User login
Bell Palsy Mimics
Facial paralysis is a common medical complaint—one that has fascinated ancient and contemporary physicians alike.1 An idiopathic facial nerve paresis involving the lower motor neuron was described in 1821 by Sir Charles Bell. This entity became known as a Bell’s palsy, the hallmark of which was weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. However, not all facial paralysis is due to Bell’s palsy.
We present a case of a patient with a Bell’s palsy mimic to facilitate and guide the differential diagnosis and distinguish conditions from the classical presentation that Bell first described to the more concerning symptoms that may not be immediately obvious. Our case further underscores the importance of performing a thorough assessment to determine the presence of other neurological findings.
Case
A 61-year-old woman presented to the ED for evaluation of right facial droop and sensation of “room spinning.” The patient stated both symptoms began approximately 36 hours prior to presentation, upon awakening.
The patient denied any headache, neck or chest pain, extremity numbness, or weakness, but stated that she felt like she was going to fall toward her right side whenever she attempted to walk. The patient’s medical history was significant for hypertension, for which she was taking losartan. Her surgical history was notable for a left oophorectomy secondary to an ovarian cyst. Regarding the social history, the patient admitted to smoking 90 packs of cigarettes per year, but denied alcohol or illicit drug use.
Upon arrival at the ED, the patient’s vital signs were: blood pressure, 164/86 mm Hg: pulse, 89 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.6°F. Oxygen saturation was 98% on room air.
Physical examination revealed the patient had a right facial droop consistent with right facial palsy. She was unable to wrinkle her right forehead or fully close her right eye. There were no field cuts on confrontation. The patient’s speech was noticeable for a mild dysarthria. The motor examination revealed mild weakness of the left upper extremity and impaired right facial sensation. There were no rashes noted on the face, head, or ears. The patient had slightly impaired hearing in the right ear, which was new in onset. The remainder of the physical examination was unremarkable.
Although the patient exhibited the classic signs of Bell’s palsy, including complete paralysis of the muscles of one side of the face, inability to wrinkle the muscle of the right forehead, and inability to fully close the right eye, she also had concerning symptoms of vertigo, dysarthria, and contralateral upper extremity weakness.
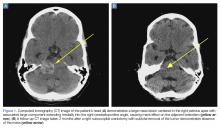
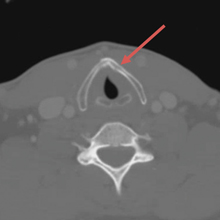
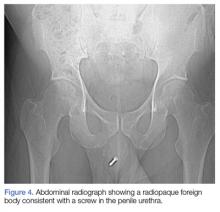
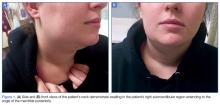
A computed tomography (CT) scan of the head was ordered, which revealed a large mass lesion centered in the right petrous apex, with an associated large component extending medially into the right cerebellopontine angle (CPA) that caused a mass effect on the adjacent brainstem (Figures 1a and 1b).
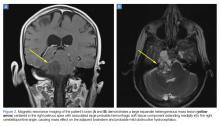
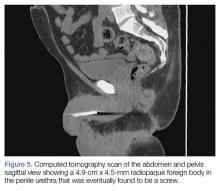
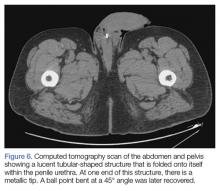
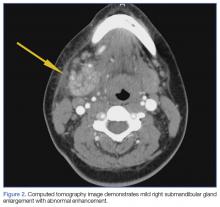
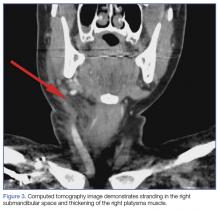
Upon these findings, the patient was transferred to another facility for neurosurgical evaluation. Magnetic resonance imaging (MRI) studies performed at the receiving hospital demonstrated a large expansile heterogeneous mass lesion centered in the right petrous apex with an associated large, probable hemorrhagic soft-tissue component extending medially into the right CPA, causing a mass effect on the adjacent brainstem and mild obstructive hydrocephalus (Figures 2a and 2b).
The patient was given dexamethasone 10 mg intravenously and taken to the operating room for a right suboccipital craniotomy with subtotal tumor removal. Intraoperative high-voltage stimulation of the fifth to eighth cranial nerves showed no response, indicating significant impairment.
While there were no intraoperative complications, the patient had significant postoperative dysphagia and resultant aspiration. A tracheostomy and percutaneous endoscopic gastrostomy tube were subsequently placed. Results of a biopsy taken during surgery identified an atypical meningioma. The patient remained in the hospital for 4 weeks, after which she was discharged to a long-term care (LTC) and rehabilitation facility.
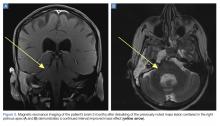
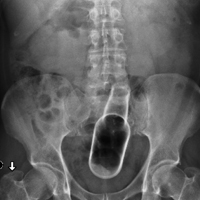
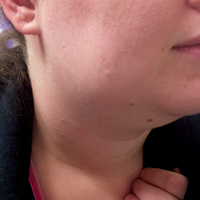
A repeat CT scan taken 2 months after surgery demonstrated absence of the previously identified large mass (Figure 1b). Three months after discharge from the LTC-rehabilitation facility, MRI of the brain showed continued interval improvement of the previously noted mass centered in the right petrous apex (Figures 3a and 3b).
Discussion
Accounts of facial paralysis and facial nerve disorders have been noted throughout history and include accounts of the condition by Hippocrates.1 Bell’s palsy was named after surgeon Sir Charles Bell, who described a peripheral-nerve paralysis of the facial nerve in 1821. Bell’s work helped to elucidate the anatomy and functional role of the facial nerve.1,2
Signs and Symptoms
The classic presentation of Bell’s palsy is weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. The eyelid on the affected side generally does not close, which can result in ocular irritation due to ineffective lubrication.
A scoring system has been developed by House and Brackmann which grades the degree impairment based on such characteristics as facial muscle function and eye closure.3,4 Approximately 96% of patients with a Bell’s palsy will improve to a House-Brackmann score of 2 or better within 1 year from diagnosis,5 and 85% of patients with Bell’s palsy will show at least some improvement within 3 weeks of onset (Table).2 Although the classic description of Bell’s palsy notes the condition as idiopathic, there is an increasing body of evidence in the literature showing a link to herpes simplex virus 1.5-7
Ramsey-Hunt Syndrome
The relationship between Bell’s palsy and Ramsey-Hunt syndrome is complex and controversial. Ramsey-Hunt syndrome is a constellation of possible complications from varicella-virus infection. Symptoms of Ramsey-Hunt syndrome include facial paralysis, tinnitus, hearing loss, vertigo, hyperacusis (increased sensitivity to certain frequencies and volume ranges of sound), and decreased ocular tearing.8 Due to the nature of symptoms associated with Ramsey-Hunt syndrome, it is apparent that the condition involves more than the seventh cranial nerve. In fact, studies have shown that Ramsey-Hunt syndrome can affect the fifth, sixth, eighth, and ninth cranial nerves.8
Ramsey-Hunt syndrome, which can present in the absence of cutaneous rash (referred to as zoster sine herpete), is estimated to occur in 8% to 20% of unilateral facial nerve palsies in adult patients.8,9 Regardless of the etiology of Bell’s palsy, a review of the literature makes it clear that facial nerve paralysis is not synonymous with Bell’s palsy.10 In one example, Yetter et al10 describe the case of a patient who, though initially diagnosed with Bell’s palsy, ultimately was found to have a facial palsy due to a parotid gland malignancy.
Likewise, Stomeo11 describes a case of a patient with facial paralysis and profound ipsilateral hearing loss who ultimately was found to have a mucoepithelial carcinoma of the parotid gland. In their report, the authors note that approximately 80% of facial nerve paralysis is due to Bell’s palsy, while 5% is due to malignancy.
In another report, Clemis12 describes a case in which a patient who initially was diagnosed with Bell’s palsy eventually was found to have an adenoid cystic carcinoma of the parotid. Thus, the authors appropriately emphasize in their report that “all that palsies is not Bell’s.”
Differential Diagnosis
Historical factors, including timing and duration of symptom onset, help to distinguish a Bell’s palsy from other disorders that can mimic this condition. In their study, Brach VanSwewaringen13 highlight the fact that “not all facial paralysis is Bell’s palsy.” In their review, the authors describe clues to help distinguish conditions that mimic Bell’s palsy. For example, maximal weakness from Bell’s Palsy typically occurs within 3 to 7 days from symptom onset, and that a more gradual onset of symptoms, with slow or negligible improvement over 6 to 12 months, is more indicative of a space-occupying lesion than Bell’s palsy.13It is, however, important to note that although the patient in our case had a central lesion, she experienced an acute onset of symptoms.
The presence of additional symptoms may also suggest an alternative diagnosis. Brach and VanSwearingen13 further noted that symptoms associated with the eighth nerve, such as vertigo, tinnitus, and hearing loss may be found in patients with a CPA tumor. In patients with larger tumors, ninth and 10th nerve symptoms, including the impaired hearing noted in our patient, may be present. Some patients with ninth and 10th nerve symptoms may perceive a sense of facial numbness, but actual sensory changes in the facial nerve distribution are unlikely in Bell’s palsy. Gustatory changes, however, are consistent with Bell’s palsy.
Ear pain is consistent with Bell’s palsy and is a signal to be vigilant for the possible emergence of an ear rash, which would suggest the diagnosis of herpes zoster oticus along the trajectory of Ramsey-Hunt syndrome. Facial pain in the area of the facial nerve is inconsistent with Bell’s palsy, while hyperacusis is consistent with Bell’s palsy. Hearing loss is an eighth nerve symptom that is inconsistent with Bell’s palsy.
Similarly, there are physical examination findings that can help distinguish a true Bell’s palsy from a mimic. Changes in tear production are consistent with Bell’s palsy, but imbalance and disequilibrium are not.14
As previously noted, the patient in this case had difficulty walking and felt as if she was falling toward her right side.
One way to organize the causes of facial paralysis has been proposed by Adour et al.15 In this system, etiologies are listed as either acute paralysis or chronic, progressive paralysis. Acute paralysis (ie, the sudden onset of symptoms with maximal severity within 2 weeks), of which Bell’s palsy is the most common, can be seen in cases of polyneuritis.
A new case of Bell’s palsy has been estimated to occur in the United States every 10 minutes.8 Guillain-Barré syndrome and Lyme disease are also in this category, as is Ramsey-Hunt syndrome. Patients with Lyme disease may have a history of a tick bite or rash.14
Trauma can also cause acute facial nerve paralysis (eg, blunt trauma-associated facial fracture, penetrating trauma, birth trauma). Unilateral central facial weakness can have a neurological cause, such as a lesion to the contralateral cortex, subcortical white matter, or internal capsule.2,15 Otitis media can sometimes cause facial paralysis.16 A cholesteatoma can cause acute facial paralysis.2 Malignancies cause 5% of all cases of facial paralysis. Primary parotid tumors of various types are in this category. Metastatic disease from breast, lung, skin, colon, and kidney may cause facial paralysis. As our case illustrates, CPA tumors can cause facial paralysis.15 It is important to also note that a patient can have both a Bell’s palsy and a concurrent disease. There are a number of case reports in the literature that describe acute onset of facial paralysis as a presenting symptom of malignancy.17 In addition, there are cases wherein a neurological finding on imaging, such as an acoustic neuroma, was presumed to be the cause of facial paralysis, yet the patient’s symptoms resolved in a manner consistent with Bell’s palsy.18
For example, Lagman et al19 described a patient in which a CPA lipoma was presumed to be the cause of the facial paralysis, but the eventual outcome showed the lipoma to have been an incidentaloma.
Conclusion
This case demonstrates a presenting symptom of facial palsy and the presence of a CPA tumor. The presence of vertigo along with other historical and physical examination findings inconsistent with Bell’s palsy prompted the CT scan of the head. A review of the literature suggests a number of important findings in patients with facial palsy to assist the clinician in distinguishing true Bell’s palsy from other diseases that can mimic this condition. This case serves as a reminder of the need to perform a thorough and diligent workup to determine the presence or absence of other neurologic findings prior to closing on the diagnosis of Bell’s palsy.
1. Glicenstein J. Ann Chir Plast Esthet. 2015;60(5):347-362. doi:10.1016/j.anplas.2015.05.007.
2. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
3. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi:10.1177/019459988509300202.
4. Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009;140(2):154-158. doi:10.1016/j.otohns.2008.11.021.
5. Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007;34(2):159-164. doi:10.1016/j.anl.2006.09.005.
6. Ahmed A. When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med. 2005;72(5):398-401, 405.
7. Gilden DH. Clinical practice. Bell’s palsy. N Engl J Med. 2004;351(13):1323-1331. doi:10.1056/NEJMcp041120.
8. Adour KK. Otological complications of herpes zoster. Ann Neurol. 1994;35:Suppl:S62-S64.
9. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55(5):708-710.
10. Yetter MF, Ogren FP, Moore GF, Yonkers AJ. Bell’s palsy: a facial nerve paralysis diagnosis of exclusion. Nebr Med J. 1990;75(5):109-116.
11. Stomeo F. Possibilities of diagnostic errors in paralysis of the 7th cranial nerve. Acta Otorhinolaryngol Ital. 1989;9(6):629-633.
12. Clemis JD. All that palsies is not Bell’s: Bell’s palsy due to adenoid cystic carcinoma of the parotid. Am J Otol. 1991;12(5):397.
13. Brach JS, VanSwearingen JM. Not all facial paralysis is Bell’s palsy: a case report. Arch Phys Med Rehabil. 1999;80(7):857-859.
14. Albers JR, Tamang S. Common questions about Bell palsy. Am Fam Physician. 2014;89(3):209-212.
15. Adour KK, Hilsinger RL Jr, Callan EJ. Facial paralysis and Bell’s palsy: a protocol for differential diagnosis. Am J Otol. 1985;Suppl:68-73.
16. Morrow MJ. Bell’s palsy and herpes zoster. Curr Treat Options Neurol. 2000;2(5):407-416.
17. Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell’s palsy: finding occult malignancy in acute-onset facial paralysis. Am J Otolaryngol. 2010;31(5):339-342. doi:10.1016/j.amjoto.2009.04.003.
