User login
Atrophic Areas on the Axillary and Anogenital Anatomy
Atrophic Areas on the Axillary and Anogenital Anatomy
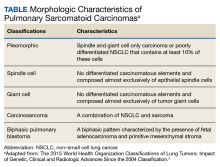
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
Atrophic Areas on the Axillary and Anogenital Anatomy
Atrophic Areas on the Axillary and Anogenital Anatomy
A 62-year-old woman presented for a fullbody skin examination and was found to have a rash in her axillae and inframammary regions. The rash was intermittently pruritic, and the patient felt that the inframammary rash had started from contact with brassiere underwires. She had no oral lesions but noted intermittent burning and itching of the vaginal folds and intermittent bleeding near her anus. Physical examination revealed confluent, shiny, white, atrophic, thin papules with surrounding pink and purple patches on bilateral axillae, bilateral inframammary folds, bilateral inner thighs, and on the clitoral hood and labia minora. There was also an hourglass-shaped erythematous patch involving the vagina and anus. A small fissure was noted perianally, and small hemorrhage was noted on the clitoral head, with fusion of the clitoral head and superior labia minora (Figures 1 and 2).
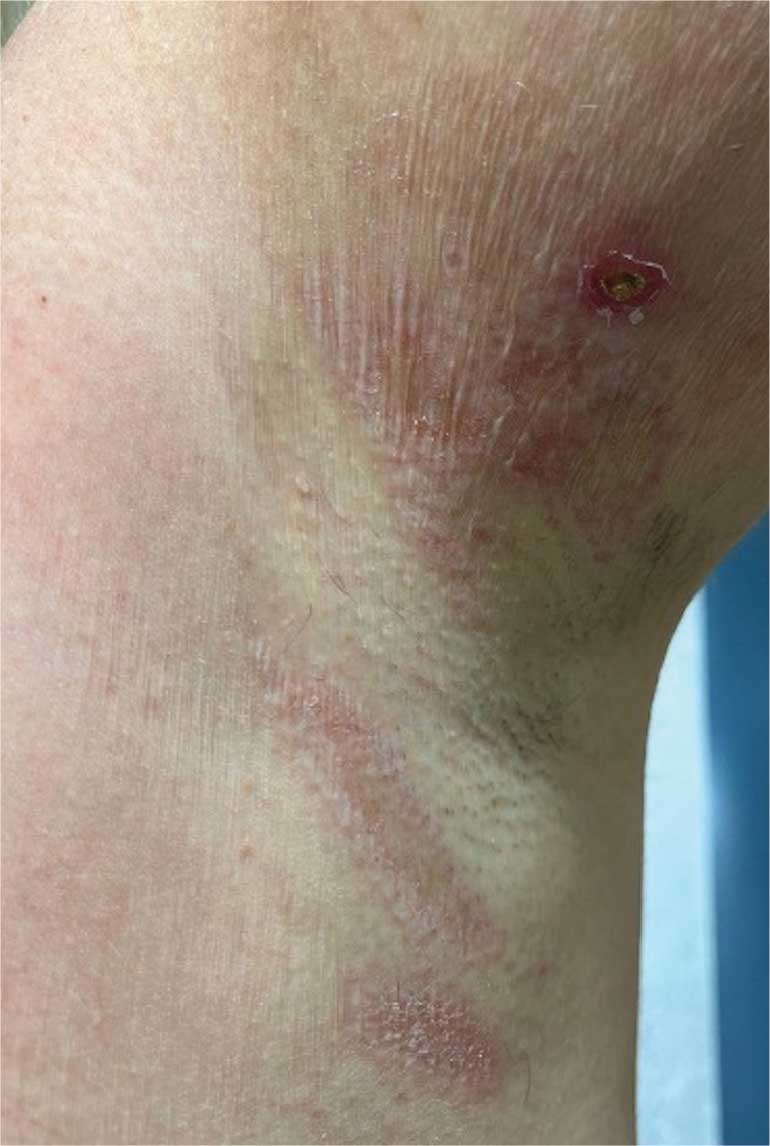
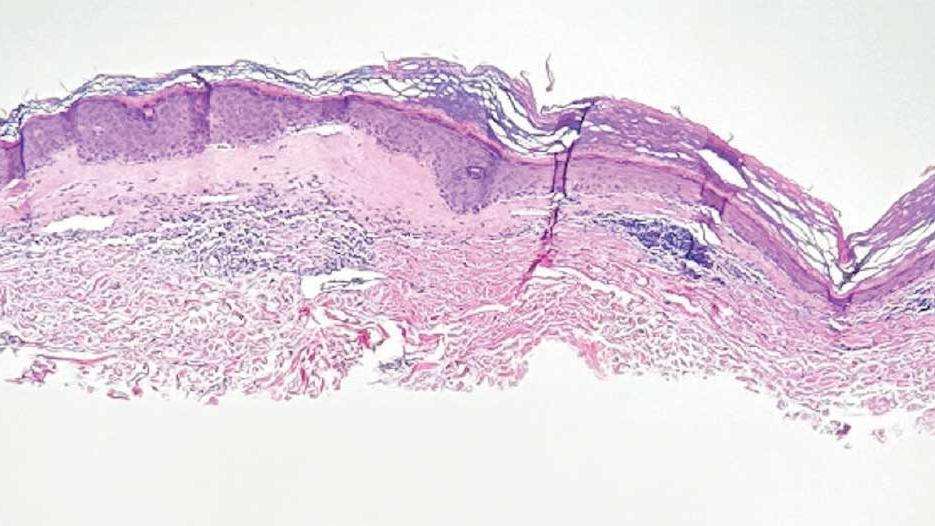
lesion from punch biopsy of the patient’s left axilla.
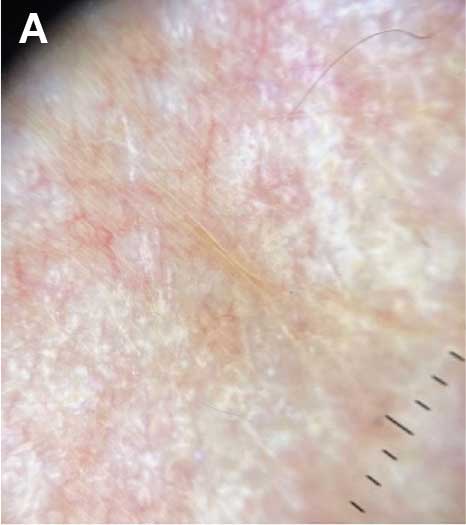
sclerosus plaque showing bright white grouped dots
on a pink background with follicular plugging and linear
branching vessels.
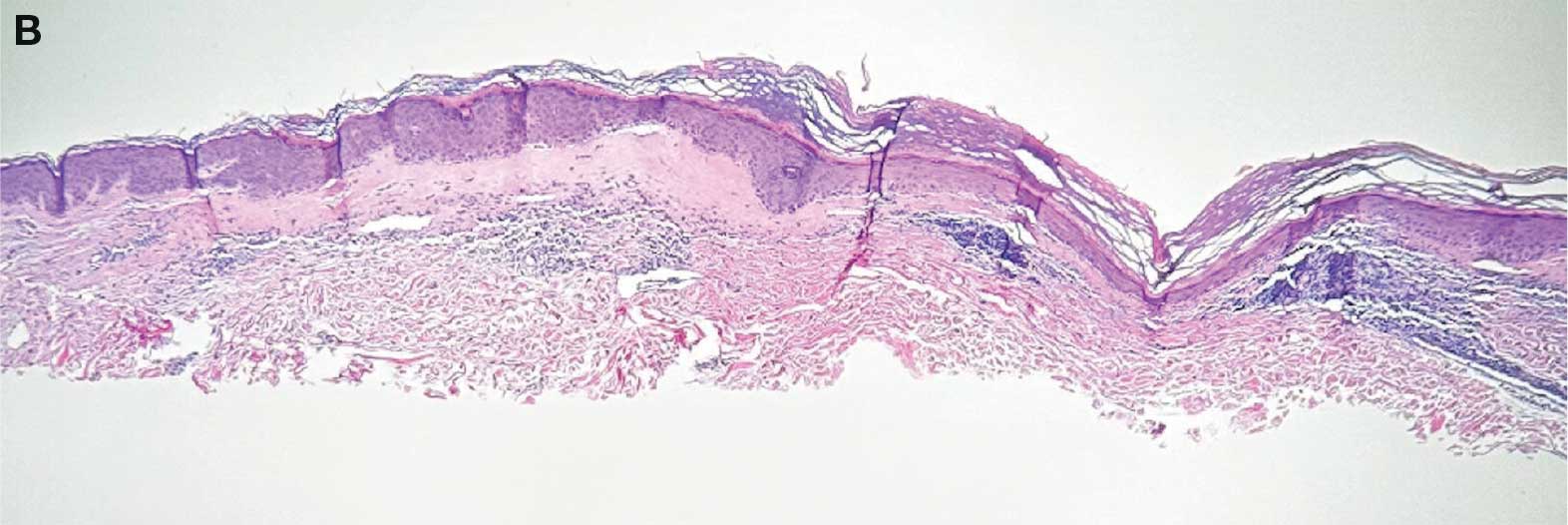
showing a compact corneal layer with a pale papillary
dermis and an underlying lymphocytic infiltrate. These
findings give the “red, white, and blue” appearance.
Low power 20× magnification.
nsbp;
Facial Angioedema, Rash, and “Mastitis” in a 31-Year-Old Female
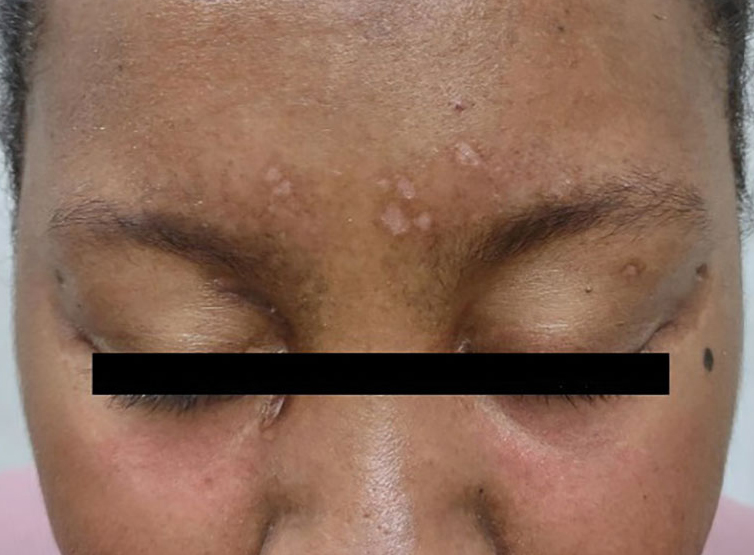
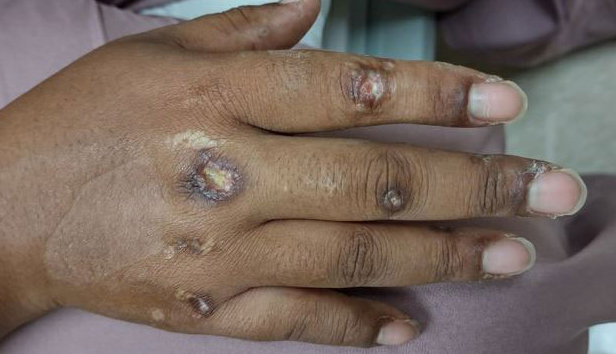
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.

DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.



DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.



DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
Persistent Flu-Like Symptoms in a Patient With Glaucoma and Osteoporosis
A 62-year-old man presented to the emergency department (ED) with 3 days of chills, myalgias, and nausea. The patient’s oral temperature at home ranged from 99.9 to 100.1 °F. He came to the ED after multiple phone discussions with primary care nursing over 3 days. His medical history included posttraumatic stress disorder, enlarged prostate, osteoporosis, gastroesophageal reflux, glaucoma, and left eye central retinal vein occlusion. Medications included fluoxetine 20 mg twice daily, omeprazole 20 mg twice daily, rosuvastatin 10 mg once daily, tamsulosin 0.4 mg nightly, and zolpidem 10 mg nightly. The patient’s glaucoma had been treated with a dexamethasone intraocular implant about 90 days earlier. The patient started on intravenous (IV) zoledronic acid for osteoporosis, with the first infusion 5 days prior to presentation.
In the ED, the patient’s temperature was 98.2 °F, blood pressure was 156/76 mm Hg, pulse was 94 bpm, respiratory rate was 16 breaths per minute, and 98% oxygen saturation on room air. He was in no acute distress, with an unremarkable physical examination reporting no abnormal respiratory sounds, no arrhythmia, normal gait, and no focal neurologic deficits. A comprehensive metabolic panel was unremarkable, creatine phosphokinase was 155 U/L (reference range, 30-240 U/L), and the complete blood count was notable only for an elevated white blood count of 15.3 × 109/L (reference range, 4.0-11.0 × 109/L), with 73.4% neutrophils, 16.2% lymphocytes, 9.1% monocytes, 0.5% eosinophils, and 0.4% basophils. The patient’s urinalysis was unremarkable.
What is your diagnosis?
How would you treat this patient?
Discussion
The ED physician considered viral infection and tested for both influenza and COVID-19. Laboratory results eliminated urinary tract infection and rhabdomyolysis as possible diagnoses. An acute phase reaction to zoledronic acid was determined to be the most likely cause. The patient was treated with IV saline in the ED, and acetaminophen both in the ED and at home.
Although initial nursing triage notes document consideration of acute phase reaction to zoledronic acid, the endocrinology service, which had recommended and arranged the zoledronic acid infusion, was not immediately notified of the reaction. It does not appear any treatment (eg, acetaminophen) was suggested, only that the patient was given advice this may resolve over 3 to 4 days. When he was seen 2 months later for an endocrinology follow-up appointment, he reported that all symptoms (chills, myalgias, and nausea) resolved gradually over 1 week. Since then, he has felt as well as he did before taking zoledronic acid. However, the patient was wary of further zoledronic acid, opting to defer deciding on a second dose until a future appointment.
Prior to starting zoledronic acid therapy, the patient was being treated for vitamin D deficiency. Four months prior to infusion, his 25-hydroxyvitamin D level was 12.0 ng/mL (reference range, 30 to 80 ng/mL). He then started taking cholecalciferol 100 mcg (4000 IU) daily. Eight days prior to infusion his 25-hydroxyvitamin D level was 29.5 ng/mL.
Federal health care practitioners, especially those working in the Veterans Health Administration (VHA), will commonly encounter patients similar to this case. Osteoporosisis is common in the United States with > 10 million diagnoses (including > 2 million men) and in VHA primary care populations.1,2 Zoledronic acid is a frequently prescribed treatment, appearing in guidelines for osteoporosis management.3-5
The acute phase reaction is a common adverse effect of both oral and IV bisphosphonates, although it’s substantially more common with IV bisphosphonates such as zoledronic acid. This reaction is characterized by flu-like symptoms of fever, myalgia, and arthralgia that occur within the first few days following bisphosphonate administration, and tends to be rated mild to moderate by patients.6 Clinical trial data from > 7000 women with postmenopausal osteoporosis found that 42% experienced ≥ 1 acute phase symptom following the first infusion (fever was most common, followed by musculoskeletal symptoms and gastrointestinal symptoms), compared with 12% for placebo. Incidence decreases with each subsequent infusion.7 Risk factors for reactions include low 25-hydroxyvitamin D levels,8,9 no prior bisphosphonate exposure,9 younger age (aged 64-67 years vs 78-89 years),7 lower body mass index,10 and higher lymphocyte levels at baseline.11 While most cases are mild and self-limited, severe consequences have been noted, such as precipitation of adrenal crisis.12,13 Additionally, more prolonged bone pain, sometimes quite severe, has been rarely reported with bisphosphonate use. However, it’s unclear whether this represents a separate adverse effect or a more severe acute phase reaction.6
The acute phase reaction is a transient inflammatory state marked by increases in proinflammatory cytokines such as C-reactive protein, interleukin-6, and tumor necrosis factor-α. Proposed mechanisms include: (1) inhibition of farnesyl pyrophosphate synthase, an enzyme of the mevalonate pathway, resulting inactivation of γϐ T cells and increased production of proinflammatory cytokines; (2) inhibition of the suppressor of cytokine signalling-3 in the macrophages, resulting in cessation of the suppression in cytokine signaling; or (3) negative regulation of γϐ T-cell expansion and interferon-c production by low serum 25-hydroxyvitamin D concentrations.11
Prevention
Can an acute phase reaction to zoledronic acid be prevented? Bourke and colleagues reported that baseline calcium and/or vitamin D intake do not appear to affect rates of acute phase reaction in data pooled from 2 trials of zoledronic acid in postmenopausal women.14 However, patients receiving zoledronic acid had 25-hydroxyvitamin D values > 20 ng/mL 86% of the time, and values > 30 ng/mL 36% of the time. Bourke and colleagues suggest that “coadministration of calcium and vitamin D with zoledronate may not be necessary for individuals not at risk of marked vitamin D deficiency.”14 However, they did not prospectively test this hypothesis.
