organization. Unfortunately, not enough attention is paid to the problem, not only in less-developed countries but also in the United States. Patients with peripheral artery disease tend to be elderly, in the lowest economic classes, and uninsured, and they often do not understand the impact of the disease on their health.
LEG ULCERS: CAUSES AND COSTS
Finding the underlying cause of leg ulcers is important, and the differential diagnosis is large (Table 1). However, knowing the cause does not necessarily lead to healing; it is still essential to assess perfusion, infection, and wound care, and to arrest edema.
Causes of leg and foot ulcers include venous insufficiency (with an estimated 2.5 million cases annually),1,2 diabetes (nearly 1 million cases),3 and pressure (ie, bedsores, occurring in up to 28% of patients in extended care),4 all at a cost in the billions of dollars.5–7
In general, peripheral artery disease itself does not cause ulcers; it is an inciting factor. It is important to find what started the process. Ill-fitting shoes, poor sensation because of diabetes, or a cut when trimming toenails can all contribute to a wound, and peripheral artery disease makes it unable to heal. The healing process requires more nutrients and oxygen than poor circulation can provide.
ACUTE LIMB ISCHEMIA
Acute limb ischemia is defined as any sudden decrease in limb perfusion causing a potential threat to limb viability.8 Although it comes on suddenly, it does not imply that the patient has not had long-standing peripheral artery disease. It is important to determine what suddenly changed to cause the onset of symptoms.
History and physical examination: The six Ps
A good history includes a thorough evaluation of the present illness, including the pain’s time of onset, abruptness, location, intensity, and change over time, and whether it is present at rest. The medical history should focus on claudication, diabetes, smoking, heart disease, palpitations, atrial fibrillation, and previous ischemic symptoms.8
The physical examination should focus on the “six Ps”:
- Pain
- Pulselessness
- Paresthesia (numbness occurs in about half of patients)
- Pallor (obstruction is typically one joint above the level of demarcation of pallor)
- Paralysis (a bad sign, particularly if the calf is tight)
- Poikilothermia (inability to regulate temperature).
A good pulse examination includes measuring the ankle-brachial index and a Doppler examination of both legs. A neurologic examination focusing on sensory and motor function is critical for determining the level of ischemia and the urgency of intervention.
Classification of acute limb ischemia
If it is determined that a patient has acute leg ischemia, it is important to categorize the condition using the classification system devised by the Society of Vascular Surgery and International Society of Cardiovascular Surgery (Table 2).9 The category establishes the type and urgency of treatment. This classification system is simple and depends on factors that can be assessed easily by nonspecialists:
- Pulses—arterial and venous pulses assessed by Doppler ultrasonography
- Sensation—the patient closes the eyes and answers if he or she can feel the examiner’s touch
- Motor function—can the patient move his or her toes?
Venous pulses can be difficult to assess. However, if the arterial pulse is present, the venous pulse should be next to it. Knowing the other criteria can determine the category, so not being certain of the venous pulse should not deter a clinician from assessing the other factors.
Category I is “viable.” Patients have intact sensory and motor functions and audible pulses. Patients in this category should be admitted and possibly started on anticoagulation therapy and referred to a vascular specialist within hours.
Category IIa is “threatened.” Sensation is starting to be lost but motor function is still present. These patients are considered to have reversible ischemia, analogous to myocardial infarction of the leg, and they require immediate attention.
Category IIb is similar and it also requires immediate attention.
Category III is usually irreversible, with loss of motor function and sensation.
CAUSES OF ACUTE LIMB ISCHEMIA
Thrombosis accounts for about 50% of cases. Underlying causes of the thrombosis are artherosclerosis (native or bypass), aneurysm, trauma, vasculitis (eg, in a rheumatologic disease such as lupus), and hypercoagulable states (particularly in patients with cancer).
Embolism accounts for about 30% of cases. Emboli usually arise from plaque rupture in atherosclerotic arteries or a clot breaking off from an aneurysm or from within the heart in patients with atrial fibrillation or another underlying heart disease. Paradoxical embolism, caused by an embolism crossing the heart through an opening such as a patent foramen ovale, is rare.
Uncommon causes include arterial dissection following trauma, adventitial cystic disease, popliteal artery entrapment, ergotism (from consuming fungus-contaminated grains), and human immunodeficiency virus arteriopathy.
The physical examination provides clues to the origin: livedo reticularis (purple discoloration in a mottled pattern) and blue nail beds indicate that an embolus is likely. Tests, including electrocardiography, echocardiography, and computed tomography of the chest and abdomen to look for an aneurysm, can help identify the cause. Ultrasonography of the popliteal arteries should also be considered to search for an aneurysm.
CRITICAL LIMB ISCHEMIA
Critical limb ischemia is more likely than acute limb ischemia to be seen in a general practice. Many aspects need to be addressed simultaneously, by different specialists: vascular and endocrine systems, infection, and wound care. The most successful management strategy is a dynamic approach using every piece of information.10
The Rutherford classification of peripheral artery disease has six categories based on the clinical presentation, with categories I through III being mild to severe claudication. We discuss here only the more severe categories: IV (pain at rest), V (tissue loss), and VI (gangrene).
Strong indicators of pain at rest are that the patient has to get up at night to dangle the leg over the bed or walk a few steps, or sleeps in a chair, or refuses to elevate the leg because of pain. The affected leg tends to appear red when the patient is standing (dependent rubor), but pale when the foot is elevated (elevation pallor).
Confirming that a patient has dependent rubor can be challenging, especially in people with dark skin. Classically, redness is seen when the leg is down and disappears with elevation, but in cellulitis, redness can also be reduced by elevating the leg. A foot that is hot to the touch is an indication of infection and not lack of perfusion alone.
The hemodynamic definition of critical limb ischemia is11:
- Ankle-brachial pressure index less than 0.4
- Reduced toebrachial pressure index, ie, less than 0.7
- Reduced transcutaneous pressure of oxygen (Tcpo2), ie, less than 40 mm Hg.
From 15% to 20% of patients with claudication will progress to critical ischemia over their lifetime, and in patients with claudication who also have diabetes, the risk is nearly 10 times higher. Without revascularization, the risk of amputation within 1 year is 73% for patients in Rutherford class IV and 95% for patients in class V or VI.
Revascularization and limb preservation
Preserving the limb is a prime goal. For patients who have an amputation, the mortality rate is 40% within 2 years.8 These patients tend to be elderly, and after an amputation, most will not learn to use a prosthesis and resume their previous level of activity. Other treatment objectives are to relieve pain, reduce cardiovascular risk, and minimize procedural complications.