18. Kaushal A, Curran WJ Jr. For whom the Bell’s palsy tolls? Am J Clin Oncol. 2009;32(4):450-451. doi:10.1097/01.coc.0000239141.22916.22.
19. Lagman C, Choy W, Lee SJ, et al. A Case of Bell’s palsy with an incidental finding of a cerebellopontine angle lipoma. Cureus. 2016;8(8):e747. doi:10.7759/cureus.747.
Facial paralysis is a common medical complaint—one that has fascinated ancient and contemporary physicians alike.1 An idiopathic facial nerve paresis involving the lower motor neuron was described in 1821 by Sir Charles Bell. This entity became known as a Bell’s palsy, the hallmark of which was weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. However, not all facial paralysis is due to Bell’s palsy.
We present a case of a patient with a Bell’s palsy mimic to facilitate and guide the differential diagnosis and distinguish conditions from the classical presentation that Bell first described to the more concerning symptoms that may not be immediately obvious. Our case further underscores the importance of performing a thorough assessment to determine the presence of other neurological findings.
Case
A 61-year-old woman presented to the ED for evaluation of right facial droop and sensation of “room spinning.” The patient stated both symptoms began approximately 36 hours prior to presentation, upon awakening.
The patient denied any headache, neck or chest pain, extremity numbness, or weakness, but stated that she felt like she was going to fall toward her right side whenever she attempted to walk. The patient’s medical history was significant for hypertension, for which she was taking losartan. Her surgical history was notable for a left oophorectomy secondary to an ovarian cyst. Regarding the social history, the patient admitted to smoking 90 packs of cigarettes per year, but denied alcohol or illicit drug use.
Upon arrival at the ED, the patient’s vital signs were: blood pressure, 164/86 mm Hg: pulse, 89 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.6°F. Oxygen saturation was 98% on room air.
Physical examination revealed the patient had a right facial droop consistent with right facial palsy. She was unable to wrinkle her right forehead or fully close her right eye. There were no field cuts on confrontation. The patient’s speech was noticeable for a mild dysarthria. The motor examination revealed mild weakness of the left upper extremity and impaired right facial sensation. There were no rashes noted on the face, head, or ears. The patient had slightly impaired hearing in the right ear, which was new in onset. The remainder of the physical examination was unremarkable.
Although the patient exhibited the classic signs of Bell’s palsy, including complete paralysis of the muscles of one side of the face, inability to wrinkle the muscle of the right forehead, and inability to fully close the right eye, she also had concerning symptoms of vertigo, dysarthria, and contralateral upper extremity weakness.
A computed tomography (CT) scan of the head was ordered, which revealed a large mass lesion centered in the right petrous apex, with an associated large component extending medially into the right cerebellopontine angle (CPA) that caused a mass effect on the adjacent brainstem (Figures 1a and 1b).
Upon these findings, the patient was transferred to another facility for neurosurgical evaluation. Magnetic resonance imaging (MRI) studies performed at the receiving hospital demonstrated a large expansile heterogeneous mass lesion centered in the right petrous apex with an associated large, probable hemorrhagic soft-tissue component extending medially into the right CPA, causing a mass effect on the adjacent brainstem and mild obstructive hydrocephalus (Figures 2a and 2b).
The patient was given dexamethasone 10 mg intravenously and taken to the operating room for a right suboccipital craniotomy with subtotal tumor removal. Intraoperative high-voltage stimulation of the fifth to eighth cranial nerves showed no response, indicating significant impairment.
While there were no intraoperative complications, the patient had significant postoperative dysphagia and resultant aspiration. A tracheostomy and percutaneous endoscopic gastrostomy tube were subsequently placed. Results of a biopsy taken during surgery identified an atypical meningioma. The patient remained in the hospital for 4 weeks, after which she was discharged to a long-term care (LTC) and rehabilitation facility.
A repeat CT scan taken 2 months after surgery demonstrated absence of the previously identified large mass (Figure 1b). Three months after discharge from the LTC-rehabilitation facility, MRI of the brain showed continued interval improvement of the previously noted mass centered in the right petrous apex (Figures 3a and 3b).
Discussion
Accounts of facial paralysis and facial nerve disorders have been noted throughout history and include accounts of the condition by Hippocrates.1 Bell’s palsy was named after surgeon Sir Charles Bell, who described a peripheral-nerve paralysis of the facial nerve in 1821. Bell’s work helped to elucidate the anatomy and functional role of the facial nerve.1,2
Signs and Symptoms
The classic presentation of Bell’s palsy is weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. The eyelid on the affected side generally does not close, which can result in ocular irritation due to ineffective lubrication.
A scoring system has been developed by House and Brackmann which grades the degree impairment based on such characteristics as facial muscle function and eye closure.3,4 Approximately 96% of patients with a Bell’s palsy will improve to a House-Brackmann score of 2 or better within 1 year from diagnosis,5 and 85% of patients with Bell’s palsy will show at least some improvement within 3 weeks of onset (Table).2 Although the classic description of Bell’s palsy notes the condition as idiopathic, there is an increasing body of evidence in the literature showing a link to herpes simplex virus 1.5-7
Ramsey-Hunt Syndrome
The relationship between Bell’s palsy and Ramsey-Hunt syndrome is complex and controversial. Ramsey-Hunt syndrome is a constellation of possible complications from varicella-virus infection. Symptoms of Ramsey-Hunt syndrome include facial paralysis, tinnitus, hearing loss, vertigo, hyperacusis (increased sensitivity to certain frequencies and volume ranges of sound), and decreased ocular tearing.8 Due to the nature of symptoms associated with Ramsey-Hunt syndrome, it is apparent that the condition involves more than the seventh cranial nerve. In fact, studies have shown that Ramsey-Hunt syndrome can affect the fifth, sixth, eighth, and ninth cranial nerves.8
Ramsey-Hunt syndrome, which can present in the absence of cutaneous rash (referred to as zoster sine herpete), is estimated to occur in 8% to 20% of unilateral facial nerve palsies in adult patients.8,9 Regardless of the etiology of Bell’s palsy, a review of the literature makes it clear that facial nerve paralysis is not synonymous with Bell’s palsy.10 In one example, Yetter et al10 describe the case of a patient who, though initially diagnosed with Bell’s palsy, ultimately was found to have a facial palsy due to a parotid gland malignancy.
Likewise, Stomeo11 describes a case of a patient with facial paralysis and profound ipsilateral hearing loss who ultimately was found to have a mucoepithelial carcinoma of the parotid gland. In their report, the authors note that approximately 80% of facial nerve paralysis is due to Bell’s palsy, while 5% is due to malignancy.
In another report, Clemis12 describes a case in which a patient who initially was diagnosed with Bell’s palsy eventually was found to have an adenoid cystic carcinoma of the parotid. Thus, the authors appropriately emphasize in their report that “all that palsies is not Bell’s.”
Differential Diagnosis
Historical factors, including timing and duration of symptom onset, help to distinguish a Bell’s palsy from other disorders that can mimic this condition. In their study, Brach VanSwewaringen13 highlight the fact that “not all facial paralysis is Bell’s palsy.” In their review, the authors describe clues to help distinguish conditions that mimic Bell’s palsy. For example, maximal weakness from Bell’s Palsy typically occurs within 3 to 7 days from symptom onset, and that a more gradual onset of symptoms, with slow or negligible improvement over 6 to 12 months, is more indicative of a space-occupying lesion than Bell’s palsy.13It is, however, important to note that although the patient in our case had a central lesion, she experienced an acute onset of symptoms.
The presence of additional symptoms may also suggest an alternative diagnosis. Brach and VanSwearingen13 further noted that symptoms associated with the eighth nerve, such as vertigo, tinnitus, and hearing loss may be found in patients with a CPA tumor. In patients with larger tumors, ninth and 10th nerve symptoms, including the impaired hearing noted in our patient, may be present. Some patients with ninth and 10th nerve symptoms may perceive a sense of facial numbness, but actual sensory changes in the facial nerve distribution are unlikely in Bell’s palsy. Gustatory changes, however, are consistent with Bell’s palsy.
Ear pain is consistent with Bell’s palsy and is a signal to be vigilant for the possible emergence of an ear rash, which would suggest the diagnosis of herpes zoster oticus along the trajectory of Ramsey-Hunt syndrome. Facial pain in the area of the facial nerve is inconsistent with Bell’s palsy, while hyperacusis is consistent with Bell’s palsy. Hearing loss is an eighth nerve symptom that is inconsistent with Bell’s palsy.
Similarly, there are physical examination findings that can help distinguish a true Bell’s palsy from a mimic. Changes in tear production are consistent with Bell’s palsy, but imbalance and disequilibrium are not.14
As previously noted, the patient in this case had difficulty walking and felt as if she was falling toward her right side.
One way to organize the causes of facial paralysis has been proposed by Adour et al.15 In this system, etiologies are listed as either acute paralysis or chronic, progressive paralysis. Acute paralysis (ie, the sudden onset of symptoms with maximal severity within 2 weeks), of which Bell’s palsy is the most common, can be seen in cases of polyneuritis.
A new case of Bell’s palsy has been estimated to occur in the United States every 10 minutes.8 Guillain-Barré syndrome and Lyme disease are also in this category, as is Ramsey-Hunt syndrome. Patients with Lyme disease may have a history of a tick bite or rash.14
Trauma can also cause acute facial nerve paralysis (eg, blunt trauma-associated facial fracture, penetrating trauma, birth trauma). Unilateral central facial weakness can have a neurological cause, such as a lesion to the contralateral cortex, subcortical white matter, or internal capsule.2,15 Otitis media can sometimes cause facial paralysis.16 A cholesteatoma can cause acute facial paralysis.2 Malignancies cause 5% of all cases of facial paralysis. Primary parotid tumors of various types are in this category. Metastatic disease from breast, lung, skin, colon, and kidney may cause facial paralysis. As our case illustrates, CPA tumors can cause facial paralysis.15 It is important to also note that a patient can have both a Bell’s palsy and a concurrent disease. There are a number of case reports in the literature that describe acute onset of facial paralysis as a presenting symptom of malignancy.17 In addition, there are cases wherein a neurological finding on imaging, such as an acoustic neuroma, was presumed to be the cause of facial paralysis, yet the patient’s symptoms resolved in a manner consistent with Bell’s palsy.18
For example, Lagman et al19 described a patient in which a CPA lipoma was presumed to be the cause of the facial paralysis, but the eventual outcome showed the lipoma to have been an incidentaloma.
Conclusion
This case demonstrates a presenting symptom of facial palsy and the presence of a CPA tumor. The presence of vertigo along with other historical and physical examination findings inconsistent with Bell’s palsy prompted the CT scan of the head. A review of the literature suggests a number of important findings in patients with facial palsy to assist the clinician in distinguishing true Bell’s palsy from other diseases that can mimic this condition. This case serves as a reminder of the need to perform a thorough and diligent workup to determine the presence or absence of other neurologic findings prior to closing on the diagnosis of Bell’s palsy.
Facial paralysis is a common medical complaint—one that has fascinated ancient and contemporary physicians alike.1 An idiopathic facial nerve paresis involving the lower motor neuron was described in 1821 by Sir Charles Bell. This entity became known as a Bell’s palsy, the hallmark of which was weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. However, not all facial paralysis is due to Bell’s palsy.
We present a case of a patient with a Bell’s palsy mimic to facilitate and guide the differential diagnosis and distinguish conditions from the classical presentation that Bell first described to the more concerning symptoms that may not be immediately obvious. Our case further underscores the importance of performing a thorough assessment to determine the presence of other neurological findings.
Case
A 61-year-old woman presented to the ED for evaluation of right facial droop and sensation of “room spinning.” The patient stated both symptoms began approximately 36 hours prior to presentation, upon awakening.
The patient denied any headache, neck or chest pain, extremity numbness, or weakness, but stated that she felt like she was going to fall toward her right side whenever she attempted to walk. The patient’s medical history was significant for hypertension, for which she was taking losartan. Her surgical history was notable for a left oophorectomy secondary to an ovarian cyst. Regarding the social history, the patient admitted to smoking 90 packs of cigarettes per year, but denied alcohol or illicit drug use.
Upon arrival at the ED, the patient’s vital signs were: blood pressure, 164/86 mm Hg: pulse, 89 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.6°F. Oxygen saturation was 98% on room air.
Physical examination revealed the patient had a right facial droop consistent with right facial palsy. She was unable to wrinkle her right forehead or fully close her right eye. There were no field cuts on confrontation. The patient’s speech was noticeable for a mild dysarthria. The motor examination revealed mild weakness of the left upper extremity and impaired right facial sensation. There were no rashes noted on the face, head, or ears. The patient had slightly impaired hearing in the right ear, which was new in onset. The remainder of the physical examination was unremarkable.
Although the patient exhibited the classic signs of Bell’s palsy, including complete paralysis of the muscles of one side of the face, inability to wrinkle the muscle of the right forehead, and inability to fully close the right eye, she also had concerning symptoms of vertigo, dysarthria, and contralateral upper extremity weakness.
A computed tomography (CT) scan of the head was ordered, which revealed a large mass lesion centered in the right petrous apex, with an associated large component extending medially into the right cerebellopontine angle (CPA) that caused a mass effect on the adjacent brainstem (Figures 1a and 1b).
Upon these findings, the patient was transferred to another facility for neurosurgical evaluation. Magnetic resonance imaging (MRI) studies performed at the receiving hospital demonstrated a large expansile heterogeneous mass lesion centered in the right petrous apex with an associated large, probable hemorrhagic soft-tissue component extending medially into the right CPA, causing a mass effect on the adjacent brainstem and mild obstructive hydrocephalus (Figures 2a and 2b).
The patient was given dexamethasone 10 mg intravenously and taken to the operating room for a right suboccipital craniotomy with subtotal tumor removal. Intraoperative high-voltage stimulation of the fifth to eighth cranial nerves showed no response, indicating significant impairment.
While there were no intraoperative complications, the patient had significant postoperative dysphagia and resultant aspiration. A tracheostomy and percutaneous endoscopic gastrostomy tube were subsequently placed. Results of a biopsy taken during surgery identified an atypical meningioma. The patient remained in the hospital for 4 weeks, after which she was discharged to a long-term care (LTC) and rehabilitation facility.
