User login
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
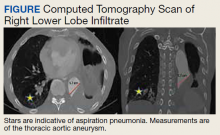
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843