In our patient, vitamin D deficiency had been identified and treated, nearly achieving 30 ng/mL. The 2020 guidelines for postmenopausal osteoporosis recommend maintaining serum 25-hydroxyvitamin D levels 30 to 50 ng/mL, advising to supplement with vitamin D3 as needed.5 The 2012 guidelines for osteoporosis in men from the Endocrine Society suggest that men with low vitamin D levels receive vitamin D supplements to raise the level > 30 ng/ml.4
Oral analgesics have been studied for the prevention of adverse effects related to zoledronic acid. Initiating 650 mg acetaminophen 45 minutes before zoledronic acid infusion and then every 6 hours over the next 3 days has been shown to significantly reduce symptoms.15 Acetaminophen or ibuprofen given every 6 hours for 3 days (starting 4 hours after zoledronic acid infusion) has been shown to reduce fever and other symptoms.16
Statins have been shown in vitro to prevent bisphosphonate-induced γϐ T cell activation.17 This has led to studies with various statins, although none have yet shown benefit in vivo. A double-blind, randomized, placebo-controlled trial of postmenopausal women for fluvastatin (single dose of 40 mg or 3 doses of 40 mg, each 24 hours apart) did not prevent acute phase reaction symptoms, nor did it prevent zoledronic acid-induced cytokine release.17 Rosuvastatin 10 mg daily starting 5 days before zoledronic acid treatment and taken for a total of 11 days did not show any difference in fever or pain.18 A protocol for pravastatin has been disseminated, but no study results have been published yet.19
Prophylactic dexamethasone has also been studied. A randomized double-blind, placebo-controlled trial of oral dexamethasone 4 mg at the time of first infusion of zoledronic acid found no significant difference in temperature change or symptom score over the following 3 days.20 Chen and colleagues compared the efficacy of acetaminophen alone vs acetaminophen plus dexamethasone over several days.21 Acetaminophen 500 mg was given on the day of infusion and 4 times daily for 3 to 7 days for both groups, while dexamethasone 4 mg was given for 3 to 7 days. The dexamethasone group reported substantially lower incidence of any acute phase reaction symptoms (34% vs 67%, P = .003). A more recent study by Murdoch and colleagues comparing dexamethasone (4 mg daily for 3 days with the first dose 90 minutes before zoledronic acid infusion) with placebo found that the dexamethasone group had a statistically significant lower mean temperature change and acute phase reaction symptom score.22
Adverse Effect Treatment
Treatment after development of acute phase reaction due to zoledronic acid infusion is generally limited to supportive care and/or nonsteroidal anti-inflammatory drugs (NSAIDs) acetaminophen or dexamethasone, largely based on extrapolation of the noted preventive trials and expert opinion.3,6 Experiencing an acute phase reaction may portend better fracture risk reduction from zoledronic acid, although there is a potential association between acute phase reaction and mortality risk.23,24
Our case was typical for acute phase reaction to zoledronic acid. The patient was already taking rosuvastatin 10 mg daily for hypercholesterolemia as prescribed by his primary care physician. Rosuvastatin was not shown to prevent symptoms, although it was not studied in patients on long-term statin therapy at the time of zoledronic acid infusion.18 The patient was also taking vitamin D3 supplementation and was nearly in the reference range.5 His ED treatment included IV fluids and acetaminophen. Pretreatment (prior to or at the time of zoledronic acid infusion) with acetaminophen or ibuprofen may have prevented his symptoms, or at least lessened them to the point that an ED visit would not have resulted. The endocrinologist who prescribed the zoledronic acid documented a detailed discussion of the adverse effects of zoledronic acid with the patient, and the initial nursing call documents consideration of acute phase reaction. It is unclear whether the persistence of symptoms or worsening of symptoms ultimately led to the ED visit. Because no treatment was offered, it is unknown whether earlier posttreatment with acetaminophen, ibuprofen, or dexamethasone might have prevented his ED visit.
Conclusions
Clinicians who treat patients with osteoporosis should be aware of several key points. First, acute phase reaction symptoms are common with bisphosphonates, especially zoledronic acid infusions. Second, the symptoms are nonspecific but should have a suggestive time course. Third, dexamethasone may be partially protective, but based on the various trials discussed, it likely needs to be given for multiple days (instead of a single dose on the day of infusion). Given that acetaminophen and NSAIDs also seem to be protective (when given for multiple days starting on the day of infusion), both have lower overall adverse effect profiles than dexamethasone, consideration may be given to using either of these prophylactically.6 Dexamethasone could then be prescribed if symptoms are severe or persistent despite the use of acetaminophen or NSAIDs.
1. Choksi P, Gay BL, Reyes-Gastelum D, Haymart MR, Papaleontiou M. Understanding osteoporosis screening practices in men: a nationwide physician survey. Endocr Pract. 2020;26(11):1237-1243. doi:10.4158/EP-2020-0123
2. Yu ZL, Fisher L, Hand J. Osteoporosis screening for male veterans in a resident based primary care clinic at Northport Veterans Affairs Medical Center. Am J Med Qual. 2023;38(5):272.doi:10.1097/JMQ.0000000000000134
3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595-1622. doi:10.1210/jc.2019-00221
4. Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822. doi:10.1210/jc.2011-3045
5. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update. Endocr Pract. 2020;26(suppl 1):1-46. doi:10.4158/GL-2020-0524SUPPL
6. Lim SY, Bolster MB. What can we do about musculoskeletal pain from bisphosphonates?. Cleve Clin J Med. 2018;85(9):675-678. doi:10.3949/ccjm.85a.18005
7. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380-4387. doi:10.1210/jc.2010-0597
8. Lu K, Shi Q, Gong YQ, Li C. Association between vitamin D and zoledronate-induced acute-phase response fever risk in osteoporotic patients. Front Endocrinol (Lausanne). 2022;13:991913. Published 2022 Oct 10. doi:10.3389/fendo.2022.991913
9. Popp AW, Senn R, Curkovic I, et al. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28(6):1995-2002. doi:10.1007/s00198-017-3992-5
10. Zheng X, Ye J, Zhan Q, et al. Prediction of musculoskeletal pain after the first intravenous zoledronic acid injection in patients with primary osteoporosis: development and evaluation of a new nomogram. BMC Musculoskelet Disord. 2023;24(1):841. Published 2023 Oct 25. doi:10.1186/s12891-023-06965-y
11. Anastasilakis AD, Polyzos SA, Delaroudis S, et al. The role of cytokines and adipocytokines in zoledronate-induced acute phase reaction in postmenopausal women with low bone mass. Clin Endocrinol (Oxf). 2012;77(6):816-822. doi:10.1111/j.1365-2265.2012.04459.x
12. Smrecnik M, Kavcic Trsinar Z, Kocjan T. Adrenal crisis after first infusion of zoledronic acid: a case report. Osteoporos Int. 2018;29(7):1675-1678. doi:10.1007/s00198-018-4508-7
13. Kuo B, Koransky A, Vaz Wicks CL. Adrenal crisis as an adverse reaction to zoledronic acid in a patient with primary adrenal insufficiency: a case report and literature review. AACE Clin Case Rep. 2022;9(2):32-34. Published 2022 Dec 17. doi:10.1016/j.aace.2022.12.003
14. Bourke S, Bolland MJ, Grey A, et al. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos Int. 2013;24(1):349-354. doi:10.1007/s00198-012-2117-4
15. Silverman SL, Kriegman A, and Goncalves J, et al. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int. 2011;22(8):2337-2345.
16. Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int. 2012;23(2):503-512. doi:10.1007/s00198-011-1563-8
17. Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone. 2011;49(1):140-145. doi:10.1016/j.bone.2010.10.177
18. Makras P, Anastasilakis AD, Polyzos SA, Bisbinas I, Sakellariou GT, Papapoulos SE. No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif Tissue Int. 2011;88(5):402-408. doi:10.1007/s00223-011-9468-2
19. Liu Q, Han G, Li R, et al. Reduction effect of oral pravastatin on the acute phase response to intravenous zoledronic acid: protocol for a real-world prospective, placebo-controlled trial. BMJ Open. 2022;12(7):e060703. Published 2022 Jul 13. doi:10.1136/bmjopen-2021-060703
20. Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int. 2017;28(6):1867-1874. doi:10.1007/s00198-017-3960-0
21. Chen FP, Fu TS, Lin YC, Lin YJ. Addition of dexamethasone to manage acute phase responses following initial zoledronic acid infusion. Osteoporos Int. 2021;32(4):663-670. doi:10.1007/s00198-020-05653-0
22. Murdoch R, Mellar A, Horne AM, et al. Effect of a three-day course of dexamethasone on acute phase response following treatment with zoledronate: a randomized controlled trial. J Bone Miner Res. 2023;38(5):631-638. doi:10.1002/jbmr.4802
23. Black DM, Reid IR, Napoli N, et al. The interaction of acute-phase reaction and efficacy for osteoporosis after zoledronic acid: HORIZON pivotal fracture trial. J Bone Miner Res. 2022;37(1):21-28. doi:10.1002/jbmr.4434
24. Lu K, Wu YM, Shi Q, Gong YQ, Zhang T, Li C. The impact of acute-phase reaction on mortality and re-fracture after zoledronic acid in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. 2023;34(9):1613-1623. doi:10.1007/s00198-023-06803-w
A 62-year-old man presented to the emergency department (ED) with 3 days of chills, myalgias, and nausea. The patient’s oral temperature at home ranged from 99.9 to 100.1 °F. He came to the ED after multiple phone discussions with primary care nursing over 3 days. His medical history included posttraumatic stress disorder, enlarged prostate, osteoporosis, gastroesophageal reflux, glaucoma, and left eye central retinal vein occlusion. Medications included fluoxetine 20 mg twice daily, omeprazole 20 mg twice daily, rosuvastatin 10 mg once daily, tamsulosin 0.4 mg nightly, and zolpidem 10 mg nightly. The patient’s glaucoma had been treated with a dexamethasone intraocular implant about 90 days earlier. The patient started on intravenous (IV) zoledronic acid for osteoporosis, with the first infusion 5 days prior to presentation.
In the ED, the patient’s temperature was 98.2 °F, blood pressure was 156/76 mm Hg, pulse was 94 bpm, respiratory rate was 16 breaths per minute, and 98% oxygen saturation on room air. He was in no acute distress, with an unremarkable physical examination reporting no abnormal respiratory sounds, no arrhythmia, normal gait, and no focal neurologic deficits. A comprehensive metabolic panel was unremarkable, creatine phosphokinase was 155 U/L (reference range, 30-240 U/L), and the complete blood count was notable only for an elevated white blood count of 15.3 × 109/L (reference range, 4.0-11.0 × 109/L), with 73.4% neutrophils, 16.2% lymphocytes, 9.1% monocytes, 0.5% eosinophils, and 0.4% basophils. The patient’s urinalysis was unremarkable.
What is your diagnosis?
How would you treat this patient?
Discussion
The ED physician considered viral infection and tested for both influenza and COVID-19. Laboratory results eliminated urinary tract infection and rhabdomyolysis as possible diagnoses. An acute phase reaction to zoledronic acid was determined to be the most likely cause. The patient was treated with IV saline in the ED, and acetaminophen both in the ED and at home.
Although initial nursing triage notes document consideration of acute phase reaction to zoledronic acid, the endocrinology service, which had recommended and arranged the zoledronic acid infusion, was not immediately notified of the reaction. It does not appear any treatment (eg, acetaminophen) was suggested, only that the patient was given advice this may resolve over 3 to 4 days. When he was seen 2 months later for an endocrinology follow-up appointment, he reported that all symptoms (chills, myalgias, and nausea) resolved gradually over 1 week. Since then, he has felt as well as he did before taking zoledronic acid. However, the patient was wary of further zoledronic acid, opting to defer deciding on a second dose until a future appointment.
Prior to starting zoledronic acid therapy, the patient was being treated for vitamin D deficiency. Four months prior to infusion, his 25-hydroxyvitamin D level was 12.0 ng/mL (reference range, 30 to 80 ng/mL). He then started taking cholecalciferol 100 mcg (4000 IU) daily. Eight days prior to infusion his 25-hydroxyvitamin D level was 29.5 ng/mL.
Federal health care practitioners, especially those working in the Veterans Health Administration (VHA), will commonly encounter patients similar to this case. Osteoporosisis is common in the United States with > 10 million diagnoses (including > 2 million men) and in VHA primary care populations.1,2 Zoledronic acid is a frequently prescribed treatment, appearing in guidelines for osteoporosis management.3-5
The acute phase reaction is a common adverse effect of both oral and IV bisphosphonates, although it’s substantially more common with IV bisphosphonates such as zoledronic acid. This reaction is characterized by flu-like symptoms of fever, myalgia, and arthralgia that occur within the first few days following bisphosphonate administration, and tends to be rated mild to moderate by patients.6 Clinical trial data from > 7000 women with postmenopausal osteoporosis found that 42% experienced ≥ 1 acute phase symptom following the first infusion (fever was most common, followed by musculoskeletal symptoms and gastrointestinal symptoms), compared with 12% for placebo. Incidence decreases with each subsequent infusion.7 Risk factors for reactions include low 25-hydroxyvitamin D levels,8,9 no prior bisphosphonate exposure,9 younger age (aged 64-67 years vs 78-89 years),7 lower body mass index,10 and higher lymphocyte levels at baseline.11 While most cases are mild and self-limited, severe consequences have been noted, such as precipitation of adrenal crisis.12,13 Additionally, more prolonged bone pain, sometimes quite severe, has been rarely reported with bisphosphonate use. However, it’s unclear whether this represents a separate adverse effect or a more severe acute phase reaction.6
The acute phase reaction is a transient inflammatory state marked by increases in proinflammatory cytokines such as C-reactive protein, interleukin-6, and tumor necrosis factor-α. Proposed mechanisms include: (1) inhibition of farnesyl pyrophosphate synthase, an enzyme of the mevalonate pathway, resulting inactivation of γϐ T cells and increased production of proinflammatory cytokines; (2) inhibition of the suppressor of cytokine signalling-3 in the macrophages, resulting in cessation of the suppression in cytokine signaling; or (3) negative regulation of γϐ T-cell expansion and interferon-c production by low serum 25-hydroxyvitamin D concentrations.11
Prevention
Can an acute phase reaction to zoledronic acid be prevented? Bourke and colleagues reported that baseline calcium and/or vitamin D intake do not appear to affect rates of acute phase reaction in data pooled from 2 trials of zoledronic acid in postmenopausal women.14 However, patients receiving zoledronic acid had 25-hydroxyvitamin D values > 20 ng/mL 86% of the time, and values > 30 ng/mL 36% of the time. Bourke and colleagues suggest that “coadministration of calcium and vitamin D with zoledronate may not be necessary for individuals not at risk of marked vitamin D deficiency.”14 However, they did not prospectively test this hypothesis.
