User login
Article Type
Changed
Display Headline
New and Emerging Treatments for MASLD/MASH
References
- Hu Y, Sun C, Chen Y, Liu Y-D, Fan J-G. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. J Clin Transl Hepatol. 2024;12(9):802-814. doi:10.14218/JCTH.2024.00123
- Petta S, Targher G, Romeo S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024;44(7):1526-1536. doi:10.14218/JCTH.2024.00123doi:10.1111/liv.15930
- Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterol Rep (Oxf). 2024;12:goae029. doi:10.1093/gastro/goae029
- Chen VL, Morgan TR, Rotman Y, et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology. 2025;81(1):312-320. doi:10.1097/HEP.0000000000001112
- Economist Impact 2024. MASLD/MASH in the US: A liver disease country profile. Published 2024. Accessed January 22, 2025. https://impact.economist.com/perspectives/sites/default/files/download/liver-disease-country-profile_united_states_final.pdf
- Tincopa MA, Anstee QM, Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36(5):912-926. doi:10.1016/j.cmet.2024.03.011
- Carpi S, Daniele S, de Almeida JFM, Gabbia D. Recent Advances in miRNA-Based Therapy for MASLD/MASH and MASH-Associated HCC. Int J Mol Sci. 2024;25(22):12229. https://www.mdpi.com/1422-0067/25/22/1222
- Wong RJ. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab Target Organ Damage. 2024;4:35. http://dx.doi.org/10.20517/mtod.2024.57
- Younossi ZM, Kalligeros M, Henry L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin Mol Hepatol. 2024. doi:10.3350/cmh.2024.0431
- Jozst L. Estimating the True Prevalence of MASH and MASLD in the US. AJMC. Published October 17, 2024. Accessed January 22, 2025. https://www.ajmc.com/view/estimating-the-true-prevalence-of-mash-and-masld-in-the-us
- Mayo Clinic website. Pediatric metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD). Published October 4, 2023. Accessed January 22, 2025. https://www.mayoclinic.org/medical-professionals/pediatrics/news/pediatric-metabolic-dysfunction-associated-steatotic-liver-disease-masld-formerly-known-as-nonalcoholic-fatty-liver-disease-nafld/mac-20555493
- Younossi ZM. Economic burden of MASLD/MASH. Conference report for NATAP. EASL 2024. Published June 5-8, 2024. Accessed January 22, 2025. https://www.natap.org/2024/EASL/EASL_41.htm
- Loomba R, Noureddin M, Kowdley KV, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol. 202;73(2):625-643. doi:10.1002/hep.31622
- Nicastro E. D’Antiga L. Nutritional Interventions, Probiotics, Synbiotics and Fecal Microbiota Transplantation in Steatoic Liver Disease. Advances in experimental medicine and Biology. Published online January 1. 202:113-133. doi:https://doi.org.10.1007/978-3-031-58572-2_7
- Shera S, Katzka W, Yang JC, et al. Bariatric-induced microbiome changes alter MASLD development in association with changes in the innate immune system. Front Microbiol. 2024;15:1407555. doi:10.3389/fmicb.2024.1407555
- Globe Newswire website. Akero Therapeutics Reports Second Quarter 2024 Financial Results and Provides Business Update [press release]. Published August 9, 2024. Accessed January 22, 2025. https://www.globenewswire.com/en/news-release/2024/08/09/2927685/0/en/Akero-Therapeutics-Reports-Second-Quarter-2024-Financial-Results-and-Provides-Business-Update.html
- Akero website. Clinical Trials Overview. We are currently enrolling three clinical trials as part of a Phase 3 SYNCHRONY program evaluating EFX for the treatment of pre-cirrhotic MASH (F2-F3) and compensated cirrhosis (F4) due to MASH [press release]. Published 2024. Accessed January 22, 2025. https://akerotx.com/clinical-trials/