A repeat CT scan taken 2 months after surgery demonstrated absence of the previously identified large mass (Figure 1b). Three months after discharge from the LTC-rehabilitation facility, MRI of the brain showed continued interval improvement of the previously noted mass centered in the right petrous apex (Figures 3a and 3b).
Discussion
Accounts of facial paralysis and facial nerve disorders have been noted throughout history and include accounts of the condition by Hippocrates.1 Bell’s palsy was named after surgeon Sir Charles Bell, who described a peripheral-nerve paralysis of the facial nerve in 1821. Bell’s work helped to elucidate the anatomy and functional role of the facial nerve.1,2
Signs and Symptoms
The classic presentation of Bell’s palsy is weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. The eyelid on the affected side generally does not close, which can result in ocular irritation due to ineffective lubrication.
A scoring system has been developed by House and Brackmann which grades the degree impairment based on such characteristics as facial muscle function and eye closure.3,4 Approximately 96% of patients with a Bell’s palsy will improve to a House-Brackmann score of 2 or better within 1 year from diagnosis,5 and 85% of patients with Bell’s palsy will show at least some improvement within 3 weeks of onset (Table).2 Although the classic description of Bell’s palsy notes the condition as idiopathic, there is an increasing body of evidence in the literature showing a link to herpes simplex virus 1.5-7
Ramsey-Hunt Syndrome
The relationship between Bell’s palsy and Ramsey-Hunt syndrome is complex and controversial. Ramsey-Hunt syndrome is a constellation of possible complications from varicella-virus infection. Symptoms of Ramsey-Hunt syndrome include facial paralysis, tinnitus, hearing loss, vertigo, hyperacusis (increased sensitivity to certain frequencies and volume ranges of sound), and decreased ocular tearing.8 Due to the nature of symptoms associated with Ramsey-Hunt syndrome, it is apparent that the condition involves more than the seventh cranial nerve. In fact, studies have shown that Ramsey-Hunt syndrome can affect the fifth, sixth, eighth, and ninth cranial nerves.8
Ramsey-Hunt syndrome, which can present in the absence of cutaneous rash (referred to as zoster sine herpete), is estimated to occur in 8% to 20% of unilateral facial nerve palsies in adult patients.8,9 Regardless of the etiology of Bell’s palsy, a review of the literature makes it clear that facial nerve paralysis is not synonymous with Bell’s palsy.10 In one example, Yetter et al10 describe the case of a patient who, though initially diagnosed with Bell’s palsy, ultimately was found to have a facial palsy due to a parotid gland malignancy.
Likewise, Stomeo11 describes a case of a patient with facial paralysis and profound ipsilateral hearing loss who ultimately was found to have a mucoepithelial carcinoma of the parotid gland. In their report, the authors note that approximately 80% of facial nerve paralysis is due to Bell’s palsy, while 5% is due to malignancy.
In another report, Clemis12 describes a case in which a patient who initially was diagnosed with Bell’s palsy eventually was found to have an adenoid cystic carcinoma of the parotid. Thus, the authors appropriately emphasize in their report that “all that palsies is not Bell’s.”
Differential Diagnosis
Historical factors, including timing and duration of symptom onset, help to distinguish a Bell’s palsy from other disorders that can mimic this condition. In their study, Brach VanSwewaringen13 highlight the fact that “not all facial paralysis is Bell’s palsy.” In their review, the authors describe clues to help distinguish conditions that mimic Bell’s palsy. For example, maximal weakness from Bell’s Palsy typically occurs within 3 to 7 days from symptom onset, and that a more gradual onset of symptoms, with slow or negligible improvement over 6 to 12 months, is more indicative of a space-occupying lesion than Bell’s palsy.13It is, however, important to note that although the patient in our case had a central lesion, she experienced an acute onset of symptoms.
The presence of additional symptoms may also suggest an alternative diagnosis. Brach and VanSwearingen13 further noted that symptoms associated with the eighth nerve, such as vertigo, tinnitus, and hearing loss may be found in patients with a CPA tumor. In patients with larger tumors, ninth and 10th nerve symptoms, including the impaired hearing noted in our patient, may be present. Some patients with ninth and 10th nerve symptoms may perceive a sense of facial numbness, but actual sensory changes in the facial nerve distribution are unlikely in Bell’s palsy. Gustatory changes, however, are consistent with Bell’s palsy.
Ear pain is consistent with Bell’s palsy and is a signal to be vigilant for the possible emergence of an ear rash, which would suggest the diagnosis of herpes zoster oticus along the trajectory of Ramsey-Hunt syndrome. Facial pain in the area of the facial nerve is inconsistent with Bell’s palsy, while hyperacusis is consistent with Bell’s palsy. Hearing loss is an eighth nerve symptom that is inconsistent with Bell’s palsy.
Similarly, there are physical examination findings that can help distinguish a true Bell’s palsy from a mimic. Changes in tear production are consistent with Bell’s palsy, but imbalance and disequilibrium are not.14
As previously noted, the patient in this case had difficulty walking and felt as if she was falling toward her right side.
One way to organize the causes of facial paralysis has been proposed by Adour et al.15 In this system, etiologies are listed as either acute paralysis or chronic, progressive paralysis. Acute paralysis (ie, the sudden onset of symptoms with maximal severity within 2 weeks), of which Bell’s palsy is the most common, can be seen in cases of polyneuritis.
A new case of Bell’s palsy has been estimated to occur in the United States every 10 minutes.8 Guillain-Barré syndrome and Lyme disease are also in this category, as is Ramsey-Hunt syndrome. Patients with Lyme disease may have a history of a tick bite or rash.14
Trauma can also cause acute facial nerve paralysis (eg, blunt trauma-associated facial fracture, penetrating trauma, birth trauma). Unilateral central facial weakness can have a neurological cause, such as a lesion to the contralateral cortex, subcortical white matter, or internal capsule.2,15 Otitis media can sometimes cause facial paralysis.16 A cholesteatoma can cause acute facial paralysis.2 Malignancies cause 5% of all cases of facial paralysis. Primary parotid tumors of various types are in this category. Metastatic disease from breast, lung, skin, colon, and kidney may cause facial paralysis. As our case illustrates, CPA tumors can cause facial paralysis.15 It is important to also note that a patient can have both a Bell’s palsy and a concurrent disease. There are a number of case reports in the literature that describe acute onset of facial paralysis as a presenting symptom of malignancy.17 In addition, there are cases wherein a neurological finding on imaging, such as an acoustic neuroma, was presumed to be the cause of facial paralysis, yet the patient’s symptoms resolved in a manner consistent with Bell’s palsy.18
For example, Lagman et al19 described a patient in which a CPA lipoma was presumed to be the cause of the facial paralysis, but the eventual outcome showed the lipoma to have been an incidentaloma.
Conclusion
This case demonstrates a presenting symptom of facial palsy and the presence of a CPA tumor. The presence of vertigo along with other historical and physical examination findings inconsistent with Bell’s palsy prompted the CT scan of the head. A review of the literature suggests a number of important findings in patients with facial palsy to assist the clinician in distinguishing true Bell’s palsy from other diseases that can mimic this condition. This case serves as a reminder of the need to perform a thorough and diligent workup to determine the presence or absence of other neurologic findings prior to closing on the diagnosis of Bell’s palsy.
1. Glicenstein J. Ann Chir Plast Esthet. 2015;60(5):347-362. doi:10.1016/j.anplas.2015.05.007.
2. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
3. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi:10.1177/019459988509300202.
4. Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009;140(2):154-158. doi:10.1016/j.otohns.2008.11.021.
5. Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007;34(2):159-164. doi:10.1016/j.anl.2006.09.005.
6. Ahmed A. When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med. 2005;72(5):398-401, 405.
7. Gilden DH. Clinical practice. Bell’s palsy. N Engl J Med. 2004;351(13):1323-1331. doi:10.1056/NEJMcp041120.
8. Adour KK. Otological complications of herpes zoster. Ann Neurol. 1994;35:Suppl:S62-S64.
9. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55(5):708-710.
10. Yetter MF, Ogren FP, Moore GF, Yonkers AJ. Bell’s palsy: a facial nerve paralysis diagnosis of exclusion. Nebr Med J. 1990;75(5):109-116.
11. Stomeo F. Possibilities of diagnostic errors in paralysis of the 7th cranial nerve. Acta Otorhinolaryngol Ital. 1989;9(6):629-633.
12. Clemis JD. All that palsies is not Bell’s: Bell’s palsy due to adenoid cystic carcinoma of the parotid. Am J Otol. 1991;12(5):397.
13. Brach JS, VanSwearingen JM. Not all facial paralysis is Bell’s palsy: a case report. Arch Phys Med Rehabil. 1999;80(7):857-859.
14. Albers JR, Tamang S. Common questions about Bell palsy. Am Fam Physician. 2014;89(3):209-212.
15. Adour KK, Hilsinger RL Jr, Callan EJ. Facial paralysis and Bell’s palsy: a protocol for differential diagnosis. Am J Otol. 1985;Suppl:68-73.
16. Morrow MJ. Bell’s palsy and herpes zoster. Curr Treat Options Neurol. 2000;2(5):407-416.
17. Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell’s palsy: finding occult malignancy in acute-onset facial paralysis. Am J Otolaryngol. 2010;31(5):339-342. doi:10.1016/j.amjoto.2009.04.003.
18. Kaushal A, Curran WJ Jr. For whom the Bell’s palsy tolls? Am J Clin Oncol. 2009;32(4):450-451. doi:10.1097/01.coc.0000239141.22916.22.
19. Lagman C, Choy W, Lee SJ, et al. A Case of Bell’s palsy with an incidental finding of a cerebellopontine angle lipoma. Cureus. 2016;8(8):e747. doi:10.7759/cureus.747.
1. Glicenstein J. Ann Chir Plast Esthet. 2015;60(5):347-362. doi:10.1016/j.anplas.2015.05.007.
2. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
3. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi:10.1177/019459988509300202.
4. Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009;140(2):154-158. doi:10.1016/j.otohns.2008.11.021.
5. Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007;34(2):159-164. doi:10.1016/j.anl.2006.09.005.
6. Ahmed A. When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med. 2005;72(5):398-401, 405.
7. Gilden DH. Clinical practice. Bell’s palsy. N Engl J Med. 2004;351(13):1323-1331. doi:10.1056/NEJMcp041120.
8. Adour KK. Otological complications of herpes zoster. Ann Neurol. 1994;35:Suppl:S62-S64.
9. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55(5):708-710.
10. Yetter MF, Ogren FP, Moore GF, Yonkers AJ. Bell’s palsy: a facial nerve paralysis diagnosis of exclusion. Nebr Med J. 1990;75(5):109-116.
11. Stomeo F. Possibilities of diagnostic errors in paralysis of the 7th cranial nerve. Acta Otorhinolaryngol Ital. 1989;9(6):629-633.
12. Clemis JD. All that palsies is not Bell’s: Bell’s palsy due to adenoid cystic carcinoma of the parotid. Am J Otol. 1991;12(5):397.
13. Brach JS, VanSwearingen JM. Not all facial paralysis is Bell’s palsy: a case report. Arch Phys Med Rehabil. 1999;80(7):857-859.
14. Albers JR, Tamang S. Common questions about Bell palsy. Am Fam Physician. 2014;89(3):209-212.
15. Adour KK, Hilsinger RL Jr, Callan EJ. Facial paralysis and Bell’s palsy: a protocol for differential diagnosis. Am J Otol. 1985;Suppl:68-73.
16. Morrow MJ. Bell’s palsy and herpes zoster. Curr Treat Options Neurol. 2000;2(5):407-416.
17. Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell’s palsy: finding occult malignancy in acute-onset facial paralysis. Am J Otolaryngol. 2010;31(5):339-342. doi:10.1016/j.amjoto.2009.04.003.
18. Kaushal A, Curran WJ Jr. For whom the Bell’s palsy tolls? Am J Clin Oncol. 2009;32(4):450-451. doi:10.1097/01.coc.0000239141.22916.22.
19. Lagman C, Choy W, Lee SJ, et al. A Case of Bell’s palsy with an incidental finding of a cerebellopontine angle lipoma. Cureus. 2016;8(8):e747. doi:10.7759/cureus.747.
Ethanol Intoxication From Hand Sanitizer Ingestion
Case
A 29-year-old man presented to the ED several hours after ingesting what he described as a “hefty” bottle of hand sanitizer. The patient stated that he ingested such a considerable quantity of liquid hand sanitizer because he was unable to obtain beer or liquor. He further admitted to drinking two 40-ounce beers daily for the past several years, noting that he last consumed drinking alcohol the preceding day.
The patient denied any other coingestants. He also denied nausea, vomiting, abdominal pain, or other somatic complaints. The patient’s medical history was significant for hypertension and hepatitis C, and his social history was significant for daily alcohol consumption, tobacco abuse, and former benzodiazepine, marijuana, and intravenous heroin abuse. His psychiatric history was significant for borderline personality disorder, major depression, and bulimia. The patient’s home medications included a daily multivitamin, folate, thiamine, sertraline, mirtazapine, and prazosin.
Initial vital signs at presentation were: blood pressure, 124/77 mm Hg; heart rate, 86 beats/min; respiratory rate, 15 breaths/min; and temperature, 98.0°F. On physical examination, he was noted to have slurred speech and nystagmus. His pupils were equal and reactive, without scleral icterus. The abdomen was nontender and nondistended, with regular bowel sounds, and without ascites or varicosities visualized. The rest of the examination was unremarkable. The patient did express thoughts of suicidality, but denied any homicidal ideation.
Laboratory studies revealed a serum ethanol concentration of 446 mg/dL. The patient’s basic metabolic panel was unremarkable, and liver function test results showed mildly elevated enzymes. The coagulation panel was within normal limits.
Is alcohol-based hand sanitizer consumption an emerging public health concern?
Excessive alcohol consumption is a recognized public health problem in the United States and is associated with an average of 88,000 deaths per year.1 In a select population of patients, an untoward effect has developed from another public health target—that of hand hygiene.
Alcohol-based liquid hand sanitizers have become ubiquitous as a weapon in the antimicrobial arsenal with recommendations for its use as an alternative to soap and water in certain clinical settings. Liquid hand sanitizers are ideal for hospital or community use as they are faster, more effective, and less irritating to the skin than traditional hand-washing techniques.2
The downside to the widespread availability of hand sanitizers is that they offer easy access to individuals in search of clandestine sources of alcohol. Prior case reports have discussed the practice of consuming alcohol-based hand sanitizers for the purpose of intoxication in institutionalized persons, such as prisoners or patients in psychiatric facilities who are restricted to conventional sources of alcohol.