In our patient, vitamin D deficiency had been identified and treated, nearly achieving 30 ng/mL. The 2020 guidelines for postmenopausal osteoporosis recommend maintaining serum 25-hydroxyvitamin D levels 30 to 50 ng/mL, advising to supplement with vitamin D3 as needed.5 The 2012 guidelines for osteoporosis in men from the Endocrine Society suggest that men with low vitamin D levels receive vitamin D supplements to raise the level > 30 ng/ml.4
Oral analgesics have been studied for the prevention of adverse effects related to zoledronic acid. Initiating 650 mg acetaminophen 45 minutes before zoledronic acid infusion and then every 6 hours over the next 3 days has been shown to significantly reduce symptoms.15 Acetaminophen or ibuprofen given every 6 hours for 3 days (starting 4 hours after zoledronic acid infusion) has been shown to reduce fever and other symptoms.16
Statins have been shown in vitro to prevent bisphosphonate-induced γϐ T cell activation.17 This has led to studies with various statins, although none have yet shown benefit in vivo. A double-blind, randomized, placebo-controlled trial of postmenopausal women for fluvastatin (single dose of 40 mg or 3 doses of 40 mg, each 24 hours apart) did not prevent acute phase reaction symptoms, nor did it prevent zoledronic acid-induced cytokine release.17 Rosuvastatin 10 mg daily starting 5 days before zoledronic acid treatment and taken for a total of 11 days did not show any difference in fever or pain.18 A protocol for pravastatin has been disseminated, but no study results have been published yet.19
Prophylactic dexamethasone has also been studied. A randomized double-blind, placebo-controlled trial of oral dexamethasone 4 mg at the time of first infusion of zoledronic acid found no significant difference in temperature change or symptom score over the following 3 days.20 Chen and colleagues compared the efficacy of acetaminophen alone vs acetaminophen plus dexamethasone over several days.21 Acetaminophen 500 mg was given on the day of infusion and 4 times daily for 3 to 7 days for both groups, while dexamethasone 4 mg was given for 3 to 7 days. The dexamethasone group reported substantially lower incidence of any acute phase reaction symptoms (34% vs 67%, P = .003). A more recent study by Murdoch and colleagues comparing dexamethasone (4 mg daily for 3 days with the first dose 90 minutes before zoledronic acid infusion) with placebo found that the dexamethasone group had a statistically significant lower mean temperature change and acute phase reaction symptom score.22
Adverse Effect Treatment
Treatment after development of acute phase reaction due to zoledronic acid infusion is generally limited to supportive care and/or nonsteroidal anti-inflammatory drugs (NSAIDs) acetaminophen or dexamethasone, largely based on extrapolation of the noted preventive trials and expert opinion.3,6 Experiencing an acute phase reaction may portend better fracture risk reduction from zoledronic acid, although there is a potential association between acute phase reaction and mortality risk.23,24
Our case was typical for acute phase reaction to zoledronic acid. The patient was already taking rosuvastatin 10 mg daily for hypercholesterolemia as prescribed by his primary care physician. Rosuvastatin was not shown to prevent symptoms, although it was not studied in patients on long-term statin therapy at the time of zoledronic acid infusion.18 The patient was also taking vitamin D3 supplementation and was nearly in the reference range.5 His ED treatment included IV fluids and acetaminophen. Pretreatment (prior to or at the time of zoledronic acid infusion) with acetaminophen or ibuprofen may have prevented his symptoms, or at least lessened them to the point that an ED visit would not have resulted. The endocrinologist who prescribed the zoledronic acid documented a detailed discussion of the adverse effects of zoledronic acid with the patient, and the initial nursing call documents consideration of acute phase reaction. It is unclear whether the persistence of symptoms or worsening of symptoms ultimately led to the ED visit. Because no treatment was offered, it is unknown whether earlier posttreatment with acetaminophen, ibuprofen, or dexamethasone might have prevented his ED visit.
Conclusions
Clinicians who treat patients with osteoporosis should be aware of several key points. First, acute phase reaction symptoms are common with bisphosphonates, especially zoledronic acid infusions. Second, the symptoms are nonspecific but should have a suggestive time course. Third, dexamethasone may be partially protective, but based on the various trials discussed, it likely needs to be given for multiple days (instead of a single dose on the day of infusion). Given that acetaminophen and NSAIDs also seem to be protective (when given for multiple days starting on the day of infusion), both have lower overall adverse effect profiles than dexamethasone, consideration may be given to using either of these prophylactically.6 Dexamethasone could then be prescribed if symptoms are severe or persistent despite the use of acetaminophen or NSAIDs.
A 62-year-old man presented to the emergency department (ED) with 3 days of chills, myalgias, and nausea. The patient’s oral temperature at home ranged from 99.9 to 100.1 °F. He came to the ED after multiple phone discussions with primary care nursing over 3 days. His medical history included posttraumatic stress disorder, enlarged prostate, osteoporosis, gastroesophageal reflux, glaucoma, and left eye central retinal vein occlusion. Medications included fluoxetine 20 mg twice daily, omeprazole 20 mg twice daily, rosuvastatin 10 mg once daily, tamsulosin 0.4 mg nightly, and zolpidem 10 mg nightly. The patient’s glaucoma had been treated with a dexamethasone intraocular implant about 90 days earlier. The patient started on intravenous (IV) zoledronic acid for osteoporosis, with the first infusion 5 days prior to presentation.
In the ED, the patient’s temperature was 98.2 °F, blood pressure was 156/76 mm Hg, pulse was 94 bpm, respiratory rate was 16 breaths per minute, and 98% oxygen saturation on room air. He was in no acute distress, with an unremarkable physical examination reporting no abnormal respiratory sounds, no arrhythmia, normal gait, and no focal neurologic deficits. A comprehensive metabolic panel was unremarkable, creatine phosphokinase was 155 U/L (reference range, 30-240 U/L), and the complete blood count was notable only for an elevated white blood count of 15.3 × 109/L (reference range, 4.0-11.0 × 109/L), with 73.4% neutrophils, 16.2% lymphocytes, 9.1% monocytes, 0.5% eosinophils, and 0.4% basophils. The patient’s urinalysis was unremarkable.
What is your diagnosis?
How would you treat this patient?
Discussion
The ED physician considered viral infection and tested for both influenza and COVID-19. Laboratory results eliminated urinary tract infection and rhabdomyolysis as possible diagnoses. An acute phase reaction to zoledronic acid was determined to be the most likely cause. The patient was treated with IV saline in the ED, and acetaminophen both in the ED and at home.
Although initial nursing triage notes document consideration of acute phase reaction to zoledronic acid, the endocrinology service, which had recommended and arranged the zoledronic acid infusion, was not immediately notified of the reaction. It does not appear any treatment (eg, acetaminophen) was suggested, only that the patient was given advice this may resolve over 3 to 4 days. When he was seen 2 months later for an endocrinology follow-up appointment, he reported that all symptoms (chills, myalgias, and nausea) resolved gradually over 1 week. Since then, he has felt as well as he did before taking zoledronic acid. However, the patient was wary of further zoledronic acid, opting to defer deciding on a second dose until a future appointment.
Prior to starting zoledronic acid therapy, the patient was being treated for vitamin D deficiency. Four months prior to infusion, his 25-hydroxyvitamin D level was 12.0 ng/mL (reference range, 30 to 80 ng/mL). He then started taking cholecalciferol 100 mcg (4000 IU) daily. Eight days prior to infusion his 25-hydroxyvitamin D level was 29.5 ng/mL.
Federal health care practitioners, especially those working in the Veterans Health Administration (VHA), will commonly encounter patients similar to this case. Osteoporosisis is common in the United States with > 10 million diagnoses (including > 2 million men) and in VHA primary care populations.1,2 Zoledronic acid is a frequently prescribed treatment, appearing in guidelines for osteoporosis management.3-5
The acute phase reaction is a common adverse effect of both oral and IV bisphosphonates, although it’s substantially more common with IV bisphosphonates such as zoledronic acid. This reaction is characterized by flu-like symptoms of fever, myalgia, and arthralgia that occur within the first few days following bisphosphonate administration, and tends to be rated mild to moderate by patients.6 Clinical trial data from > 7000 women with postmenopausal osteoporosis found that 42% experienced ≥ 1 acute phase symptom following the first infusion (fever was most common, followed by musculoskeletal symptoms and gastrointestinal symptoms), compared with 12% for placebo. Incidence decreases with each subsequent infusion.7 Risk factors for reactions include low 25-hydroxyvitamin D levels,8,9 no prior bisphosphonate exposure,9 younger age (aged 64-67 years vs 78-89 years),7 lower body mass index,10 and higher lymphocyte levels at baseline.11 While most cases are mild and self-limited, severe consequences have been noted, such as precipitation of adrenal crisis.12,13 Additionally, more prolonged bone pain, sometimes quite severe, has been rarely reported with bisphosphonate use. However, it’s unclear whether this represents a separate adverse effect or a more severe acute phase reaction.6
The acute phase reaction is a transient inflammatory state marked by increases in proinflammatory cytokines such as C-reactive protein, interleukin-6, and tumor necrosis factor-α. Proposed mechanisms include: (1) inhibition of farnesyl pyrophosphate synthase, an enzyme of the mevalonate pathway, resulting inactivation of γϐ T cells and increased production of proinflammatory cytokines; (2) inhibition of the suppressor of cytokine signalling-3 in the macrophages, resulting in cessation of the suppression in cytokine signaling; or (3) negative regulation of γϐ T-cell expansion and interferon-c production by low serum 25-hydroxyvitamin D concentrations.11
Prevention
Can an acute phase reaction to zoledronic acid be prevented? Bourke and colleagues reported that baseline calcium and/or vitamin D intake do not appear to affect rates of acute phase reaction in data pooled from 2 trials of zoledronic acid in postmenopausal women.14 However, patients receiving zoledronic acid had 25-hydroxyvitamin D values > 20 ng/mL 86% of the time, and values > 30 ng/mL 36% of the time. Bourke and colleagues suggest that “coadministration of calcium and vitamin D with zoledronate may not be necessary for individuals not at risk of marked vitamin D deficiency.”14 However, they did not prospectively test this hypothesis.
In our patient, vitamin D deficiency had been identified and treated, nearly achieving 30 ng/mL. The 2020 guidelines for postmenopausal osteoporosis recommend maintaining serum 25-hydroxyvitamin D levels 30 to 50 ng/mL, advising to supplement with vitamin D3 as needed.5 The 2012 guidelines for osteoporosis in men from the Endocrine Society suggest that men with low vitamin D levels receive vitamin D supplements to raise the level > 30 ng/ml.4
Oral analgesics have been studied for the prevention of adverse effects related to zoledronic acid. Initiating 650 mg acetaminophen 45 minutes before zoledronic acid infusion and then every 6 hours over the next 3 days has been shown to significantly reduce symptoms.15 Acetaminophen or ibuprofen given every 6 hours for 3 days (starting 4 hours after zoledronic acid infusion) has been shown to reduce fever and other symptoms.16
Statins have been shown in vitro to prevent bisphosphonate-induced γϐ T cell activation.17 This has led to studies with various statins, although none have yet shown benefit in vivo. A double-blind, randomized, placebo-controlled trial of postmenopausal women for fluvastatin (single dose of 40 mg or 3 doses of 40 mg, each 24 hours apart) did not prevent acute phase reaction symptoms, nor did it prevent zoledronic acid-induced cytokine release.17 Rosuvastatin 10 mg daily starting 5 days before zoledronic acid treatment and taken for a total of 11 days did not show any difference in fever or pain.18 A protocol for pravastatin has been disseminated, but no study results have been published yet.19
Prophylactic dexamethasone has also been studied. A randomized double-blind, placebo-controlled trial of oral dexamethasone 4 mg at the time of first infusion of zoledronic acid found no significant difference in temperature change or symptom score over the following 3 days.20 Chen and colleagues compared the efficacy of acetaminophen alone vs acetaminophen plus dexamethasone over several days.21 Acetaminophen 500 mg was given on the day of infusion and 4 times daily for 3 to 7 days for both groups, while dexamethasone 4 mg was given for 3 to 7 days. The dexamethasone group reported substantially lower incidence of any acute phase reaction symptoms (34% vs 67%, P = .003). A more recent study by Murdoch and colleagues comparing dexamethasone (4 mg daily for 3 days with the first dose 90 minutes before zoledronic acid infusion) with placebo found that the dexamethasone group had a statistically significant lower mean temperature change and acute phase reaction symptom score.22
Adverse Effect Treatment
Treatment after development of acute phase reaction due to zoledronic acid infusion is generally limited to supportive care and/or nonsteroidal anti-inflammatory drugs (NSAIDs) acetaminophen or dexamethasone, largely based on extrapolation of the noted preventive trials and expert opinion.3,6 Experiencing an acute phase reaction may portend better fracture risk reduction from zoledronic acid, although there is a potential association between acute phase reaction and mortality risk.23,24
Our case was typical for acute phase reaction to zoledronic acid. The patient was already taking rosuvastatin 10 mg daily for hypercholesterolemia as prescribed by his primary care physician. Rosuvastatin was not shown to prevent symptoms, although it was not studied in patients on long-term statin therapy at the time of zoledronic acid infusion.18 The patient was also taking vitamin D3 supplementation and was nearly in the reference range.5 His ED treatment included IV fluids and acetaminophen. Pretreatment (prior to or at the time of zoledronic acid infusion) with acetaminophen or ibuprofen may have prevented his symptoms, or at least lessened them to the point that an ED visit would not have resulted. The endocrinologist who prescribed the zoledronic acid documented a detailed discussion of the adverse effects of zoledronic acid with the patient, and the initial nursing call documents consideration of acute phase reaction. It is unclear whether the persistence of symptoms or worsening of symptoms ultimately led to the ED visit. Because no treatment was offered, it is unknown whether earlier posttreatment with acetaminophen, ibuprofen, or dexamethasone might have prevented his ED visit.
Conclusions
Clinicians who treat patients with osteoporosis should be aware of several key points. First, acute phase reaction symptoms are common with bisphosphonates, especially zoledronic acid infusions. Second, the symptoms are nonspecific but should have a suggestive time course. Third, dexamethasone may be partially protective, but based on the various trials discussed, it likely needs to be given for multiple days (instead of a single dose on the day of infusion). Given that acetaminophen and NSAIDs also seem to be protective (when given for multiple days starting on the day of infusion), both have lower overall adverse effect profiles than dexamethasone, consideration may be given to using either of these prophylactically.6 Dexamethasone could then be prescribed if symptoms are severe or persistent despite the use of acetaminophen or NSAIDs.
1. Choksi P, Gay BL, Reyes-Gastelum D, Haymart MR, Papaleontiou M. Understanding osteoporosis screening practices in men: a nationwide physician survey. Endocr Pract. 2020;26(11):1237-1243. doi:10.4158/EP-2020-0123
2. Yu ZL, Fisher L, Hand J. Osteoporosis screening for male veterans in a resident based primary care clinic at Northport Veterans Affairs Medical Center. Am J Med Qual. 2023;38(5):272.doi:10.1097/JMQ.0000000000000134
3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595-1622. doi:10.1210/jc.2019-00221
4. Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822. doi:10.1210/jc.2011-3045
5. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update. Endocr Pract. 2020;26(suppl 1):1-46. doi:10.4158/GL-2020-0524SUPPL
6. Lim SY, Bolster MB. What can we do about musculoskeletal pain from bisphosphonates?. Cleve Clin J Med. 2018;85(9):675-678. doi:10.3949/ccjm.85a.18005
7. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380-4387. doi:10.1210/jc.2010-0597
8. Lu K, Shi Q, Gong YQ, Li C. Association between vitamin D and zoledronate-induced acute-phase response fever risk in osteoporotic patients. Front Endocrinol (Lausanne). 2022;13:991913. Published 2022 Oct 10. doi:10.3389/fendo.2022.991913
9. Popp AW, Senn R, Curkovic I, et al. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28(6):1995-2002. doi:10.1007/s00198-017-3992-5
10. Zheng X, Ye J, Zhan Q, et al. Prediction of musculoskeletal pain after the first intravenous zoledronic acid injection in patients with primary osteoporosis: development and evaluation of a new nomogram. BMC Musculoskelet Disord. 2023;24(1):841. Published 2023 Oct 25. doi:10.1186/s12891-023-06965-y
11. Anastasilakis AD, Polyzos SA, Delaroudis S, et al. The role of cytokines and adipocytokines in zoledronate-induced acute phase reaction in postmenopausal women with low bone mass. Clin Endocrinol (Oxf). 2012;77(6):816-822. doi:10.1111/j.1365-2265.2012.04459.x
12. Smrecnik M, Kavcic Trsinar Z, Kocjan T. Adrenal crisis after first infusion of zoledronic acid: a case report. Osteoporos Int. 2018;29(7):1675-1678. doi:10.1007/s00198-018-4508-7
13. Kuo B, Koransky A, Vaz Wicks CL. Adrenal crisis as an adverse reaction to zoledronic acid in a patient with primary adrenal insufficiency: a case report and literature review. AACE Clin Case Rep. 2022;9(2):32-34. Published 2022 Dec 17. doi:10.1016/j.aace.2022.12.003
14. Bourke S, Bolland MJ, Grey A, et al. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos Int. 2013;24(1):349-354. doi:10.1007/s00198-012-2117-4
15. Silverman SL, Kriegman A, and Goncalves J, et al. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int. 2011;22(8):2337-2345.
16. Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int. 2012;23(2):503-512. doi:10.1007/s00198-011-1563-8
17. Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone. 2011;49(1):140-145. doi:10.1016/j.bone.2010.10.177
18. Makras P, Anastasilakis AD, Polyzos SA, Bisbinas I, Sakellariou GT, Papapoulos SE. No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif Tissue Int. 2011;88(5):402-408. doi:10.1007/s00223-011-9468-2
19. Liu Q, Han G, Li R, et al. Reduction effect of oral pravastatin on the acute phase response to intravenous zoledronic acid: protocol for a real-world prospective, placebo-controlled trial. BMJ Open. 2022;12(7):e060703. Published 2022 Jul 13. doi:10.1136/bmjopen-2021-060703
20. Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int. 2017;28(6):1867-1874. doi:10.1007/s00198-017-3960-0
21. Chen FP, Fu TS, Lin YC, Lin YJ. Addition of dexamethasone to manage acute phase responses following initial zoledronic acid infusion. Osteoporos Int. 2021;32(4):663-670. doi:10.1007/s00198-020-05653-0
22. Murdoch R, Mellar A, Horne AM, et al. Effect of a three-day course of dexamethasone on acute phase response following treatment with zoledronate: a randomized controlled trial. J Bone Miner Res. 2023;38(5):631-638. doi:10.1002/jbmr.4802
23. Black DM, Reid IR, Napoli N, et al. The interaction of acute-phase reaction and efficacy for osteoporosis after zoledronic acid: HORIZON pivotal fracture trial. J Bone Miner Res. 2022;37(1):21-28. doi:10.1002/jbmr.4434
24. Lu K, Wu YM, Shi Q, Gong YQ, Zhang T, Li C. The impact of acute-phase reaction on mortality and re-fracture after zoledronic acid in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. 2023;34(9):1613-1623. doi:10.1007/s00198-023-06803-w
1. Choksi P, Gay BL, Reyes-Gastelum D, Haymart MR, Papaleontiou M. Understanding osteoporosis screening practices in men: a nationwide physician survey. Endocr Pract. 2020;26(11):1237-1243. doi:10.4158/EP-2020-0123
2. Yu ZL, Fisher L, Hand J. Osteoporosis screening for male veterans in a resident based primary care clinic at Northport Veterans Affairs Medical Center. Am J Med Qual. 2023;38(5):272.doi:10.1097/JMQ.0000000000000134
3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595-1622. doi:10.1210/jc.2019-00221
4. Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822. doi:10.1210/jc.2011-3045
5. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update. Endocr Pract. 2020;26(suppl 1):1-46. doi:10.4158/GL-2020-0524SUPPL
6. Lim SY, Bolster MB. What can we do about musculoskeletal pain from bisphosphonates?. Cleve Clin J Med. 2018;85(9):675-678. doi:10.3949/ccjm.85a.18005
7. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380-4387. doi:10.1210/jc.2010-0597
8. Lu K, Shi Q, Gong YQ, Li C. Association between vitamin D and zoledronate-induced acute-phase response fever risk in osteoporotic patients. Front Endocrinol (Lausanne). 2022;13:991913. Published 2022 Oct 10. doi:10.3389/fendo.2022.991913
9. Popp AW, Senn R, Curkovic I, et al. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28(6):1995-2002. doi:10.1007/s00198-017-3992-5
10. Zheng X, Ye J, Zhan Q, et al. Prediction of musculoskeletal pain after the first intravenous zoledronic acid injection in patients with primary osteoporosis: development and evaluation of a new nomogram. BMC Musculoskelet Disord. 2023;24(1):841. Published 2023 Oct 25. doi:10.1186/s12891-023-06965-y
11. Anastasilakis AD, Polyzos SA, Delaroudis S, et al. The role of cytokines and adipocytokines in zoledronate-induced acute phase reaction in postmenopausal women with low bone mass. Clin Endocrinol (Oxf). 2012;77(6):816-822. doi:10.1111/j.1365-2265.2012.04459.x
12. Smrecnik M, Kavcic Trsinar Z, Kocjan T. Adrenal crisis after first infusion of zoledronic acid: a case report. Osteoporos Int. 2018;29(7):1675-1678. doi:10.1007/s00198-018-4508-7
13. Kuo B, Koransky A, Vaz Wicks CL. Adrenal crisis as an adverse reaction to zoledronic acid in a patient with primary adrenal insufficiency: a case report and literature review. AACE Clin Case Rep. 2022;9(2):32-34. Published 2022 Dec 17. doi:10.1016/j.aace.2022.12.003
14. Bourke S, Bolland MJ, Grey A, et al. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos Int. 2013;24(1):349-354. doi:10.1007/s00198-012-2117-4
15. Silverman SL, Kriegman A, and Goncalves J, et al. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int. 2011;22(8):2337-2345.
16. Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int. 2012;23(2):503-512. doi:10.1007/s00198-011-1563-8
17. Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone. 2011;49(1):140-145. doi:10.1016/j.bone.2010.10.177
18. Makras P, Anastasilakis AD, Polyzos SA, Bisbinas I, Sakellariou GT, Papapoulos SE. No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif Tissue Int. 2011;88(5):402-408. doi:10.1007/s00223-011-9468-2
19. Liu Q, Han G, Li R, et al. Reduction effect of oral pravastatin on the acute phase response to intravenous zoledronic acid: protocol for a real-world prospective, placebo-controlled trial. BMJ Open. 2022;12(7):e060703. Published 2022 Jul 13. doi:10.1136/bmjopen-2021-060703
20. Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int. 2017;28(6):1867-1874. doi:10.1007/s00198-017-3960-0
21. Chen FP, Fu TS, Lin YC, Lin YJ. Addition of dexamethasone to manage acute phase responses following initial zoledronic acid infusion. Osteoporos Int. 2021;32(4):663-670. doi:10.1007/s00198-020-05653-0
22. Murdoch R, Mellar A, Horne AM, et al. Effect of a three-day course of dexamethasone on acute phase response following treatment with zoledronate: a randomized controlled trial. J Bone Miner Res. 2023;38(5):631-638. doi:10.1002/jbmr.4802
23. Black DM, Reid IR, Napoli N, et al. The interaction of acute-phase reaction and efficacy for osteoporosis after zoledronic acid: HORIZON pivotal fracture trial. J Bone Miner Res. 2022;37(1):21-28. doi:10.1002/jbmr.4434
24. Lu K, Wu YM, Shi Q, Gong YQ, Zhang T, Li C. The impact of acute-phase reaction on mortality and re-fracture after zoledronic acid in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. 2023;34(9):1613-1623. doi:10.1007/s00198-023-06803-w
Coronary Artery Bypass Graft Saphenous Vein Harvest Site Hyperpigmentation
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
Abdominal Pain and Fever 48 Hours After Hysterosalpingography
A 37-year-old woman presented to the emergency department (ED) with 12 hours of fever and lower abdominal cramp pain. She had a history significant for hypothyroidism, infertility, and dysmenorrhea and had a hysterosalpingography (HSG) 48 hours prior for a comprehensive infertility workup.
On examination, the patient’s vital signs were a 94 bpm heart rate; 109/64 mm Hg blood pressure; 14 breaths per minute respiratory rate; 99% oxygen saturation on room air; and 101.2 °F temperature. The patient reported pain in the bilateral lower abdominal quadrants and no history of sexually transmitted infection, pelvic inflammatory disease, vaginal discharge or bleeding, dysuria, hematuria, melena, or bright red blood per rectum. A human chorionic gonadotropin urine test was negative on intake. The HSG 48 hours prior showed no concerning findings with normal uterine cavity and normal caliber fallopian tubes bilaterally.
On physical examination, the patient’s abdomen was nondistended, nonperitonitic, and without evidence of acute trauma or surgical scars. On palpation, the patient was tender in her suprapubic region and lower abdominal quadrants without evidence of guarding or rebound
The patient’s initial laboratory tests were as follows: white blood cells, 20.1 × 103/μL (reference range, 4-11 × 103/μL); hemoglobin, 12.1 g/dL (reference range, 12.1-15.1 g/dL); hematocrit, 37.1%, (reference range, 36%-48%); alanine aminotransferase, 84 U/L (reference range, 7-56 U/L); aspartate aminotransferase, 66 U/L (reference range, 8-33 U/L); and lipase, 25 U/L (5-60 U/L). Urinalysis was notable for only 5 red blood cells and negative for white blood cells, leukocyte esterase, and nitrites. The patient’s pain and fever were controlled with 1 g IV acetaminophen.
The patient’s fever, leukocytosis, and physical examination were concerning for possible intra-abdominal processes, so a
Discussion
The patient was diagnosed with bilateral tubo-ovarian abscess (TOA) likely secondary to her HSG procedure 48 hours before. TOA is a severe infectious, inflammatory condition involving a mass of the ovaries, fallopian tubes, or adjacent tissues of the upper female genital tract.1 Traditionally, TOAs are sequelae of undiagnosed or subclinical acute or chronic pelvic inflammatory disease (PID). This is known to occur via pathogen ascension from the lower to the upper female genital tract resulting in cervicitis, endometritis, salpingitis, oophoritis, and if left untreated, peritonitis.1 About 70,000 women are diagnosed with TOAs in the US every year. These patients require hospitalization as well as IV antibiotics for gold-standard treatment; however, some cases may require percutaneous drainage based on size, severity, and location.2
Diagnostic Considerations
Clinically, patients with TOAs present with fever, chills, lower abdominal pain, vaginal discharge with cervical motion tenderness, and an adnexal mass on examination.3 When a TOA is suspected, a urine human chorionic gonadotropin test and testing for C trachomatis and N gonorrhoeae are warranted. An ED workup often reveals leukocytosis, elevated C-reactive protein, and elevated erythrocyte sedimentation rate. Imaging is recommended once a TOA is suspected. Ultrasound is the gold-standard imaging modality and boasts a sensitivity of 93% and specificity of 98% for the detection of TOAs; however, CT has also been shown to be an effective diagnostic modality.4
Despite being common, TOAs are difficult to predict, detect, and diagnose; thus the clinician must often rely on thorough history taking and physical examination to raise suspicion.5 Although most frequently associated with sexual transmission, TOAs occur in not sexually active women in adolescence and adulthood. Specifically, TOAs also can present secondary to other intra-abdominal pathologies, such as appendicitis, diverticulitis, and pyelonephritis, as well as a complication of intrauterine procedures, such as an HSG, or less commonly, following intrauterine device (IUD) insertion.5-7
Given that sexually transmitted infections are the most common etiology of TOAs, C trachomatis and N gonorrhoeae are the most likely microorganisms to be isolated (Table).8-12 In not sexually active populations, Escherichia coli and Gardnerella vaginalis should be considered instead. Although rare, women with IUDs have been shown to have an increased incidence of PID/TOA secondary to Actinomyces israleii relative to women without IUDs.12 In patients with TOAs secondary to intraabdominal surgery, anaerobic bacteria, such as Bacterioides and Peptostreptococcus species in addition to Escherichia coli, are likely culprits.11
In patients after HSG, infectious complications are uncommon enough that the American College of Obstetricians and Gynecologists recommends against antibiotic prophylaxis unless there are risk factors of dilated fallopian tubes or a history of PID.13 Identifying a precise percentage of TOA as a complication of HSG is rather elusive in the literature, though, it is frequently noted that infection in general is uncommon, and the risk of developing PID is about 1.4% to 3.4%.14 However, in the retrospective study most often cited, all women who developed PID following HSG had evidence of dilated fallopian tubes. Given our patient had no history of PID or dilated fallopian tubes, her risk of developing infection (PID or postprocedural abscess) would be considered very low; therefore, the index of suspicion also was low.15
Following diagnosis, the patient was promptly admitted and treated with a course of IV ceftriaxone 1 g every 24 hours, IV doxycycline 100 mg every 12 hours, and a single dose of IV metronidazole 500 mg. Her leukocytosis, fever, and pain improved within 48 hours without the need for percutaneous drainage and the patient made a complete recovery.
Complications
TOAs can carry significant morbidity and mortality, and there are both acute and chronic complications associated. Even if properly treated, TOAs can rupture leading to severe illness, such as peritonitis and septic shock. This often requires surgical intervention and hemodynamic pressure support in the intensive care unit setting.16 One of the most feared long-term complications of TOAs is infertility secondary to structural abnormalities of the female reproductive tract.10 Adhesions, strictures, and scarring are associated with TOAs irrespective of medical or surgical management, and thus any women with a history of PID or TOA require advanced fertility workup if they are having difficulties with conception or implantation.17
Minimizing infectious transmission also is essential in the treatment of TOA/PID. All women who receive a diagnosis of PID or TOA should be evaluated for gonorrhea, chlamydia, HIV, and syphilis. Women should be instructed to abstain from sexual intercourse until therapy is complete, symptoms have resolved, and sex partners have been treated for potential chlamydial or gonococcal infections. All contraceptive methods can be continued during treatment.
Conclusions
This case presented several challenges as the bilateral TOAs developed postprocedure in a patient without risk factors. Furthermore, this case did not follow the classic presentation of ascending bacterial translocation to the ovaries over days to weeks. The diagnosis was complicated by a largely benign physical examination and a pelvic examination without evidence of abnormal vaginal discharge or cervicitis. The only indicators were fever, leukocytosis, and abdominal pain in the setting of a recent, uncomplicated HSG procedure. Vigilance is required to obtain the necessary history, and the differential of TOA must be broadened to include women without a history or symptoms of a sexually transmitted infection (contrary to the classic association). We aim to encourage heightened clinical suspicion for TOAs in patients who present with fever, leukocytosis, and abdominal pain after recent HSG or other intrauterine instrumentation procedures and therefore improve patient outcomes.
1. Gkrozou F, Tsonis O, Daniilidis A, Navrozoglou I, Paschopoulos M. Tubo-ovarian abscess: exploring optimal treatment options based on current evidence. J Endometr Pelvic Pain Disord. 2020;13(1):10-19. doi:10.1177/2284026520960649
2. Taylor KJ, Wasson JF, De Graaff C, Rosenfield AT, Andriole VT. Accuracy of grey-scale ultrasound diagnosis of abdominal and pelvic abscesses in 220 patients. Lancet. 1978;1(8055):83-84. doi:10.1016/s0140-6736(78)90016-8
3. Bridwell RE, Koyfman A, Long B. High risk and low prevalence diseases: tubo-ovarian abscess. Am J Emerg Med. 2022;57:70-75. doi:10.1016/j.ajem.2022.04.026
4. Lambert MJ, Villa M. Gynecologic ultrasound in emergency medicine. Emerg Med Clin North Am. 2004;22(3):683-696. doi:10.1016/j.emc.2004.04.016
5. Munro K, Gharaibeh A, Nagabushanam S, Martin C. Diagnosis and management of tubo-ovarian abscesses. Obstet Gynaecol. 2018;20(1):11-19. doi:10.1111/tog.12447
6. Fink D, Lim PPC, Desai A, Stephans AB, Wien MA. Recurrent tubo-ovarian abscess in a nonsexually active adolescent. Consultant. 2022;62(1):e26-e28. doi:10.25270/con.2021.04.00010
7. Hiller N, Fux T, Finkelstein A, Mezeh H, Simanovsky N. CT differentiation between tubo-ovarian and appendiceal origin of right lower quadrant abscess: CT, clinical, and laboratory correlation. Emerg Radiol. 2016;23:133-139. doi:10.1007/s10140-015-1372-z
8. Kairys N, Roepke C. Tubo-ovarian abscess. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 12, 2023.
9. Gao Y, Qu P, Zhou Y, Ding W. Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case-control study. BMC Womens Health. 2021:21:43. doi:10.1186/s12905-021-01188-6
10. Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019;100(6):357-364.
11. Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876-884. doi:10.1093/clinids/5.5.876
12. Burkman R, Schlesselman S, McCaffrey L, Gupta PK, Spence M. The relationship of genital tract actinomycetes and the development of pelvic inflammatory disease. Am J Obstet Gynecol. 1982;143(5):585-589. doi:10.1016/0002-9378(82)90552-x
13. Armstrong C. ACOG releases guidelines on antibiotic prophylaxis for gynecologic procedures. Am Fam Physician. 2007;75(7):1094-1096.