- 89bio website. 89bio Initiates Phase 3 ENLIGHTEN-Fibrosis Trial of Pegozafermin in Non-Cirrhotic Metabolic Dysfunction-Associated Steatohepatitis (MASH) Patients with Fibrosis [press release]. Published March 12, 2024. Accessed January 22, 2025. https://www.89bio.com/news/89bio-initiates-phase-3-enlighten-fibrosis-trial-of-pegozafermin-in-non-cirrhotic-metabolic-dysfunction-associated-steatohepatitis-mash-patients-with-fibrosis/
- 89bio website. 89bio Reaches Alignment with the FDA and EMA on Phase 3 Program for Pegozafermin in Nonalcoholic Steatohepatitis (NASH); Program Initiation Planned in the First Half of 2024 [press release]. Published December 4, 2023. Accessed January 22, 2025. https://www.89bio.com/news/89bio-reaches-alignment-with-the-fda-and-ema-on-phase-3-program-for-pegozafermin-in-nonalcoholic-steatohepatitis-nash-program-initiation-planned-in-the-first-half-of-2024/
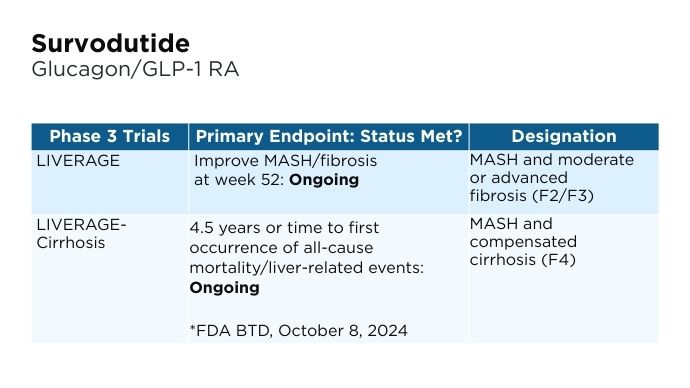
- Boehringer Ingelheim website. Boehringer receives U.S. FDA Breakthrough Therapy designation and initiates two phase III trials in MASH for survodutide [press release]. Published October 8, 2024. Accessed January 22, 2025. https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash
Publications
Topics
References
- Hu Y, Sun C, Chen Y, Liu Y-D, Fan J-G. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. J Clin Transl Hepatol. 2024;12(9):802-814. doi:10.14218/JCTH.2024.00123
- Petta S, Targher G, Romeo S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024;44(7):1526-1536. doi:10.14218/JCTH.2024.00123doi:10.1111/liv.15930
- Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterol Rep (Oxf). 2024;12:goae029. doi:10.1093/gastro/goae029
- Chen VL, Morgan TR, Rotman Y, et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology. 2025;81(1):312-320. doi:10.1097/HEP.0000000000001112
- Economist Impact 2024. MASLD/MASH in the US: A liver disease country profile. Published 2024. Accessed January 22, 2025. https://impact.economist.com/perspectives/sites/default/files/download/liver-disease-country-profile_united_states_final.pdf
- Tincopa MA, Anstee QM, Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36(5):912-926. doi:10.1016/j.cmet.2024.03.011
- Carpi S, Daniele S, de Almeida JFM, Gabbia D. Recent Advances in miRNA-Based Therapy for MASLD/MASH and MASH-Associated HCC. Int J Mol Sci. 2024;25(22):12229. https://www.mdpi.com/1422-0067/25/22/1222
- Wong RJ. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab Target Organ Damage. 2024;4:35. http://dx.doi.org/10.20517/mtod.2024.57
- Younossi ZM, Kalligeros M, Henry L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin Mol Hepatol. 2024. doi:10.3350/cmh.2024.0431
- Jozst L. Estimating the True Prevalence of MASH and MASLD in the US. AJMC. Published October 17, 2024. Accessed January 22, 2025. https://www.ajmc.com/view/estimating-the-true-prevalence-of-mash-and-masld-in-the-us
- Mayo Clinic website. Pediatric metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD). Published October 4, 2023. Accessed January 22, 2025. https://www.mayoclinic.org/medical-professionals/pediatrics/news/pediatric-metabolic-dysfunction-associated-steatotic-liver-disease-masld-formerly-known-as-nonalcoholic-fatty-liver-disease-nafld/mac-20555493
- Younossi ZM. Economic burden of MASLD/MASH. Conference report for NATAP. EASL 2024. Published June 5-8, 2024. Accessed January 22, 2025. https://www.natap.org/2024/EASL/EASL_41.htm
- Loomba R, Noureddin M, Kowdley KV, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol. 202;73(2):625-643. doi:10.1002/hep.31622
- Nicastro E. D’Antiga L. Nutritional Interventions, Probiotics, Synbiotics and Fecal Microbiota Transplantation in Steatoic Liver Disease. Advances in experimental medicine and Biology. Published online January 1. 202:113-133. doi:https://doi.org.10.1007/978-3-031-58572-2_7