Although limb preservation is not a controversial goal, best practices to preserve limbs are not universally available. Goodney et al12 studied variation in the United States in the use of lower-extremity vascular procedures for critical limb ischemia. They defined “low-intensity” to “high-intensity” regions of the country depending on the proportion of patients who underwent a vascular procedure in the year before amputation. They found considerable variation, but even in the region of highest intensity, more than 40% of patients did not have a vascular procedure in the year before amputation.
Similarly, Jones et al13 mapped amputation rates by US state and found significant variation even after adjusting for risk factors such as tobacco use and obesity.
Controversy surrounds the specifics of revascularization treatment, as in many fields in vascular medicine. However, most experts agree that improved perfusion is the goal.
The Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Artery Disease recommends revascularization as the best treatment for patients with critical limb ischemia.8 In addition, the American College of Cardiology and American Heart Association Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic) state that the tibial or pedal artery that is capable of providing continuous and uncompromised outflow to the foot should be used as the site of distal anastomosis.14 These guidelines do not yet mention endovascular therapy.
Angiosomes guide revascularization
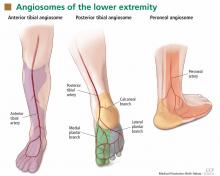
In the past few years, the ability to facilitate healing of foot ulcers has improved. Angiosomes—regions of vascularization supplied by specific arteries—can be mapped on the skin, similar to the way dermatomes are mapped for neural innervation (Figure 1). The foot and lower leg region has six angiosomes perfused by three arteries that branch off the popliteal artery after it passes behind the knee:
- The anterior tibial artery supplies the dorsum of the foot and the front of the lower limb.
- The posterior tibial artery supplies the plantar surface of the foot via three branches—the medial plantar, lateral plantar, and calcaneal branches.
- The peroneal artery supplies the lateral part of the foot with collaterals to the anterior and posterior tibial arteries if they are compromised.
Studies have compared angiosome-based treatment vs revascularizing the best available artery (thus depending on collateral flow to compensate to surrounding areas). They have found that regardless of whether an endovascular or bypass method of revascularization was used, an angiosome-based approach led to significantly higher amputation-free survival rates.15–17
Patients typically do not have blockage of only a single tibial artery. Graziani et al18 assessed the vascular lesions in 417 patients with critical limb ischemia and found that multiple below-knee arteries were frequently involved. This makes it difficult to decide where to target revascularization efforts, and the angiosome concept helps with that.
ASSESSING WOUND PERFUSION
Ankle- and toe-brachial indices assess perfusion
The ankle-brachial index19 is a good superficial assessment of perfusion. Multiple epidemiologic studies have shown the prognostic value of the ankle brachial index beyond the traditional risk factors and even the Framingham risk score.19 Values:
- Normal 1.1–1.30 (> 1.31 is abnormal and consistent with calcified vessels, and is an unreliable measure)
- Low normal 0.91–1.00
- Mild disease 0.71–0.90
- Moderate disease 0.41–0.70
- Severe disease ≤ 0.40.
However, the ankle-brachial index assesses perfusion only to the ankle, and many patients have ulcers in the toes and distal foot. The toe-brachial index must be specifically ordered in most institutions (if the first toe has an ulcer, the second toe should be assessed). The toe-brachial index is also important if the ankle-brachial index cannot be obtained because of calcified, noncompressible arteries in the ankle. A normal toe-brachial index is greater than 0.7.
The segmental blood pressure examination compares blood pressure measurements at multiple sites in the lower extremity. A drop of more than 20 mm Hg between segments indicates obstruction at that location. The test is simple and noninvasive and often can replace computed tomography.20
Transcutaneous oximetry
Transcutaneous oximetry measures the Tcpo2 from 1 to 2 mm deep in the skin from local capillaries. Measured adjacent to an ulcer, it is useful to predict wound healing and to assess the response to hyperbaric oxygen therapy.21 The values are:
- Normal > 70 mm Hg
- Impaired wound healing < 40 mm Hg
- Critical limb ischemia < 30 mm Hg.
Although most agree that a Tcpo2 below 40 mm Hg requires revascularization, low values can arise from many causes other than peripheral artery disease, including high altitude, pulmonary disease, heart failure, edema, inflammation, callus, and skin diseases such as scleroderma.
Skin perfusion pressure better predicts healing
Skin perfusion pressure is a measure of the capillary opening pressure after occlusion and is another way to assess perfusion. This test is not routinely done and must be specially requested.
The test is performed by inflating a blood pressure cuff on the leg until blood flow is occluded, then using laser Doppler to determine reactive hyperemia, ie, the gradual return of blood flow during controlled pressure release. The pressure at which movement is detected is the skin perfusion pressure.22
The laser Doppler probe emits and detects light scattered in the tissue. Light hitting moving blood cells undergoes a change in frequency, ie, a Doppler shift. An algorithm converts the optical information in the skin perfusion pressure by capturing the onset of capillary flow return and determining the pressure at which flow returns. Categories of results:
- > 50 mm Hg—normal
- 40–50 mm Hg—mild ischemia (wound healing probable)
- 30–40 mm Hg—moderate ischemia (wound healing uncertain)
- < 30 mm Hg—critical limb ischemia (wound healing unlikely).
Skin perfusion pressure testing has the advantages of not being affected by vessel calcification, thickened skin, or edema. It can be used on the plantar aspect of the foot and on digits. Recent small studies indicate that it is more sensitive for predicting wound healing than Tcpo2 measures.
On the other hand, skin perfusion pressure testing is not useful for predicting response to hyperbaric oxygen therapy. Also, blood flow occlusion by the cuff may be painful.
Intraoperative fluorescence angiography
Intraoperative fluorescence angiography is used to assess flap viability during reconstructive surgery and is being studied to determine its usefulness for assessing tissue viability in limb ischemia.
The test provides real-time assessment of capillary perfusion, determining surface tissue viability. The imaging head contains a digital camera, a laser light source, and a distance sensor. The test requires intravenous administration of indocyanine green, which binds to plasma proteins and is cleared through the liver, making it safe for patients with renal dysfunction. It cannot be used in patients with allergies to iodine contrast, penicillin, or sulfa.23
PREVENTION TARGETS CARDIOVASCULAR RISK FACTORS
Preventive measures are the same as for cardiovascular disease, ie, aggressive risk-factor modification: quitting smoking, lowering low-density lipoprotein cholesterol, reducing blood pressure, controlling diabetes, and managing heart failure.
Dual antiplatelet therapy should be instituted with aspirin and clopidogrel (Plavix) in patients undergoing revascularization. One can also consider cilostazol (Pletal); however, the role of this agent in patients with critical limb ischemia is less defined.
BYPASS OR ANGIOPLASTY?
The Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial24 randomly assigned 452 patients with severe limb ischemia due to infrainguinal atherosclerosis to receive either surgery-first or angioplasty-first care and followed them for 5.5 years.