14. Pittaway DE, Winfield AC, Maxson W, Daniell J, Herbert C, Wentz AC. Prevention of acute pelvic inflammatory disease after hysterosalpingography: efficacy of doxycycline prophylaxis. Am J Obstet Gynecol. 1983;147(6):623-626. doi:10.1016/0002-9378(83)90438-6
15. Committee on Practice Bulletins—Gynecology. Prevention of Infection After Gynecologic Procedures: ACOG Practice Bulletin, Number 195. Obstet Gynecol. 2018;131(6):e172-e189. doi:10.1097/AOG.0000000000002670
16. Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo). 2018;73:e364. Published 2018 Aug 9. doi:10.6061/clinics/2018/e364
17. Fouks Y, Azem F, Many A, Cohen Y, Levin I, Cohen A. Fertility outcomes in patients with tubo-ovarian abscesses after an oocyte retrieval: a longitudinal cohort analysis. Arch Gynecol Obstet. 2019;300(3):763-769. doi:10.1007/s00404-019-05230-9
A 37-year-old woman presented to the emergency department (ED) with 12 hours of fever and lower abdominal cramp pain. She had a history significant for hypothyroidism, infertility, and dysmenorrhea and had a hysterosalpingography (HSG) 48 hours prior for a comprehensive infertility workup.
On examination, the patient’s vital signs were a 94 bpm heart rate; 109/64 mm Hg blood pressure; 14 breaths per minute respiratory rate; 99% oxygen saturation on room air; and 101.2 °F temperature. The patient reported pain in the bilateral lower abdominal quadrants and no history of sexually transmitted infection, pelvic inflammatory disease, vaginal discharge or bleeding, dysuria, hematuria, melena, or bright red blood per rectum. A human chorionic gonadotropin urine test was negative on intake. The HSG 48 hours prior showed no concerning findings with normal uterine cavity and normal caliber fallopian tubes bilaterally.
On physical examination, the patient’s abdomen was nondistended, nonperitonitic, and without evidence of acute trauma or surgical scars. On palpation, the patient was tender in her suprapubic region and lower abdominal quadrants without evidence of guarding or rebound
The patient’s initial laboratory tests were as follows: white blood cells, 20.1 × 103/μL (reference range, 4-11 × 103/μL); hemoglobin, 12.1 g/dL (reference range, 12.1-15.1 g/dL); hematocrit, 37.1%, (reference range, 36%-48%); alanine aminotransferase, 84 U/L (reference range, 7-56 U/L); aspartate aminotransferase, 66 U/L (reference range, 8-33 U/L); and lipase, 25 U/L (5-60 U/L). Urinalysis was notable for only 5 red blood cells and negative for white blood cells, leukocyte esterase, and nitrites. The patient’s pain and fever were controlled with 1 g IV acetaminophen.
The patient’s fever, leukocytosis, and physical examination were concerning for possible intra-abdominal processes, so a
Discussion
The patient was diagnosed with bilateral tubo-ovarian abscess (TOA) likely secondary to her HSG procedure 48 hours before. TOA is a severe infectious, inflammatory condition involving a mass of the ovaries, fallopian tubes, or adjacent tissues of the upper female genital tract.1 Traditionally, TOAs are sequelae of undiagnosed or subclinical acute or chronic pelvic inflammatory disease (PID). This is known to occur via pathogen ascension from the lower to the upper female genital tract resulting in cervicitis, endometritis, salpingitis, oophoritis, and if left untreated, peritonitis.1 About 70,000 women are diagnosed with TOAs in the US every year. These patients require hospitalization as well as IV antibiotics for gold-standard treatment; however, some cases may require percutaneous drainage based on size, severity, and location.2
Diagnostic Considerations
Clinically, patients with TOAs present with fever, chills, lower abdominal pain, vaginal discharge with cervical motion tenderness, and an adnexal mass on examination.3 When a TOA is suspected, a urine human chorionic gonadotropin test and testing for C trachomatis and N gonorrhoeae are warranted. An ED workup often reveals leukocytosis, elevated C-reactive protein, and elevated erythrocyte sedimentation rate. Imaging is recommended once a TOA is suspected. Ultrasound is the gold-standard imaging modality and boasts a sensitivity of 93% and specificity of 98% for the detection of TOAs; however, CT has also been shown to be an effective diagnostic modality.4
Despite being common, TOAs are difficult to predict, detect, and diagnose; thus the clinician must often rely on thorough history taking and physical examination to raise suspicion.5 Although most frequently associated with sexual transmission, TOAs occur in not sexually active women in adolescence and adulthood. Specifically, TOAs also can present secondary to other intra-abdominal pathologies, such as appendicitis, diverticulitis, and pyelonephritis, as well as a complication of intrauterine procedures, such as an HSG, or less commonly, following intrauterine device (IUD) insertion.5-7
Given that sexually transmitted infections are the most common etiology of TOAs, C trachomatis and N gonorrhoeae are the most likely microorganisms to be isolated (Table).8-12 In not sexually active populations, Escherichia coli and Gardnerella vaginalis should be considered instead. Although rare, women with IUDs have been shown to have an increased incidence of PID/TOA secondary to Actinomyces israleii relative to women without IUDs.12 In patients with TOAs secondary to intraabdominal surgery, anaerobic bacteria, such as Bacterioides and Peptostreptococcus species in addition to Escherichia coli, are likely culprits.11
In patients after HSG, infectious complications are uncommon enough that the American College of Obstetricians and Gynecologists recommends against antibiotic prophylaxis unless there are risk factors of dilated fallopian tubes or a history of PID.13 Identifying a precise percentage of TOA as a complication of HSG is rather elusive in the literature, though, it is frequently noted that infection in general is uncommon, and the risk of developing PID is about 1.4% to 3.4%.14 However, in the retrospective study most often cited, all women who developed PID following HSG had evidence of dilated fallopian tubes. Given our patient had no history of PID or dilated fallopian tubes, her risk of developing infection (PID or postprocedural abscess) would be considered very low; therefore, the index of suspicion also was low.15
Following diagnosis, the patient was promptly admitted and treated with a course of IV ceftriaxone 1 g every 24 hours, IV doxycycline 100 mg every 12 hours, and a single dose of IV metronidazole 500 mg. Her leukocytosis, fever, and pain improved within 48 hours without the need for percutaneous drainage and the patient made a complete recovery.
Complications
TOAs can carry significant morbidity and mortality, and there are both acute and chronic complications associated. Even if properly treated, TOAs can rupture leading to severe illness, such as peritonitis and septic shock. This often requires surgical intervention and hemodynamic pressure support in the intensive care unit setting.16 One of the most feared long-term complications of TOAs is infertility secondary to structural abnormalities of the female reproductive tract.10 Adhesions, strictures, and scarring are associated with TOAs irrespective of medical or surgical management, and thus any women with a history of PID or TOA require advanced fertility workup if they are having difficulties with conception or implantation.17
Minimizing infectious transmission also is essential in the treatment of TOA/PID. All women who receive a diagnosis of PID or TOA should be evaluated for gonorrhea, chlamydia, HIV, and syphilis. Women should be instructed to abstain from sexual intercourse until therapy is complete, symptoms have resolved, and sex partners have been treated for potential chlamydial or gonococcal infections. All contraceptive methods can be continued during treatment.
Conclusions
This case presented several challenges as the bilateral TOAs developed postprocedure in a patient without risk factors. Furthermore, this case did not follow the classic presentation of ascending bacterial translocation to the ovaries over days to weeks. The diagnosis was complicated by a largely benign physical examination and a pelvic examination without evidence of abnormal vaginal discharge or cervicitis. The only indicators were fever, leukocytosis, and abdominal pain in the setting of a recent, uncomplicated HSG procedure. Vigilance is required to obtain the necessary history, and the differential of TOA must be broadened to include women without a history or symptoms of a sexually transmitted infection (contrary to the classic association). We aim to encourage heightened clinical suspicion for TOAs in patients who present with fever, leukocytosis, and abdominal pain after recent HSG or other intrauterine instrumentation procedures and therefore improve patient outcomes.
A 37-year-old woman presented to the emergency department (ED) with 12 hours of fever and lower abdominal cramp pain. She had a history significant for hypothyroidism, infertility, and dysmenorrhea and had a hysterosalpingography (HSG) 48 hours prior for a comprehensive infertility workup.
On examination, the patient’s vital signs were a 94 bpm heart rate; 109/64 mm Hg blood pressure; 14 breaths per minute respiratory rate; 99% oxygen saturation on room air; and 101.2 °F temperature. The patient reported pain in the bilateral lower abdominal quadrants and no history of sexually transmitted infection, pelvic inflammatory disease, vaginal discharge or bleeding, dysuria, hematuria, melena, or bright red blood per rectum. A human chorionic gonadotropin urine test was negative on intake. The HSG 48 hours prior showed no concerning findings with normal uterine cavity and normal caliber fallopian tubes bilaterally.
On physical examination, the patient’s abdomen was nondistended, nonperitonitic, and without evidence of acute trauma or surgical scars. On palpation, the patient was tender in her suprapubic region and lower abdominal quadrants without evidence of guarding or rebound
The patient’s initial laboratory tests were as follows: white blood cells, 20.1 × 103/μL (reference range, 4-11 × 103/μL); hemoglobin, 12.1 g/dL (reference range, 12.1-15.1 g/dL); hematocrit, 37.1%, (reference range, 36%-48%); alanine aminotransferase, 84 U/L (reference range, 7-56 U/L); aspartate aminotransferase, 66 U/L (reference range, 8-33 U/L); and lipase, 25 U/L (5-60 U/L). Urinalysis was notable for only 5 red blood cells and negative for white blood cells, leukocyte esterase, and nitrites. The patient’s pain and fever were controlled with 1 g IV acetaminophen.
The patient’s fever, leukocytosis, and physical examination were concerning for possible intra-abdominal processes, so a
Discussion
The patient was diagnosed with bilateral tubo-ovarian abscess (TOA) likely secondary to her HSG procedure 48 hours before. TOA is a severe infectious, inflammatory condition involving a mass of the ovaries, fallopian tubes, or adjacent tissues of the upper female genital tract.1 Traditionally, TOAs are sequelae of undiagnosed or subclinical acute or chronic pelvic inflammatory disease (PID). This is known to occur via pathogen ascension from the lower to the upper female genital tract resulting in cervicitis, endometritis, salpingitis, oophoritis, and if left untreated, peritonitis.1 About 70,000 women are diagnosed with TOAs in the US every year. These patients require hospitalization as well as IV antibiotics for gold-standard treatment; however, some cases may require percutaneous drainage based on size, severity, and location.2
Diagnostic Considerations
Clinically, patients with TOAs present with fever, chills, lower abdominal pain, vaginal discharge with cervical motion tenderness, and an adnexal mass on examination.3 When a TOA is suspected, a urine human chorionic gonadotropin test and testing for C trachomatis and N gonorrhoeae are warranted. An ED workup often reveals leukocytosis, elevated C-reactive protein, and elevated erythrocyte sedimentation rate. Imaging is recommended once a TOA is suspected. Ultrasound is the gold-standard imaging modality and boasts a sensitivity of 93% and specificity of 98% for the detection of TOAs; however, CT has also been shown to be an effective diagnostic modality.4
Despite being common, TOAs are difficult to predict, detect, and diagnose; thus the clinician must often rely on thorough history taking and physical examination to raise suspicion.5 Although most frequently associated with sexual transmission, TOAs occur in not sexually active women in adolescence and adulthood. Specifically, TOAs also can present secondary to other intra-abdominal pathologies, such as appendicitis, diverticulitis, and pyelonephritis, as well as a complication of intrauterine procedures, such as an HSG, or less commonly, following intrauterine device (IUD) insertion.5-7
Given that sexually transmitted infections are the most common etiology of TOAs, C trachomatis and N gonorrhoeae are the most likely microorganisms to be isolated (Table).8-12 In not sexually active populations, Escherichia coli and Gardnerella vaginalis should be considered instead. Although rare, women with IUDs have been shown to have an increased incidence of PID/TOA secondary to Actinomyces israleii relative to women without IUDs.12 In patients with TOAs secondary to intraabdominal surgery, anaerobic bacteria, such as Bacterioides and Peptostreptococcus species in addition to Escherichia coli, are likely culprits.11
In patients after HSG, infectious complications are uncommon enough that the American College of Obstetricians and Gynecologists recommends against antibiotic prophylaxis unless there are risk factors of dilated fallopian tubes or a history of PID.13 Identifying a precise percentage of TOA as a complication of HSG is rather elusive in the literature, though, it is frequently noted that infection in general is uncommon, and the risk of developing PID is about 1.4% to 3.4%.14 However, in the retrospective study most often cited, all women who developed PID following HSG had evidence of dilated fallopian tubes. Given our patient had no history of PID or dilated fallopian tubes, her risk of developing infection (PID or postprocedural abscess) would be considered very low; therefore, the index of suspicion also was low.15
Following diagnosis, the patient was promptly admitted and treated with a course of IV ceftriaxone 1 g every 24 hours, IV doxycycline 100 mg every 12 hours, and a single dose of IV metronidazole 500 mg. Her leukocytosis, fever, and pain improved within 48 hours without the need for percutaneous drainage and the patient made a complete recovery.
Complications
TOAs can carry significant morbidity and mortality, and there are both acute and chronic complications associated. Even if properly treated, TOAs can rupture leading to severe illness, such as peritonitis and septic shock. This often requires surgical intervention and hemodynamic pressure support in the intensive care unit setting.16 One of the most feared long-term complications of TOAs is infertility secondary to structural abnormalities of the female reproductive tract.10 Adhesions, strictures, and scarring are associated with TOAs irrespective of medical or surgical management, and thus any women with a history of PID or TOA require advanced fertility workup if they are having difficulties with conception or implantation.17
Minimizing infectious transmission also is essential in the treatment of TOA/PID. All women who receive a diagnosis of PID or TOA should be evaluated for gonorrhea, chlamydia, HIV, and syphilis. Women should be instructed to abstain from sexual intercourse until therapy is complete, symptoms have resolved, and sex partners have been treated for potential chlamydial or gonococcal infections. All contraceptive methods can be continued during treatment.
Conclusions
This case presented several challenges as the bilateral TOAs developed postprocedure in a patient without risk factors. Furthermore, this case did not follow the classic presentation of ascending bacterial translocation to the ovaries over days to weeks. The diagnosis was complicated by a largely benign physical examination and a pelvic examination without evidence of abnormal vaginal discharge or cervicitis. The only indicators were fever, leukocytosis, and abdominal pain in the setting of a recent, uncomplicated HSG procedure. Vigilance is required to obtain the necessary history, and the differential of TOA must be broadened to include women without a history or symptoms of a sexually transmitted infection (contrary to the classic association). We aim to encourage heightened clinical suspicion for TOAs in patients who present with fever, leukocytosis, and abdominal pain after recent HSG or other intrauterine instrumentation procedures and therefore improve patient outcomes.
1. Gkrozou F, Tsonis O, Daniilidis A, Navrozoglou I, Paschopoulos M. Tubo-ovarian abscess: exploring optimal treatment options based on current evidence. J Endometr Pelvic Pain Disord. 2020;13(1):10-19. doi:10.1177/2284026520960649
2. Taylor KJ, Wasson JF, De Graaff C, Rosenfield AT, Andriole VT. Accuracy of grey-scale ultrasound diagnosis of abdominal and pelvic abscesses in 220 patients. Lancet. 1978;1(8055):83-84. doi:10.1016/s0140-6736(78)90016-8
3. Bridwell RE, Koyfman A, Long B. High risk and low prevalence diseases: tubo-ovarian abscess. Am J Emerg Med. 2022;57:70-75. doi:10.1016/j.ajem.2022.04.026
4. Lambert MJ, Villa M. Gynecologic ultrasound in emergency medicine. Emerg Med Clin North Am. 2004;22(3):683-696. doi:10.1016/j.emc.2004.04.016
5. Munro K, Gharaibeh A, Nagabushanam S, Martin C. Diagnosis and management of tubo-ovarian abscesses. Obstet Gynaecol. 2018;20(1):11-19. doi:10.1111/tog.12447
6. Fink D, Lim PPC, Desai A, Stephans AB, Wien MA. Recurrent tubo-ovarian abscess in a nonsexually active adolescent. Consultant. 2022;62(1):e26-e28. doi:10.25270/con.2021.04.00010
7. Hiller N, Fux T, Finkelstein A, Mezeh H, Simanovsky N. CT differentiation between tubo-ovarian and appendiceal origin of right lower quadrant abscess: CT, clinical, and laboratory correlation. Emerg Radiol. 2016;23:133-139. doi:10.1007/s10140-015-1372-z
8. Kairys N, Roepke C. Tubo-ovarian abscess. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 12, 2023.
9. Gao Y, Qu P, Zhou Y, Ding W. Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case-control study. BMC Womens Health. 2021:21:43. doi:10.1186/s12905-021-01188-6
10. Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019;100(6):357-364.
11. Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876-884. doi:10.1093/clinids/5.5.876
12. Burkman R, Schlesselman S, McCaffrey L, Gupta PK, Spence M. The relationship of genital tract actinomycetes and the development of pelvic inflammatory disease. Am J Obstet Gynecol. 1982;143(5):585-589. doi:10.1016/0002-9378(82)90552-x
13. Armstrong C. ACOG releases guidelines on antibiotic prophylaxis for gynecologic procedures. Am Fam Physician. 2007;75(7):1094-1096.
14. Pittaway DE, Winfield AC, Maxson W, Daniell J, Herbert C, Wentz AC. Prevention of acute pelvic inflammatory disease after hysterosalpingography: efficacy of doxycycline prophylaxis. Am J Obstet Gynecol. 1983;147(6):623-626. doi:10.1016/0002-9378(83)90438-6
15. Committee on Practice Bulletins—Gynecology. Prevention of Infection After Gynecologic Procedures: ACOG Practice Bulletin, Number 195. Obstet Gynecol. 2018;131(6):e172-e189. doi:10.1097/AOG.0000000000002670
16. Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo). 2018;73:e364. Published 2018 Aug 9. doi:10.6061/clinics/2018/e364
17. Fouks Y, Azem F, Many A, Cohen Y, Levin I, Cohen A. Fertility outcomes in patients with tubo-ovarian abscesses after an oocyte retrieval: a longitudinal cohort analysis. Arch Gynecol Obstet. 2019;300(3):763-769. doi:10.1007/s00404-019-05230-9
1. Gkrozou F, Tsonis O, Daniilidis A, Navrozoglou I, Paschopoulos M. Tubo-ovarian abscess: exploring optimal treatment options based on current evidence. J Endometr Pelvic Pain Disord. 2020;13(1):10-19. doi:10.1177/2284026520960649
2. Taylor KJ, Wasson JF, De Graaff C, Rosenfield AT, Andriole VT. Accuracy of grey-scale ultrasound diagnosis of abdominal and pelvic abscesses in 220 patients. Lancet. 1978;1(8055):83-84. doi:10.1016/s0140-6736(78)90016-8
3. Bridwell RE, Koyfman A, Long B. High risk and low prevalence diseases: tubo-ovarian abscess. Am J Emerg Med. 2022;57:70-75. doi:10.1016/j.ajem.2022.04.026
4. Lambert MJ, Villa M. Gynecologic ultrasound in emergency medicine. Emerg Med Clin North Am. 2004;22(3):683-696. doi:10.1016/j.emc.2004.04.016
5. Munro K, Gharaibeh A, Nagabushanam S, Martin C. Diagnosis and management of tubo-ovarian abscesses. Obstet Gynaecol. 2018;20(1):11-19. doi:10.1111/tog.12447
6. Fink D, Lim PPC, Desai A, Stephans AB, Wien MA. Recurrent tubo-ovarian abscess in a nonsexually active adolescent. Consultant. 2022;62(1):e26-e28. doi:10.25270/con.2021.04.00010
7. Hiller N, Fux T, Finkelstein A, Mezeh H, Simanovsky N. CT differentiation between tubo-ovarian and appendiceal origin of right lower quadrant abscess: CT, clinical, and laboratory correlation. Emerg Radiol. 2016;23:133-139. doi:10.1007/s10140-015-1372-z
8. Kairys N, Roepke C. Tubo-ovarian abscess. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 12, 2023.
9. Gao Y, Qu P, Zhou Y, Ding W. Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case-control study. BMC Womens Health. 2021:21:43. doi:10.1186/s12905-021-01188-6
10. Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019;100(6):357-364.
11. Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876-884. doi:10.1093/clinids/5.5.876
12. Burkman R, Schlesselman S, McCaffrey L, Gupta PK, Spence M. The relationship of genital tract actinomycetes and the development of pelvic inflammatory disease. Am J Obstet Gynecol. 1982;143(5):585-589. doi:10.1016/0002-9378(82)90552-x
13. Armstrong C. ACOG releases guidelines on antibiotic prophylaxis for gynecologic procedures. Am Fam Physician. 2007;75(7):1094-1096.
14. Pittaway DE, Winfield AC, Maxson W, Daniell J, Herbert C, Wentz AC. Prevention of acute pelvic inflammatory disease after hysterosalpingography: efficacy of doxycycline prophylaxis. Am J Obstet Gynecol. 1983;147(6):623-626. doi:10.1016/0002-9378(83)90438-6
15. Committee on Practice Bulletins—Gynecology. Prevention of Infection After Gynecologic Procedures: ACOG Practice Bulletin, Number 195. Obstet Gynecol. 2018;131(6):e172-e189. doi:10.1097/AOG.0000000000002670
16. Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo). 2018;73:e364. Published 2018 Aug 9. doi:10.6061/clinics/2018/e364
17. Fouks Y, Azem F, Many A, Cohen Y, Levin I, Cohen A. Fertility outcomes in patients with tubo-ovarian abscesses after an oocyte retrieval: a longitudinal cohort analysis. Arch Gynecol Obstet. 2019;300(3):763-769. doi:10.1007/s00404-019-05230-9
Patient With Leukocytosis and Persistent Dry Cough
Discussion
An interdisciplinary discussion regarding the diagnosis considered the clinical features of the patient along with the imaging characteristics. The histological examination demonstrating sarcomatoid features with the supporting immunohistochemistry that confirmed both mesenchymal and epithelioid presence and was used to make the diagnosis of pulmonary sarcomatoid carcinoma (PSC).
The incidence of PSC ranges between 0.1% and 0.4% of all lung malignancies.1,4-7 PSC usually occurs in older men whose weight is moderate to heavy and who smoke. PSC appears to have an upper lobe predilection; also, these tumors tend to be bulky with invasive tendency, early recurrence, and systemic metastases. PSC frequently involves the adjacent lung, chest wall, diaphragm, pericardium, and other tissues.1-5 The source of the sarcoma component of the PSC remains uncertain. However, prior research suggests that it is associated with a clonal evolution that induces epidermal and mesenchymal tumor histological characteristics.1,8,9 The tumor cell epithelial-mesenchymal transition may induce transformation of the carcinoma component of PSC to into a sarcoma component. The epithelial-mesenchymal transition is associated with the PSC high risk for invasiveness and induces metastasis sites, such as the esophagus, colon, rectum, kidneys, and the common sites of NSCLC.
The most common symptoms include productive cough, chest congestion, and chest pain.1,7 In view of PSC’s clinical presentation and imaging, numerous differential diagnoses should be considered, such as sarcomatoid carcinomas, primary or secondary metastatic sarcomas, malignant melanoma, and pleural mesothelioma.6,10
The tumor is initially identified by a chest CT, confirmed by histology and immunohistochemistry. Several biomarkers are useful for diagnosis and classification of an undifferentiated neoplasm/tumor of uncertain origin. Those biomarkers help to understand the tumor pathobiology, to select the therapeutic regimen, and to predict the patient’s outcome. Although immunohistochemical staining of epithelial and mesenchymal markers can be helpful, a reliable diagnosis requires a precise histopathological examination. This is often difficult on small biopsy samples, such as fine-needle aspiration, as all the histological elements of PSC required to make the correct evaluation may not be present. Adequate sampling to generate a considerable number of histological slides is essential for an accurate diagnosis, which can be reached only with surgical resection.5
Due to rarity, rapid progression, short survival, and heterogeneous pathological qualities, PSC has been difficult to formulate treatment recommendations. Compared with other histological subtypes of NSCLC, PSC is more aggressive and has a poor prognosis. Survival time on average is about 13.3 months due to early metastasis, lower than other types of NSCLC. The greatest overall survival (OS) benefit has been shown with surgery in early-stage operable PSC, which remains the standard of care. Because most patients with PSC present in the advanced stage, they lose their opportunity for curative surgery. Auxiliary methods of treatment include radiotherapy and chemotherapy. Prior studies have shown that systemic chemotherapy efficacy has varied, some showing no OS benefit; others showing a modest benefit. It has also been noted that advanced-stage PSC has minimal response to chemotherapy. Further larger prospective studies are needed to outline the efficacy and role of systemic chemotherapy and other therapeutic agents, including targeted therapies and immunotherapy.1,4,11-13 However, two-thirds of patients are not sensitive to conventional chemotherapy. In comparison with other types of NSCLC, PSC carries a poor prognosis even in early-stage disease or if tumor metastasis is present. Therefore, further research on novel treatment options is needed to improve long-term survival.1,3-8
Conclusions
PSC is diagnostically challenging because it is rare and has an aggressive progression. Identification of this tumor requires knowledge of histological criteria to identify their subtypes. Immunohistochemistry has an important role in the classification and to rule out differential diagnoses, including metastatic spread. Nevertheless, a reliable diagnosis requires precise histopathological examination, reached with surgical resection. Therefore, a detailed history, physical examination, systematic investigation, and correlation with chest imaging are needed to avoid misdiagnosis.
Our case highlights the importance of keeping this rare, aggressive tumor as part of the differential diagnosis. In view of its natural history, heterogeneity, and low incidence, published cases of PSC are limited. Thus, further investigation could optimize rapid identification and treatment options.
1. Qin Z, Huang B, Yu G, Zheng Y, Zhao K. Gingival metastasis of a mediastinal pulmonary sarcomatoid carcinoma: a case report. J Cardiothorac Surg. 2019;14(1):161. Published 2019 Sep 9. doi:10.1186/s13019-019-0991-y
2. Travis WD, Brambilla E, Nicholson AG, et al; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243-1260. doi:10.1097/JTO.0000000000000630
3. Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152(3):397-402. doi:10.1016/j.surg.2012.05.007
4. Karim NA, Schuster J, Eldessouki I, et al. Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget. 2017;9(3):4102-4108. Published 2017 Dec 18. doi:10.18632/oncotarget.23468
5. Weissferdt A. Pulmonary sarcomatoid carcinomas: a review. Adv Anat Pathol. 2018;25(5):304-313. doi:10.1097/PAP.0000000000000202
6. Roesel C, Terjung S, Weinreich G, et al. Sarcomatoid carcinoma of the lung: a rare histological subtype of non-small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact Cardiovasc Thorac Surg. 2017;24(3):407-413. doi:10.1093/icvts/ivw392
7. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54. doi:10.5858/2008-0547-RAR.1
8. Thomas VT, Hinson S, Konduri K. Epithelial-mesenchymal transition in pulmonary carcinosarcoma: case report and literature review. Ther Adv Med Oncol. 2012;4(1):31-37. doi:10.1177/1758834011421949
9. Chang YL, Wu CT, Shih JY, Lee YC. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol. 2011;18(10):2952-2960. doi:10.1245/s10434-011-1621-7
10. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, eds. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. International Agency for Research on Cancer; 2015:88-94.
11. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol. 2013;11:252. Published 2013 Oct 2. doi:10.1186/1477-7819-11-252
12. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120. doi:10.1177/1066896908330049
13. Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med. 2014;138(12):1583-1610. doi:10.5858/arpa.2014-0061-RA
Discussion
An interdisciplinary discussion regarding the diagnosis considered the clinical features of the patient along with the imaging characteristics. The histological examination demonstrating sarcomatoid features with the supporting immunohistochemistry that confirmed both mesenchymal and epithelioid presence and was used to make the diagnosis of pulmonary sarcomatoid carcinoma (PSC).
The incidence of PSC ranges between 0.1% and 0.4% of all lung malignancies.1,4-7 PSC usually occurs in older men whose weight is moderate to heavy and who smoke. PSC appears to have an upper lobe predilection; also, these tumors tend to be bulky with invasive tendency, early recurrence, and systemic metastases. PSC frequently involves the adjacent lung, chest wall, diaphragm, pericardium, and other tissues.1-5 The source of the sarcoma component of the PSC remains uncertain. However, prior research suggests that it is associated with a clonal evolution that induces epidermal and mesenchymal tumor histological characteristics.1,8,9 The tumor cell epithelial-mesenchymal transition may induce transformation of the carcinoma component of PSC to into a sarcoma component. The epithelial-mesenchymal transition is associated with the PSC high risk for invasiveness and induces metastasis sites, such as the esophagus, colon, rectum, kidneys, and the common sites of NSCLC.
The most common symptoms include productive cough, chest congestion, and chest pain.1,7 In view of PSC’s clinical presentation and imaging, numerous differential diagnoses should be considered, such as sarcomatoid carcinomas, primary or secondary metastatic sarcomas, malignant melanoma, and pleural mesothelioma.6,10
The tumor is initially identified by a chest CT, confirmed by histology and immunohistochemistry. Several biomarkers are useful for diagnosis and classification of an undifferentiated neoplasm/tumor of uncertain origin. Those biomarkers help to understand the tumor pathobiology, to select the therapeutic regimen, and to predict the patient’s outcome. Although immunohistochemical staining of epithelial and mesenchymal markers can be helpful, a reliable diagnosis requires a precise histopathological examination. This is often difficult on small biopsy samples, such as fine-needle aspiration, as all the histological elements of PSC required to make the correct evaluation may not be present. Adequate sampling to generate a considerable number of histological slides is essential for an accurate diagnosis, which can be reached only with surgical resection.5
Due to rarity, rapid progression, short survival, and heterogeneous pathological qualities, PSC has been difficult to formulate treatment recommendations. Compared with other histological subtypes of NSCLC, PSC is more aggressive and has a poor prognosis. Survival time on average is about 13.3 months due to early metastasis, lower than other types of NSCLC. The greatest overall survival (OS) benefit has been shown with surgery in early-stage operable PSC, which remains the standard of care. Because most patients with PSC present in the advanced stage, they lose their opportunity for curative surgery. Auxiliary methods of treatment include radiotherapy and chemotherapy. Prior studies have shown that systemic chemotherapy efficacy has varied, some showing no OS benefit; others showing a modest benefit. It has also been noted that advanced-stage PSC has minimal response to chemotherapy. Further larger prospective studies are needed to outline the efficacy and role of systemic chemotherapy and other therapeutic agents, including targeted therapies and immunotherapy.1,4,11-13 However, two-thirds of patients are not sensitive to conventional chemotherapy. In comparison with other types of NSCLC, PSC carries a poor prognosis even in early-stage disease or if tumor metastasis is present. Therefore, further research on novel treatment options is needed to improve long-term survival.1,3-8
Conclusions
PSC is diagnostically challenging because it is rare and has an aggressive progression. Identification of this tumor requires knowledge of histological criteria to identify their subtypes. Immunohistochemistry has an important role in the classification and to rule out differential diagnoses, including metastatic spread. Nevertheless, a reliable diagnosis requires precise histopathological examination, reached with surgical resection. Therefore, a detailed history, physical examination, systematic investigation, and correlation with chest imaging are needed to avoid misdiagnosis.
Our case highlights the importance of keeping this rare, aggressive tumor as part of the differential diagnosis. In view of its natural history, heterogeneity, and low incidence, published cases of PSC are limited. Thus, further investigation could optimize rapid identification and treatment options.
Discussion
An interdisciplinary discussion regarding the diagnosis considered the clinical features of the patient along with the imaging characteristics. The histological examination demonstrating sarcomatoid features with the supporting immunohistochemistry that confirmed both mesenchymal and epithelioid presence and was used to make the diagnosis of pulmonary sarcomatoid carcinoma (PSC).
The incidence of PSC ranges between 0.1% and 0.4% of all lung malignancies.1,4-7 PSC usually occurs in older men whose weight is moderate to heavy and who smoke. PSC appears to have an upper lobe predilection; also, these tumors tend to be bulky with invasive tendency, early recurrence, and systemic metastases. PSC frequently involves the adjacent lung, chest wall, diaphragm, pericardium, and other tissues.1-5 The source of the sarcoma component of the PSC remains uncertain. However, prior research suggests that it is associated with a clonal evolution that induces epidermal and mesenchymal tumor histological characteristics.1,8,9 The tumor cell epithelial-mesenchymal transition may induce transformation of the carcinoma component of PSC to into a sarcoma component. The epithelial-mesenchymal transition is associated with the PSC high risk for invasiveness and induces metastasis sites, such as the esophagus, colon, rectum, kidneys, and the common sites of NSCLC.