Children and confused elderly patients are also at risk for unintentional ingestions.3,4 An article reviewed exposures reported to the American Association of Poison Control Center’s National Poison Data System over a 5-year period from 2005 to 2009.3 Of the 68,712 reported cases in this cohort, 80.5% were in children younger than 6 years of age. The investigators also noted an increased incidence of exposure over this period with an average of 1,894 additional cases per year.3There were 17,154 children aged 12 years and younger reported in 2017 to poison centers with exposures to hand sanitizers. Young children may be enticed by the bright colorful packaging and similarity to food and candy smells.5
What are the clinical manifestations of alcohol-based hand sanitizer ingestion?
Significant hazards exist from ingesting liquid hand sanitizer, including the high alcohol content, which varies from 40% to 85%.2 Because isopropanol is commonly one of the components (if not the sole component) of many hand-sanitizer preparations, isopropanol toxicity may occur when ingested. The effects of isopropanol are similar to those of ethanol, with clinical effects reported after ingestion of as little as 100 mL of 70% isopropanol solution.4
Hand sanitizer formulations vary by manufacturer and contain different concentrations of ethanol and/or isopropanol, as well as additional potential inactive ingredients such as acetone, 1-propanol, 2-propanol, benzyl alcohol, hydrogen peroxide, glycerin, water, and different perfumes.3,4
Persons who consume hand sanitizers recreationally are often unaware of the large alcohol content by volume that they are consuming. Recreational ingestion of hand sanitizer is believed to be the cause of at least one case of lethal ethanol intoxication. An articlereported a case of a male patient who suffered respiratory arrest after consuming an ethanol-based hand sanitizer.6 This patient was noted to have a serum ethanol of 536 mg/dL after consuming an unknown quantity of a 354 mL container of a 62% ethanol by volume hand sanitizer.6
Institutionalized individuals seeking alcohol through this source have discovered novel ways to yield a stronger product. Through the use of table salt and a cotton sock, it is possible to extract a liquid from a gel hand sanitizer preparation, yielding an alcohol context 30% higher by volume than the parent mixture.7
Alcohol intoxication poses a host of health effects. In nonhabituated individuals, a lethal load of alcohol can be achieved by consuming a volume of as little as 400 mL of an 80% alcohol-based solution.4 Symptoms from ingestion of an alcohol-based liquid hand sanitizer typically appear 1 to 2 hours after ingestion and mirror that of the alcohol toxidrome. Most commonly, this includes nausea, vomiting, epigastric pain, and varying degrees of central nervous system (CNS) depression.4 The life-threatening clinical manifestation of alcohol intoxication includes severe CNS and respiratory depression resulting in respiratory arrest, hypothermia, cardiac dysrhythmias with possible cardiac arrest, hypoglycemia, ketoacidosis, and hypotension.3
How is alcohol-based hand sanitizer ingestion managed?
The management of patients with alcohol-based hand sanitizer ingestion is the same as the management of alcohol ingestion from more socially acceptable sources and is mainly supportive.3,4 These measures are directed at managing the patient’s airway with intubation and mechanical ventilation when appropriate, as well as supportive measures to address any underlying metabolic derangement or hypotension.2 While hemodialysis has been used in some patients who had severe organ dysfunction and did not respond to supportive measures, it is usually not necessary.1,3
Case Conclusion
The patient in this case was subsequently admitted to an intermediate level of care. He did not require intubation or further hemodynamic support during his initial acute intoxication. Later in the patient’s hospital course, he was noted to be in alcohol withdrawal, and proper management was initiated. He also required therapeutic one-to-one supervision after members of the nursing staff observed the patient consuming the hand sanitizer gel present in patient-care areas. He was later seen by psychiatry services. The psychiatrist recommended transfer to an inpatient psychiatric facility upon medical clearance for treatment of his psychiatric illness as well as alcohol dependence.
1. Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009-2011. Prev Chronic Dis. 2014;11:E206. doi:10.5888/pcd11.140329.
2. Pittet D, Boyce JM. Revolutionizing hand hygiene in health-care settings: guidelines revisted. Lancet Infect Dis. 2003;3(5):269-270.
3. Gormley NJ, Bronstein AC, Rasimas JJ, et al. The rising incidence of intentional ingestion of ethanol-containing hand sanitizers. Crit Care Med. 2012:40(1):290-294. doi:10.1097/CCM.0b013e31822f09c0.
4. Archer JR, Wood DM, Tizzard Z, Jones AL, Dargan PI. Alcohol hand rubs: hygiene and hazard. BMJ. 2007;335(7630):1154-1155.
5. Hand sanitizer. American Association of Poison Control Centers Web site. http://www.aapcc.org/alerts/hand-sanitizer/. Accessed December 27, 2017.
6. Schneir AB, Clark RF. Death caused by ingestion of an ethanol-based hand sanitizer. J Emerg Med. 2013;45(3):358-360. doi:10.1016/j.jemermed.2013.03.018.
7. Darracq MA, Ghafouri N, Pesce A, Cantrell FL. Hand sanitizer intoxication following a crude extraction method. Am J Drug Alcohol Abuse. 2013;39(3):217-218. doi:10.3109/00952990.2013.773335.
Case
A 29-year-old man presented to the ED several hours after ingesting what he described as a “hefty” bottle of hand sanitizer. The patient stated that he ingested such a considerable quantity of liquid hand sanitizer because he was unable to obtain beer or liquor. He further admitted to drinking two 40-ounce beers daily for the past several years, noting that he last consumed drinking alcohol the preceding day.
The patient denied any other coingestants. He also denied nausea, vomiting, abdominal pain, or other somatic complaints. The patient’s medical history was significant for hypertension and hepatitis C, and his social history was significant for daily alcohol consumption, tobacco abuse, and former benzodiazepine, marijuana, and intravenous heroin abuse. His psychiatric history was significant for borderline personality disorder, major depression, and bulimia. The patient’s home medications included a daily multivitamin, folate, thiamine, sertraline, mirtazapine, and prazosin.
Initial vital signs at presentation were: blood pressure, 124/77 mm Hg; heart rate, 86 beats/min; respiratory rate, 15 breaths/min; and temperature, 98.0°F. On physical examination, he was noted to have slurred speech and nystagmus. His pupils were equal and reactive, without scleral icterus. The abdomen was nontender and nondistended, with regular bowel sounds, and without ascites or varicosities visualized. The rest of the examination was unremarkable. The patient did express thoughts of suicidality, but denied any homicidal ideation.
Laboratory studies revealed a serum ethanol concentration of 446 mg/dL. The patient’s basic metabolic panel was unremarkable, and liver function test results showed mildly elevated enzymes. The coagulation panel was within normal limits.
Is alcohol-based hand sanitizer consumption an emerging public health concern?
Excessive alcohol consumption is a recognized public health problem in the United States and is associated with an average of 88,000 deaths per year.1 In a select population of patients, an untoward effect has developed from another public health target—that of hand hygiene.
Alcohol-based liquid hand sanitizers have become ubiquitous as a weapon in the antimicrobial arsenal with recommendations for its use as an alternative to soap and water in certain clinical settings. Liquid hand sanitizers are ideal for hospital or community use as they are faster, more effective, and less irritating to the skin than traditional hand-washing techniques.2
The downside to the widespread availability of hand sanitizers is that they offer easy access to individuals in search of clandestine sources of alcohol. Prior case reports have discussed the practice of consuming alcohol-based hand sanitizers for the purpose of intoxication in institutionalized persons, such as prisoners or patients in psychiatric facilities who are restricted to conventional sources of alcohol.
Children and confused elderly patients are also at risk for unintentional ingestions.3,4 An article reviewed exposures reported to the American Association of Poison Control Center’s National Poison Data System over a 5-year period from 2005 to 2009.3 Of the 68,712 reported cases in this cohort, 80.5% were in children younger than 6 years of age. The investigators also noted an increased incidence of exposure over this period with an average of 1,894 additional cases per year.3There were 17,154 children aged 12 years and younger reported in 2017 to poison centers with exposures to hand sanitizers. Young children may be enticed by the bright colorful packaging and similarity to food and candy smells.5
What are the clinical manifestations of alcohol-based hand sanitizer ingestion?
Significant hazards exist from ingesting liquid hand sanitizer, including the high alcohol content, which varies from 40% to 85%.2 Because isopropanol is commonly one of the components (if not the sole component) of many hand-sanitizer preparations, isopropanol toxicity may occur when ingested. The effects of isopropanol are similar to those of ethanol, with clinical effects reported after ingestion of as little as 100 mL of 70% isopropanol solution.4
Hand sanitizer formulations vary by manufacturer and contain different concentrations of ethanol and/or isopropanol, as well as additional potential inactive ingredients such as acetone, 1-propanol, 2-propanol, benzyl alcohol, hydrogen peroxide, glycerin, water, and different perfumes.3,4
Persons who consume hand sanitizers recreationally are often unaware of the large alcohol content by volume that they are consuming. Recreational ingestion of hand sanitizer is believed to be the cause of at least one case of lethal ethanol intoxication. An articlereported a case of a male patient who suffered respiratory arrest after consuming an ethanol-based hand sanitizer.6 This patient was noted to have a serum ethanol of 536 mg/dL after consuming an unknown quantity of a 354 mL container of a 62% ethanol by volume hand sanitizer.6
Institutionalized individuals seeking alcohol through this source have discovered novel ways to yield a stronger product. Through the use of table salt and a cotton sock, it is possible to extract a liquid from a gel hand sanitizer preparation, yielding an alcohol context 30% higher by volume than the parent mixture.7
Alcohol intoxication poses a host of health effects. In nonhabituated individuals, a lethal load of alcohol can be achieved by consuming a volume of as little as 400 mL of an 80% alcohol-based solution.4 Symptoms from ingestion of an alcohol-based liquid hand sanitizer typically appear 1 to 2 hours after ingestion and mirror that of the alcohol toxidrome. Most commonly, this includes nausea, vomiting, epigastric pain, and varying degrees of central nervous system (CNS) depression.4 The life-threatening clinical manifestation of alcohol intoxication includes severe CNS and respiratory depression resulting in respiratory arrest, hypothermia, cardiac dysrhythmias with possible cardiac arrest, hypoglycemia, ketoacidosis, and hypotension.3
How is alcohol-based hand sanitizer ingestion managed?
The management of patients with alcohol-based hand sanitizer ingestion is the same as the management of alcohol ingestion from more socially acceptable sources and is mainly supportive.3,4 These measures are directed at managing the patient’s airway with intubation and mechanical ventilation when appropriate, as well as supportive measures to address any underlying metabolic derangement or hypotension.2 While hemodialysis has been used in some patients who had severe organ dysfunction and did not respond to supportive measures, it is usually not necessary.1,3
Case Conclusion
The patient in this case was subsequently admitted to an intermediate level of care. He did not require intubation or further hemodynamic support during his initial acute intoxication. Later in the patient’s hospital course, he was noted to be in alcohol withdrawal, and proper management was initiated. He also required therapeutic one-to-one supervision after members of the nursing staff observed the patient consuming the hand sanitizer gel present in patient-care areas. He was later seen by psychiatry services. The psychiatrist recommended transfer to an inpatient psychiatric facility upon medical clearance for treatment of his psychiatric illness as well as alcohol dependence.
Case
A 29-year-old man presented to the ED several hours after ingesting what he described as a “hefty” bottle of hand sanitizer. The patient stated that he ingested such a considerable quantity of liquid hand sanitizer because he was unable to obtain beer or liquor. He further admitted to drinking two 40-ounce beers daily for the past several years, noting that he last consumed drinking alcohol the preceding day.
The patient denied any other coingestants. He also denied nausea, vomiting, abdominal pain, or other somatic complaints. The patient’s medical history was significant for hypertension and hepatitis C, and his social history was significant for daily alcohol consumption, tobacco abuse, and former benzodiazepine, marijuana, and intravenous heroin abuse. His psychiatric history was significant for borderline personality disorder, major depression, and bulimia. The patient’s home medications included a daily multivitamin, folate, thiamine, sertraline, mirtazapine, and prazosin.
Initial vital signs at presentation were: blood pressure, 124/77 mm Hg; heart rate, 86 beats/min; respiratory rate, 15 breaths/min; and temperature, 98.0°F. On physical examination, he was noted to have slurred speech and nystagmus. His pupils were equal and reactive, without scleral icterus. The abdomen was nontender and nondistended, with regular bowel sounds, and without ascites or varicosities visualized. The rest of the examination was unremarkable. The patient did express thoughts of suicidality, but denied any homicidal ideation.
Laboratory studies revealed a serum ethanol concentration of 446 mg/dL. The patient’s basic metabolic panel was unremarkable, and liver function test results showed mildly elevated enzymes. The coagulation panel was within normal limits.
Is alcohol-based hand sanitizer consumption an emerging public health concern?
Excessive alcohol consumption is a recognized public health problem in the United States and is associated with an average of 88,000 deaths per year.1 In a select population of patients, an untoward effect has developed from another public health target—that of hand hygiene.
Alcohol-based liquid hand sanitizers have become ubiquitous as a weapon in the antimicrobial arsenal with recommendations for its use as an alternative to soap and water in certain clinical settings. Liquid hand sanitizers are ideal for hospital or community use as they are faster, more effective, and less irritating to the skin than traditional hand-washing techniques.2
The downside to the widespread availability of hand sanitizers is that they offer easy access to individuals in search of clandestine sources of alcohol. Prior case reports have discussed the practice of consuming alcohol-based hand sanitizers for the purpose of intoxication in institutionalized persons, such as prisoners or patients in psychiatric facilities who are restricted to conventional sources of alcohol.
Children and confused elderly patients are also at risk for unintentional ingestions.3,4 An article reviewed exposures reported to the American Association of Poison Control Center’s National Poison Data System over a 5-year period from 2005 to 2009.3 Of the 68,712 reported cases in this cohort, 80.5% were in children younger than 6 years of age. The investigators also noted an increased incidence of exposure over this period with an average of 1,894 additional cases per year.3There were 17,154 children aged 12 years and younger reported in 2017 to poison centers with exposures to hand sanitizers. Young children may be enticed by the bright colorful packaging and similarity to food and candy smells.5
What are the clinical manifestations of alcohol-based hand sanitizer ingestion?
