User login
Ondansetron Quells Methotrexate-Induced Nausea
LISBON – Getting an earful of complaints of nausea from your patients on oral methotrexate therapy?
Consider bracketing the antifolate drug with the antiemetic ondansetron before and afterward, Dr. Richard B. Warren suggested at the congress.
"One successful means of helping patients overcome their nausea that we’ve used a lot in Manchester is ondansetron. For some people it can literally sort out the nausea altogether," said Dr. Warren, senior lecturer and honorary consultant dermatologist at the University of Manchester (England).
The dosing is 8 mg of ondansetron (Zofran) – a serotonin receptor antagonist used to treat nausea caused by chemotherapy, radiation therapy, and/or surgery – administered 2 hours before and once again 12 hours after taking methotrexate.
Nausea is a common nuisance side effect of the drug. It affects roughly one-quarter of patients, typically beginning about 12 hours after they take their weekly dose. The nausea can last for up to 72 hours.
In addition to prophylactic ondansetron, other strategies that may be effective in overcoming methotrexate-induced nausea include the following:
– Divide the dose.
– Have patients take their oral methotrexate with the evening meal.
– Consider switching to subcutaneous methotrexate. "It has been shown to be more efficacious, and with less in the way of adverse events than oral methotrexate," Dr. Warren said.
He declared having no relevant financial interests.
LISBON – Getting an earful of complaints of nausea from your patients on oral methotrexate therapy?
Consider bracketing the antifolate drug with the antiemetic ondansetron before and afterward, Dr. Richard B. Warren suggested at the congress.
"One successful means of helping patients overcome their nausea that we’ve used a lot in Manchester is ondansetron. For some people it can literally sort out the nausea altogether," said Dr. Warren, senior lecturer and honorary consultant dermatologist at the University of Manchester (England).
The dosing is 8 mg of ondansetron (Zofran) – a serotonin receptor antagonist used to treat nausea caused by chemotherapy, radiation therapy, and/or surgery – administered 2 hours before and once again 12 hours after taking methotrexate.
Nausea is a common nuisance side effect of the drug. It affects roughly one-quarter of patients, typically beginning about 12 hours after they take their weekly dose. The nausea can last for up to 72 hours.
In addition to prophylactic ondansetron, other strategies that may be effective in overcoming methotrexate-induced nausea include the following:
– Divide the dose.
– Have patients take their oral methotrexate with the evening meal.
– Consider switching to subcutaneous methotrexate. "It has been shown to be more efficacious, and with less in the way of adverse events than oral methotrexate," Dr. Warren said.
He declared having no relevant financial interests.
LISBON – Getting an earful of complaints of nausea from your patients on oral methotrexate therapy?
Consider bracketing the antifolate drug with the antiemetic ondansetron before and afterward, Dr. Richard B. Warren suggested at the congress.
"One successful means of helping patients overcome their nausea that we’ve used a lot in Manchester is ondansetron. For some people it can literally sort out the nausea altogether," said Dr. Warren, senior lecturer and honorary consultant dermatologist at the University of Manchester (England).
The dosing is 8 mg of ondansetron (Zofran) – a serotonin receptor antagonist used to treat nausea caused by chemotherapy, radiation therapy, and/or surgery – administered 2 hours before and once again 12 hours after taking methotrexate.
Nausea is a common nuisance side effect of the drug. It affects roughly one-quarter of patients, typically beginning about 12 hours after they take their weekly dose. The nausea can last for up to 72 hours.
In addition to prophylactic ondansetron, other strategies that may be effective in overcoming methotrexate-induced nausea include the following:
– Divide the dose.
– Have patients take their oral methotrexate with the evening meal.
– Consider switching to subcutaneous methotrexate. "It has been shown to be more efficacious, and with less in the way of adverse events than oral methotrexate," Dr. Warren said.
He declared having no relevant financial interests.
EXPERT OPINION FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
The Whys and How of Stopping Biologics
The vast majority of medical talks on biologic therapy for psoriasis focus on starting and maintaining the medications. Stopping biologics is a seldom-discussed topic, yet one that physicians often need to address.
"We’ve all got patients who are on biologic therapy who are completely clear of their psoriasis, and you’re constantly wondering, ‘Should I stop the biologic? Do they need to have that treatment anymore? Can I reduce the dosing frequency?’ The simplest answer is that in most cases, it’s probably not a good idea to stop unless there’s a good reason for doing so. It has been shown in most of the big clinical trials that if you stop therapy, the psoriasis will relapse at some point," Dr. Christopher Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
"With long-term therapy beyond 6-12 months, can biologics be stopped or produce remission? In most cases, no. And there’s no biomarker for disease activity as of yet to guide us," added Dr. Griffiths, professor of dermatology and associate dean for research at the University of Manchester (England).
Compelling reasons to stop biologic therapy include failure to achieve or maintain significant clinical improvement, serious adverse events, impending elective major surgery, certain vaccinations, and pregnancy. Dr. Griffiths highlighted the following points:
• Lack of Effectiveness. Today’s biologics are not curative. In a recent French report, only 31 of 86 psoriasis patients (36%) who started on etanercept (Enbrel), infliximab (Remicade), or efalizumab were still on the same biologic agent 24 months later (J. Dermatol. Treat. 2011;22:151-2). Similarly, the Danish national psoriasis database experience has been that roughly 40% of patients started on etanercept or adalimumab (Humira) were still on the medication 4 years later, as were 70% of those who started on infliximab (Br. J. Dermatol. 2011;164:1091-6).
The good news, Dr. Griffiths continued, is that lack of effectiveness for anti–tumor necrosis factor agents is not a class effect. He and his coinvestigators have reported that 21 of 31 psoriasis patients who switched to adalimumab from another anti-TNF biologic for lack of efficacy met the NICE (U.K. National Institute for Health and Clinical Excellence) criteria for adalimumab treatment continuation at 16 weeks (Br. J. Dermatol. 2010;163:859-62).
"Each anti-TNF drug is different, so if you fail one it doesn’t mean you’re going to fail another. That’s a very important point, and a very strong argument in your favor when dealing with payers. And it’s an observation that can only come from real-life data from a cohort study; you’re not going to get that information from clinical trials," the dermatologist explained.
The NICE criteria for continuation of biologic therapy bring an element of strict objectivity to treatment decision making in a rationed health care system. For British psoriasis patients to continue on a given biologic agent, they have to demonstrate a PASI 75 response (that is, a 75% improvement over the baseline Psoriasis Area and Severity Index score) at 16 weeks, or a PASI 50 response plus a 5-point drop in the Dermatology Life Quality Index (DLQI).
When physicians switch biologics, Dr. Griffiths recommends that they skip a lengthy washout period because of the associated risk of a severe, hard-to-control flare. His practice is to stop one and start another.
• Major Elective Surgery. There is a dearth of data on stopping biologics in psoriasis patients who plan to undergo elective surgery. The best practice for now, in Dr. Griffiths’ view, is to follow the British Society for Rheumatology guidance, which is based on an extensive biologics register the group maintains for rheumatoid arthritis.