No significant differences between the two groups were found in amputation-free survival, deaths, or health-related quality of life. However, hospital costs associated with the surgery-first strategy were about one-third higher. As expected, more patients in the surgery group developed a wound infection, and more patients in the angioplasty group required bypass surgery at some point.
The conclusion that can be reached from this study is that patients presenting with severe limb ischemia due to infrainguinal atherosclerotic occlusive disease who are suitable for both surgical and interventional procedures can be treated with either method. However, most experts consider endovascular therapy as the first option in many patients. The National Institutes of Health recently funded a study to compare contemporary endovascular therapy vs surgery in patients with critical limb ischemia.
TAKE-HOME POINTS
In the last decade, significant endovascular advances have been made. New devices and techniques have enhanced our ability to treat high-risk patients who have critical limb ischemia. The combination of risk factor modification, accurate diagnosis, and aggressive revascularization should prevent limb loss in many of these patients. For the primary care physician, a low threshold for assessing perfusion in patients with critical limb ischemia is important using a screening ankle-brachial index and toe-brachial index. These patients should promptly be referred to a vascular specialist for further evaluation and treatment.
- Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994; 31:49–53.
- Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg 2004; 188(1A suppl):1–8.
- Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22:382–387.
- Cuddigan J, Berlowitz DR, Ayello E; National Pressure Ulcer Advisory Panel. Pressure ulcers in America: Prevalence, incidence, and implications for the future: an executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care 2001; 14:208–215.
- Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med 1999; 4:1–7.
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26:1790–1795.
- Kumar RN, Gupchup GV, Dodd MA, et al. Direct health care costs of 4 common skin ulcers in New Mexico Medicaid fee-for-service patients. Adv Skin Wound Care 2004; 17:143–149.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(suppl):S5–S67.
- Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26:517–538. Erratum in J Vasc Surg 2001; 33:805.
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363:301–304. Erratum in N Engl J Med 2010; 363:1092.
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000; 31:S1–S296.
- Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes 2012; 5:94–102.
- Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol 2012; 60:2230–2236.
- Hirsch AT, Haskal ZJ, Mertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Alexandrescu VA, Hubermont G, Philips Y, et al. Selective primary angioplasty following an angiosome model of reperfusion in the treatment of Wagner 1–4 diabetic foot lesions: practice in a multidisciplinary diabetic limb service. J Endovasc Ther 2008; 15:580–593.
- Neville RF, Attinger CE, Bulan EJ, Ducic I, Thomassen M, Sidawy AN. Revascularization of a specific angiosome for limb salvage: does the target artery matter? Ann Vasc Surg 2009; 23:367–373.
- Iida O, Soga Y, Hirano K, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg 2012; 55:363–370.
- Graziani L, Silvestro A, Bertone V, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg 2007; 33:453–460.
- Newman AB, Siscovick DS, Manolio TA, et al., Cardiovascular Heart Study (CHS) Collaborative Research Group. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation 1993; 88:837–845.
- Cronenwett JL, Johnston KW. Rutherford’s Vascular Surgery. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010.
- Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med 2009; 36:43–53.
- Lo T, Sample R, Moore P, Gold P. Prediction of wound healing outcome using skin perfusion pressure and transcutaneous oximetry. Wounds 2009; 21:310–316.
- Perry D, Bharara M, Armstrong DG, Mills J. Intraoperative fluorescence vascular angiography: during tibial bypass. J Diabetes Sci Technol 2012; 6:204–208.
- Adam DJ, Beard JD, Cleveland T, et al; BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005; 366:1925–1934.
In many ways, vascular disease in the leg is similar to that in the heart. The risk factors, underlying conditions, and pathogenetic processes are the same, and in many cases, patients have both conditions. And just as cardiologists and emergency physicians have learned that in acute myocardial infarction “time is muscle,” we are coming to appreciate that in many cases of limb ischemia, “time is limb.”
Most physicians well understand the clinical spectrum of coronary artery disease, which ranges from stable angina to ST-elevation myocardial infarction. In the leg, the same situation exists: at the more benign end of the spectrum, patients experience no symptoms, but often that is because they lead a sedentary lifestyle, modifying their activity level to avoid pain. As the disease worsens, they can develop claudication and critical leg ischemia, comparable to non-ST-elevation myocardial infarction. The most severe condition is acute limb ischemia, analagous to ST-elevation myocardial infarction.
Distinguishing acute from critical limb ischemia is essential in patients who present with leg problems, whether it be leg pain or ulcers. The farther along the clinical spectrum the patient’s condition is, the more important it is to be aggressive in diagnosis and treatment. The history and physical examination are the most important first steps, focusing on the onset of symptoms, history, risk factors, and past interventions.
Peripheral artery disease is increasingly becoming a worldwide problem that is now being emphasized by the World Health Organization. Unfortunately, not enough attention is paid to the problem, not only in less-developed countries but also in the United States. Patients with peripheral artery disease tend to be elderly, in the lowest economic classes, and uninsured, and they often do not understand the impact of the disease on their health.
LEG ULCERS: CAUSES AND COSTS
Finding the underlying cause of leg ulcers is important, and the differential diagnosis is large (Table 1). However, knowing the cause does not necessarily lead to healing; it is still essential to assess perfusion, infection, and wound care, and to arrest edema.
Causes of leg and foot ulcers include venous insufficiency (with an estimated 2.5 million cases annually),1,2 diabetes (nearly 1 million cases),3 and pressure (ie, bedsores, occurring in up to 28% of patients in extended care),4 all at a cost in the billions of dollars.5–7
In general, peripheral artery disease itself does not cause ulcers; it is an inciting factor. It is important to find what started the process. Ill-fitting shoes, poor sensation because of diabetes, or a cut when trimming toenails can all contribute to a wound, and peripheral artery disease makes it unable to heal. The healing process requires more nutrients and oxygen than poor circulation can provide.
ACUTE LIMB ISCHEMIA
Acute limb ischemia is defined as any sudden decrease in limb perfusion causing a potential threat to limb viability.8 Although it comes on suddenly, it does not imply that the patient has not had long-standing peripheral artery disease. It is important to determine what suddenly changed to cause the onset of symptoms.
History and physical examination: The six Ps
A good history includes a thorough evaluation of the present illness, including the pain’s time of onset, abruptness, location, intensity, and change over time, and whether it is present at rest. The medical history should focus on claudication, diabetes, smoking, heart disease, palpitations, atrial fibrillation, and previous ischemic symptoms.8
The physical examination should focus on the “six Ps”:
- Pain
- Pulselessness
- Paresthesia (numbness occurs in about half of patients)
- Pallor (obstruction is typically one joint above the level of demarcation of pallor)
- Paralysis (a bad sign, particularly if the calf is tight)
- Poikilothermia (inability to regulate temperature).