The most common symptoms include productive cough, chest congestion, and chest pain.1,7 In view of PSC’s clinical presentation and imaging, numerous differential diagnoses should be considered, such as sarcomatoid carcinomas, primary or secondary metastatic sarcomas, malignant melanoma, and pleural mesothelioma.6,10
The tumor is initially identified by a chest CT, confirmed by histology and immunohistochemistry. Several biomarkers are useful for diagnosis and classification of an undifferentiated neoplasm/tumor of uncertain origin. Those biomarkers help to understand the tumor pathobiology, to select the therapeutic regimen, and to predict the patient’s outcome. Although immunohistochemical staining of epithelial and mesenchymal markers can be helpful, a reliable diagnosis requires a precise histopathological examination. This is often difficult on small biopsy samples, such as fine-needle aspiration, as all the histological elements of PSC required to make the correct evaluation may not be present. Adequate sampling to generate a considerable number of histological slides is essential for an accurate diagnosis, which can be reached only with surgical resection.5
Due to rarity, rapid progression, short survival, and heterogeneous pathological qualities, PSC has been difficult to formulate treatment recommendations. Compared with other histological subtypes of NSCLC, PSC is more aggressive and has a poor prognosis. Survival time on average is about 13.3 months due to early metastasis, lower than other types of NSCLC. The greatest overall survival (OS) benefit has been shown with surgery in early-stage operable PSC, which remains the standard of care. Because most patients with PSC present in the advanced stage, they lose their opportunity for curative surgery. Auxiliary methods of treatment include radiotherapy and chemotherapy. Prior studies have shown that systemic chemotherapy efficacy has varied, some showing no OS benefit; others showing a modest benefit. It has also been noted that advanced-stage PSC has minimal response to chemotherapy. Further larger prospective studies are needed to outline the efficacy and role of systemic chemotherapy and other therapeutic agents, including targeted therapies and immunotherapy.1,4,11-13 However, two-thirds of patients are not sensitive to conventional chemotherapy. In comparison with other types of NSCLC, PSC carries a poor prognosis even in early-stage disease or if tumor metastasis is present. Therefore, further research on novel treatment options is needed to improve long-term survival.1,3-8
Conclusions
PSC is diagnostically challenging because it is rare and has an aggressive progression. Identification of this tumor requires knowledge of histological criteria to identify their subtypes. Immunohistochemistry has an important role in the classification and to rule out differential diagnoses, including metastatic spread. Nevertheless, a reliable diagnosis requires precise histopathological examination, reached with surgical resection. Therefore, a detailed history, physical examination, systematic investigation, and correlation with chest imaging are needed to avoid misdiagnosis.
Our case highlights the importance of keeping this rare, aggressive tumor as part of the differential diagnosis. In view of its natural history, heterogeneity, and low incidence, published cases of PSC are limited. Thus, further investigation could optimize rapid identification and treatment options.
1. Qin Z, Huang B, Yu G, Zheng Y, Zhao K. Gingival metastasis of a mediastinal pulmonary sarcomatoid carcinoma: a case report. J Cardiothorac Surg. 2019;14(1):161. Published 2019 Sep 9. doi:10.1186/s13019-019-0991-y
2. Travis WD, Brambilla E, Nicholson AG, et al; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243-1260. doi:10.1097/JTO.0000000000000630
3. Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152(3):397-402. doi:10.1016/j.surg.2012.05.007
4. Karim NA, Schuster J, Eldessouki I, et al. Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget. 2017;9(3):4102-4108. Published 2017 Dec 18. doi:10.18632/oncotarget.23468
5. Weissferdt A. Pulmonary sarcomatoid carcinomas: a review. Adv Anat Pathol. 2018;25(5):304-313. doi:10.1097/PAP.0000000000000202
6. Roesel C, Terjung S, Weinreich G, et al. Sarcomatoid carcinoma of the lung: a rare histological subtype of non-small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact Cardiovasc Thorac Surg. 2017;24(3):407-413. doi:10.1093/icvts/ivw392
7. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54. doi:10.5858/2008-0547-RAR.1
8. Thomas VT, Hinson S, Konduri K. Epithelial-mesenchymal transition in pulmonary carcinosarcoma: case report and literature review. Ther Adv Med Oncol. 2012;4(1):31-37. doi:10.1177/1758834011421949
9. Chang YL, Wu CT, Shih JY, Lee YC. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol. 2011;18(10):2952-2960. doi:10.1245/s10434-011-1621-7
10. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, eds. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. International Agency for Research on Cancer; 2015:88-94.
11. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol. 2013;11:252. Published 2013 Oct 2. doi:10.1186/1477-7819-11-252
12. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120. doi:10.1177/1066896908330049
13. Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med. 2014;138(12):1583-1610. doi:10.5858/arpa.2014-0061-RA
1. Qin Z, Huang B, Yu G, Zheng Y, Zhao K. Gingival metastasis of a mediastinal pulmonary sarcomatoid carcinoma: a case report. J Cardiothorac Surg. 2019;14(1):161. Published 2019 Sep 9. doi:10.1186/s13019-019-0991-y
2. Travis WD, Brambilla E, Nicholson AG, et al; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243-1260. doi:10.1097/JTO.0000000000000630
3. Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152(3):397-402. doi:10.1016/j.surg.2012.05.007
4. Karim NA, Schuster J, Eldessouki I, et al. Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget. 2017;9(3):4102-4108. Published 2017 Dec 18. doi:10.18632/oncotarget.23468
5. Weissferdt A. Pulmonary sarcomatoid carcinomas: a review. Adv Anat Pathol. 2018;25(5):304-313. doi:10.1097/PAP.0000000000000202
6. Roesel C, Terjung S, Weinreich G, et al. Sarcomatoid carcinoma of the lung: a rare histological subtype of non-small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact Cardiovasc Thorac Surg. 2017;24(3):407-413. doi:10.1093/icvts/ivw392
7. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54. doi:10.5858/2008-0547-RAR.1
8. Thomas VT, Hinson S, Konduri K. Epithelial-mesenchymal transition in pulmonary carcinosarcoma: case report and literature review. Ther Adv Med Oncol. 2012;4(1):31-37. doi:10.1177/1758834011421949
9. Chang YL, Wu CT, Shih JY, Lee YC. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol. 2011;18(10):2952-2960. doi:10.1245/s10434-011-1621-7
10. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, eds. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. International Agency for Research on Cancer; 2015:88-94.
11. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol. 2013;11:252. Published 2013 Oct 2. doi:10.1186/1477-7819-11-252
12. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120. doi:10.1177/1066896908330049
13. Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med. 2014;138(12):1583-1610. doi:10.5858/arpa.2014-0061-RA
Patient With Severe Headache After IV Immunoglobulin
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?
Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
A Patient Presenting With Shortness of Breath, Fever, and Eosinophilia
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?
In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
Postprandial Right Upper Quadrant Abdominal Pain
A 53-year-old male patient presented to the emergency department following a primary care office visit with sudden onset right upper quadrant abdominal pain that persisted for 3 weeks, worsening over the last 2 days. The abdominal pain worsened after eating or drinking and mildly improved with omeprazole. Associated symptoms included intermittent fever, night sweats, fatigue, and bloating since onset without vomiting or diarrhea. He reported a “complicated” cholecystectomy at an outside facility 6 months prior and that his “gallbladder was adhered to his duodenum,” though outside records were not available. Additional medical history included diverticulosis with prior flares of diverticulitis but no recent flares or treatments. His home medications included acetaminophen, naproxen, intranasal fluticasone, omeprazole, gabapentin, baclofen, trazodone, and antihistamines. He reported no tobacco or illicit drug use and stated he consumed a 6 pack of beer every 6 weeks.
Initial vital signs in the emergency department demonstrated an afebrile oral temperature with unremarkable blood pressure and pulse. He was alert and oriented and did not appear in significant acute distress. Physical examination of the abdomen demonstrated a nondistended abdomen, normal active bowel sounds in all 4 quadrants, and mild right upper and lower quadrant tenderness to soft and deep palpation with release.
Significant laboratory values included elevated C-reactive protein of 44.1 mg/L and mild leukocytosis of 11.1 K/µL (reference range, 4.00-10.60 K/µL). The basic metabolic panel, liver-associated enzymes, and lipase levels were within normal limits.
The initial imaging study was a computed tomography (CT) of the abdomen and pelvis with oral and IV contrast. The radiology report depicted a thin, needle-like hypodense foreign body approximately 8 cm in length in the proximal duodenum, slightly protruding extraluminally, and at least a moderate amount of surrounding inflammation without abscess or free air (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
Our Diagnosis
Based on the clinical history of postprandial abdominal pain with prior cholecystectomy and leukocytosis, the initial differential diagnosis included peptic ulcer disease, gastroesophageal reflux, or delayed sequela of the cholecystectomy 6 months prior. Although suspicion remained for possible delayed postoperative complications from the cholecystectomy, ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were not pursued based on CT imaging findings. The needle-like hypodensity in the duodenum with surrounding inflammation visualized on CT was concerning for an unidentified penetrating foreign body with a possible retroperitoneal microperforation.
After these imaging findings were relayed from Radiology to the Gastroenterology Service, the patient underwent an upper gastrointestinal (GI) endoscopy to further evaluate the duodenum. Inspection revealed mild gastritis and a linear, clear piece of plastic with both ends firmly lodged within the mucosa from the distal duodenal bulb to the second portion of the duodenum; a significant mucosal defect of the bowel wall was visualized after careful extraction of the foreign body (Figure 2). The patient was diagnosed with a small duodenal perforation, which was sealed endoscopically with 2 endoclips. The extracted piece of plastic was examined and determined to be a broken cocktail pick (Figure 3). During discussion with the patient postprocedure, he stated that he ingested several olive martinis (which were served with cocktail picks) approximately 3 weeks prior to presentation and did not recall ingesting the cocktail pick. A repeat abdominal CT following the endoscopy demonstrated no leak or free air from the site of the repaired duodenal perforation (Figure 4). The patient avoided surgery and was permitted to resume a liquid diet prior to discharge.
Discussion
Foreign body ingestion in adults is most commonly unintentional with fish bones being the most common culprit.1 In unintentional instances of foreign body ingestion, many patients are not aware of the event, with dentures posing a significant well-known risk factor due to lack of palatal sensory feedback.2 Most ingested foreign bodies pass uninhibited through the GI tract without complications. However, less than 1% of ingested foreign bodies cause potentially life-threatening GI perforations.3
The risk of GI perforation due to foreign body ingestion is greatest with elongated, sharp objects, such as needles, bones, toothpicks, and cocktail picks. These objects tend to lodge at areas of narrowing or angulation, such as the appendix, ileocecal region, or as in this case, the duodenum.3 Passage of a foreign body through the duodenum is more likely to be inhibited if the object is longer than 6 cm and with a diameter > 2.5 cm.4 Signs of duodenal perforation are often subtle compared with jejunal or ileal perforations. Patients are commonly afebrile with normal white blood cell counts and are more likely to have chronic symptoms for > 3 days before the appropriate diagnosis of foreign body ingestion is made.1 Duodenal perforations may be more stable clinically compared with distal GI perforations in part due to the retroperitoneal location with relatively fewer bacteria present intraluminally. GI perforations may not occur acutely during passage of the foreign body but can present weeks, months, or even years later.5 Delayed onset of symptoms may happen when the foreign body becomes lodged and only partially perforates the bowel wall, resulting in a chronic inflammatory process. Other possible complications include fistulization and abscess formation from migrating linear sharp objects through the bowel wall, which is most observed with toothpicks and cocktail picks, specifically.5
Foreign bodies identified on plain radiographs commonly include radiopaque objects, such as glass, metallic objects, most animal bones and some fish bones, and some medications. However, radiolucent objects, such as toothpicks and cocktail picks, wood, plastic, most fish bones, and most medicines, often will not appear on radiographs. The diagnosis of ingested foreign body can therefore easily be delayed or overlooked on plain radiographs due to ingestion of radiolucent objects or lack of adequate patient history. A high index of suspicion is needed in such instances. The modality of choice for identifying GI perforation due to ingested foreign objects is CT.5 All of these commonly missed materials on radiographs will be visible on CT with variable densities. As an added benefit, CT also may reveal ingested objects not visualized on radiographs and show ancillary signs of perforation, such as extraluminal free air, localized inflammation, and fluid collections or abscess surrounding a segment of thickened bowel.5
Most ingested foreign bodies will pass through the GI system and can be managed with careful observation alone. However, upper endoscopy is emergently indicated in 3 scenarios of foreign body ingestion: (1) complete occlusion of the esophagus with salivary pooling due to risk of aspiration; (2) ingestion of batteries due to toxic substances; and (3) ingestion of sharp or pointed foreign bodies due to risk of perforation.4 Overall, endoscopic intervention is required in 20% of cases and surgical intervention remains rare at 1%.4 In the case of this patient, an emergent upper endoscopy was needed due to suspected duodenal perforation.
Treatment of duodenal perforations due to foreign bodies may involve conservative, surgical, or endoscopic management. Contained, small perforations in a stable patient may be treated conservatively with IV fluids, antibiotics, and proton pump inhibitors as they self-seal with omentum if the foreign body has passed.6 Retained duodenal foreign bodies pose a risk of persistent perforation or fistulization and must be removed. Anterior duodenal perforations pose a risk of peritonitis, whereas posterior duodenal perforations, although retroperitoneal and sparing the peritoneal cavity, may result in localized abscess formation necessitating foreign body removal. Endoscopic clipping is a modernized, less invasive way to close GI perforations. Through-the-scope clips (TTSCs) can close luminal defects < 2 cm in size.7 Defects > 1 cm may be repaired with combined TTSCs and endoloop or omental patching. Over-the-scope clips can close full thickness defects up to 2 to 3 cm with the advantage of being able to close leaks and fistulas involving inflamed or indurated tissue.7
Conclusions
Intestinal perforations related to foreign body ingestion are a rare complication occurring in < 1% of patients. Although most ingested foreign objects will pass through the GI tract, elongated or sharp objects pose a risk for perforation. In many cases, a history of foreign body ingestion is not obtained, and a high index of suspicion is required. Duodenal perforations due to foreign body ingestion should be included in the differential among the more common diagnoses of peptic ulcers, pancreatitis, and gallbladder disease in the setting of postprandial right upper quadrant abdominal pain. CT is the best modality for identifying foreign bodies, including objects that may be missed on plain radiographs.
1. Goh BK, Chow PK, Quah HM, et al. Perforation of the gastrointestinal tract secondary to ingestion of foreign bodies. World J Surg. 2006;(30)372-377. doi:10.1007/s00268-005-0490-2
2. Bunker PG. The role of dentistry in problems of foreign body in the air and food passage. J Am Dent Assoc. 1962;(64):782-787. doi:10.14219/jada.archive.1962.0160
3. Hunter TB, Taljanovic MS. Foreign bodies. Radiographics. 2003;23(3):731-757. doi:10.1148/rg.233025137
4. Ambe P, Weber SA, Schauer M, Knoefel WT. Swallowed foreign bodies in adults. Dtsch Arztebl Int. 2012;109(50):869-875. doi:10.3238/arztebl.2012.0869
5. Kuzmich S, Burke CJ, Harvey CJ, et al. Perforation of gastrointestinal tract by poorly conspicuous ingested foreign bodies: radiological diagnosis. Br J Radiol. 2015;88(1050):20150086. doi:10.1259/bjr.20150086
6. Hill AG. Management of perforated duodenal ulcer. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001.
7. Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21(37):10542-10552. doi:10.3748/wjg.v21.i37.10542
A 53-year-old male patient presented to the emergency department following a primary care office visit with sudden onset right upper quadrant abdominal pain that persisted for 3 weeks, worsening over the last 2 days. The abdominal pain worsened after eating or drinking and mildly improved with omeprazole. Associated symptoms included intermittent fever, night sweats, fatigue, and bloating since onset without vomiting or diarrhea. He reported a “complicated” cholecystectomy at an outside facility 6 months prior and that his “gallbladder was adhered to his duodenum,” though outside records were not available. Additional medical history included diverticulosis with prior flares of diverticulitis but no recent flares or treatments. His home medications included acetaminophen, naproxen, intranasal fluticasone, omeprazole, gabapentin, baclofen, trazodone, and antihistamines. He reported no tobacco or illicit drug use and stated he consumed a 6 pack of beer every 6 weeks.
Initial vital signs in the emergency department demonstrated an afebrile oral temperature with unremarkable blood pressure and pulse. He was alert and oriented and did not appear in significant acute distress. Physical examination of the abdomen demonstrated a nondistended abdomen, normal active bowel sounds in all 4 quadrants, and mild right upper and lower quadrant tenderness to soft and deep palpation with release.