The rheumatologists’ advice is to halt effective biologic therapy only for truly major surgery, and to stop anti-TNF drugs more than four half-lives before the operation. That’s 2-3 weeks beforehand for etanercept, 6-8 weeks for adalimumab, and 4-6 weeks for infliximab. There are no firm data for ustekinumab (Stelara) as yet, but experts advise halting it 12 weeks before surgery. Biologic therapy should be resumed as soon as possible postoperatively.
• Pregnancy. Again, a paucity of data exists on biologics for psoriasis in pregnancy. But the data accumulating in the British Society for Rheumatology Biologics Register (BSRBR) is reassuring. Although the rheumatologists recommend that pregnancy be avoided in patients on biologics, the experience to date in pregnant rheumatoid arthritis patients suggests there is little to no risk to the fetus. Breastfeeding should be avoided by women on biologic therapy other than infliximab, which is not excreted in breast milk, Dr. Griffiths continued.
• Vaccinations. British Association of Dermatologists guidelines on the use of biologics for psoriasis (Br. J. Dermatol. 2009;161:987-1019), which Dr. Griffiths coauthored and which will be updated in late 2012, recommend avoiding giving live or attenuated virus vaccines within 2 weeks prior to starting a patient on biologic therapy, or while the patient is on a biologic, or for up to 6 months after the patient stops the biologic. Inactivated virus vaccines are safe for patients on biologic therapy, although the antibody response will be somewhat less robust in a patient on an anti-TNF agent than in other individuals.
"But we advise – as should you – that all patients on biologics should receive influenza and pneumococcal vaccines. It’s just good clinical practice because these patients are by definition in a high-risk category," he said.
• Stopping and Restarting Biologics. It’s well established that etanercept can be used intermittently with good results. That is, a patient might use etanercept to good effect for 6 months, stop it, then restart when relapse occurs, and the agent will still remain effective. The same typically holds true for alefacept (Amevive).
In contrast, infliximab can realistically be used for only a single course of treatment. If a patient goes off the drug and later goes back on it, the chances of regaining a response are very low because of the formation of blocking antibodies.
The picture regarding the intermittent use of adalimumab is less clear. There are documented cases in which this agent hasn’t been effective any longer upon second usage.
There is good new evidence, presented by Dr. Griffiths elsewhere during the congress, that ustekinumab can be restarted after a hiatus with very good results.
Dr. Griffiths disclosed that he serves on the advisory boards for and has received research grants from numerous pharmaceutical companies.
The vast majority of medical talks on biologic therapy for psoriasis focus on starting and maintaining the medications. Stopping biologics is a seldom-discussed topic, yet one that physicians often need to address.
"We’ve all got patients who are on biologic therapy who are completely clear of their psoriasis, and you’re constantly wondering, ‘Should I stop the biologic? Do they need to have that treatment anymore? Can I reduce the dosing frequency?’ The simplest answer is that in most cases, it’s probably not a good idea to stop unless there’s a good reason for doing so. It has been shown in most of the big clinical trials that if you stop therapy, the psoriasis will relapse at some point," Dr. Christopher Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
"With long-term therapy beyond 6-12 months, can biologics be stopped or produce remission? In most cases, no. And there’s no biomarker for disease activity as of yet to guide us," added Dr. Griffiths, professor of dermatology and associate dean for research at the University of Manchester (England).
Compelling reasons to stop biologic therapy include failure to achieve or maintain significant clinical improvement, serious adverse events, impending elective major surgery, certain vaccinations, and pregnancy. Dr. Griffiths highlighted the following points:
• Lack of Effectiveness. Today’s biologics are not curative. In a recent French report, only 31 of 86 psoriasis patients (36%) who started on etanercept (Enbrel), infliximab (Remicade), or efalizumab were still on the same biologic agent 24 months later (J. Dermatol. Treat. 2011;22:151-2). Similarly, the Danish national psoriasis database experience has been that roughly 40% of patients started on etanercept or adalimumab (Humira) were still on the medication 4 years later, as were 70% of those who started on infliximab (Br. J. Dermatol. 2011;164:1091-6).
The good news, Dr. Griffiths continued, is that lack of effectiveness for anti–tumor necrosis factor agents is not a class effect. He and his coinvestigators have reported that 21 of 31 psoriasis patients who switched to adalimumab from another anti-TNF biologic for lack of efficacy met the NICE (U.K. National Institute for Health and Clinical Excellence) criteria for adalimumab treatment continuation at 16 weeks (Br. J. Dermatol. 2010;163:859-62).
"Each anti-TNF drug is different, so if you fail one it doesn’t mean you’re going to fail another. That’s a very important point, and a very strong argument in your favor when dealing with payers. And it’s an observation that can only come from real-life data from a cohort study; you’re not going to get that information from clinical trials," the dermatologist explained.
The NICE criteria for continuation of biologic therapy bring an element of strict objectivity to treatment decision making in a rationed health care system. For British psoriasis patients to continue on a given biologic agent, they have to demonstrate a PASI 75 response (that is, a 75% improvement over the baseline Psoriasis Area and Severity Index score) at 16 weeks, or a PASI 50 response plus a 5-point drop in the Dermatology Life Quality Index (DLQI).
When physicians switch biologics, Dr. Griffiths recommends that they skip a lengthy washout period because of the associated risk of a severe, hard-to-control flare. His practice is to stop one and start another.
• Major Elective Surgery. There is a dearth of data on stopping biologics in psoriasis patients who plan to undergo elective surgery. The best practice for now, in Dr. Griffiths’ view, is to follow the British Society for Rheumatology guidance, which is based on an extensive biologics register the group maintains for rheumatoid arthritis.
The rheumatologists’ advice is to halt effective biologic therapy only for truly major surgery, and to stop anti-TNF drugs more than four half-lives before the operation. That’s 2-3 weeks beforehand for etanercept, 6-8 weeks for adalimumab, and 4-6 weeks for infliximab. There are no firm data for ustekinumab (Stelara) as yet, but experts advise halting it 12 weeks before surgery. Biologic therapy should be resumed as soon as possible postoperatively.
• Pregnancy. Again, a paucity of data exists on biologics for psoriasis in pregnancy. But the data accumulating in the British Society for Rheumatology Biologics Register (BSRBR) is reassuring. Although the rheumatologists recommend that pregnancy be avoided in patients on biologics, the experience to date in pregnant rheumatoid arthritis patients suggests there is little to no risk to the fetus. Breastfeeding should be avoided by women on biologic therapy other than infliximab, which is not excreted in breast milk, Dr. Griffiths continued.
• Vaccinations. British Association of Dermatologists guidelines on the use of biologics for psoriasis (Br. J. Dermatol. 2009;161:987-1019), which Dr. Griffiths coauthored and which will be updated in late 2012, recommend avoiding giving live or attenuated virus vaccines within 2 weeks prior to starting a patient on biologic therapy, or while the patient is on a biologic, or for up to 6 months after the patient stops the biologic. Inactivated virus vaccines are safe for patients on biologic therapy, although the antibody response will be somewhat less robust in a patient on an anti-TNF agent than in other individuals.
"But we advise – as should you – that all patients on biologics should receive influenza and pneumococcal vaccines. It’s just good clinical practice because these patients are by definition in a high-risk category," he said.
• Stopping and Restarting Biologics. It’s well established that etanercept can be used intermittently with good results. That is, a patient might use etanercept to good effect for 6 months, stop it, then restart when relapse occurs, and the agent will still remain effective. The same typically holds true for alefacept (Amevive).