Significant hazards exist from ingesting liquid hand sanitizer, including the high alcohol content, which varies from 40% to 85%.2 Because isopropanol is commonly one of the components (if not the sole component) of many hand-sanitizer preparations, isopropanol toxicity may occur when ingested. The effects of isopropanol are similar to those of ethanol, with clinical effects reported after ingestion of as little as 100 mL of 70% isopropanol solution.4
Hand sanitizer formulations vary by manufacturer and contain different concentrations of ethanol and/or isopropanol, as well as additional potential inactive ingredients such as acetone, 1-propanol, 2-propanol, benzyl alcohol, hydrogen peroxide, glycerin, water, and different perfumes.3,4
Persons who consume hand sanitizers recreationally are often unaware of the large alcohol content by volume that they are consuming. Recreational ingestion of hand sanitizer is believed to be the cause of at least one case of lethal ethanol intoxication. An articlereported a case of a male patient who suffered respiratory arrest after consuming an ethanol-based hand sanitizer.6 This patient was noted to have a serum ethanol of 536 mg/dL after consuming an unknown quantity of a 354 mL container of a 62% ethanol by volume hand sanitizer.6
Institutionalized individuals seeking alcohol through this source have discovered novel ways to yield a stronger product. Through the use of table salt and a cotton sock, it is possible to extract a liquid from a gel hand sanitizer preparation, yielding an alcohol context 30% higher by volume than the parent mixture.7
Alcohol intoxication poses a host of health effects. In nonhabituated individuals, a lethal load of alcohol can be achieved by consuming a volume of as little as 400 mL of an 80% alcohol-based solution.4 Symptoms from ingestion of an alcohol-based liquid hand sanitizer typically appear 1 to 2 hours after ingestion and mirror that of the alcohol toxidrome. Most commonly, this includes nausea, vomiting, epigastric pain, and varying degrees of central nervous system (CNS) depression.4 The life-threatening clinical manifestation of alcohol intoxication includes severe CNS and respiratory depression resulting in respiratory arrest, hypothermia, cardiac dysrhythmias with possible cardiac arrest, hypoglycemia, ketoacidosis, and hypotension.3
How is alcohol-based hand sanitizer ingestion managed?
The management of patients with alcohol-based hand sanitizer ingestion is the same as the management of alcohol ingestion from more socially acceptable sources and is mainly supportive.3,4 These measures are directed at managing the patient’s airway with intubation and mechanical ventilation when appropriate, as well as supportive measures to address any underlying metabolic derangement or hypotension.2 While hemodialysis has been used in some patients who had severe organ dysfunction and did not respond to supportive measures, it is usually not necessary.1,3
Case Conclusion
The patient in this case was subsequently admitted to an intermediate level of care. He did not require intubation or further hemodynamic support during his initial acute intoxication. Later in the patient’s hospital course, he was noted to be in alcohol withdrawal, and proper management was initiated. He also required therapeutic one-to-one supervision after members of the nursing staff observed the patient consuming the hand sanitizer gel present in patient-care areas. He was later seen by psychiatry services. The psychiatrist recommended transfer to an inpatient psychiatric facility upon medical clearance for treatment of his psychiatric illness as well as alcohol dependence.
1. Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009-2011. Prev Chronic Dis. 2014;11:E206. doi:10.5888/pcd11.140329.
2. Pittet D, Boyce JM. Revolutionizing hand hygiene in health-care settings: guidelines revisted. Lancet Infect Dis. 2003;3(5):269-270.
3. Gormley NJ, Bronstein AC, Rasimas JJ, et al. The rising incidence of intentional ingestion of ethanol-containing hand sanitizers. Crit Care Med. 2012:40(1):290-294. doi:10.1097/CCM.0b013e31822f09c0.
4. Archer JR, Wood DM, Tizzard Z, Jones AL, Dargan PI. Alcohol hand rubs: hygiene and hazard. BMJ. 2007;335(7630):1154-1155.
5. Hand sanitizer. American Association of Poison Control Centers Web site. http://www.aapcc.org/alerts/hand-sanitizer/. Accessed December 27, 2017.
6. Schneir AB, Clark RF. Death caused by ingestion of an ethanol-based hand sanitizer. J Emerg Med. 2013;45(3):358-360. doi:10.1016/j.jemermed.2013.03.018.
7. Darracq MA, Ghafouri N, Pesce A, Cantrell FL. Hand sanitizer intoxication following a crude extraction method. Am J Drug Alcohol Abuse. 2013;39(3):217-218. doi:10.3109/00952990.2013.773335.
1. Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009-2011. Prev Chronic Dis. 2014;11:E206. doi:10.5888/pcd11.140329.
2. Pittet D, Boyce JM. Revolutionizing hand hygiene in health-care settings: guidelines revisted. Lancet Infect Dis. 2003;3(5):269-270.
3. Gormley NJ, Bronstein AC, Rasimas JJ, et al. The rising incidence of intentional ingestion of ethanol-containing hand sanitizers. Crit Care Med. 2012:40(1):290-294. doi:10.1097/CCM.0b013e31822f09c0.
4. Archer JR, Wood DM, Tizzard Z, Jones AL, Dargan PI. Alcohol hand rubs: hygiene and hazard. BMJ. 2007;335(7630):1154-1155.
5. Hand sanitizer. American Association of Poison Control Centers Web site. http://www.aapcc.org/alerts/hand-sanitizer/. Accessed December 27, 2017.
6. Schneir AB, Clark RF. Death caused by ingestion of an ethanol-based hand sanitizer. J Emerg Med. 2013;45(3):358-360. doi:10.1016/j.jemermed.2013.03.018.
7. Darracq MA, Ghafouri N, Pesce A, Cantrell FL. Hand sanitizer intoxication following a crude extraction method. Am J Drug Alcohol Abuse. 2013;39(3):217-218. doi:10.3109/00952990.2013.773335.
Back to Basics: An Uncommon, Unrelated Presentation of a Common Disease
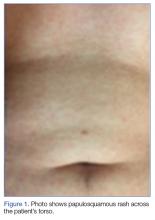
The early initial ulcerative lesion (chancre) caused by Treponema pallidum infection, has a median incubation period of 21 days (primary syphilis). When untreated, secondary syphilis will develop within weeks to months and is characterized by generalized symptoms such as malaise, fevers, headaches, sore throat, and myalgia. However, the most characteristic finding in secondary syphilis remains a rash that is classically identified as symmetric, macular, or papular, and involving the entire trunk and extremities, including the palms and soles.
When secondary syphilis is left untreated, late syphilis or tertiary syphilis can develop, which is characterized by cardiovascular involvement, including aortitis, gummatous syphilis (granulomatous nodules in a variety of organs but typically the skin and bones), or central nervous system involvement.1-3 The following case describes a patient with nondescript symptoms, including malaise and cough, who had a characteristic rash of secondary syphilis that was diagnosed and treated in our Houston-area community hospital.
Case
In late autumn, a 30-year-old man presented to our community ED for evaluation of a cough productive of green sputum along with mild chest discomfort, malaise, and generalized myalgia, which were intermittent over the course of the past month. The patient denied rhinorrhea, fevers, chills, dyspnea, or any other systemic complaints. He also denied any sick contacts, but noted that his influenza vaccine was not up to date.
The patient denied any remote or recent medical or surgical history. He further denied taking any medications, and noted that his only medical allergy was to penicillin. His family history was noncontributory. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and to a daily alcohol intake of approximately one 6-pack of beer. He also admitted to frequently smoking crystal methamphetamine, which he stated he had last used 2 days prior to presentation. The patient said his current chest pain was similar to prior episodes, noting that when the pain occurred, he would temporarily stop smoking crystal methamphetamine.
Plain chest radiography, electrocardiogram, complete metabolic panel, complete blood count, B-natriuretic peptide, and troponin levels were all unremarkable. Due to the presence and nature of the patient’s rash, a rapid plasma reagin (RPR) screen was also taken, the results of which were reactive.
On further questioning, the patient admitted to having multiple female sexual partners with whom he used barrier protection sporadically. A more detailed physical examination revealed multiple painless ulcerations/chancres over the penile shaft and scrotum, without urethral drainage or inguinal lymphadenopathy. The patient denied dysuria or hematuria.
Since the patient was allergic to penicillin, he was given a single oral dose of azithromycin 2 g, and started on a 2-week course of oral doxycycline 100 mg. Further laboratory studies included gonorrhea and chlamydia cultures, both of which were negative. He was instructed to follow-up with his primary care physician for extended sexually transmitted infection (STI) panel-testing, including HIV, hepatitis, and confirmatory syphilis testing. Unfortunately, it is not known whether the patient complied with discharge instructions as he was lost to follow-up.
Discussion
Diagnostic algorithms for syphilis, one of the best studied STIs, have changed with technological advancement, but diagnosis and treatment for the most part has remained mostly the same. The uniqueness of this case is really focused around the patient’s chief complaint. While it is classic to present with malaise, headache, and rash, our patient complained of cough productive of sputum with chest pain—a rare presentation of secondary syphilis. The fortuitous key finding of the truncal rash directed the emergency physician toward the appropriate diagnosis.
Diagnosis
In the ED, where patients such as the one in our case are often lost to follow-up, and consistent infectious disease and primary care follow-up is unavailable, prompt treatment based on history and physical examination alone is recommended. Patients should be tested for syphilis, as well as other STIs including chlamydia, gonorrhea, hepatitis, and HIV as an outpatient. In addition, any partners with whom the patient has had sexual contact within the last 90 days should also undergo STI testing; sexual partners from over 90 days should be notified of the patient’s status and evaluated with testing as indicated.4 All positive test results should be reported to the Centers for Disease Control and Prevention (CDC).5
Nontreponemal and Treponemal Testing
For patients with clinical signs and symptoms of syphilis, recommended laboratory evaluation includes both nontreponemal and treponemal testing. Nontreponemal tests include RPR, venereal disease research laboratory test, and toluidine red unheated serum test. Treponemal tests include fluorescent treponemal antibody absorption, microhemagglutination test for antibodies to T pallidum, T pallidum particle agglutination assay, T pallidum enzyme immunoassay, and chemiluminescence immunoassay. Patients who test positive for treponemal antibody will typically remain reactive for life.5,6
In the setting of discordant test results, patients with a nonreactive treponemal result are generally considered to be negative for syphilis. Discordant results with a negative nontreponemal test are more complicated, and recommendations are based on symptomatology and repeat testing.5
Treatment
When a patient has a positive nontreponemal and treponemal test, treatment is usually indicated. As with the patient in this case, treatment is always indicated for patients who have no prior history of syphilis. For patients who have a history of treated syphilis, attention must be given to titer levels on previous testing and to patient symptomatology.
The treatment for early (primary and secondary) syphilis in patients with no penicillin allergy is a single dose of penicillin G benzathine intramuscularly, at a dose of 2.4 million U. Alternative regimens include doxycycline 100 mg orally twice daily for 14 days, and azithromycin 2 g orally as a single dose; however, there is an association of treatment failure with azithromycin due to macrolide resistance.5 The patient in this case received empiric treatment targeting syphilis, gonorrhea, and chlamydia.
Conclusion
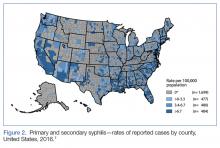
Ten years ago, the rates of primary and secondary syphilis were low, leading the infectious disease community to believe that preventive efforts had been effective. According to the CDC, however, “[current] rates…are the highest they have been in more than 20 years.”5Figure 2 demonstrates the geographic distribution of syphilis cases in the United States in 2016.7
Heightened concern has prompted the CDC to promote the theme “Syphilis Strikes Back” in April 2017, which was STI Awareness Month.8 Identification of disease is critical in the ED, especially when a previously common disease has become uncommon, like the resurgence of syphilis we are now seeing.
1. Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: An epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
2. Rockwell DH, Yobs AR, Moore MB Jr. The Tuskegee study of untreated syphilis; the 30th year of observation. Arch Intern Med. 1964;114:792-798.
3. Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 1999:661-684.
4. Birnbaumer DM. Sexually transmitted diseases. In: Marx JA, Hockberger RS, Walls RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014:1312-1325.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
6. Larsen SA. Syphilis. Clin Lab Med. 1989;9(3):545-557.
7. Centers for Disease Control Prevention. Primary and secondary syphilis—rates of reported cases by county, United States, 2016. https://www.cdc.gov/std/stats16/figures/33.htm. Updated September 26, 2017. Accessed October 31 2017.]
8. Centers for Disease Control and Prevention. STD Awareness Month. Syphilis Strikes Back. https://www.cdc.gov/std/sam/index.htm?s_cid=tw_SAM_17001. Updated April 6, 2017. Accessed October 31, 2017.
The early initial ulcerative lesion (chancre) caused by Treponema pallidum infection, has a median incubation period of 21 days (primary syphilis). When untreated, secondary syphilis will develop within weeks to months and is characterized by generalized symptoms such as malaise, fevers, headaches, sore throat, and myalgia. However, the most characteristic finding in secondary syphilis remains a rash that is classically identified as symmetric, macular, or papular, and involving the entire trunk and extremities, including the palms and soles.
When secondary syphilis is left untreated, late syphilis or tertiary syphilis can develop, which is characterized by cardiovascular involvement, including aortitis, gummatous syphilis (granulomatous nodules in a variety of organs but typically the skin and bones), or central nervous system involvement.1-3 The following case describes a patient with nondescript symptoms, including malaise and cough, who had a characteristic rash of secondary syphilis that was diagnosed and treated in our Houston-area community hospital.
Case
In late autumn, a 30-year-old man presented to our community ED for evaluation of a cough productive of green sputum along with mild chest discomfort, malaise, and generalized myalgia, which were intermittent over the course of the past month. The patient denied rhinorrhea, fevers, chills, dyspnea, or any other systemic complaints. He also denied any sick contacts, but noted that his influenza vaccine was not up to date.
The patient denied any remote or recent medical or surgical history. He further denied taking any medications, and noted that his only medical allergy was to penicillin. His family history was noncontributory. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and to a daily alcohol intake of approximately one 6-pack of beer. He also admitted to frequently smoking crystal methamphetamine, which he stated he had last used 2 days prior to presentation. The patient said his current chest pain was similar to prior episodes, noting that when the pain occurred, he would temporarily stop smoking crystal methamphetamine.