A good pulse examination includes measuring the ankle-brachial index and a Doppler examination of both legs. A neurologic examination focusing on sensory and motor function is critical for determining the level of ischemia and the urgency of intervention.
Classification of acute limb ischemia
If it is determined that a patient has acute leg ischemia, it is important to categorize the condition using the classification system devised by the Society of Vascular Surgery and International Society of Cardiovascular Surgery (Table 2).9 The category establishes the type and urgency of treatment. This classification system is simple and depends on factors that can be assessed easily by nonspecialists:
- Pulses—arterial and venous pulses assessed by Doppler ultrasonography
- Sensation—the patient closes the eyes and answers if he or she can feel the examiner’s touch
- Motor function—can the patient move his or her toes?
Venous pulses can be difficult to assess. However, if the arterial pulse is present, the venous pulse should be next to it. Knowing the other criteria can determine the category, so not being certain of the venous pulse should not deter a clinician from assessing the other factors.
Category I is “viable.” Patients have intact sensory and motor functions and audible pulses. Patients in this category should be admitted and possibly started on anticoagulation therapy and referred to a vascular specialist within hours.
Category IIa is “threatened.” Sensation is starting to be lost but motor function is still present. These patients are considered to have reversible ischemia, analogous to myocardial infarction of the leg, and they require immediate attention.
Category IIb is similar and it also requires immediate attention.
Category III is usually irreversible, with loss of motor function and sensation.
CAUSES OF ACUTE LIMB ISCHEMIA
Thrombosis accounts for about 50% of cases. Underlying causes of the thrombosis are artherosclerosis (native or bypass), aneurysm, trauma, vasculitis (eg, in a rheumatologic disease such as lupus), and hypercoagulable states (particularly in patients with cancer).
Embolism accounts for about 30% of cases. Emboli usually arise from plaque rupture in atherosclerotic arteries or a clot breaking off from an aneurysm or from within the heart in patients with atrial fibrillation or another underlying heart disease. Paradoxical embolism, caused by an embolism crossing the heart through an opening such as a patent foramen ovale, is rare.
Uncommon causes include arterial dissection following trauma, adventitial cystic disease, popliteal artery entrapment, ergotism (from consuming fungus-contaminated grains), and human immunodeficiency virus arteriopathy.
The physical examination provides clues to the origin: livedo reticularis (purple discoloration in a mottled pattern) and blue nail beds indicate that an embolus is likely. Tests, including electrocardiography, echocardiography, and computed tomography of the chest and abdomen to look for an aneurysm, can help identify the cause. Ultrasonography of the popliteal arteries should also be considered to search for an aneurysm.
CRITICAL LIMB ISCHEMIA
Critical limb ischemia is more likely than acute limb ischemia to be seen in a general practice. Many aspects need to be addressed simultaneously, by different specialists: vascular and endocrine systems, infection, and wound care. The most successful management strategy is a dynamic approach using every piece of information.10
The Rutherford classification of peripheral artery disease has six categories based on the clinical presentation, with categories I through III being mild to severe claudication. We discuss here only the more severe categories: IV (pain at rest), V (tissue loss), and VI (gangrene).
Strong indicators of pain at rest are that the patient has to get up at night to dangle the leg over the bed or walk a few steps, or sleeps in a chair, or refuses to elevate the leg because of pain. The affected leg tends to appear red when the patient is standing (dependent rubor), but pale when the foot is elevated (elevation pallor).
Confirming that a patient has dependent rubor can be challenging, especially in people with dark skin. Classically, redness is seen when the leg is down and disappears with elevation, but in cellulitis, redness can also be reduced by elevating the leg. A foot that is hot to the touch is an indication of infection and not lack of perfusion alone.
The hemodynamic definition of critical limb ischemia is11:
- Ankle-brachial pressure index less than 0.4
- Reduced toebrachial pressure index, ie, less than 0.7
- Reduced transcutaneous pressure of oxygen (Tcpo2), ie, less than 40 mm Hg.
From 15% to 20% of patients with claudication will progress to critical ischemia over their lifetime, and in patients with claudication who also have diabetes, the risk is nearly 10 times higher. Without revascularization, the risk of amputation within 1 year is 73% for patients in Rutherford class IV and 95% for patients in class V or VI.
Revascularization and limb preservation
Preserving the limb is a prime goal. For patients who have an amputation, the mortality rate is 40% within 2 years.8 These patients tend to be elderly, and after an amputation, most will not learn to use a prosthesis and resume their previous level of activity. Other treatment objectives are to relieve pain, reduce cardiovascular risk, and minimize procedural complications.
Although limb preservation is not a controversial goal, best practices to preserve limbs are not universally available. Goodney et al12 studied variation in the United States in the use of lower-extremity vascular procedures for critical limb ischemia. They defined “low-intensity” to “high-intensity” regions of the country depending on the proportion of patients who underwent a vascular procedure in the year before amputation. They found considerable variation, but even in the region of highest intensity, more than 40% of patients did not have a vascular procedure in the year before amputation.
Similarly, Jones et al13 mapped amputation rates by US state and found significant variation even after adjusting for risk factors such as tobacco use and obesity.
Controversy surrounds the specifics of revascularization treatment, as in many fields in vascular medicine. However, most experts agree that improved perfusion is the goal.
The Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Artery Disease recommends revascularization as the best treatment for patients with critical limb ischemia.8 In addition, the American College of Cardiology and American Heart Association Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic) state that the tibial or pedal artery that is capable of providing continuous and uncompromised outflow to the foot should be used as the site of distal anastomosis.14 These guidelines do not yet mention endovascular therapy.
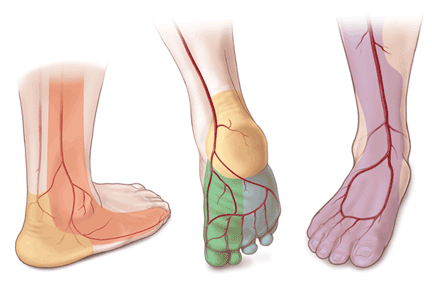
Angiosomes guide revascularization
In the past few years, the ability to facilitate healing of foot ulcers has improved. Angiosomes—regions of vascularization supplied by specific arteries—can be mapped on the skin, similar to the way dermatomes are mapped for neural innervation (Figure 1). The foot and lower leg region has six angiosomes perfused by three arteries that branch off the popliteal artery after it passes behind the knee:
- The anterior tibial artery supplies the dorsum of the foot and the front of the lower limb.
- The posterior tibial artery supplies the plantar surface of the foot via three branches—the medial plantar, lateral plantar, and calcaneal branches.
- The peroneal artery supplies the lateral part of the foot with collaterals to the anterior and posterior tibial arteries if they are compromised.