In contrast, infliximab can realistically be used for only a single course of treatment. If a patient goes off the drug and later goes back on it, the chances of regaining a response are very low because of the formation of blocking antibodies.
The picture regarding the intermittent use of adalimumab is less clear. There are documented cases in which this agent hasn’t been effective any longer upon second usage.
There is good new evidence, presented by Dr. Griffiths elsewhere during the congress, that ustekinumab can be restarted after a hiatus with very good results.
Dr. Griffiths disclosed that he serves on the advisory boards for and has received research grants from numerous pharmaceutical companies.
The vast majority of medical talks on biologic therapy for psoriasis focus on starting and maintaining the medications. Stopping biologics is a seldom-discussed topic, yet one that physicians often need to address.
"We’ve all got patients who are on biologic therapy who are completely clear of their psoriasis, and you’re constantly wondering, ‘Should I stop the biologic? Do they need to have that treatment anymore? Can I reduce the dosing frequency?’ The simplest answer is that in most cases, it’s probably not a good idea to stop unless there’s a good reason for doing so. It has been shown in most of the big clinical trials that if you stop therapy, the psoriasis will relapse at some point," Dr. Christopher Griffiths said at the annual congress of the European Academy of Dermatology and Venereology.
"With long-term therapy beyond 6-12 months, can biologics be stopped or produce remission? In most cases, no. And there’s no biomarker for disease activity as of yet to guide us," added Dr. Griffiths, professor of dermatology and associate dean for research at the University of Manchester (England).
Compelling reasons to stop biologic therapy include failure to achieve or maintain significant clinical improvement, serious adverse events, impending elective major surgery, certain vaccinations, and pregnancy. Dr. Griffiths highlighted the following points:
• Lack of Effectiveness. Today’s biologics are not curative. In a recent French report, only 31 of 86 psoriasis patients (36%) who started on etanercept (Enbrel), infliximab (Remicade), or efalizumab were still on the same biologic agent 24 months later (J. Dermatol. Treat. 2011;22:151-2). Similarly, the Danish national psoriasis database experience has been that roughly 40% of patients started on etanercept or adalimumab (Humira) were still on the medication 4 years later, as were 70% of those who started on infliximab (Br. J. Dermatol. 2011;164:1091-6).
The good news, Dr. Griffiths continued, is that lack of effectiveness for anti–tumor necrosis factor agents is not a class effect. He and his coinvestigators have reported that 21 of 31 psoriasis patients who switched to adalimumab from another anti-TNF biologic for lack of efficacy met the NICE (U.K. National Institute for Health and Clinical Excellence) criteria for adalimumab treatment continuation at 16 weeks (Br. J. Dermatol. 2010;163:859-62).
"Each anti-TNF drug is different, so if you fail one it doesn’t mean you’re going to fail another. That’s a very important point, and a very strong argument in your favor when dealing with payers. And it’s an observation that can only come from real-life data from a cohort study; you’re not going to get that information from clinical trials," the dermatologist explained.
The NICE criteria for continuation of biologic therapy bring an element of strict objectivity to treatment decision making in a rationed health care system. For British psoriasis patients to continue on a given biologic agent, they have to demonstrate a PASI 75 response (that is, a 75% improvement over the baseline Psoriasis Area and Severity Index score) at 16 weeks, or a PASI 50 response plus a 5-point drop in the Dermatology Life Quality Index (DLQI).
When physicians switch biologics, Dr. Griffiths recommends that they skip a lengthy washout period because of the associated risk of a severe, hard-to-control flare. His practice is to stop one and start another.
• Major Elective Surgery. There is a dearth of data on stopping biologics in psoriasis patients who plan to undergo elective surgery. The best practice for now, in Dr. Griffiths’ view, is to follow the British Society for Rheumatology guidance, which is based on an extensive biologics register the group maintains for rheumatoid arthritis.
The rheumatologists’ advice is to halt effective biologic therapy only for truly major surgery, and to stop anti-TNF drugs more than four half-lives before the operation. That’s 2-3 weeks beforehand for etanercept, 6-8 weeks for adalimumab, and 4-6 weeks for infliximab. There are no firm data for ustekinumab (Stelara) as yet, but experts advise halting it 12 weeks before surgery. Biologic therapy should be resumed as soon as possible postoperatively.
• Pregnancy. Again, a paucity of data exists on biologics for psoriasis in pregnancy. But the data accumulating in the British Society for Rheumatology Biologics Register (BSRBR) is reassuring. Although the rheumatologists recommend that pregnancy be avoided in patients on biologics, the experience to date in pregnant rheumatoid arthritis patients suggests there is little to no risk to the fetus. Breastfeeding should be avoided by women on biologic therapy other than infliximab, which is not excreted in breast milk, Dr. Griffiths continued.
• Vaccinations. British Association of Dermatologists guidelines on the use of biologics for psoriasis (Br. J. Dermatol. 2009;161:987-1019), which Dr. Griffiths coauthored and which will be updated in late 2012, recommend avoiding giving live or attenuated virus vaccines within 2 weeks prior to starting a patient on biologic therapy, or while the patient is on a biologic, or for up to 6 months after the patient stops the biologic. Inactivated virus vaccines are safe for patients on biologic therapy, although the antibody response will be somewhat less robust in a patient on an anti-TNF agent than in other individuals.
"But we advise – as should you – that all patients on biologics should receive influenza and pneumococcal vaccines. It’s just good clinical practice because these patients are by definition in a high-risk category," he said.
• Stopping and Restarting Biologics. It’s well established that etanercept can be used intermittently with good results. That is, a patient might use etanercept to good effect for 6 months, stop it, then restart when relapse occurs, and the agent will still remain effective. The same typically holds true for alefacept (Amevive).
In contrast, infliximab can realistically be used for only a single course of treatment. If a patient goes off the drug and later goes back on it, the chances of regaining a response are very low because of the formation of blocking antibodies.
The picture regarding the intermittent use of adalimumab is less clear. There are documented cases in which this agent hasn’t been effective any longer upon second usage.
There is good new evidence, presented by Dr. Griffiths elsewhere during the congress, that ustekinumab can be restarted after a hiatus with very good results.
Dr. Griffiths disclosed that he serves on the advisory boards for and has received research grants from numerous pharmaceutical companies.
'Transcutaneous Systemic' Clobetasol Reverses Bullous Pemphigoid
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Disease control was achieved in a mean of 26 days in 90% of patients with mild disease and in 73.5% with severe disease.
Data Source: A series of 74 bullous pemphigoid patients.
Disclosures: Dr. Jonkman reported no relevant financial disclosures.
For Etanercept Nonresponders, Switch or Escalate?
LISBON – Switching to adalimumab in psoriasis patients who are not responding adequately to etanercept is significantly more cost effective than escalating the etanercept dose, an analysis has shown.
During the first 6 months following the decision to either switch or escalate, 372 etaner cept (Enbrel) dose escalators incurred an adjusted average of $2,451 more in incremental total health care costs than the $12,943 average in 728 patients who instead switched to adalimumab (Humira), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
Driving the markedly higher total costs in the etanercept dose escalation group was their significantly greater utilization of both inpatient and outpatient services, including urgent care. Also, psoriasis drug costs, which accounted for 75% of total health care costs, were significantly lower in the group that switched to adalimumab. The cost of adalimumab over the course of 6 months averaged an adjusted $1,573 less per patient than etanercept therapy, according to Dr. Papp, director of research at Probity Medical Research in Waterloo, Ont.