Plain chest radiography, electrocardiogram, complete metabolic panel, complete blood count, B-natriuretic peptide, and troponin levels were all unremarkable. Due to the presence and nature of the patient’s rash, a rapid plasma reagin (RPR) screen was also taken, the results of which were reactive.
On further questioning, the patient admitted to having multiple female sexual partners with whom he used barrier protection sporadically. A more detailed physical examination revealed multiple painless ulcerations/chancres over the penile shaft and scrotum, without urethral drainage or inguinal lymphadenopathy. The patient denied dysuria or hematuria.
Since the patient was allergic to penicillin, he was given a single oral dose of azithromycin 2 g, and started on a 2-week course of oral doxycycline 100 mg. Further laboratory studies included gonorrhea and chlamydia cultures, both of which were negative. He was instructed to follow-up with his primary care physician for extended sexually transmitted infection (STI) panel-testing, including HIV, hepatitis, and confirmatory syphilis testing. Unfortunately, it is not known whether the patient complied with discharge instructions as he was lost to follow-up.
Discussion
Diagnostic algorithms for syphilis, one of the best studied STIs, have changed with technological advancement, but diagnosis and treatment for the most part has remained mostly the same. The uniqueness of this case is really focused around the patient’s chief complaint. While it is classic to present with malaise, headache, and rash, our patient complained of cough productive of sputum with chest pain—a rare presentation of secondary syphilis. The fortuitous key finding of the truncal rash directed the emergency physician toward the appropriate diagnosis.
Diagnosis
In the ED, where patients such as the one in our case are often lost to follow-up, and consistent infectious disease and primary care follow-up is unavailable, prompt treatment based on history and physical examination alone is recommended. Patients should be tested for syphilis, as well as other STIs including chlamydia, gonorrhea, hepatitis, and HIV as an outpatient. In addition, any partners with whom the patient has had sexual contact within the last 90 days should also undergo STI testing; sexual partners from over 90 days should be notified of the patient’s status and evaluated with testing as indicated.4 All positive test results should be reported to the Centers for Disease Control and Prevention (CDC).5
Nontreponemal and Treponemal Testing
For patients with clinical signs and symptoms of syphilis, recommended laboratory evaluation includes both nontreponemal and treponemal testing. Nontreponemal tests include RPR, venereal disease research laboratory test, and toluidine red unheated serum test. Treponemal tests include fluorescent treponemal antibody absorption, microhemagglutination test for antibodies to T pallidum, T pallidum particle agglutination assay, T pallidum enzyme immunoassay, and chemiluminescence immunoassay. Patients who test positive for treponemal antibody will typically remain reactive for life.5,6
In the setting of discordant test results, patients with a nonreactive treponemal result are generally considered to be negative for syphilis. Discordant results with a negative nontreponemal test are more complicated, and recommendations are based on symptomatology and repeat testing.5
Treatment
When a patient has a positive nontreponemal and treponemal test, treatment is usually indicated. As with the patient in this case, treatment is always indicated for patients who have no prior history of syphilis. For patients who have a history of treated syphilis, attention must be given to titer levels on previous testing and to patient symptomatology.
The treatment for early (primary and secondary) syphilis in patients with no penicillin allergy is a single dose of penicillin G benzathine intramuscularly, at a dose of 2.4 million U. Alternative regimens include doxycycline 100 mg orally twice daily for 14 days, and azithromycin 2 g orally as a single dose; however, there is an association of treatment failure with azithromycin due to macrolide resistance.5 The patient in this case received empiric treatment targeting syphilis, gonorrhea, and chlamydia.
Conclusion
Ten years ago, the rates of primary and secondary syphilis were low, leading the infectious disease community to believe that preventive efforts had been effective. According to the CDC, however, “[current] rates…are the highest they have been in more than 20 years.”5Figure 2 demonstrates the geographic distribution of syphilis cases in the United States in 2016.7
Heightened concern has prompted the CDC to promote the theme “Syphilis Strikes Back” in April 2017, which was STI Awareness Month.8 Identification of disease is critical in the ED, especially when a previously common disease has become uncommon, like the resurgence of syphilis we are now seeing.
The early initial ulcerative lesion (chancre) caused by Treponema pallidum infection, has a median incubation period of 21 days (primary syphilis). When untreated, secondary syphilis will develop within weeks to months and is characterized by generalized symptoms such as malaise, fevers, headaches, sore throat, and myalgia. However, the most characteristic finding in secondary syphilis remains a rash that is classically identified as symmetric, macular, or papular, and involving the entire trunk and extremities, including the palms and soles.
When secondary syphilis is left untreated, late syphilis or tertiary syphilis can develop, which is characterized by cardiovascular involvement, including aortitis, gummatous syphilis (granulomatous nodules in a variety of organs but typically the skin and bones), or central nervous system involvement.1-3 The following case describes a patient with nondescript symptoms, including malaise and cough, who had a characteristic rash of secondary syphilis that was diagnosed and treated in our Houston-area community hospital.
Case
In late autumn, a 30-year-old man presented to our community ED for evaluation of a cough productive of green sputum along with mild chest discomfort, malaise, and generalized myalgia, which were intermittent over the course of the past month. The patient denied rhinorrhea, fevers, chills, dyspnea, or any other systemic complaints. He also denied any sick contacts, but noted that his influenza vaccine was not up to date.
The patient denied any remote or recent medical or surgical history. He further denied taking any medications, and noted that his only medical allergy was to penicillin. His family history was noncontributory. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and to a daily alcohol intake of approximately one 6-pack of beer. He also admitted to frequently smoking crystal methamphetamine, which he stated he had last used 2 days prior to presentation. The patient said his current chest pain was similar to prior episodes, noting that when the pain occurred, he would temporarily stop smoking crystal methamphetamine.
Plain chest radiography, electrocardiogram, complete metabolic panel, complete blood count, B-natriuretic peptide, and troponin levels were all unremarkable. Due to the presence and nature of the patient’s rash, a rapid plasma reagin (RPR) screen was also taken, the results of which were reactive.
On further questioning, the patient admitted to having multiple female sexual partners with whom he used barrier protection sporadically. A more detailed physical examination revealed multiple painless ulcerations/chancres over the penile shaft and scrotum, without urethral drainage or inguinal lymphadenopathy. The patient denied dysuria or hematuria.
Since the patient was allergic to penicillin, he was given a single oral dose of azithromycin 2 g, and started on a 2-week course of oral doxycycline 100 mg. Further laboratory studies included gonorrhea and chlamydia cultures, both of which were negative. He was instructed to follow-up with his primary care physician for extended sexually transmitted infection (STI) panel-testing, including HIV, hepatitis, and confirmatory syphilis testing. Unfortunately, it is not known whether the patient complied with discharge instructions as he was lost to follow-up.
Discussion
Diagnostic algorithms for syphilis, one of the best studied STIs, have changed with technological advancement, but diagnosis and treatment for the most part has remained mostly the same. The uniqueness of this case is really focused around the patient’s chief complaint. While it is classic to present with malaise, headache, and rash, our patient complained of cough productive of sputum with chest pain—a rare presentation of secondary syphilis. The fortuitous key finding of the truncal rash directed the emergency physician toward the appropriate diagnosis.
Diagnosis
In the ED, where patients such as the one in our case are often lost to follow-up, and consistent infectious disease and primary care follow-up is unavailable, prompt treatment based on history and physical examination alone is recommended. Patients should be tested for syphilis, as well as other STIs including chlamydia, gonorrhea, hepatitis, and HIV as an outpatient. In addition, any partners with whom the patient has had sexual contact within the last 90 days should also undergo STI testing; sexual partners from over 90 days should be notified of the patient’s status and evaluated with testing as indicated.4 All positive test results should be reported to the Centers for Disease Control and Prevention (CDC).5
Nontreponemal and Treponemal Testing
For patients with clinical signs and symptoms of syphilis, recommended laboratory evaluation includes both nontreponemal and treponemal testing. Nontreponemal tests include RPR, venereal disease research laboratory test, and toluidine red unheated serum test. Treponemal tests include fluorescent treponemal antibody absorption, microhemagglutination test for antibodies to T pallidum, T pallidum particle agglutination assay, T pallidum enzyme immunoassay, and chemiluminescence immunoassay. Patients who test positive for treponemal antibody will typically remain reactive for life.5,6
In the setting of discordant test results, patients with a nonreactive treponemal result are generally considered to be negative for syphilis. Discordant results with a negative nontreponemal test are more complicated, and recommendations are based on symptomatology and repeat testing.5
Treatment
When a patient has a positive nontreponemal and treponemal test, treatment is usually indicated. As with the patient in this case, treatment is always indicated for patients who have no prior history of syphilis. For patients who have a history of treated syphilis, attention must be given to titer levels on previous testing and to patient symptomatology.
The treatment for early (primary and secondary) syphilis in patients with no penicillin allergy is a single dose of penicillin G benzathine intramuscularly, at a dose of 2.4 million U. Alternative regimens include doxycycline 100 mg orally twice daily for 14 days, and azithromycin 2 g orally as a single dose; however, there is an association of treatment failure with azithromycin due to macrolide resistance.5 The patient in this case received empiric treatment targeting syphilis, gonorrhea, and chlamydia.
Conclusion
Ten years ago, the rates of primary and secondary syphilis were low, leading the infectious disease community to believe that preventive efforts had been effective. According to the CDC, however, “[current] rates…are the highest they have been in more than 20 years.”5Figure 2 demonstrates the geographic distribution of syphilis cases in the United States in 2016.7
Heightened concern has prompted the CDC to promote the theme “Syphilis Strikes Back” in April 2017, which was STI Awareness Month.8 Identification of disease is critical in the ED, especially when a previously common disease has become uncommon, like the resurgence of syphilis we are now seeing.
1. Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: An epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
2. Rockwell DH, Yobs AR, Moore MB Jr. The Tuskegee study of untreated syphilis; the 30th year of observation. Arch Intern Med. 1964;114:792-798.
3. Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 1999:661-684.
4. Birnbaumer DM. Sexually transmitted diseases. In: Marx JA, Hockberger RS, Walls RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014:1312-1325.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
6. Larsen SA. Syphilis. Clin Lab Med. 1989;9(3):545-557.
7. Centers for Disease Control Prevention. Primary and secondary syphilis—rates of reported cases by county, United States, 2016. https://www.cdc.gov/std/stats16/figures/33.htm. Updated September 26, 2017. Accessed October 31 2017.]
8. Centers for Disease Control and Prevention. STD Awareness Month. Syphilis Strikes Back. https://www.cdc.gov/std/sam/index.htm?s_cid=tw_SAM_17001. Updated April 6, 2017. Accessed October 31, 2017.
1. Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: An epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
2. Rockwell DH, Yobs AR, Moore MB Jr. The Tuskegee study of untreated syphilis; the 30th year of observation. Arch Intern Med. 1964;114:792-798.
3. Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 1999:661-684.
4. Birnbaumer DM. Sexually transmitted diseases. In: Marx JA, Hockberger RS, Walls RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014:1312-1325.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
6. Larsen SA. Syphilis. Clin Lab Med. 1989;9(3):545-557.
7. Centers for Disease Control Prevention. Primary and secondary syphilis—rates of reported cases by county, United States, 2016. https://www.cdc.gov/std/stats16/figures/33.htm. Updated September 26, 2017. Accessed October 31 2017.]
8. Centers for Disease Control and Prevention. STD Awareness Month. Syphilis Strikes Back. https://www.cdc.gov/std/sam/index.htm?s_cid=tw_SAM_17001. Updated April 6, 2017. Accessed October 31, 2017.
Thyroid Cartilage Fracture in Context of Noncompetitive "Horseplay" Wrestling
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
Foreign Body Insertions: A Review
Anorectal and urethral foreign body insertions (polyembolokoilamania) are not infrequent presentations to the ED. The motivations behind these insertions vary, ranging from autoeroticism to reckless behavior. These insertions can lead to major complications and even death. Though ED staff members are used to the unpredictability of human behavior, foreign body insertions bring a mixture of responses from the staff, ranging from awe and incredulousness to anger and frustration. A knowledge and comfort in managing these cases includes a nonjudgmental triage assessment, collective professionalism, and self-awareness of the staff’s reaction.
Case 1
A 58-year-old man presented to the ED for evaluation of a foreign body in his rectum. He admitted to placing a beer bottle in his rectum, but was unable to remove it at home. The staff reported that the patient was previously seen in the ED for removal of a vibrator from his rectum.
Radiographic evaluation in the form of an acute abdominal series was obtained and confirmed a beer bottle in the rectum (Figures 1 and 2).
Case 2
A 55-year-old man presented to the ED after he inserted a pen cap into his urethra to aid in obtaining an erection. A pelvic X-ray was obtained and showed a radiolucent structure in the penis (Figure 3).
The patient was admitted to the hospital and taken to the OR by the consulting urologist. Using a rigid cystoscope and flexible graspers, the pen cap was removed from the proximal urethra under monitored anesthesia control. The procedure went without any complications.
A psychiatrist was consulted, and during the encounter, the patient admitted that his behavior was pathological. He revealed that he was a victim of child abuse and reported he had been having mixed emotions of anxiety, guilt, and embarrassment because of his behavior.
Discussion
Foreign body insertions are seen in patients with a wide variety of backgrounds, ages, and lifestyles. Approximately 80,000 cases of foreign body ingestion are seen annually in children under age 20 years. Young males have a higher predilection of swallowing foreign bodies when compared to young females,1 and rectal foreign body insertions are seen more commonly in males than in females.2 In this age group, intentional foreign body insertion may be an initial manifestation of psychiatric illness.
Rectal Insertions
The earliest published report of a rectal foreign body insertion was in 1919 by Smiley.6 The typical age at presentation ranges from 20 to 90 years old, with a mean age of 44 years old.2 Household objects such as bottles and glasses are the most commonly seen, but a long list of other items have also been reported in the literature, including toothbrushes, knives, deodorant bottles, food articles, sports equipment, cell phones, flashlights, wooden rods, broomsticks, sex toys, light bulbs, construction tools, nails, ornaments, aerosol canisters, cocaine packets, jewelry, batteries, guitar picks, and many other items.1,2,7
In nearly half of the reported cases, the reasons for rectal insertion was for sexual arousal/stimulation.1,7 Other reasons include nonsuicidal injurious behavior (eg, borderline personality disorder); suicide attempt; psychosis; depression; factitious disorder; malingering; cognitive disorders, including dementia and delirium; treatment of constipation and hemorrhoids; concealment; attention-seeking behavior; “accidental”; assault; and the consequences of drunken wagers.1,2 Additionally, abuse should be considered, especially in patients with developmental delay and/or psychiatric illness.