Studies have compared angiosome-based treatment vs revascularizing the best available artery (thus depending on collateral flow to compensate to surrounding areas). They have found that regardless of whether an endovascular or bypass method of revascularization was used, an angiosome-based approach led to significantly higher amputation-free survival rates.15–17
Patients typically do not have blockage of only a single tibial artery. Graziani et al18 assessed the vascular lesions in 417 patients with critical limb ischemia and found that multiple below-knee arteries were frequently involved. This makes it difficult to decide where to target revascularization efforts, and the angiosome concept helps with that.
ASSESSING WOUND PERFUSION
Ankle- and toe-brachial indices assess perfusion
The ankle-brachial index19 is a good superficial assessment of perfusion. Multiple epidemiologic studies have shown the prognostic value of the ankle brachial index beyond the traditional risk factors and even the Framingham risk score.19 Values:
- Normal 1.1–1.30 (> 1.31 is abnormal and consistent with calcified vessels, and is an unreliable measure)
- Low normal 0.91–1.00
- Mild disease 0.71–0.90
- Moderate disease 0.41–0.70
- Severe disease ≤ 0.40.
However, the ankle-brachial index assesses perfusion only to the ankle, and many patients have ulcers in the toes and distal foot. The toe-brachial index must be specifically ordered in most institutions (if the first toe has an ulcer, the second toe should be assessed). The toe-brachial index is also important if the ankle-brachial index cannot be obtained because of calcified, noncompressible arteries in the ankle. A normal toe-brachial index is greater than 0.7.
The segmental blood pressure examination compares blood pressure measurements at multiple sites in the lower extremity. A drop of more than 20 mm Hg between segments indicates obstruction at that location. The test is simple and noninvasive and often can replace computed tomography.20
Transcutaneous oximetry
Transcutaneous oximetry measures the Tcpo2 from 1 to 2 mm deep in the skin from local capillaries. Measured adjacent to an ulcer, it is useful to predict wound healing and to assess the response to hyperbaric oxygen therapy.21 The values are:
- Normal > 70 mm Hg
- Impaired wound healing < 40 mm Hg
- Critical limb ischemia < 30 mm Hg.
Although most agree that a Tcpo2 below 40 mm Hg requires revascularization, low values can arise from many causes other than peripheral artery disease, including high altitude, pulmonary disease, heart failure, edema, inflammation, callus, and skin diseases such as scleroderma.
Skin perfusion pressure better predicts healing
Skin perfusion pressure is a measure of the capillary opening pressure after occlusion and is another way to assess perfusion. This test is not routinely done and must be specially requested.
The test is performed by inflating a blood pressure cuff on the leg until blood flow is occluded, then using laser Doppler to determine reactive hyperemia, ie, the gradual return of blood flow during controlled pressure release. The pressure at which movement is detected is the skin perfusion pressure.22
The laser Doppler probe emits and detects light scattered in the tissue. Light hitting moving blood cells undergoes a change in frequency, ie, a Doppler shift. An algorithm converts the optical information in the skin perfusion pressure by capturing the onset of capillary flow return and determining the pressure at which flow returns. Categories of results:
- > 50 mm Hg—normal
- 40–50 mm Hg—mild ischemia (wound healing probable)
- 30–40 mm Hg—moderate ischemia (wound healing uncertain)
- < 30 mm Hg—critical limb ischemia (wound healing unlikely).
Skin perfusion pressure testing has the advantages of not being affected by vessel calcification, thickened skin, or edema. It can be used on the plantar aspect of the foot and on digits. Recent small studies indicate that it is more sensitive for predicting wound healing than Tcpo2 measures.
On the other hand, skin perfusion pressure testing is not useful for predicting response to hyperbaric oxygen therapy. Also, blood flow occlusion by the cuff may be painful.
Intraoperative fluorescence angiography
Intraoperative fluorescence angiography is used to assess flap viability during reconstructive surgery and is being studied to determine its usefulness for assessing tissue viability in limb ischemia.
The test provides real-time assessment of capillary perfusion, determining surface tissue viability. The imaging head contains a digital camera, a laser light source, and a distance sensor. The test requires intravenous administration of indocyanine green, which binds to plasma proteins and is cleared through the liver, making it safe for patients with renal dysfunction. It cannot be used in patients with allergies to iodine contrast, penicillin, or sulfa.23
PREVENTION TARGETS CARDIOVASCULAR RISK FACTORS
Preventive measures are the same as for cardiovascular disease, ie, aggressive risk-factor modification: quitting smoking, lowering low-density lipoprotein cholesterol, reducing blood pressure, controlling diabetes, and managing heart failure.
Dual antiplatelet therapy should be instituted with aspirin and clopidogrel (Plavix) in patients undergoing revascularization. One can also consider cilostazol (Pletal); however, the role of this agent in patients with critical limb ischemia is less defined.
BYPASS OR ANGIOPLASTY?
The Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial24 randomly assigned 452 patients with severe limb ischemia due to infrainguinal atherosclerosis to receive either surgery-first or angioplasty-first care and followed them for 5.5 years.
No significant differences between the two groups were found in amputation-free survival, deaths, or health-related quality of life. However, hospital costs associated with the surgery-first strategy were about one-third higher. As expected, more patients in the surgery group developed a wound infection, and more patients in the angioplasty group required bypass surgery at some point.
The conclusion that can be reached from this study is that patients presenting with severe limb ischemia due to infrainguinal atherosclerotic occlusive disease who are suitable for both surgical and interventional procedures can be treated with either method. However, most experts consider endovascular therapy as the first option in many patients. The National Institutes of Health recently funded a study to compare contemporary endovascular therapy vs surgery in patients with critical limb ischemia.
TAKE-HOME POINTS
In the last decade, significant endovascular advances have been made. New devices and techniques have enhanced our ability to treat high-risk patients who have critical limb ischemia. The combination of risk factor modification, accurate diagnosis, and aggressive revascularization should prevent limb loss in many of these patients. For the primary care physician, a low threshold for assessing perfusion in patients with critical limb ischemia is important using a screening ankle-brachial index and toe-brachial index. These patients should promptly be referred to a vascular specialist for further evaluation and treatment.
In many ways, vascular disease in the leg is similar to that in the heart. The risk factors, underlying conditions, and pathogenetic processes are the same, and in many cases, patients have both conditions. And just as cardiologists and emergency physicians have learned that in acute myocardial infarction “time is muscle,” we are coming to appreciate that in many cases of limb ischemia, “time is limb.”
Most physicians well understand the clinical spectrum of coronary artery disease, which ranges from stable angina to ST-elevation myocardial infarction. In the leg, the same situation exists: at the more benign end of the spectrum, patients experience no symptoms, but often that is because they lead a sedentary lifestyle, modifying their activity level to avoid pain. As the disease worsens, they can develop claudication and critical leg ischemia, comparable to non-ST-elevation myocardial infarction. The most severe condition is acute limb ischemia, analagous to ST-elevation myocardial infarction.