- Shera S, Katzka W, Yang JC, et al. Bariatric-induced microbiome changes alter MASLD development in association with changes in the innate immune system. Front Microbiol. 2024;15:1407555. doi:10.3389/fmicb.2024.1407555
- Globe Newswire website. Akero Therapeutics Reports Second Quarter 2024 Financial Results and Provides Business Update [press release]. Published August 9, 2024. Accessed January 22, 2025. https://www.globenewswire.com/en/news-release/2024/08/09/2927685/0/en/Akero-Therapeutics-Reports-Second-Quarter-2024-Financial-Results-and-Provides-Business-Update.html
- Akero website. Clinical Trials Overview. We are currently enrolling three clinical trials as part of a Phase 3 SYNCHRONY program evaluating EFX for the treatment of pre-cirrhotic MASH (F2-F3) and compensated cirrhosis (F4) due to MASH [press release]. Published 2024. Accessed January 22, 2025. https://akerotx.com/clinical-trials/
- 89bio website. 89bio Initiates Phase 3 ENLIGHTEN-Fibrosis Trial of Pegozafermin in Non-Cirrhotic Metabolic Dysfunction-Associated Steatohepatitis (MASH) Patients with Fibrosis [press release]. Published March 12, 2024. Accessed January 22, 2025. https://www.89bio.com/news/89bio-initiates-phase-3-enlighten-fibrosis-trial-of-pegozafermin-in-non-cirrhotic-metabolic-dysfunction-associated-steatohepatitis-mash-patients-with-fibrosis/
- 89bio website. 89bio Reaches Alignment with the FDA and EMA on Phase 3 Program for Pegozafermin in Nonalcoholic Steatohepatitis (NASH); Program Initiation Planned in the First Half of 2024 [press release]. Published December 4, 2023. Accessed January 22, 2025. https://www.89bio.com/news/89bio-reaches-alignment-with-the-fda-and-ema-on-phase-3-program-for-pegozafermin-in-nonalcoholic-steatohepatitis-nash-program-initiation-planned-in-the-first-half-of-2024/
- Boehringer Ingelheim website. Boehringer receives U.S. FDA Breakthrough Therapy designation and initiates two phase III trials in MASH for survodutide [press release]. Published October 8, 2024. Accessed January 22, 2025. https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash
References
- Hu Y, Sun C, Chen Y, Liu Y-D, Fan J-G. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. J Clin Transl Hepatol. 2024;12(9):802-814. doi:10.14218/JCTH.2024.00123
- Petta S, Targher G, Romeo S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024;44(7):1526-1536. doi:10.14218/JCTH.2024.00123doi:10.1111/liv.15930
- Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterol Rep (Oxf). 2024;12:goae029. doi:10.1093/gastro/goae029
- Chen VL, Morgan TR, Rotman Y, et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology. 2025;81(1):312-320. doi:10.1097/HEP.0000000000001112
- Economist Impact 2024. MASLD/MASH in the US: A liver disease country profile. Published 2024. Accessed January 22, 2025. https://impact.economist.com/perspectives/sites/default/files/download/liver-disease-country-profile_united_states_final.pdf
- Tincopa MA, Anstee QM, Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36(5):912-926. doi:10.1016/j.cmet.2024.03.011
- Carpi S, Daniele S, de Almeida JFM, Gabbia D. Recent Advances in miRNA-Based Therapy for MASLD/MASH and MASH-Associated HCC. Int J Mol Sci. 2024;25(22):12229. https://www.mdpi.com/1422-0067/25/22/1222
- Wong RJ. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab Target Organ Damage. 2024;4:35. http://dx.doi.org/10.20517/mtod.2024.57
- Younossi ZM, Kalligeros M, Henry L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin Mol Hepatol. 2024. doi:10.3350/cmh.2024.0431