Close to 20% of all traumatic rectal injuries are due to foreign body insertions. In most cases, foreign bodies fail to cause significant anorectal injuries. Complications, however, can result from the process of insertion, removal, or from the contents introduced into the orifice.1 Any rectal examination should be preceded by an anatomical survey utilizing radiographic modalities to evaluate the integrity and orientation of the object in question. Any sharp object can injure the examining physician if this is not done prior. All examinations should be chaperoned.2,7 The most obvious and dangerous complication is perforation, and the patient’s care should proceed in the same manner as any other trauma patient. Additionally, resulting sepsis should be managed with the same standards as any other septic patient.7
Treatment. The method of object removal is determined by the presence or absence of a surgical abdomen and the need for general anesthesia. The location and shape of the object, however, may not equate with successful retrieval. Objects placed in the sigmoid colon are more than twice as likely to require surgical intervention compared to items placed distally.2 Once it is determined that the patient is clinically stable and does not have an acute abdomen, attempts in removing the rectal foreign object can be done in the ED or, if anesthesia is needed, in the OR. Any attempts at transanal removal require optimal patient relaxation, which can be achieved via procedural sedation. The patient should be placed in a lithotomy or left lateral decubitus position to allow palpation of the object in the lower gastrointestinal tract. From here, several methods of removal can be employed. Blunt objects can be grasped and removed by a gloved hand or with a clamp. A Foley catheter can also be passed alongside the object and the balloon inflated above the foreign body to aid in extraction as the Foley is pulled out slowly. Sengstaken-Blakemore tubes, obstetric forceps, and vacuum extractors have also been utilized.7
While bedside extraction is advocated by many authors, Cawich et al8 recently reported that transanal extraction in the ED failed in 89% of cases. Additionally, these researchers reported that in 63% of the failed extractions, the objects were inadvertently pushed higher into the rectosigmoid region, and therefore recommended early mobilization of the OR team so that exploration under anesthesia can be performed under optimal conditions.8
Once the foreign body is successfully removed, follow-up imaging or postextraction endoscopy is warranted. Close observation in the hospital is recommended to facilitate serial abdominal examination.7
Urethral Insertions
Sexual exploration, efforts at contraception, transport of illicit drugs, assault or sexual violence, and accidental insertion have all been described as reasons for genitourinary (GU) insertion.1 The motives, however, mirror those who insert foreign bodies rectally.
Most presentations are due to pain or inability to void. Aggressive treatment should be undertaken because even when the penis appears dark or necrotic, salvage rates have been high. Complications include urinary tract infections, hematuria, urinary retention, urethral tears, abscess, ascending GU infections, and diverticula and fistula formations.1,3 In women, vaginal insertions can lead to pelvic pain and septic shock.1 Foreign bodies can also lead to a condition first described in the ancient literature as strangury—the process of slow and painful discharge of urine due to a significant inflammatory component or stricture. The term strangury has been replaced with the more general term bladder spasms.9
Treatment. Removal of urethral foreign bodies typically is done in conjunction with a urologist. A cystoscopic procedure is usually successful in removing the foreign body and is an effective method to minimize urethral and bladder injuries. However, more invasive surgical options, including perineal urethrotomy, suprapubic cystostomy, cystolithotomy, and external urethrotomy, have been used in more complicated cases or when the foreign body prevents urethral access of an endoscopic instrument.10
Patient and Staff Reactions
When patients realize they are unable to remove the inserted object, some present immediately to the ED for evaluation. Interestingly, others may wait up to 2 weeks after insertion before seeking help.2 Patients report feelings of being ashamed and report a feeling of being despised, frowned upon, and being talked about during the course of their ED evaluation. As a consequence, these patients may not readily come to the ED or if they do come, may not be open to conversation and hide the true reason of why they came in the first place.1,4 The amplified paranoia and perceived prejudice may delay diagnosis and lifesaving measures, or worse, lead patients to leave prior to a medical screening examination. Therefore, creating a nonjudgmental environment is essential, even when the presenting story appears to be fabricated.2
Once the patient’s foreign body is removed, and complications are excluded or properly managed, the goal is to understand the motivation behind the insertion, mitigate the consequences of the behavior, and prevent future recurrence. A psychiatric evaluation should be obtained in the ED, or if the patient is admitted, during hospitalization. Psychiatric behavior leading to insertions can be unmasked, treated, and harm-reduction strategies can be taught and instituted.1,3
Experienced ED staff members are used to the unpredictability of human behavior. However, patients who present with foreign body insertions can elicit a mixture of responses, ranging from awe and incredulousness to anger and frustration. It is not unusual for staff members to not understand or recognize their own reactions. The unique nature of the presentation, along with the astonishing radiographic images, can lead to a breach of privacy and dissemination of the digital photographs by cell phones and into social media sites.1 Staff members should be encouraged to foster open-mindedness and indifference. Ensuring privacy, professionalism, and empathy can go a long way to helping these patients. Moreover, ED staff members should be educated about countertransference reactions,1 as these actions are necessary to ensure the singular purpose of optimum patient-staff relationship.
Conclusion
Patients with foreign body insertions challenge the ED staff, as the presenting complaint not only tests the collective technical know-how of the staff, but also their emotional competencies. A nonjudgmental and open-minded approach is crucial, with the tone set during triage. Coordination with surgical specialties should be done early to ensure safe removal and to identify and manage complications. Psychiatric evaluation should be strongly considered prior to disposition in an attempt to prevent future recurrences.
1. Unruh BT, Nejad SH, Stern TW, Stern TA. Insertion of foreign bodies (polyembolokoilamania): underpinnings and management strategies. Prim Care Companion CNS Disord. 2012;14(1). doi:10.4088/PCC.11f01192.
2. Cologne KG, Ault GT. Rectal foreign bodies: what is the current standard? Clin Colon Rectal Surg. 2012;25(4):214-218. doi:10.1055/s-0032-1329392.3. Naidu K, Chung A, Mulcahy M. An unusual urethral foreign body. Int J Surg Case Rep. 2013;4(11):1052-1054. doi:10.1016/j.ijscr.2013.07.017.4. Rahman NU, Elliott SP, McAninch JW. Self-inflicted male urethral foreign body insertion: endoscopic management and complications. BJU Int. 2004;94(7):1051-1053. 10.1111/j.1464-410X.2004.05103.x.
5. SaiSwaroop Y, Darakh P, Amlani D. Self insertion of urethral foreign body in a child: a rare case report. Int J Curr Med Appl Sci. 2015;9(1):22-24.
6. Smiley O. A glass tumbler in the rectum. JAMA. 1919;72:1285.
7. Coskun A, Erkan N, Yakan S, Yıldirim M, Cengiz F. Management of rectal foreign bodies. World J Emerg Surg. 2013;8(1):11. doi:10.1186/1749-7922-8-11.
8. Cawich SO, Thomas DA, Mohammed F, Bobb NJ, Williams D, Naraynsingh V. A management algorithm for retained rectal foreign bodies. Am J Mens Health. 2017;11(3):684-692. doi:10.1177/1557988316680929.
9. Wright B, Husbands E. Strangury: the case of a symptom with ancient origins. BMJ Support Palliat Care. 2011;1(1):49-50. doi:10.1136/bmjspcare-2011-000030.
10. Moon SJ, Kim DH, Chung JH, et al. Unusual foreign bodies in the urinary bladder and urethra due to autoerotism. Int Neurourol J. 2010;14(3):186-189. doi:10.5213/inj.2010.14.3.186.
Anorectal and urethral foreign body insertions (polyembolokoilamania) are not infrequent presentations to the ED. The motivations behind these insertions vary, ranging from autoeroticism to reckless behavior. These insertions can lead to major complications and even death. Though ED staff members are used to the unpredictability of human behavior, foreign body insertions bring a mixture of responses from the staff, ranging from awe and incredulousness to anger and frustration. A knowledge and comfort in managing these cases includes a nonjudgmental triage assessment, collective professionalism, and self-awareness of the staff’s reaction.
Case 1
A 58-year-old man presented to the ED for evaluation of a foreign body in his rectum. He admitted to placing a beer bottle in his rectum, but was unable to remove it at home. The staff reported that the patient was previously seen in the ED for removal of a vibrator from his rectum.
Radiographic evaluation in the form of an acute abdominal series was obtained and confirmed a beer bottle in the rectum (Figures 1 and 2).
Case 2
A 55-year-old man presented to the ED after he inserted a pen cap into his urethra to aid in obtaining an erection. A pelvic X-ray was obtained and showed a radiolucent structure in the penis (Figure 3).
The patient was admitted to the hospital and taken to the OR by the consulting urologist. Using a rigid cystoscope and flexible graspers, the pen cap was removed from the proximal urethra under monitored anesthesia control. The procedure went without any complications.
A psychiatrist was consulted, and during the encounter, the patient admitted that his behavior was pathological. He revealed that he was a victim of child abuse and reported he had been having mixed emotions of anxiety, guilt, and embarrassment because of his behavior.
Discussion
Foreign body insertions are seen in patients with a wide variety of backgrounds, ages, and lifestyles. Approximately 80,000 cases of foreign body ingestion are seen annually in children under age 20 years. Young males have a higher predilection of swallowing foreign bodies when compared to young females,1 and rectal foreign body insertions are seen more commonly in males than in females.2 In this age group, intentional foreign body insertion may be an initial manifestation of psychiatric illness.
Rectal Insertions
The earliest published report of a rectal foreign body insertion was in 1919 by Smiley.6 The typical age at presentation ranges from 20 to 90 years old, with a mean age of 44 years old.2 Household objects such as bottles and glasses are the most commonly seen, but a long list of other items have also been reported in the literature, including toothbrushes, knives, deodorant bottles, food articles, sports equipment, cell phones, flashlights, wooden rods, broomsticks, sex toys, light bulbs, construction tools, nails, ornaments, aerosol canisters, cocaine packets, jewelry, batteries, guitar picks, and many other items.1,2,7
In nearly half of the reported cases, the reasons for rectal insertion was for sexual arousal/stimulation.1,7 Other reasons include nonsuicidal injurious behavior (eg, borderline personality disorder); suicide attempt; psychosis; depression; factitious disorder; malingering; cognitive disorders, including dementia and delirium; treatment of constipation and hemorrhoids; concealment; attention-seeking behavior; “accidental”; assault; and the consequences of drunken wagers.1,2 Additionally, abuse should be considered, especially in patients with developmental delay and/or psychiatric illness.
Close to 20% of all traumatic rectal injuries are due to foreign body insertions. In most cases, foreign bodies fail to cause significant anorectal injuries. Complications, however, can result from the process of insertion, removal, or from the contents introduced into the orifice.1 Any rectal examination should be preceded by an anatomical survey utilizing radiographic modalities to evaluate the integrity and orientation of the object in question. Any sharp object can injure the examining physician if this is not done prior. All examinations should be chaperoned.2,7 The most obvious and dangerous complication is perforation, and the patient’s care should proceed in the same manner as any other trauma patient. Additionally, resulting sepsis should be managed with the same standards as any other septic patient.7
Treatment. The method of object removal is determined by the presence or absence of a surgical abdomen and the need for general anesthesia. The location and shape of the object, however, may not equate with successful retrieval. Objects placed in the sigmoid colon are more than twice as likely to require surgical intervention compared to items placed distally.2 Once it is determined that the patient is clinically stable and does not have an acute abdomen, attempts in removing the rectal foreign object can be done in the ED or, if anesthesia is needed, in the OR. Any attempts at transanal removal require optimal patient relaxation, which can be achieved via procedural sedation. The patient should be placed in a lithotomy or left lateral decubitus position to allow palpation of the object in the lower gastrointestinal tract. From here, several methods of removal can be employed. Blunt objects can be grasped and removed by a gloved hand or with a clamp. A Foley catheter can also be passed alongside the object and the balloon inflated above the foreign body to aid in extraction as the Foley is pulled out slowly. Sengstaken-Blakemore tubes, obstetric forceps, and vacuum extractors have also been utilized.7
While bedside extraction is advocated by many authors, Cawich et al8 recently reported that transanal extraction in the ED failed in 89% of cases. Additionally, these researchers reported that in 63% of the failed extractions, the objects were inadvertently pushed higher into the rectosigmoid region, and therefore recommended early mobilization of the OR team so that exploration under anesthesia can be performed under optimal conditions.8
Once the foreign body is successfully removed, follow-up imaging or postextraction endoscopy is warranted. Close observation in the hospital is recommended to facilitate serial abdominal examination.7
Urethral Insertions
Sexual exploration, efforts at contraception, transport of illicit drugs, assault or sexual violence, and accidental insertion have all been described as reasons for genitourinary (GU) insertion.1 The motives, however, mirror those who insert foreign bodies rectally.