Distinguishing acute from critical limb ischemia is essential in patients who present with leg problems, whether it be leg pain or ulcers. The farther along the clinical spectrum the patient’s condition is, the more important it is to be aggressive in diagnosis and treatment. The history and physical examination are the most important first steps, focusing on the onset of symptoms, history, risk factors, and past interventions.
Peripheral artery disease is increasingly becoming a worldwide problem that is now being emphasized by the World Health Organization. Unfortunately, not enough attention is paid to the problem, not only in less-developed countries but also in the United States. Patients with peripheral artery disease tend to be elderly, in the lowest economic classes, and uninsured, and they often do not understand the impact of the disease on their health.
LEG ULCERS: CAUSES AND COSTS
Finding the underlying cause of leg ulcers is important, and the differential diagnosis is large (Table 1). However, knowing the cause does not necessarily lead to healing; it is still essential to assess perfusion, infection, and wound care, and to arrest edema.
Causes of leg and foot ulcers include venous insufficiency (with an estimated 2.5 million cases annually),1,2 diabetes (nearly 1 million cases),3 and pressure (ie, bedsores, occurring in up to 28% of patients in extended care),4 all at a cost in the billions of dollars.5–7
In general, peripheral artery disease itself does not cause ulcers; it is an inciting factor. It is important to find what started the process. Ill-fitting shoes, poor sensation because of diabetes, or a cut when trimming toenails can all contribute to a wound, and peripheral artery disease makes it unable to heal. The healing process requires more nutrients and oxygen than poor circulation can provide.
ACUTE LIMB ISCHEMIA
Acute limb ischemia is defined as any sudden decrease in limb perfusion causing a potential threat to limb viability.8 Although it comes on suddenly, it does not imply that the patient has not had long-standing peripheral artery disease. It is important to determine what suddenly changed to cause the onset of symptoms.
History and physical examination: The six Ps
A good history includes a thorough evaluation of the present illness, including the pain’s time of onset, abruptness, location, intensity, and change over time, and whether it is present at rest. The medical history should focus on claudication, diabetes, smoking, heart disease, palpitations, atrial fibrillation, and previous ischemic symptoms.8
The physical examination should focus on the “six Ps”:
- Pain
- Pulselessness
- Paresthesia (numbness occurs in about half of patients)
- Pallor (obstruction is typically one joint above the level of demarcation of pallor)
- Paralysis (a bad sign, particularly if the calf is tight)
- Poikilothermia (inability to regulate temperature).
A good pulse examination includes measuring the ankle-brachial index and a Doppler examination of both legs. A neurologic examination focusing on sensory and motor function is critical for determining the level of ischemia and the urgency of intervention.
Classification of acute limb ischemia
If it is determined that a patient has acute leg ischemia, it is important to categorize the condition using the classification system devised by the Society of Vascular Surgery and International Society of Cardiovascular Surgery (Table 2).9 The category establishes the type and urgency of treatment. This classification system is simple and depends on factors that can be assessed easily by nonspecialists:
- Pulses—arterial and venous pulses assessed by Doppler ultrasonography
- Sensation—the patient closes the eyes and answers if he or she can feel the examiner’s touch
- Motor function—can the patient move his or her toes?
Venous pulses can be difficult to assess. However, if the arterial pulse is present, the venous pulse should be next to it. Knowing the other criteria can determine the category, so not being certain of the venous pulse should not deter a clinician from assessing the other factors.
Category I is “viable.” Patients have intact sensory and motor functions and audible pulses. Patients in this category should be admitted and possibly started on anticoagulation therapy and referred to a vascular specialist within hours.
Category IIa is “threatened.” Sensation is starting to be lost but motor function is still present. These patients are considered to have reversible ischemia, analogous to myocardial infarction of the leg, and they require immediate attention.
Category IIb is similar and it also requires immediate attention.
Category III is usually irreversible, with loss of motor function and sensation.
CAUSES OF ACUTE LIMB ISCHEMIA
Thrombosis accounts for about 50% of cases. Underlying causes of the thrombosis are artherosclerosis (native or bypass), aneurysm, trauma, vasculitis (eg, in a rheumatologic disease such as lupus), and hypercoagulable states (particularly in patients with cancer).
Embolism accounts for about 30% of cases. Emboli usually arise from plaque rupture in atherosclerotic arteries or a clot breaking off from an aneurysm or from within the heart in patients with atrial fibrillation or another underlying heart disease. Paradoxical embolism, caused by an embolism crossing the heart through an opening such as a patent foramen ovale, is rare.
Uncommon causes include arterial dissection following trauma, adventitial cystic disease, popliteal artery entrapment, ergotism (from consuming fungus-contaminated grains), and human immunodeficiency virus arteriopathy.
The physical examination provides clues to the origin: livedo reticularis (purple discoloration in a mottled pattern) and blue nail beds indicate that an embolus is likely. Tests, including electrocardiography, echocardiography, and computed tomography of the chest and abdomen to look for an aneurysm, can help identify the cause. Ultrasonography of the popliteal arteries should also be considered to search for an aneurysm.
CRITICAL LIMB ISCHEMIA
Critical limb ischemia is more likely than acute limb ischemia to be seen in a general practice. Many aspects need to be addressed simultaneously, by different specialists: vascular and endocrine systems, infection, and wound care. The most successful management strategy is a dynamic approach using every piece of information.10
The Rutherford classification of peripheral artery disease has six categories based on the clinical presentation, with categories I through III being mild to severe claudication. We discuss here only the more severe categories: IV (pain at rest), V (tissue loss), and VI (gangrene).
Strong indicators of pain at rest are that the patient has to get up at night to dangle the leg over the bed or walk a few steps, or sleeps in a chair, or refuses to elevate the leg because of pain. The affected leg tends to appear red when the patient is standing (dependent rubor), but pale when the foot is elevated (elevation pallor).
Confirming that a patient has dependent rubor can be challenging, especially in people with dark skin. Classically, redness is seen when the leg is down and disappears with elevation, but in cellulitis, redness can also be reduced by elevating the leg. A foot that is hot to the touch is an indication of infection and not lack of perfusion alone.
The hemodynamic definition of critical limb ischemia is11:
- Ankle-brachial pressure index less than 0.4
- Reduced toebrachial pressure index, ie, less than 0.7
- Reduced transcutaneous pressure of oxygen (Tcpo2), ie, less than 40 mm Hg.
From 15% to 20% of patients with claudication will progress to critical ischemia over their lifetime, and in patients with claudication who also have diabetes, the risk is nearly 10 times higher. Without revascularization, the risk of amputation within 1 year is 73% for patients in Rutherford class IV and 95% for patients in class V or VI.