- Jozst L. Estimating the True Prevalence of MASH and MASLD in the US. AJMC. Published October 17, 2024. Accessed January 22, 2025. https://www.ajmc.com/view/estimating-the-true-prevalence-of-mash-and-masld-in-the-us
- Mayo Clinic website. Pediatric metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD). Published October 4, 2023. Accessed January 22, 2025. https://www.mayoclinic.org/medical-professionals/pediatrics/news/pediatric-metabolic-dysfunction-associated-steatotic-liver-disease-masld-formerly-known-as-nonalcoholic-fatty-liver-disease-nafld/mac-20555493
- Younossi ZM. Economic burden of MASLD/MASH. Conference report for NATAP. EASL 2024. Published June 5-8, 2024. Accessed January 22, 2025. https://www.natap.org/2024/EASL/EASL_41.htm
- Loomba R, Noureddin M, Kowdley KV, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol. 202;73(2):625-643. doi:10.1002/hep.31622
- Nicastro E. D’Antiga L. Nutritional Interventions, Probiotics, Synbiotics and Fecal Microbiota Transplantation in Steatoic Liver Disease. Advances in experimental medicine and Biology. Published online January 1. 202:113-133. doi:https://doi.org.10.1007/978-3-031-58572-2_7
- Shera S, Katzka W, Yang JC, et al. Bariatric-induced microbiome changes alter MASLD development in association with changes in the innate immune system. Front Microbiol. 2024;15:1407555. doi:10.3389/fmicb.2024.1407555
- Globe Newswire website. Akero Therapeutics Reports Second Quarter 2024 Financial Results and Provides Business Update [press release]. Published August 9, 2024. Accessed January 22, 2025. https://www.globenewswire.com/en/news-release/2024/08/09/2927685/0/en/Akero-Therapeutics-Reports-Second-Quarter-2024-Financial-Results-and-Provides-Business-Update.html
- Akero website. Clinical Trials Overview. We are currently enrolling three clinical trials as part of a Phase 3 SYNCHRONY program evaluating EFX for the treatment of pre-cirrhotic MASH (F2-F3) and compensated cirrhosis (F4) due to MASH [press release]. Published 2024. Accessed January 22, 2025. https://akerotx.com/clinical-trials/
- 89bio website. 89bio Initiates Phase 3 ENLIGHTEN-Fibrosis Trial of Pegozafermin in Non-Cirrhotic Metabolic Dysfunction-Associated Steatohepatitis (MASH) Patients with Fibrosis [press release]. Published March 12, 2024. Accessed January 22, 2025. https://www.89bio.com/news/89bio-initiates-phase-3-enlighten-fibrosis-trial-of-pegozafermin-in-non-cirrhotic-metabolic-dysfunction-associated-steatohepatitis-mash-patients-with-fibrosis/
- 89bio website. 89bio Reaches Alignment with the FDA and EMA on Phase 3 Program for Pegozafermin in Nonalcoholic Steatohepatitis (NASH); Program Initiation Planned in the First Half of 2024 [press release]. Published December 4, 2023. Accessed January 22, 2025. https://www.89bio.com/news/89bio-reaches-alignment-with-the-fda-and-ema-on-phase-3-program-for-pegozafermin-in-nonalcoholic-steatohepatitis-nash-program-initiation-planned-in-the-first-half-of-2024/
- Boehringer Ingelheim website. Boehringer receives U.S. FDA Breakthrough Therapy designation and initiates two phase III trials in MASH for survodutide [press release]. Published October 8, 2024. Accessed January 22, 2025. https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash
Publications
Publications
Topics
Article Type
Display Headline
New and Emerging Treatments for MASLD/MASH
Display Headline
New and Emerging Treatments for MASLD/MASH
Disallow All Ads
Content Gating
No Gating (article Unlocked/Free)
Alternative CME
Disqus Comments
Default
Eyebrow Default
SLIDESHOW
Consolidated Pubs: Do Not Show Source Publication Logo
Use ProPublica
Conference Recap Checkbox
Not Conference Recap
Clinical Edge
Medscape Article
Display survey writer
Reuters content
Disable Inline Native ads
WebMD Article