Significant laboratory values included elevated C-reactive protein of 44.1 mg/L and mild leukocytosis of 11.1 K/µL (reference range, 4.00-10.60 K/µL). The basic metabolic panel, liver-associated enzymes, and lipase levels were within normal limits.
The initial imaging study was a computed tomography (CT) of the abdomen and pelvis with oral and IV contrast. The radiology report depicted a thin, needle-like hypodense foreign body approximately 8 cm in length in the proximal duodenum, slightly protruding extraluminally, and at least a moderate amount of surrounding inflammation without abscess or free air (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
Our Diagnosis
Based on the clinical history of postprandial abdominal pain with prior cholecystectomy and leukocytosis, the initial differential diagnosis included peptic ulcer disease, gastroesophageal reflux, or delayed sequela of the cholecystectomy 6 months prior. Although suspicion remained for possible delayed postoperative complications from the cholecystectomy, ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were not pursued based on CT imaging findings. The needle-like hypodensity in the duodenum with surrounding inflammation visualized on CT was concerning for an unidentified penetrating foreign body with a possible retroperitoneal microperforation.
After these imaging findings were relayed from Radiology to the Gastroenterology Service, the patient underwent an upper gastrointestinal (GI) endoscopy to further evaluate the duodenum. Inspection revealed mild gastritis and a linear, clear piece of plastic with both ends firmly lodged within the mucosa from the distal duodenal bulb to the second portion of the duodenum; a significant mucosal defect of the bowel wall was visualized after careful extraction of the foreign body (Figure 2). The patient was diagnosed with a small duodenal perforation, which was sealed endoscopically with 2 endoclips. The extracted piece of plastic was examined and determined to be a broken cocktail pick (Figure 3). During discussion with the patient postprocedure, he stated that he ingested several olive martinis (which were served with cocktail picks) approximately 3 weeks prior to presentation and did not recall ingesting the cocktail pick. A repeat abdominal CT following the endoscopy demonstrated no leak or free air from the site of the repaired duodenal perforation (Figure 4). The patient avoided surgery and was permitted to resume a liquid diet prior to discharge.
Discussion
Foreign body ingestion in adults is most commonly unintentional with fish bones being the most common culprit.1 In unintentional instances of foreign body ingestion, many patients are not aware of the event, with dentures posing a significant well-known risk factor due to lack of palatal sensory feedback.2 Most ingested foreign bodies pass uninhibited through the GI tract without complications. However, less than 1% of ingested foreign bodies cause potentially life-threatening GI perforations.3
The risk of GI perforation due to foreign body ingestion is greatest with elongated, sharp objects, such as needles, bones, toothpicks, and cocktail picks. These objects tend to lodge at areas of narrowing or angulation, such as the appendix, ileocecal region, or as in this case, the duodenum.3 Passage of a foreign body through the duodenum is more likely to be inhibited if the object is longer than 6 cm and with a diameter > 2.5 cm.4 Signs of duodenal perforation are often subtle compared with jejunal or ileal perforations. Patients are commonly afebrile with normal white blood cell counts and are more likely to have chronic symptoms for > 3 days before the appropriate diagnosis of foreign body ingestion is made.1 Duodenal perforations may be more stable clinically compared with distal GI perforations in part due to the retroperitoneal location with relatively fewer bacteria present intraluminally. GI perforations may not occur acutely during passage of the foreign body but can present weeks, months, or even years later.5 Delayed onset of symptoms may happen when the foreign body becomes lodged and only partially perforates the bowel wall, resulting in a chronic inflammatory process. Other possible complications include fistulization and abscess formation from migrating linear sharp objects through the bowel wall, which is most observed with toothpicks and cocktail picks, specifically.5
Foreign bodies identified on plain radiographs commonly include radiopaque objects, such as glass, metallic objects, most animal bones and some fish bones, and some medications. However, radiolucent objects, such as toothpicks and cocktail picks, wood, plastic, most fish bones, and most medicines, often will not appear on radiographs. The diagnosis of ingested foreign body can therefore easily be delayed or overlooked on plain radiographs due to ingestion of radiolucent objects or lack of adequate patient history. A high index of suspicion is needed in such instances. The modality of choice for identifying GI perforation due to ingested foreign objects is CT.5 All of these commonly missed materials on radiographs will be visible on CT with variable densities. As an added benefit, CT also may reveal ingested objects not visualized on radiographs and show ancillary signs of perforation, such as extraluminal free air, localized inflammation, and fluid collections or abscess surrounding a segment of thickened bowel.5
Most ingested foreign bodies will pass through the GI system and can be managed with careful observation alone. However, upper endoscopy is emergently indicated in 3 scenarios of foreign body ingestion: (1) complete occlusion of the esophagus with salivary pooling due to risk of aspiration; (2) ingestion of batteries due to toxic substances; and (3) ingestion of sharp or pointed foreign bodies due to risk of perforation.4 Overall, endoscopic intervention is required in 20% of cases and surgical intervention remains rare at 1%.4 In the case of this patient, an emergent upper endoscopy was needed due to suspected duodenal perforation.
Treatment of duodenal perforations due to foreign bodies may involve conservative, surgical, or endoscopic management. Contained, small perforations in a stable patient may be treated conservatively with IV fluids, antibiotics, and proton pump inhibitors as they self-seal with omentum if the foreign body has passed.6 Retained duodenal foreign bodies pose a risk of persistent perforation or fistulization and must be removed. Anterior duodenal perforations pose a risk of peritonitis, whereas posterior duodenal perforations, although retroperitoneal and sparing the peritoneal cavity, may result in localized abscess formation necessitating foreign body removal. Endoscopic clipping is a modernized, less invasive way to close GI perforations. Through-the-scope clips (TTSCs) can close luminal defects < 2 cm in size.7 Defects > 1 cm may be repaired with combined TTSCs and endoloop or omental patching. Over-the-scope clips can close full thickness defects up to 2 to 3 cm with the advantage of being able to close leaks and fistulas involving inflamed or indurated tissue.7
Conclusions
Intestinal perforations related to foreign body ingestion are a rare complication occurring in < 1% of patients. Although most ingested foreign objects will pass through the GI tract, elongated or sharp objects pose a risk for perforation. In many cases, a history of foreign body ingestion is not obtained, and a high index of suspicion is required. Duodenal perforations due to foreign body ingestion should be included in the differential among the more common diagnoses of peptic ulcers, pancreatitis, and gallbladder disease in the setting of postprandial right upper quadrant abdominal pain. CT is the best modality for identifying foreign bodies, including objects that may be missed on plain radiographs.
A 53-year-old male patient presented to the emergency department following a primary care office visit with sudden onset right upper quadrant abdominal pain that persisted for 3 weeks, worsening over the last 2 days. The abdominal pain worsened after eating or drinking and mildly improved with omeprazole. Associated symptoms included intermittent fever, night sweats, fatigue, and bloating since onset without vomiting or diarrhea. He reported a “complicated” cholecystectomy at an outside facility 6 months prior and that his “gallbladder was adhered to his duodenum,” though outside records were not available. Additional medical history included diverticulosis with prior flares of diverticulitis but no recent flares or treatments. His home medications included acetaminophen, naproxen, intranasal fluticasone, omeprazole, gabapentin, baclofen, trazodone, and antihistamines. He reported no tobacco or illicit drug use and stated he consumed a 6 pack of beer every 6 weeks.
Initial vital signs in the emergency department demonstrated an afebrile oral temperature with unremarkable blood pressure and pulse. He was alert and oriented and did not appear in significant acute distress. Physical examination of the abdomen demonstrated a nondistended abdomen, normal active bowel sounds in all 4 quadrants, and mild right upper and lower quadrant tenderness to soft and deep palpation with release.
Significant laboratory values included elevated C-reactive protein of 44.1 mg/L and mild leukocytosis of 11.1 K/µL (reference range, 4.00-10.60 K/µL). The basic metabolic panel, liver-associated enzymes, and lipase levels were within normal limits.
The initial imaging study was a computed tomography (CT) of the abdomen and pelvis with oral and IV contrast. The radiology report depicted a thin, needle-like hypodense foreign body approximately 8 cm in length in the proximal duodenum, slightly protruding extraluminally, and at least a moderate amount of surrounding inflammation without abscess or free air (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
Our Diagnosis
Based on the clinical history of postprandial abdominal pain with prior cholecystectomy and leukocytosis, the initial differential diagnosis included peptic ulcer disease, gastroesophageal reflux, or delayed sequela of the cholecystectomy 6 months prior. Although suspicion remained for possible delayed postoperative complications from the cholecystectomy, ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were not pursued based on CT imaging findings. The needle-like hypodensity in the duodenum with surrounding inflammation visualized on CT was concerning for an unidentified penetrating foreign body with a possible retroperitoneal microperforation.
After these imaging findings were relayed from Radiology to the Gastroenterology Service, the patient underwent an upper gastrointestinal (GI) endoscopy to further evaluate the duodenum. Inspection revealed mild gastritis and a linear, clear piece of plastic with both ends firmly lodged within the mucosa from the distal duodenal bulb to the second portion of the duodenum; a significant mucosal defect of the bowel wall was visualized after careful extraction of the foreign body (Figure 2). The patient was diagnosed with a small duodenal perforation, which was sealed endoscopically with 2 endoclips. The extracted piece of plastic was examined and determined to be a broken cocktail pick (Figure 3). During discussion with the patient postprocedure, he stated that he ingested several olive martinis (which were served with cocktail picks) approximately 3 weeks prior to presentation and did not recall ingesting the cocktail pick. A repeat abdominal CT following the endoscopy demonstrated no leak or free air from the site of the repaired duodenal perforation (Figure 4). The patient avoided surgery and was permitted to resume a liquid diet prior to discharge.
Discussion
Foreign body ingestion in adults is most commonly unintentional with fish bones being the most common culprit.1 In unintentional instances of foreign body ingestion, many patients are not aware of the event, with dentures posing a significant well-known risk factor due to lack of palatal sensory feedback.2 Most ingested foreign bodies pass uninhibited through the GI tract without complications. However, less than 1% of ingested foreign bodies cause potentially life-threatening GI perforations.3
The risk of GI perforation due to foreign body ingestion is greatest with elongated, sharp objects, such as needles, bones, toothpicks, and cocktail picks. These objects tend to lodge at areas of narrowing or angulation, such as the appendix, ileocecal region, or as in this case, the duodenum.3 Passage of a foreign body through the duodenum is more likely to be inhibited if the object is longer than 6 cm and with a diameter > 2.5 cm.4 Signs of duodenal perforation are often subtle compared with jejunal or ileal perforations. Patients are commonly afebrile with normal white blood cell counts and are more likely to have chronic symptoms for > 3 days before the appropriate diagnosis of foreign body ingestion is made.1 Duodenal perforations may be more stable clinically compared with distal GI perforations in part due to the retroperitoneal location with relatively fewer bacteria present intraluminally. GI perforations may not occur acutely during passage of the foreign body but can present weeks, months, or even years later.5 Delayed onset of symptoms may happen when the foreign body becomes lodged and only partially perforates the bowel wall, resulting in a chronic inflammatory process. Other possible complications include fistulization and abscess formation from migrating linear sharp objects through the bowel wall, which is most observed with toothpicks and cocktail picks, specifically.5
Foreign bodies identified on plain radiographs commonly include radiopaque objects, such as glass, metallic objects, most animal bones and some fish bones, and some medications. However, radiolucent objects, such as toothpicks and cocktail picks, wood, plastic, most fish bones, and most medicines, often will not appear on radiographs. The diagnosis of ingested foreign body can therefore easily be delayed or overlooked on plain radiographs due to ingestion of radiolucent objects or lack of adequate patient history. A high index of suspicion is needed in such instances. The modality of choice for identifying GI perforation due to ingested foreign objects is CT.5 All of these commonly missed materials on radiographs will be visible on CT with variable densities. As an added benefit, CT also may reveal ingested objects not visualized on radiographs and show ancillary signs of perforation, such as extraluminal free air, localized inflammation, and fluid collections or abscess surrounding a segment of thickened bowel.5
Most ingested foreign bodies will pass through the GI system and can be managed with careful observation alone. However, upper endoscopy is emergently indicated in 3 scenarios of foreign body ingestion: (1) complete occlusion of the esophagus with salivary pooling due to risk of aspiration; (2) ingestion of batteries due to toxic substances; and (3) ingestion of sharp or pointed foreign bodies due to risk of perforation.4 Overall, endoscopic intervention is required in 20% of cases and surgical intervention remains rare at 1%.4 In the case of this patient, an emergent upper endoscopy was needed due to suspected duodenal perforation.
Treatment of duodenal perforations due to foreign bodies may involve conservative, surgical, or endoscopic management. Contained, small perforations in a stable patient may be treated conservatively with IV fluids, antibiotics, and proton pump inhibitors as they self-seal with omentum if the foreign body has passed.6 Retained duodenal foreign bodies pose a risk of persistent perforation or fistulization and must be removed. Anterior duodenal perforations pose a risk of peritonitis, whereas posterior duodenal perforations, although retroperitoneal and sparing the peritoneal cavity, may result in localized abscess formation necessitating foreign body removal. Endoscopic clipping is a modernized, less invasive way to close GI perforations. Through-the-scope clips (TTSCs) can close luminal defects < 2 cm in size.7 Defects > 1 cm may be repaired with combined TTSCs and endoloop or omental patching. Over-the-scope clips can close full thickness defects up to 2 to 3 cm with the advantage of being able to close leaks and fistulas involving inflamed or indurated tissue.7
Conclusions
Intestinal perforations related to foreign body ingestion are a rare complication occurring in < 1% of patients. Although most ingested foreign objects will pass through the GI tract, elongated or sharp objects pose a risk for perforation. In many cases, a history of foreign body ingestion is not obtained, and a high index of suspicion is required. Duodenal perforations due to foreign body ingestion should be included in the differential among the more common diagnoses of peptic ulcers, pancreatitis, and gallbladder disease in the setting of postprandial right upper quadrant abdominal pain. CT is the best modality for identifying foreign bodies, including objects that may be missed on plain radiographs.
1. Goh BK, Chow PK, Quah HM, et al. Perforation of the gastrointestinal tract secondary to ingestion of foreign bodies. World J Surg. 2006;(30)372-377. doi:10.1007/s00268-005-0490-2
2. Bunker PG. The role of dentistry in problems of foreign body in the air and food passage. J Am Dent Assoc. 1962;(64):782-787. doi:10.14219/jada.archive.1962.0160
3. Hunter TB, Taljanovic MS. Foreign bodies. Radiographics. 2003;23(3):731-757. doi:10.1148/rg.233025137
4. Ambe P, Weber SA, Schauer M, Knoefel WT. Swallowed foreign bodies in adults. Dtsch Arztebl Int. 2012;109(50):869-875. doi:10.3238/arztebl.2012.0869
5. Kuzmich S, Burke CJ, Harvey CJ, et al. Perforation of gastrointestinal tract by poorly conspicuous ingested foreign bodies: radiological diagnosis. Br J Radiol. 2015;88(1050):20150086. doi:10.1259/bjr.20150086
6. Hill AG. Management of perforated duodenal ulcer. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001.
7. Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21(37):10542-10552. doi:10.3748/wjg.v21.i37.10542
1. Goh BK, Chow PK, Quah HM, et al. Perforation of the gastrointestinal tract secondary to ingestion of foreign bodies. World J Surg. 2006;(30)372-377. doi:10.1007/s00268-005-0490-2
2. Bunker PG. The role of dentistry in problems of foreign body in the air and food passage. J Am Dent Assoc. 1962;(64):782-787. doi:10.14219/jada.archive.1962.0160
3. Hunter TB, Taljanovic MS. Foreign bodies. Radiographics. 2003;23(3):731-757. doi:10.1148/rg.233025137
4. Ambe P, Weber SA, Schauer M, Knoefel WT. Swallowed foreign bodies in adults. Dtsch Arztebl Int. 2012;109(50):869-875. doi:10.3238/arztebl.2012.0869
5. Kuzmich S, Burke CJ, Harvey CJ, et al. Perforation of gastrointestinal tract by poorly conspicuous ingested foreign bodies: radiological diagnosis. Br J Radiol. 2015;88(1050):20150086. doi:10.1259/bjr.20150086
6. Hill AG. Management of perforated duodenal ulcer. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001.
7. Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21(37):10542-10552. doi:10.3748/wjg.v21.i37.10542
Cavitary Lung Lesion in a Tuberculosis-Negative Patient
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326