Subjects for this analysis were drawn from the MarketScan and Ingenix Impact National Managed Care databases. All participants were etanercept-treated psoriasis patients with full health care utilization and cost data accessible for the 6 months immediately before the escalate or switch decision and for 6 months afterward.
The two groups had nearly identical total health care costs during the baseline first 6 months: an average of $11,264 in patients prior to etanercept dose escalation, compared with $11,628 in those who went on to switch to adalimumab. The two groups were similar in age, sex, and comorbidities. However, the switchers appeared to have more severe psoriasis. They had a greater prevalence of psoriatic arthritis. Moreover, during the baseline period, they had significantly more outpatient visits, and 41% of them used antimicrobial medications, compared with 33% in the dose escalation group. These potential confounders were adjusted for in the analysis.
This switch or escalate decision point is a common but underappreciated aspect of etanercept therapy. Prior studies have established that 30%-50% of psoriasis patients placed on etanercept require a dosing increase during the first year of therapy, according to Dr. Papp.
This study was funded by Abbott Laboratories. Dr. Papp disclosed that he serves as an adviser to Abbott as well as numerous other pharmaceutical companies funding psoriasis research.
LISBON – Switching to adalimumab in psoriasis patients who are not responding adequately to etanercept is significantly more cost effective than escalating the etanercept dose, an analysis has shown.
During the first 6 months following the decision to either switch or escalate, 372 etaner cept (Enbrel) dose escalators incurred an adjusted average of $2,451 more in incremental total health care costs than the $12,943 average in 728 patients who instead switched to adalimumab (Humira), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
Driving the markedly higher total costs in the etanercept dose escalation group was their significantly greater utilization of both inpatient and outpatient services, including urgent care. Also, psoriasis drug costs, which accounted for 75% of total health care costs, were significantly lower in the group that switched to adalimumab. The cost of adalimumab over the course of 6 months averaged an adjusted $1,573 less per patient than etanercept therapy, according to Dr. Papp, director of research at Probity Medical Research in Waterloo, Ont.
Subjects for this analysis were drawn from the MarketScan and Ingenix Impact National Managed Care databases. All participants were etanercept-treated psoriasis patients with full health care utilization and cost data accessible for the 6 months immediately before the escalate or switch decision and for 6 months afterward.
The two groups had nearly identical total health care costs during the baseline first 6 months: an average of $11,264 in patients prior to etanercept dose escalation, compared with $11,628 in those who went on to switch to adalimumab. The two groups were similar in age, sex, and comorbidities. However, the switchers appeared to have more severe psoriasis. They had a greater prevalence of psoriatic arthritis. Moreover, during the baseline period, they had significantly more outpatient visits, and 41% of them used antimicrobial medications, compared with 33% in the dose escalation group. These potential confounders were adjusted for in the analysis.
This switch or escalate decision point is a common but underappreciated aspect of etanercept therapy. Prior studies have established that 30%-50% of psoriasis patients placed on etanercept require a dosing increase during the first year of therapy, according to Dr. Papp.
This study was funded by Abbott Laboratories. Dr. Papp disclosed that he serves as an adviser to Abbott as well as numerous other pharmaceutical companies funding psoriasis research.
LISBON – Switching to adalimumab in psoriasis patients who are not responding adequately to etanercept is significantly more cost effective than escalating the etanercept dose, an analysis has shown.
During the first 6 months following the decision to either switch or escalate, 372 etaner cept (Enbrel) dose escalators incurred an adjusted average of $2,451 more in incremental total health care costs than the $12,943 average in 728 patients who instead switched to adalimumab (Humira), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
Driving the markedly higher total costs in the etanercept dose escalation group was their significantly greater utilization of both inpatient and outpatient services, including urgent care. Also, psoriasis drug costs, which accounted for 75% of total health care costs, were significantly lower in the group that switched to adalimumab. The cost of adalimumab over the course of 6 months averaged an adjusted $1,573 less per patient than etanercept therapy, according to Dr. Papp, director of research at Probity Medical Research in Waterloo, Ont.
Subjects for this analysis were drawn from the MarketScan and Ingenix Impact National Managed Care databases. All participants were etanercept-treated psoriasis patients with full health care utilization and cost data accessible for the 6 months immediately before the escalate or switch decision and for 6 months afterward.
The two groups had nearly identical total health care costs during the baseline first 6 months: an average of $11,264 in patients prior to etanercept dose escalation, compared with $11,628 in those who went on to switch to adalimumab. The two groups were similar in age, sex, and comorbidities. However, the switchers appeared to have more severe psoriasis. They had a greater prevalence of psoriatic arthritis. Moreover, during the baseline period, they had significantly more outpatient visits, and 41% of them used antimicrobial medications, compared with 33% in the dose escalation group. These potential confounders were adjusted for in the analysis.
This switch or escalate decision point is a common but underappreciated aspect of etanercept therapy. Prior studies have established that 30%-50% of psoriasis patients placed on etanercept require a dosing increase during the first year of therapy, according to Dr. Papp.
This study was funded by Abbott Laboratories. Dr. Papp disclosed that he serves as an adviser to Abbott as well as numerous other pharmaceutical companies funding psoriasis research.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: During the first 6 months after psoriasis patients were switched from etanercept to adalimumab because of an inadequate response to etanercept, total health care costs averaged $2,451 less than in nonresponders whose etanercept dose was escalated.
Data Source: Analysis of administrative databases containing complete data on health care costs and resource utilization for 728 switchers and 372 dose escalators.
Disclosures: The study was sponsored by Abbott Laboratories. Dr. Papp disclosed that he serves as an adviser to Abbott as well as numerous other pharmaceutical companies funding psoriasis research.
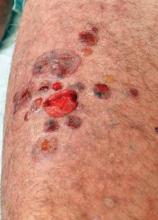
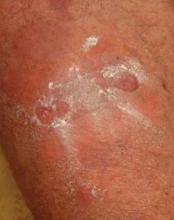
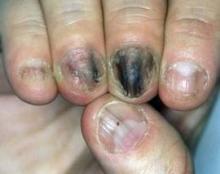
Pulsed Dye Laser Zaps Nail Psoriasis in Small Study
LISBON – Pulsed dye laser therapy may be an attractive new option for treating nail psoriasis, according to Dr. Veronique Blatiere.
Nail psoriasis is challenging to treat because the psoriatic disease process damages the nails while they are still being formed. But Turkish investigators have reported positive results with three once-monthly pulsed dye laser (PDL) treatment sessions in a small uncontrolled patient series, Dr. Blatiere reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Yasemin Oram and coworkers at the American Hospital in Istanbul, Turkey, reported on five patients with nail psoriasis treated using PDL. The laser therapy was applied at 595 nm with a pulse duration of 1.5 milliseconds, a beam diameter of 7 mm, and an energy fluence of 8-10 J/cm2. A treatment session was continued until a purple discoloration appeared.
The hypothesized mechanism of action involves destruction of the abnormal vasculature, according to the investigators (Dermatol. Surg. 2010;36:377-81).
