User login
LISBON – Physicians outside the United States are turning cartwheels over the outcomes they're obtaining with oral alitretinoin for chronic hand eczema.
New data from the postmarketing observational TOCCATA trial demonstrate that oral alitretinoin reduced eczema-related work absences by 50% in German workers, Dr. Thomas Diepgen reported at the annual congress of the European Academy of Dermatology and Venereology.
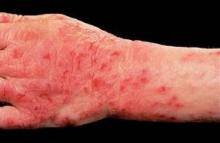
The TOCCATA study involved 680 German patients with chronic hand eczema rated as severe in two-thirds of participants and moderate in the remainder. TOCCATA was designed to determine whether alitretinoin’s performance in real-world clinical practice is similar to that in the pivotal 1,032-patient randomized BACH (Benefits of Alitretinoin in Chronic Hand Eczema) trial, which resulted in European approval of the novel retinoid (Br. J. Dermatol. 2008;158:808-17).
TOCCATA participants received either 10 mg or 30 mg of alitretinoin per day at their physician’s discretion for up to 24 weeks, with follow-up evaluations at 4-week intervals. Treatment outcomes in TOCCATA proved comparable to those in the BACH trial: a 56.7% rating of clear or almost clear by Physician Global Assessment, as compared with 47.7% in BACH.
As in BACH, the greatest response in TOCCATA was in patients with the hyperkeratotic rhagadiform form of hand eczema, with a 59.2% rate of clear or almost clear. But all the morphologic types showed a favorable response: a 47.9% clear or almost clear rate in dyshidrosiform hand eczema, and a 52.2% rate in fingertip eczema.
In TOCCATA, 522 participants were employed, mostly as steel or other metal workers, or in food processing. At baseline, 80 reported a work absence because of their chronic hand eczema within the previous 4 weeks. By week 8, this number had dropped to 53 workers. At weeks 12 and 16 it was 43 workers, at week 20 it was 41, and by week 24 only 37 TOCCATA participants had missed work during the previous 4 weeks, according to Dr. Diepgen, director of the department of social medicine, occupational, and environmental dermatology at the University of Heidelberg (Germany).
Not only was the number of workers taking sick leave reduced by slightly more than half, but total sick days were even more dramatically impacted. At baseline, workers who had taken hand eczema-related sick leave within the prior 4 weeks had missed an average of 17.6 work days during that period. By week 8, that figure had dropped to an average 10.7 days of absence in the last 4 weeks. At week 24, it was 7.6 days, meaning that after 6 months of daily alitretinoin workers who were off the job because of their hand eczema were taking 57% fewer sick days.
The reduction in work absenteeism in TOCCATA was paralleled by the clinical improvement. It took about 4 weeks of alitretinoin therapy to see a significant impact on Physician Global Assessment ratings. The bulk of the clinical improvement was achieved by week 12, Dr. Diepgen noted.
The most common adverse events in TOCCATA were mild to moderate mucocutaneous reactions, seen in about 10% of patients, which is a lower rate than reported with other retinoids. Headache occurred in 8% of patients, and flushing in 1%.
Alitretinoin is a potent teratogen, like other retinoids. However, its relatively short half-life of 2-10 hours means that women of childbearing potential must continue on contraception for 1 month posttreatment, as compared to 3 years with acitretin.
Asked how much oral alitretinoin costs, Dr. Diepgen said it’s about 600 euros (US $811) per month in Germany. But he added that in another study, he and his coworkers calculated that the yearly cost of severe chronic hand eczema was about 9,000 euros (US $12,170), mostly from sick leave and resultant lost workplace productivity. And that’s a conservative figure, he added; the indirect costs of severe hand eczema run nearly twice as high in expertly skilled, higher-paid workers.
The U.K. National Institute for Health and Clinical Excellence guidelines recommend oral 9-cis-retinoic acid as first-line therapy for patients who fail to respond to potent topical corticosteroids, as do Italian and Canadian guidelines and a German dermatology association. A phase III clinical trial (HANDEL) is currently underway in the United States.
Dr. Diepgen is an adviser to Basilea Pharmaceutica, which markets oral alitretinoin.