Most presentations are due to pain or inability to void. Aggressive treatment should be undertaken because even when the penis appears dark or necrotic, salvage rates have been high. Complications include urinary tract infections, hematuria, urinary retention, urethral tears, abscess, ascending GU infections, and diverticula and fistula formations.1,3 In women, vaginal insertions can lead to pelvic pain and septic shock.1 Foreign bodies can also lead to a condition first described in the ancient literature as strangury—the process of slow and painful discharge of urine due to a significant inflammatory component or stricture. The term strangury has been replaced with the more general term bladder spasms.9
Treatment. Removal of urethral foreign bodies typically is done in conjunction with a urologist. A cystoscopic procedure is usually successful in removing the foreign body and is an effective method to minimize urethral and bladder injuries. However, more invasive surgical options, including perineal urethrotomy, suprapubic cystostomy, cystolithotomy, and external urethrotomy, have been used in more complicated cases or when the foreign body prevents urethral access of an endoscopic instrument.10
Patient and Staff Reactions
When patients realize they are unable to remove the inserted object, some present immediately to the ED for evaluation. Interestingly, others may wait up to 2 weeks after insertion before seeking help.2 Patients report feelings of being ashamed and report a feeling of being despised, frowned upon, and being talked about during the course of their ED evaluation. As a consequence, these patients may not readily come to the ED or if they do come, may not be open to conversation and hide the true reason of why they came in the first place.1,4 The amplified paranoia and perceived prejudice may delay diagnosis and lifesaving measures, or worse, lead patients to leave prior to a medical screening examination. Therefore, creating a nonjudgmental environment is essential, even when the presenting story appears to be fabricated.2
Once the patient’s foreign body is removed, and complications are excluded or properly managed, the goal is to understand the motivation behind the insertion, mitigate the consequences of the behavior, and prevent future recurrence. A psychiatric evaluation should be obtained in the ED, or if the patient is admitted, during hospitalization. Psychiatric behavior leading to insertions can be unmasked, treated, and harm-reduction strategies can be taught and instituted.1,3
Experienced ED staff members are used to the unpredictability of human behavior. However, patients who present with foreign body insertions can elicit a mixture of responses, ranging from awe and incredulousness to anger and frustration. It is not unusual for staff members to not understand or recognize their own reactions. The unique nature of the presentation, along with the astonishing radiographic images, can lead to a breach of privacy and dissemination of the digital photographs by cell phones and into social media sites.1 Staff members should be encouraged to foster open-mindedness and indifference. Ensuring privacy, professionalism, and empathy can go a long way to helping these patients. Moreover, ED staff members should be educated about countertransference reactions,1 as these actions are necessary to ensure the singular purpose of optimum patient-staff relationship.
Conclusion
Patients with foreign body insertions challenge the ED staff, as the presenting complaint not only tests the collective technical know-how of the staff, but also their emotional competencies. A nonjudgmental and open-minded approach is crucial, with the tone set during triage. Coordination with surgical specialties should be done early to ensure safe removal and to identify and manage complications. Psychiatric evaluation should be strongly considered prior to disposition in an attempt to prevent future recurrences.
Anorectal and urethral foreign body insertions (polyembolokoilamania) are not infrequent presentations to the ED. The motivations behind these insertions vary, ranging from autoeroticism to reckless behavior. These insertions can lead to major complications and even death. Though ED staff members are used to the unpredictability of human behavior, foreign body insertions bring a mixture of responses from the staff, ranging from awe and incredulousness to anger and frustration. A knowledge and comfort in managing these cases includes a nonjudgmental triage assessment, collective professionalism, and self-awareness of the staff’s reaction.
Case 1
A 58-year-old man presented to the ED for evaluation of a foreign body in his rectum. He admitted to placing a beer bottle in his rectum, but was unable to remove it at home. The staff reported that the patient was previously seen in the ED for removal of a vibrator from his rectum.
Radiographic evaluation in the form of an acute abdominal series was obtained and confirmed a beer bottle in the rectum (Figures 1 and 2).
Case 2
A 55-year-old man presented to the ED after he inserted a pen cap into his urethra to aid in obtaining an erection. A pelvic X-ray was obtained and showed a radiolucent structure in the penis (Figure 3).
The patient was admitted to the hospital and taken to the OR by the consulting urologist. Using a rigid cystoscope and flexible graspers, the pen cap was removed from the proximal urethra under monitored anesthesia control. The procedure went without any complications.
A psychiatrist was consulted, and during the encounter, the patient admitted that his behavior was pathological. He revealed that he was a victim of child abuse and reported he had been having mixed emotions of anxiety, guilt, and embarrassment because of his behavior.
Discussion
Foreign body insertions are seen in patients with a wide variety of backgrounds, ages, and lifestyles. Approximately 80,000 cases of foreign body ingestion are seen annually in children under age 20 years. Young males have a higher predilection of swallowing foreign bodies when compared to young females,1 and rectal foreign body insertions are seen more commonly in males than in females.2 In this age group, intentional foreign body insertion may be an initial manifestation of psychiatric illness.
Rectal Insertions
The earliest published report of a rectal foreign body insertion was in 1919 by Smiley.6 The typical age at presentation ranges from 20 to 90 years old, with a mean age of 44 years old.2 Household objects such as bottles and glasses are the most commonly seen, but a long list of other items have also been reported in the literature, including toothbrushes, knives, deodorant bottles, food articles, sports equipment, cell phones, flashlights, wooden rods, broomsticks, sex toys, light bulbs, construction tools, nails, ornaments, aerosol canisters, cocaine packets, jewelry, batteries, guitar picks, and many other items.1,2,7
In nearly half of the reported cases, the reasons for rectal insertion was for sexual arousal/stimulation.1,7 Other reasons include nonsuicidal injurious behavior (eg, borderline personality disorder); suicide attempt; psychosis; depression; factitious disorder; malingering; cognitive disorders, including dementia and delirium; treatment of constipation and hemorrhoids; concealment; attention-seeking behavior; “accidental”; assault; and the consequences of drunken wagers.1,2 Additionally, abuse should be considered, especially in patients with developmental delay and/or psychiatric illness.
Close to 20% of all traumatic rectal injuries are due to foreign body insertions. In most cases, foreign bodies fail to cause significant anorectal injuries. Complications, however, can result from the process of insertion, removal, or from the contents introduced into the orifice.1 Any rectal examination should be preceded by an anatomical survey utilizing radiographic modalities to evaluate the integrity and orientation of the object in question. Any sharp object can injure the examining physician if this is not done prior. All examinations should be chaperoned.2,7 The most obvious and dangerous complication is perforation, and the patient’s care should proceed in the same manner as any other trauma patient. Additionally, resulting sepsis should be managed with the same standards as any other septic patient.7
Treatment. The method of object removal is determined by the presence or absence of a surgical abdomen and the need for general anesthesia. The location and shape of the object, however, may not equate with successful retrieval. Objects placed in the sigmoid colon are more than twice as likely to require surgical intervention compared to items placed distally.2 Once it is determined that the patient is clinically stable and does not have an acute abdomen, attempts in removing the rectal foreign object can be done in the ED or, if anesthesia is needed, in the OR. Any attempts at transanal removal require optimal patient relaxation, which can be achieved via procedural sedation. The patient should be placed in a lithotomy or left lateral decubitus position to allow palpation of the object in the lower gastrointestinal tract. From here, several methods of removal can be employed. Blunt objects can be grasped and removed by a gloved hand or with a clamp. A Foley catheter can also be passed alongside the object and the balloon inflated above the foreign body to aid in extraction as the Foley is pulled out slowly. Sengstaken-Blakemore tubes, obstetric forceps, and vacuum extractors have also been utilized.7
While bedside extraction is advocated by many authors, Cawich et al8 recently reported that transanal extraction in the ED failed in 89% of cases. Additionally, these researchers reported that in 63% of the failed extractions, the objects were inadvertently pushed higher into the rectosigmoid region, and therefore recommended early mobilization of the OR team so that exploration under anesthesia can be performed under optimal conditions.8
Once the foreign body is successfully removed, follow-up imaging or postextraction endoscopy is warranted. Close observation in the hospital is recommended to facilitate serial abdominal examination.7
Urethral Insertions
Sexual exploration, efforts at contraception, transport of illicit drugs, assault or sexual violence, and accidental insertion have all been described as reasons for genitourinary (GU) insertion.1 The motives, however, mirror those who insert foreign bodies rectally.
Most presentations are due to pain or inability to void. Aggressive treatment should be undertaken because even when the penis appears dark or necrotic, salvage rates have been high. Complications include urinary tract infections, hematuria, urinary retention, urethral tears, abscess, ascending GU infections, and diverticula and fistula formations.1,3 In women, vaginal insertions can lead to pelvic pain and septic shock.1 Foreign bodies can also lead to a condition first described in the ancient literature as strangury—the process of slow and painful discharge of urine due to a significant inflammatory component or stricture. The term strangury has been replaced with the more general term bladder spasms.9
Treatment. Removal of urethral foreign bodies typically is done in conjunction with a urologist. A cystoscopic procedure is usually successful in removing the foreign body and is an effective method to minimize urethral and bladder injuries. However, more invasive surgical options, including perineal urethrotomy, suprapubic cystostomy, cystolithotomy, and external urethrotomy, have been used in more complicated cases or when the foreign body prevents urethral access of an endoscopic instrument.10
Patient and Staff Reactions
When patients realize they are unable to remove the inserted object, some present immediately to the ED for evaluation. Interestingly, others may wait up to 2 weeks after insertion before seeking help.2 Patients report feelings of being ashamed and report a feeling of being despised, frowned upon, and being talked about during the course of their ED evaluation. As a consequence, these patients may not readily come to the ED or if they do come, may not be open to conversation and hide the true reason of why they came in the first place.1,4 The amplified paranoia and perceived prejudice may delay diagnosis and lifesaving measures, or worse, lead patients to leave prior to a medical screening examination. Therefore, creating a nonjudgmental environment is essential, even when the presenting story appears to be fabricated.2
Once the patient’s foreign body is removed, and complications are excluded or properly managed, the goal is to understand the motivation behind the insertion, mitigate the consequences of the behavior, and prevent future recurrence. A psychiatric evaluation should be obtained in the ED, or if the patient is admitted, during hospitalization. Psychiatric behavior leading to insertions can be unmasked, treated, and harm-reduction strategies can be taught and instituted.1,3
Experienced ED staff members are used to the unpredictability of human behavior. However, patients who present with foreign body insertions can elicit a mixture of responses, ranging from awe and incredulousness to anger and frustration. It is not unusual for staff members to not understand or recognize their own reactions. The unique nature of the presentation, along with the astonishing radiographic images, can lead to a breach of privacy and dissemination of the digital photographs by cell phones and into social media sites.1 Staff members should be encouraged to foster open-mindedness and indifference. Ensuring privacy, professionalism, and empathy can go a long way to helping these patients. Moreover, ED staff members should be educated about countertransference reactions,1 as these actions are necessary to ensure the singular purpose of optimum patient-staff relationship.
Conclusion
Patients with foreign body insertions challenge the ED staff, as the presenting complaint not only tests the collective technical know-how of the staff, but also their emotional competencies. A nonjudgmental and open-minded approach is crucial, with the tone set during triage. Coordination with surgical specialties should be done early to ensure safe removal and to identify and manage complications. Psychiatric evaluation should be strongly considered prior to disposition in an attempt to prevent future recurrences.
1. Unruh BT, Nejad SH, Stern TW, Stern TA. Insertion of foreign bodies (polyembolokoilamania): underpinnings and management strategies. Prim Care Companion CNS Disord. 2012;14(1). doi:10.4088/PCC.11f01192.
2. Cologne KG, Ault GT. Rectal foreign bodies: what is the current standard? Clin Colon Rectal Surg. 2012;25(4):214-218. doi:10.1055/s-0032-1329392.3. Naidu K, Chung A, Mulcahy M. An unusual urethral foreign body. Int J Surg Case Rep. 2013;4(11):1052-1054. doi:10.1016/j.ijscr.2013.07.017.4. Rahman NU, Elliott SP, McAninch JW. Self-inflicted male urethral foreign body insertion: endoscopic management and complications. BJU Int. 2004;94(7):1051-1053. 10.1111/j.1464-410X.2004.05103.x.
5. SaiSwaroop Y, Darakh P, Amlani D. Self insertion of urethral foreign body in a child: a rare case report. Int J Curr Med Appl Sci. 2015;9(1):22-24.
6. Smiley O. A glass tumbler in the rectum. JAMA. 1919;72:1285.
7. Coskun A, Erkan N, Yakan S, Yıldirim M, Cengiz F. Management of rectal foreign bodies. World J Emerg Surg. 2013;8(1):11. doi:10.1186/1749-7922-8-11.
8. Cawich SO, Thomas DA, Mohammed F, Bobb NJ, Williams D, Naraynsingh V. A management algorithm for retained rectal foreign bodies. Am J Mens Health. 2017;11(3):684-692. doi:10.1177/1557988316680929.
9. Wright B, Husbands E. Strangury: the case of a symptom with ancient origins. BMJ Support Palliat Care. 2011;1(1):49-50. doi:10.1136/bmjspcare-2011-000030.
10. Moon SJ, Kim DH, Chung JH, et al. Unusual foreign bodies in the urinary bladder and urethra due to autoerotism. Int Neurourol J. 2010;14(3):186-189. doi:10.5213/inj.2010.14.3.186.
1. Unruh BT, Nejad SH, Stern TW, Stern TA. Insertion of foreign bodies (polyembolokoilamania): underpinnings and management strategies. Prim Care Companion CNS Disord. 2012;14(1). doi:10.4088/PCC.11f01192.
2. Cologne KG, Ault GT. Rectal foreign bodies: what is the current standard? Clin Colon Rectal Surg. 2012;25(4):214-218. doi:10.1055/s-0032-1329392.3. Naidu K, Chung A, Mulcahy M. An unusual urethral foreign body. Int J Surg Case Rep. 2013;4(11):1052-1054. doi:10.1016/j.ijscr.2013.07.017.4. Rahman NU, Elliott SP, McAninch JW. Self-inflicted male urethral foreign body insertion: endoscopic management and complications. BJU Int. 2004;94(7):1051-1053. 10.1111/j.1464-410X.2004.05103.x.
5. SaiSwaroop Y, Darakh P, Amlani D. Self insertion of urethral foreign body in a child: a rare case report. Int J Curr Med Appl Sci. 2015;9(1):22-24.
6. Smiley O. A glass tumbler in the rectum. JAMA. 1919;72:1285.
7. Coskun A, Erkan N, Yakan S, Yıldirim M, Cengiz F. Management of rectal foreign bodies. World J Emerg Surg. 2013;8(1):11. doi:10.1186/1749-7922-8-11.
8. Cawich SO, Thomas DA, Mohammed F, Bobb NJ, Williams D, Naraynsingh V. A management algorithm for retained rectal foreign bodies. Am J Mens Health. 2017;11(3):684-692. doi:10.1177/1557988316680929.
9. Wright B, Husbands E. Strangury: the case of a symptom with ancient origins. BMJ Support Palliat Care. 2011;1(1):49-50. doi:10.1136/bmjspcare-2011-000030.
10. Moon SJ, Kim DH, Chung JH, et al. Unusual foreign bodies in the urinary bladder and urethra due to autoerotism. Int Neurourol J. 2010;14(3):186-189. doi:10.5213/inj.2010.14.3.186.
Acute Submandibular Sialadenitis
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.