Nail bed lesions, particularly onycholysis and subungual hyperkeratosis, responded to PDL better than did nail matrix lesions. After three treatment sessions, the average Nail Psoriasis Severity Index (NAPSI) score for nail bed lesions dropped from 14.8 to 8.
While the Turkish report is certainly encouraging, it should be viewed as a proof of concept pilot study, said Dr. Blatiere of Saint Eloi University Hospital in Montpellier, France. It needs confirmation with larger numbers of patients, a control arm, and blinded investigator assessment.
Dr. Blatiere noted that interest has been mounting in evaluating biologic agents for nail psoriasis. Favorable clinical experiences, albeit all of them open label, have recently been reported for the use of infliximab (J. Eur. Acad. Dermatol. Venereol. 2011;25:549-53); adalimumab (J. Eur. Acad. Dermatol. Venereol. 2010;24:530-4); ustekinumab (Arch. Dermatol. 2010;146:1315-6); and etanercept for nail psoriasis (J. Eur. Acad. Dermatol. Venereol. 2009;23:896-904).
But important questions remain about biologics for nail psoriasis, such as the appropriate duration of treatment, length of response, and whether they will help prevent psoriatic arthritis. And then there are the still incompletely answered questions regarding the long-term safety of the agents, as well as the issue of their considerable expense, Dr. Blatiere said.
She reported having no relevant financial disclosures.
psoriatic disease, nail damage, PDL, the European Academy of Dermatology and Venereology, Dr. Yasemin Oram Nail Psoriasis Severity Index, NAPSI, nail bed lesions
LISBON – Pulsed dye laser therapy may be an attractive new option for treating nail psoriasis, according to Dr. Veronique Blatiere.
Nail psoriasis is challenging to treat because the psoriatic disease process damages the nails while they are still being formed. But Turkish investigators have reported positive results with three once-monthly pulsed dye laser (PDL) treatment sessions in a small uncontrolled patient series, Dr. Blatiere reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Yasemin Oram and coworkers at the American Hospital in Istanbul, Turkey, reported on five patients with nail psoriasis treated using PDL. The laser therapy was applied at 595 nm with a pulse duration of 1.5 milliseconds, a beam diameter of 7 mm, and an energy fluence of 8-10 J/cm2. A treatment session was continued until a purple discoloration appeared.
The hypothesized mechanism of action involves destruction of the abnormal vasculature, according to the investigators (Dermatol. Surg. 2010;36:377-81).
Nail bed lesions, particularly onycholysis and subungual hyperkeratosis, responded to PDL better than did nail matrix lesions. After three treatment sessions, the average Nail Psoriasis Severity Index (NAPSI) score for nail bed lesions dropped from 14.8 to 8.
While the Turkish report is certainly encouraging, it should be viewed as a proof of concept pilot study, said Dr. Blatiere of Saint Eloi University Hospital in Montpellier, France. It needs confirmation with larger numbers of patients, a control arm, and blinded investigator assessment.
Dr. Blatiere noted that interest has been mounting in evaluating biologic agents for nail psoriasis. Favorable clinical experiences, albeit all of them open label, have recently been reported for the use of infliximab (J. Eur. Acad. Dermatol. Venereol. 2011;25:549-53); adalimumab (J. Eur. Acad. Dermatol. Venereol. 2010;24:530-4); ustekinumab (Arch. Dermatol. 2010;146:1315-6); and etanercept for nail psoriasis (J. Eur. Acad. Dermatol. Venereol. 2009;23:896-904).
But important questions remain about biologics for nail psoriasis, such as the appropriate duration of treatment, length of response, and whether they will help prevent psoriatic arthritis. And then there are the still incompletely answered questions regarding the long-term safety of the agents, as well as the issue of their considerable expense, Dr. Blatiere said.
She reported having no relevant financial disclosures.
LISBON – Pulsed dye laser therapy may be an attractive new option for treating nail psoriasis, according to Dr. Veronique Blatiere.
Nail psoriasis is challenging to treat because the psoriatic disease process damages the nails while they are still being formed. But Turkish investigators have reported positive results with three once-monthly pulsed dye laser (PDL) treatment sessions in a small uncontrolled patient series, Dr. Blatiere reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Yasemin Oram and coworkers at the American Hospital in Istanbul, Turkey, reported on five patients with nail psoriasis treated using PDL. The laser therapy was applied at 595 nm with a pulse duration of 1.5 milliseconds, a beam diameter of 7 mm, and an energy fluence of 8-10 J/cm2. A treatment session was continued until a purple discoloration appeared.
The hypothesized mechanism of action involves destruction of the abnormal vasculature, according to the investigators (Dermatol. Surg. 2010;36:377-81).
Nail bed lesions, particularly onycholysis and subungual hyperkeratosis, responded to PDL better than did nail matrix lesions. After three treatment sessions, the average Nail Psoriasis Severity Index (NAPSI) score for nail bed lesions dropped from 14.8 to 8.
While the Turkish report is certainly encouraging, it should be viewed as a proof of concept pilot study, said Dr. Blatiere of Saint Eloi University Hospital in Montpellier, France. It needs confirmation with larger numbers of patients, a control arm, and blinded investigator assessment.
Dr. Blatiere noted that interest has been mounting in evaluating biologic agents for nail psoriasis. Favorable clinical experiences, albeit all of them open label, have recently been reported for the use of infliximab (J. Eur. Acad. Dermatol. Venereol. 2011;25:549-53); adalimumab (J. Eur. Acad. Dermatol. Venereol. 2010;24:530-4); ustekinumab (Arch. Dermatol. 2010;146:1315-6); and etanercept for nail psoriasis (J. Eur. Acad. Dermatol. Venereol. 2009;23:896-904).
But important questions remain about biologics for nail psoriasis, such as the appropriate duration of treatment, length of response, and whether they will help prevent psoriatic arthritis. And then there are the still incompletely answered questions regarding the long-term safety of the agents, as well as the issue of their considerable expense, Dr. Blatiere said.
She reported having no relevant financial disclosures.
psoriatic disease, nail damage, PDL, the European Academy of Dermatology and Venereology, Dr. Yasemin Oram Nail Psoriasis Severity Index, NAPSI, nail bed lesions
psoriatic disease, nail damage, PDL, the European Academy of Dermatology and Venereology, Dr. Yasemin Oram Nail Psoriasis Severity Index, NAPSI, nail bed lesions
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Comorbid Psoriasis/Diabetes Carries Hefty Economic Burden
LISBON – Psoriasis and diabetes are expensive diseases, and patients with both conditions experience a synergistic increase in health care utilization and costs that is significantly greater than the incremental economic burden imposed by each disease individually.
In other words, patients with comorbid psoriasis and diabetes have more hospitalizations and outpatient visits over the course of a year than would be expected simply from adding together the increased use typical of patients with psoriasis to that of patients with diabetes, compared with health care use by individuals with neither condition, Dr. Frank Zhang reported at the annual congress of the European Academy of Dermatology and Venereology.
This is an observation with important implications for health economics. These are two common diseases. Psoriasis affects 2%-3% of the world’s population, with 260,000 new cases arising per year in the United States alone. Psoriasis predisposes to insulin resistance, and psoriasis patients have a one-third greater risk of diabetes than the general population, noted Dr. Zhang of Celgene Corporation, Summit, N.J.