Revascularization and limb preservation
Preserving the limb is a prime goal. For patients who have an amputation, the mortality rate is 40% within 2 years.8 These patients tend to be elderly, and after an amputation, most will not learn to use a prosthesis and resume their previous level of activity. Other treatment objectives are to relieve pain, reduce cardiovascular risk, and minimize procedural complications.
Although limb preservation is not a controversial goal, best practices to preserve limbs are not universally available. Goodney et al12 studied variation in the United States in the use of lower-extremity vascular procedures for critical limb ischemia. They defined “low-intensity” to “high-intensity” regions of the country depending on the proportion of patients who underwent a vascular procedure in the year before amputation. They found considerable variation, but even in the region of highest intensity, more than 40% of patients did not have a vascular procedure in the year before amputation.
Similarly, Jones et al13 mapped amputation rates by US state and found significant variation even after adjusting for risk factors such as tobacco use and obesity.
Controversy surrounds the specifics of revascularization treatment, as in many fields in vascular medicine. However, most experts agree that improved perfusion is the goal.
The Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Artery Disease recommends revascularization as the best treatment for patients with critical limb ischemia.8 In addition, the American College of Cardiology and American Heart Association Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic) state that the tibial or pedal artery that is capable of providing continuous and uncompromised outflow to the foot should be used as the site of distal anastomosis.14 These guidelines do not yet mention endovascular therapy.
Angiosomes guide revascularization
In the past few years, the ability to facilitate healing of foot ulcers has improved. Angiosomes—regions of vascularization supplied by specific arteries—can be mapped on the skin, similar to the way dermatomes are mapped for neural innervation (Figure 1). The foot and lower leg region has six angiosomes perfused by three arteries that branch off the popliteal artery after it passes behind the knee:
- The anterior tibial artery supplies the dorsum of the foot and the front of the lower limb.
- The posterior tibial artery supplies the plantar surface of the foot via three branches—the medial plantar, lateral plantar, and calcaneal branches.
- The peroneal artery supplies the lateral part of the foot with collaterals to the anterior and posterior tibial arteries if they are compromised.
Studies have compared angiosome-based treatment vs revascularizing the best available artery (thus depending on collateral flow to compensate to surrounding areas). They have found that regardless of whether an endovascular or bypass method of revascularization was used, an angiosome-based approach led to significantly higher amputation-free survival rates.15–17
Patients typically do not have blockage of only a single tibial artery. Graziani et al18 assessed the vascular lesions in 417 patients with critical limb ischemia and found that multiple below-knee arteries were frequently involved. This makes it difficult to decide where to target revascularization efforts, and the angiosome concept helps with that.
ASSESSING WOUND PERFUSION
Ankle- and toe-brachial indices assess perfusion
The ankle-brachial index19 is a good superficial assessment of perfusion. Multiple epidemiologic studies have shown the prognostic value of the ankle brachial index beyond the traditional risk factors and even the Framingham risk score.19 Values:
- Normal 1.1–1.30 (> 1.31 is abnormal and consistent with calcified vessels, and is an unreliable measure)
- Low normal 0.91–1.00
- Mild disease 0.71–0.90
- Moderate disease 0.41–0.70
- Severe disease ≤ 0.40.
However, the ankle-brachial index assesses perfusion only to the ankle, and many patients have ulcers in the toes and distal foot. The toe-brachial index must be specifically ordered in most institutions (if the first toe has an ulcer, the second toe should be assessed). The toe-brachial index is also important if the ankle-brachial index cannot be obtained because of calcified, noncompressible arteries in the ankle. A normal toe-brachial index is greater than 0.7.
The segmental blood pressure examination compares blood pressure measurements at multiple sites in the lower extremity. A drop of more than 20 mm Hg between segments indicates obstruction at that location. The test is simple and noninvasive and often can replace computed tomography.20
Transcutaneous oximetry
Transcutaneous oximetry measures the Tcpo2 from 1 to 2 mm deep in the skin from local capillaries. Measured adjacent to an ulcer, it is useful to predict wound healing and to assess the response to hyperbaric oxygen therapy.21 The values are:
- Normal > 70 mm Hg
- Impaired wound healing < 40 mm Hg
- Critical limb ischemia < 30 mm Hg.
Although most agree that a Tcpo2 below 40 mm Hg requires revascularization, low values can arise from many causes other than peripheral artery disease, including high altitude, pulmonary disease, heart failure, edema, inflammation, callus, and skin diseases such as scleroderma.
Skin perfusion pressure better predicts healing
Skin perfusion pressure is a measure of the capillary opening pressure after occlusion and is another way to assess perfusion. This test is not routinely done and must be specially requested.
The test is performed by inflating a blood pressure cuff on the leg until blood flow is occluded, then using laser Doppler to determine reactive hyperemia, ie, the gradual return of blood flow during controlled pressure release. The pressure at which movement is detected is the skin perfusion pressure.22
The laser Doppler probe emits and detects light scattered in the tissue. Light hitting moving blood cells undergoes a change in frequency, ie, a Doppler shift. An algorithm converts the optical information in the skin perfusion pressure by capturing the onset of capillary flow return and determining the pressure at which flow returns. Categories of results:
- > 50 mm Hg—normal
- 40–50 mm Hg—mild ischemia (wound healing probable)
- 30–40 mm Hg—moderate ischemia (wound healing uncertain)
- < 30 mm Hg—critical limb ischemia (wound healing unlikely).
Skin perfusion pressure testing has the advantages of not being affected by vessel calcification, thickened skin, or edema. It can be used on the plantar aspect of the foot and on digits. Recent small studies indicate that it is more sensitive for predicting wound healing than Tcpo2 measures.
On the other hand, skin perfusion pressure testing is not useful for predicting response to hyperbaric oxygen therapy. Also, blood flow occlusion by the cuff may be painful.
Intraoperative fluorescence angiography
Intraoperative fluorescence angiography is used to assess flap viability during reconstructive surgery and is being studied to determine its usefulness for assessing tissue viability in limb ischemia.
The test provides real-time assessment of capillary perfusion, determining surface tissue viability. The imaging head contains a digital camera, a laser light source, and a distance sensor. The test requires intravenous administration of indocyanine green, which binds to plasma proteins and is cleared through the liver, making it safe for patients with renal dysfunction. It cannot be used in patients with allergies to iodine contrast, penicillin, or sulfa.23
PREVENTION TARGETS CARDIOVASCULAR RISK FACTORS
Preventive measures are the same as for cardiovascular disease, ie, aggressive risk-factor modification: quitting smoking, lowering low-density lipoprotein cholesterol, reducing blood pressure, controlling diabetes, and managing heart failure.
Dual antiplatelet therapy should be instituted with aspirin and clopidogrel (Plavix) in patients undergoing revascularization. One can also consider cilostazol (Pletal); however, the role of this agent in patients with critical limb ischemia is less defined.