LISBON – Physicians outside the United States are turning cartwheels over the outcomes they're obtaining with oral alitretinoin for chronic hand eczema.
New data from the postmarketing observational TOCCATA trial demonstrate that oral alitretinoin reduced eczema-related work absences by 50% in German workers, Dr. Thomas Diepgen reported at the annual congress of the European Academy of Dermatology and Venereology.
The TOCCATA study involved 680 German patients with chronic hand eczema rated as severe in two-thirds of participants and moderate in the remainder. TOCCATA was designed to determine whether alitretinoin’s performance in real-world clinical practice is similar to that in the pivotal 1,032-patient randomized BACH (Benefits of Alitretinoin in Chronic Hand Eczema) trial, which resulted in European approval of the novel retinoid (Br. J. Dermatol. 2008;158:808-17).
TOCCATA participants received either 10 mg or 30 mg of alitretinoin per day at their physician’s discretion for up to 24 weeks, with follow-up evaluations at 4-week intervals. Treatment outcomes in TOCCATA proved comparable to those in the BACH trial: a 56.7% rating of clear or almost clear by Physician Global Assessment, as compared with 47.7% in BACH.
As in BACH, the greatest response in TOCCATA was in patients with the hyperkeratotic rhagadiform form of hand eczema, with a 59.2% rate of clear or almost clear. But all the morphologic types showed a favorable response: a 47.9% clear or almost clear rate in dyshidrosiform hand eczema, and a 52.2% rate in fingertip eczema.
In TOCCATA, 522 participants were employed, mostly as steel or other metal workers, or in food processing. At baseline, 80 reported a work absence because of their chronic hand eczema within the previous 4 weeks. By week 8, this number had dropped to 53 workers. At weeks 12 and 16 it was 43 workers, at week 20 it was 41, and by week 24 only 37 TOCCATA participants had missed work during the previous 4 weeks, according to Dr. Diepgen, director of the department of social medicine, occupational, and environmental dermatology at the University of Heidelberg (Germany).
Not only was the number of workers taking sick leave reduced by slightly more than half, but total sick days were even more dramatically impacted. At baseline, workers who had taken hand eczema-related sick leave within the prior 4 weeks had missed an average of 17.6 work days during that period. By week 8, that figure had dropped to an average 10.7 days of absence in the last 4 weeks. At week 24, it was 7.6 days, meaning that after 6 months of daily alitretinoin workers who were off the job because of their hand eczema were taking 57% fewer sick days.
The reduction in work absenteeism in TOCCATA was paralleled by the clinical improvement. It took about 4 weeks of alitretinoin therapy to see a significant impact on Physician Global Assessment ratings. The bulk of the clinical improvement was achieved by week 12, Dr. Diepgen noted.
The most common adverse events in TOCCATA were mild to moderate mucocutaneous reactions, seen in about 10% of patients, which is a lower rate than reported with other retinoids. Headache occurred in 8% of patients, and flushing in 1%.
Alitretinoin is a potent teratogen, like other retinoids. However, its relatively short half-life of 2-10 hours means that women of childbearing potential must continue on contraception for 1 month posttreatment, as compared to 3 years with acitretin.
Asked how much oral alitretinoin costs, Dr. Diepgen said it’s about 600 euros (US $811) per month in Germany. But he added that in another study, he and his coworkers calculated that the yearly cost of severe chronic hand eczema was about 9,000 euros (US $12,170), mostly from sick leave and resultant lost workplace productivity. And that’s a conservative figure, he added; the indirect costs of severe hand eczema run nearly twice as high in expertly skilled, higher-paid workers.
The U.K. National Institute for Health and Clinical Excellence guidelines recommend oral 9-cis-retinoic acid as first-line therapy for patients who fail to respond to potent topical corticosteroids, as do Italian and Canadian guidelines and a German dermatology association. A phase III clinical trial (HANDEL) is currently underway in the United States.
Dr. Diepgen is an adviser to Basilea Pharmaceutica, which markets oral alitretinoin.
LISBON – Physicians outside the United States are turning cartwheels over the outcomes they're obtaining with oral alitretinoin for chronic hand eczema.