Because the economic impact of having both diseases had not been addressed, he and his colleagues conducted a retrospective study of 106,128 patient pairs matched for age and gender, one member of each pair having psoriasis and the other free of the disease. The patients were drawn from the Thomson Reuters MarketScan Research Databases for 2004 through June 2009.
Sixteen percent of the psoriasis patients had diabetes, significantly greater than the 13% with diabetes in the control group.
Psoriasis patients with diabetes averaged 17 more hospitalizations per 100 patient-years and 5.8 more outpatient visits per year than did psoriasis patients without diabetes. And patients with diabetes with psoriasis averaged five more hospitalizations per 100 patient-years and 6.3 additional outpatient visits per year, compared with non–psoriatic patients with diabetes.
The most likely explanation for this synergistic health care burden in the dual-diagnosis patient lies in the complexity entailed in managing the two diseases simultaneously, said Dr. Zhang.
Total annual health care costs in patients with both psoriasis and diabetes averaged $19,536, compared with $13,589 for psoriasis-free patients with diabetes and $5,539 in those who had psoriasis but not diabetes.
The study was funded by Celgene, where Dr. Zhang is employed.
LISBON – Psoriasis and diabetes are expensive diseases, and patients with both conditions experience a synergistic increase in health care utilization and costs that is significantly greater than the incremental economic burden imposed by each disease individually.
In other words, patients with comorbid psoriasis and diabetes have more hospitalizations and outpatient visits over the course of a year than would be expected simply from adding together the increased use typical of patients with psoriasis to that of patients with diabetes, compared with health care use by individuals with neither condition, Dr. Frank Zhang reported at the annual congress of the European Academy of Dermatology and Venereology.
This is an observation with important implications for health economics. These are two common diseases. Psoriasis affects 2%-3% of the world’s population, with 260,000 new cases arising per year in the United States alone. Psoriasis predisposes to insulin resistance, and psoriasis patients have a one-third greater risk of diabetes than the general population, noted Dr. Zhang of Celgene Corporation, Summit, N.J.
Because the economic impact of having both diseases had not been addressed, he and his colleagues conducted a retrospective study of 106,128 patient pairs matched for age and gender, one member of each pair having psoriasis and the other free of the disease. The patients were drawn from the Thomson Reuters MarketScan Research Databases for 2004 through June 2009.
Sixteen percent of the psoriasis patients had diabetes, significantly greater than the 13% with diabetes in the control group.
Psoriasis patients with diabetes averaged 17 more hospitalizations per 100 patient-years and 5.8 more outpatient visits per year than did psoriasis patients without diabetes. And patients with diabetes with psoriasis averaged five more hospitalizations per 100 patient-years and 6.3 additional outpatient visits per year, compared with non–psoriatic patients with diabetes.
The most likely explanation for this synergistic health care burden in the dual-diagnosis patient lies in the complexity entailed in managing the two diseases simultaneously, said Dr. Zhang.
Total annual health care costs in patients with both psoriasis and diabetes averaged $19,536, compared with $13,589 for psoriasis-free patients with diabetes and $5,539 in those who had psoriasis but not diabetes.
The study was funded by Celgene, where Dr. Zhang is employed.
LISBON – Psoriasis and diabetes are expensive diseases, and patients with both conditions experience a synergistic increase in health care utilization and costs that is significantly greater than the incremental economic burden imposed by each disease individually.
In other words, patients with comorbid psoriasis and diabetes have more hospitalizations and outpatient visits over the course of a year than would be expected simply from adding together the increased use typical of patients with psoriasis to that of patients with diabetes, compared with health care use by individuals with neither condition, Dr. Frank Zhang reported at the annual congress of the European Academy of Dermatology and Venereology.
This is an observation with important implications for health economics. These are two common diseases. Psoriasis affects 2%-3% of the world’s population, with 260,000 new cases arising per year in the United States alone. Psoriasis predisposes to insulin resistance, and psoriasis patients have a one-third greater risk of diabetes than the general population, noted Dr. Zhang of Celgene Corporation, Summit, N.J.
Because the economic impact of having both diseases had not been addressed, he and his colleagues conducted a retrospective study of 106,128 patient pairs matched for age and gender, one member of each pair having psoriasis and the other free of the disease. The patients were drawn from the Thomson Reuters MarketScan Research Databases for 2004 through June 2009.
Sixteen percent of the psoriasis patients had diabetes, significantly greater than the 13% with diabetes in the control group.
Psoriasis patients with diabetes averaged 17 more hospitalizations per 100 patient-years and 5.8 more outpatient visits per year than did psoriasis patients without diabetes. And patients with diabetes with psoriasis averaged five more hospitalizations per 100 patient-years and 6.3 additional outpatient visits per year, compared with non–psoriatic patients with diabetes.
The most likely explanation for this synergistic health care burden in the dual-diagnosis patient lies in the complexity entailed in managing the two diseases simultaneously, said Dr. Zhang.
Total annual health care costs in patients with both psoriasis and diabetes averaged $19,536, compared with $13,589 for psoriasis-free patients with diabetes and $5,539 in those who had psoriasis but not diabetes.
The study was funded by Celgene, where Dr. Zhang is employed.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Psoriasis patients with diabetes averaged 17 more hospitalizations per 100 patient-years and 5.8 more outpatient visits per patient-year than psoriasis patients without diabetes.
Data Source: A retrospective study of 106,128 matched patient pairs, one with psoriasis and the other without.
Disclosures: The study was funded by Celgene, where Dr. Zhang is employed.
Novel Filler Scores Well For Deep Nasolabial Folds
LISBON – A novel, intradermally-injected, monophasic hyaluronic acid filler known as Belotero Intense outperformed Perlane for the treatment of moderate to deep nasolabial folds in a 12-month, double-blind, randomized trial, according to Dr. Martina Kerscher.
Merz Pharmaceuticals’ Belotero Intense is manufactured using a proprietary Cohesive Polydensified Matrix technology. The resultant product, a monophasic, polydensified hyaluronic acid filler, was designed to provide greater elasticity, less risk of lumping, and longer-lasting improvements than achievable with biphasic hyaluronic acid fillers, said Dr. Kerscher at the annual congress of the European Academy of Dermatology and Venereology.
She reported on 20 patients aged 35-65 years, with nasolabial folds (NLFs) that were grades 3-4 on a 5-point scale, meaning the defects were either moderately deep or very long and deep. Participants were randomized double-blindly to a single intradermal injection on one side of the face with Belotero Intense, while the NLFs on the other side of the face were treated with Medicis Aesthetics’ Perlane, a biphasic hyaluronic acid filler. Both products are gels of nonanimal origin. No touch-ups were permitted during the 12 months of follow-up.
Physician- and patient-rated scores on the Wrinkle Severity Rating Scale improved with both products; however, the degree of improvement was significantly greater through 24 weeks with the monophasic hyaluronic acid filler.
Belotero Intense is designed to provide greater elasticity, less risk of lumping, and longer-lasting improvements than is achievable with biphasic hyaluronic acid fillers, said Dr. Martina Kerscher.
