User login
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
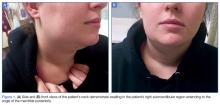
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
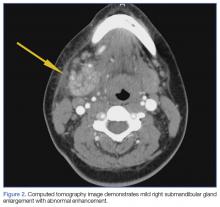
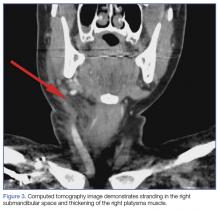
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.