New data from the postmarketing observational TOCCATA trial demonstrate that oral alitretinoin reduced eczema-related work absences by 50% in German workers, Dr. Thomas Diepgen reported at the annual congress of the European Academy of Dermatology and Venereology.
The TOCCATA study involved 680 German patients with chronic hand eczema rated as severe in two-thirds of participants and moderate in the remainder. TOCCATA was designed to determine whether alitretinoin’s performance in real-world clinical practice is similar to that in the pivotal 1,032-patient randomized BACH (Benefits of Alitretinoin in Chronic Hand Eczema) trial, which resulted in European approval of the novel retinoid (Br. J. Dermatol. 2008;158:808-17).
TOCCATA participants received either 10 mg or 30 mg of alitretinoin per day at their physician’s discretion for up to 24 weeks, with follow-up evaluations at 4-week intervals. Treatment outcomes in TOCCATA proved comparable to those in the BACH trial: a 56.7% rating of clear or almost clear by Physician Global Assessment, as compared with 47.7% in BACH.
As in BACH, the greatest response in TOCCATA was in patients with the hyperkeratotic rhagadiform form of hand eczema, with a 59.2% rate of clear or almost clear. But all the morphologic types showed a favorable response: a 47.9% clear or almost clear rate in dyshidrosiform hand eczema, and a 52.2% rate in fingertip eczema.
In TOCCATA, 522 participants were employed, mostly as steel or other metal workers, or in food processing. At baseline, 80 reported a work absence because of their chronic hand eczema within the previous 4 weeks. By week 8, this number had dropped to 53 workers. At weeks 12 and 16 it was 43 workers, at week 20 it was 41, and by week 24 only 37 TOCCATA participants had missed work during the previous 4 weeks, according to Dr. Diepgen, director of the department of social medicine, occupational, and environmental dermatology at the University of Heidelberg (Germany).
Not only was the number of workers taking sick leave reduced by slightly more than half, but total sick days were even more dramatically impacted. At baseline, workers who had taken hand eczema-related sick leave within the prior 4 weeks had missed an average of 17.6 work days during that period. By week 8, that figure had dropped to an average 10.7 days of absence in the last 4 weeks. At week 24, it was 7.6 days, meaning that after 6 months of daily alitretinoin workers who were off the job because of their hand eczema were taking 57% fewer sick days.
The reduction in work absenteeism in TOCCATA was paralleled by the clinical improvement. It took about 4 weeks of alitretinoin therapy to see a significant impact on Physician Global Assessment ratings. The bulk of the clinical improvement was achieved by week 12, Dr. Diepgen noted.
The most common adverse events in TOCCATA were mild to moderate mucocutaneous reactions, seen in about 10% of patients, which is a lower rate than reported with other retinoids. Headache occurred in 8% of patients, and flushing in 1%.
Alitretinoin is a potent teratogen, like other retinoids. However, its relatively short half-life of 2-10 hours means that women of childbearing potential must continue on contraception for 1 month posttreatment, as compared to 3 years with acitretin.
Asked how much oral alitretinoin costs, Dr. Diepgen said it’s about 600 euros (US $811) per month in Germany. But he added that in another study, he and his coworkers calculated that the yearly cost of severe chronic hand eczema was about 9,000 euros (US $12,170), mostly from sick leave and resultant lost workplace productivity. And that’s a conservative figure, he added; the indirect costs of severe hand eczema run nearly twice as high in expertly skilled, higher-paid workers.
The U.K. National Institute for Health and Clinical Excellence guidelines recommend oral 9-cis-retinoic acid as first-line therapy for patients who fail to respond to potent topical corticosteroids, as do Italian and Canadian guidelines and a German dermatology association. A phase III clinical trial (HANDEL) is currently underway in the United States.
Dr. Diepgen is an adviser to Basilea Pharmaceutica, which markets oral alitretinoin.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: The number of German workers who took sick leave for their chronic hand eczema was reduced by half after they went on daily therapy with oral alliteration.
Data Source: The observational 680-patient TOCCATA study.
Disclosures: The study was funded by Basilea Pharmaceuticals, which markets oral alitretinoin in Europe and Canada. Dr. Diepgen is an adviser to the company.