Mean scores on the 0-4 scale went from a baseline of 4.0 to 2.4 with the biphasic filler and to 2.1 with the monophasic filler at week 2; at week 24, mean scores were 2.7 for the biphasic filler, compared with 2.4 for the monophasic filler. At week 48, however, both products showed similar effects.
Investigators and patients gave the monophasic hyaluronic acid gel higher marks on the Global Aesthetic Improvement Scale through 24 weeks. After week 24, scores for the two products merged, reported Dr. Kerscher of the University of Hamburg (Germany) division of cosmetic science.
Standardized measurements of wrinkle depth for the Belotero Intense-treated NLFs went from a baseline of 271 mm to 172 mm at week 2, 194 mm at week 24, and 213 mm at week 48. Depth of the Perlane-treated NLFs improved from 222 mm at baseline to 152 mm at week 2, 177 mm at week 24, and 184 mm at week 48. This translated to a 28% reduction in wrinkle depth at week 24 in the monophasic filler-treated lesions, compared with a 20% decrease in the biphasic filler-treated folds. The week 48 improvement was 21% in the monophasic- and 17% in the biphasic-treated NLFs.
Self-rated patient satisfaction was scored on a 0-10 scale, with a lower score showing a higher level of satisfaction. From a baseline of 7.0, scores improved to 2.3 for the biphasic filler and 2.1 for the monophasic filler at week 2, to 4.9 for biphasic and 3.8 for monophasic at week 24, and to 6.7 for biphasic and 5.7 for monophasic at week 48.
Of note, the blinded patients consistently recorded significantly less injection site pain with the monophasic hyaluronic acid filler.
Belotero Intense is licensed in the United Kingdom and several European countries as a medical device for the correction of deep folds and for volume augmentation. A sister product, Belotero Balance, earned marketing approval from the Food and Drug Administration in November 2011 for correction of moderate to severe wrinkles and folds. Belotero Intense is intended for deeper injection and correction of more pronounced defects than is Belotero Balance.
Merz is seeking FDA approval of Belotero Intense.
The study presented by Dr. Kerscher was sponsored by Merz, which markets the Belotero product line. She is a consultant to the company.
LISBON – A novel, intradermally-injected, monophasic hyaluronic acid filler known as Belotero Intense outperformed Perlane for the treatment of moderate to deep nasolabial folds in a 12-month, double-blind, randomized trial, according to Dr. Martina Kerscher.
Merz Pharmaceuticals’ Belotero Intense is manufactured using a proprietary Cohesive Polydensified Matrix technology. The resultant product, a monophasic, polydensified hyaluronic acid filler, was designed to provide greater elasticity, less risk of lumping, and longer-lasting improvements than achievable with biphasic hyaluronic acid fillers, said Dr. Kerscher at the annual congress of the European Academy of Dermatology and Venereology.
She reported on 20 patients aged 35-65 years, with nasolabial folds (NLFs) that were grades 3-4 on a 5-point scale, meaning the defects were either moderately deep or very long and deep. Participants were randomized double-blindly to a single intradermal injection on one side of the face with Belotero Intense, while the NLFs on the other side of the face were treated with Medicis Aesthetics’ Perlane, a biphasic hyaluronic acid filler. Both products are gels of nonanimal origin. No touch-ups were permitted during the 12 months of follow-up.
Physician- and patient-rated scores on the Wrinkle Severity Rating Scale improved with both products; however, the degree of improvement was significantly greater through 24 weeks with the monophasic hyaluronic acid filler.
Belotero Intense is designed to provide greater elasticity, less risk of lumping, and longer-lasting improvements than is achievable with biphasic hyaluronic acid fillers, said Dr. Martina Kerscher.
Mean scores on the 0-4 scale went from a baseline of 4.0 to 2.4 with the biphasic filler and to 2.1 with the monophasic filler at week 2; at week 24, mean scores were 2.7 for the biphasic filler, compared with 2.4 for the monophasic filler. At week 48, however, both products showed similar effects.
Investigators and patients gave the monophasic hyaluronic acid gel higher marks on the Global Aesthetic Improvement Scale through 24 weeks. After week 24, scores for the two products merged, reported Dr. Kerscher of the University of Hamburg (Germany) division of cosmetic science.
Standardized measurements of wrinkle depth for the Belotero Intense-treated NLFs went from a baseline of 271 mm to 172 mm at week 2, 194 mm at week 24, and 213 mm at week 48. Depth of the Perlane-treated NLFs improved from 222 mm at baseline to 152 mm at week 2, 177 mm at week 24, and 184 mm at week 48. This translated to a 28% reduction in wrinkle depth at week 24 in the monophasic filler-treated lesions, compared with a 20% decrease in the biphasic filler-treated folds. The week 48 improvement was 21% in the monophasic- and 17% in the biphasic-treated NLFs.
Self-rated patient satisfaction was scored on a 0-10 scale, with a lower score showing a higher level of satisfaction. From a baseline of 7.0, scores improved to 2.3 for the biphasic filler and 2.1 for the monophasic filler at week 2, to 4.9 for biphasic and 3.8 for monophasic at week 24, and to 6.7 for biphasic and 5.7 for monophasic at week 48.
Of note, the blinded patients consistently recorded significantly less injection site pain with the monophasic hyaluronic acid filler.
Belotero Intense is licensed in the United Kingdom and several European countries as a medical device for the correction of deep folds and for volume augmentation. A sister product, Belotero Balance, earned marketing approval from the Food and Drug Administration in November 2011 for correction of moderate to severe wrinkles and folds. Belotero Intense is intended for deeper injection and correction of more pronounced defects than is Belotero Balance.
Merz is seeking FDA approval of Belotero Intense.
The study presented by Dr. Kerscher was sponsored by Merz, which markets the Belotero product line. She is a consultant to the company.
LISBON – A novel, intradermally-injected, monophasic hyaluronic acid filler known as Belotero Intense outperformed Perlane for the treatment of moderate to deep nasolabial folds in a 12-month, double-blind, randomized trial, according to Dr. Martina Kerscher.
Merz Pharmaceuticals’ Belotero Intense is manufactured using a proprietary Cohesive Polydensified Matrix technology. The resultant product, a monophasic, polydensified hyaluronic acid filler, was designed to provide greater elasticity, less risk of lumping, and longer-lasting improvements than achievable with biphasic hyaluronic acid fillers, said Dr. Kerscher at the annual congress of the European Academy of Dermatology and Venereology.
She reported on 20 patients aged 35-65 years, with nasolabial folds (NLFs) that were grades 3-4 on a 5-point scale, meaning the defects were either moderately deep or very long and deep. Participants were randomized double-blindly to a single intradermal injection on one side of the face with Belotero Intense, while the NLFs on the other side of the face were treated with Medicis Aesthetics’ Perlane, a biphasic hyaluronic acid filler. Both products are gels of nonanimal origin. No touch-ups were permitted during the 12 months of follow-up.
Physician- and patient-rated scores on the Wrinkle Severity Rating Scale improved with both products; however, the degree of improvement was significantly greater through 24 weeks with the monophasic hyaluronic acid filler.
Belotero Intense is designed to provide greater elasticity, less risk of lumping, and longer-lasting improvements than is achievable with biphasic hyaluronic acid fillers, said Dr. Martina Kerscher.