BYPASS OR ANGIOPLASTY?
The Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial24 randomly assigned 452 patients with severe limb ischemia due to infrainguinal atherosclerosis to receive either surgery-first or angioplasty-first care and followed them for 5.5 years.
No significant differences between the two groups were found in amputation-free survival, deaths, or health-related quality of life. However, hospital costs associated with the surgery-first strategy were about one-third higher. As expected, more patients in the surgery group developed a wound infection, and more patients in the angioplasty group required bypass surgery at some point.
The conclusion that can be reached from this study is that patients presenting with severe limb ischemia due to infrainguinal atherosclerotic occlusive disease who are suitable for both surgical and interventional procedures can be treated with either method. However, most experts consider endovascular therapy as the first option in many patients. The National Institutes of Health recently funded a study to compare contemporary endovascular therapy vs surgery in patients with critical limb ischemia.
TAKE-HOME POINTS
In the last decade, significant endovascular advances have been made. New devices and techniques have enhanced our ability to treat high-risk patients who have critical limb ischemia. The combination of risk factor modification, accurate diagnosis, and aggressive revascularization should prevent limb loss in many of these patients. For the primary care physician, a low threshold for assessing perfusion in patients with critical limb ischemia is important using a screening ankle-brachial index and toe-brachial index. These patients should promptly be referred to a vascular specialist for further evaluation and treatment.
- Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994; 31:49–53.
- Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg 2004; 188(1A suppl):1–8.
- Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22:382–387.
- Cuddigan J, Berlowitz DR, Ayello E; National Pressure Ulcer Advisory Panel. Pressure ulcers in America: Prevalence, incidence, and implications for the future: an executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care 2001; 14:208–215.
- Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med 1999; 4:1–7.
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26:1790–1795.
- Kumar RN, Gupchup GV, Dodd MA, et al. Direct health care costs of 4 common skin ulcers in New Mexico Medicaid fee-for-service patients. Adv Skin Wound Care 2004; 17:143–149.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(suppl):S5–S67.
- Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26:517–538. Erratum in J Vasc Surg 2001; 33:805.
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363:301–304. Erratum in N Engl J Med 2010; 363:1092.
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000; 31:S1–S296.
- Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes 2012; 5:94–102.
- Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol 2012; 60:2230–2236.
- Hirsch AT, Haskal ZJ, Mertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Alexandrescu VA, Hubermont G, Philips Y, et al. Selective primary angioplasty following an angiosome model of reperfusion in the treatment of Wagner 1–4 diabetic foot lesions: practice in a multidisciplinary diabetic limb service. J Endovasc Ther 2008; 15:580–593.
- Neville RF, Attinger CE, Bulan EJ, Ducic I, Thomassen M, Sidawy AN. Revascularization of a specific angiosome for limb salvage: does the target artery matter? Ann Vasc Surg 2009; 23:367–373.
- Iida O, Soga Y, Hirano K, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg 2012; 55:363–370.
- Graziani L, Silvestro A, Bertone V, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg 2007; 33:453–460.
- Newman AB, Siscovick DS, Manolio TA, et al., Cardiovascular Heart Study (CHS) Collaborative Research Group. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation 1993; 88:837–845.
- Cronenwett JL, Johnston KW. Rutherford’s Vascular Surgery. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010.
- Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med 2009; 36:43–53.
- Lo T, Sample R, Moore P, Gold P. Prediction of wound healing outcome using skin perfusion pressure and transcutaneous oximetry. Wounds 2009; 21:310–316.
- Perry D, Bharara M, Armstrong DG, Mills J. Intraoperative fluorescence vascular angiography: during tibial bypass. J Diabetes Sci Technol 2012; 6:204–208.
- Adam DJ, Beard JD, Cleveland T, et al; BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005; 366:1925–1934.
- Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994; 31:49–53.
- Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg 2004; 188(1A suppl):1–8.
- Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22:382–387.
- Cuddigan J, Berlowitz DR, Ayello E; National Pressure Ulcer Advisory Panel. Pressure ulcers in America: Prevalence, incidence, and implications for the future: an executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care 2001; 14:208–215.
- Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med 1999; 4:1–7.
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26:1790–1795.
- Kumar RN, Gupchup GV, Dodd MA, et al. Direct health care costs of 4 common skin ulcers in New Mexico Medicaid fee-for-service patients. Adv Skin Wound Care 2004; 17:143–149.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(suppl):S5–S67.
- Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26:517–538. Erratum in J Vasc Surg 2001; 33:805.
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363:301–304. Erratum in N Engl J Med 2010; 363:1092.
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000; 31:S1–S296.
- Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes 2012; 5:94–102.
- Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol 2012; 60:2230–2236.
- Hirsch AT, Haskal ZJ, Mertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Alexandrescu VA, Hubermont G, Philips Y, et al. Selective primary angioplasty following an angiosome model of reperfusion in the treatment of Wagner 1–4 diabetic foot lesions: practice in a multidisciplinary diabetic limb service. J Endovasc Ther 2008; 15:580–593.
- Neville RF, Attinger CE, Bulan EJ, Ducic I, Thomassen M, Sidawy AN. Revascularization of a specific angiosome for limb salvage: does the target artery matter? Ann Vasc Surg 2009; 23:367–373.
- Iida O, Soga Y, Hirano K, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg 2012; 55:363–370.
- Graziani L, Silvestro A, Bertone V, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg 2007; 33:453–460.
- Newman AB, Siscovick DS, Manolio TA, et al., Cardiovascular Heart Study (CHS) Collaborative Research Group. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation 1993; 88:837–845.
- Cronenwett JL, Johnston KW. Rutherford’s Vascular Surgery. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010.
- Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med 2009; 36:43–53.
- Lo T, Sample R, Moore P, Gold P. Prediction of wound healing outcome using skin perfusion pressure and transcutaneous oximetry. Wounds 2009; 21:310–316.
- Perry D, Bharara M, Armstrong DG, Mills J. Intraoperative fluorescence vascular angiography: during tibial bypass. J Diabetes Sci Technol 2012; 6:204–208.
- Adam DJ, Beard JD, Cleveland T, et al; BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005; 366:1925–1934.
KEY POINTS
- In assessing peripheral artery disease, perform a thorough history and physical examination, paying close attention to the onset and characteristics of pain, activity level, history, and pulses, and the condition of the feet.
- Acute limb ischemia is a sudden decrease in limb perfusion, potentially threatening limb viability. Patients who have acute cessation of blood flow, sensation, or motor function need immediate revascularization to avoid amputation.
- Critical limb ischemia ranges from rest pain to gangrene and must be addressed with a multidisciplinary approach.
- The ankle-brachial index is a noninvasive, inexpensive test that can be done in the office with a hand-held Doppler device to assess the presence and severity of peripheral artery disease.