Mean scores on the 0-4 scale went from a baseline of 4.0 to 2.4 with the biphasic filler and to 2.1 with the monophasic filler at week 2; at week 24, mean scores were 2.7 for the biphasic filler, compared with 2.4 for the monophasic filler. At week 48, however, both products showed similar effects.
Investigators and patients gave the monophasic hyaluronic acid gel higher marks on the Global Aesthetic Improvement Scale through 24 weeks. After week 24, scores for the two products merged, reported Dr. Kerscher of the University of Hamburg (Germany) division of cosmetic science.
Standardized measurements of wrinkle depth for the Belotero Intense-treated NLFs went from a baseline of 271 mm to 172 mm at week 2, 194 mm at week 24, and 213 mm at week 48. Depth of the Perlane-treated NLFs improved from 222 mm at baseline to 152 mm at week 2, 177 mm at week 24, and 184 mm at week 48. This translated to a 28% reduction in wrinkle depth at week 24 in the monophasic filler-treated lesions, compared with a 20% decrease in the biphasic filler-treated folds. The week 48 improvement was 21% in the monophasic- and 17% in the biphasic-treated NLFs.
Self-rated patient satisfaction was scored on a 0-10 scale, with a lower score showing a higher level of satisfaction. From a baseline of 7.0, scores improved to 2.3 for the biphasic filler and 2.1 for the monophasic filler at week 2, to 4.9 for biphasic and 3.8 for monophasic at week 24, and to 6.7 for biphasic and 5.7 for monophasic at week 48.
Of note, the blinded patients consistently recorded significantly less injection site pain with the monophasic hyaluronic acid filler.
Belotero Intense is licensed in the United Kingdom and several European countries as a medical device for the correction of deep folds and for volume augmentation. A sister product, Belotero Balance, earned marketing approval from the Food and Drug Administration in November 2011 for correction of moderate to severe wrinkles and folds. Belotero Intense is intended for deeper injection and correction of more pronounced defects than is Belotero Balance.
Merz is seeking FDA approval of Belotero Intense.
The study presented by Dr. Kerscher was sponsored by Merz, which markets the Belotero product line. She is a consultant to the company.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: A monophasic, polydensified hyaluronic acid gel dermal filler resulted in significantly less injection pain, greater efficacy through 24 weeks of follow-up, and higher patient satisfaction scores through 48 weeks than a commercially available, biphasic, stabilized hyaluronic acid gel.
Data Source: A 12-month, double-blind, randomized, facial side-to-side comparative clinical trial.
Disclosures: The study presented by Dr. Kerscher was sponsored by Merz, which markets the Belotero product line. She is a consultant to the company.
New Technique Builds Mighty Towers of Facial Hyaluronic Acid
LISBON – The tower technique is a novel method of injecting hyaluronic acid fillers that is particularly well suited for anatomic locations where underlying structural support for soft tissue has been lost because of aging.
The tower technique creates a deep base of scaffolding that extends through the entire subcutis. It reintroduces lost structural support for the overlying facial soft tissue rather than simply filling lines, Dr. C. William Hanke explained in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
"The Big Three with facial aging are skin quality, facial volume, and laxity. Volume trumps the other two. ... You can improve skin quality and laxity with volume alone," said Dr. Hanke of the Laser and Skin Surgery Center of Indiana, Carmel.
Areas particularly amenable to the tower technique include the nasolabial folds, marionette lines, the brow region, the chin compartment, and the prejowl sulcus. The best areas in which to employ the tower technique have underlying bony structural support, a thick subcutis, or an overlying thick dermis.
The technique is easily mastered, he said. "You take your syringe of hyaluronic acid and point it straight down at the bone, perpendicular, and then you inject as you withdraw. You deposit decreasing amounts of the filler as you withdraw the needle, creating a pyramid-like tower," he explained. "This is really an amazing thing in that it props up the skin right before your eyes. It’s minimally painful, there are a minimum number of injections, and the complications – such as bruising – are very minimal as well."
Dr. Hanke generally utilizes 30-gauge half-inch needles for the injections. For a nasolabial fold or superior marionette line he creates two or three towers, injecting 0.1-0.2 mL of filler into each. Inferior marionette lines get one or two towers, with 0.1-0.3 mL of product placed in each. The lateral brow lift entails two or three towers, each with 0.1 mL of filler.
Restoring support of the mental area requires one to three towers, each constructed of 0.2-0.3 mL of filler placed from the periosteum to the deep dermis. The prejowl sulcus can be shored up with one or two towers, each containing 0.2-0.3 mL of filler.
The tower technique is for use with hyaluronic acid fillers. It’s not appropriate for poly-L-lactic acid or calcium hydroxyapatite fillers, which need to be placed deeper or in a single plane.
Dr. Hanke noted that he didn’t come up with the tower technique.
"I first heard about it in Germany. I think other people were using it, too. I’ve [also] heard about this technique from the Canadians. They call it the tent pole technique," he said.
Dr. Hanke and his colleagues recently wrote a detailed how-to guide to the tower technique (J. Drugs Dermatol. 2011;10:1277-80).
He reported having no relevant financial interests.
LISBON – The tower technique is a novel method of injecting hyaluronic acid fillers that is particularly well suited for anatomic locations where underlying structural support for soft tissue has been lost because of aging.
The tower technique creates a deep base of scaffolding that extends through the entire subcutis. It reintroduces lost structural support for the overlying facial soft tissue rather than simply filling lines, Dr. C. William Hanke explained in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
"The Big Three with facial aging are skin quality, facial volume, and laxity. Volume trumps the other two. ... You can improve skin quality and laxity with volume alone," said Dr. Hanke of the Laser and Skin Surgery Center of Indiana, Carmel.
Areas particularly amenable to the tower technique include the nasolabial folds, marionette lines, the brow region, the chin compartment, and the prejowl sulcus. The best areas in which to employ the tower technique have underlying bony structural support, a thick subcutis, or an overlying thick dermis.
The technique is easily mastered, he said. "You take your syringe of hyaluronic acid and point it straight down at the bone, perpendicular, and then you inject as you withdraw. You deposit decreasing amounts of the filler as you withdraw the needle, creating a pyramid-like tower," he explained. "This is really an amazing thing in that it props up the skin right before your eyes. It’s minimally painful, there are a minimum number of injections, and the complications – such as bruising – are very minimal as well."
Dr. Hanke generally utilizes 30-gauge half-inch needles for the injections. For a nasolabial fold or superior marionette line he creates two or three towers, injecting 0.1-0.2 mL of filler into each. Inferior marionette lines get one or two towers, with 0.1-0.3 mL of product placed in each. The lateral brow lift entails two or three towers, each with 0.1 mL of filler.
Restoring support of the mental area requires one to three towers, each constructed of 0.2-0.3 mL of filler placed from the periosteum to the deep dermis. The prejowl sulcus can be shored up with one or two towers, each containing 0.2-0.3 mL of filler.
The tower technique is for use with hyaluronic acid fillers. It’s not appropriate for poly-L-lactic acid or calcium hydroxyapatite fillers, which need to be placed deeper or in a single plane.
Dr. Hanke noted that he didn’t come up with the tower technique.
"I first heard about it in Germany. I think other people were using it, too. I’ve [also] heard about this technique from the Canadians. They call it the tent pole technique," he said.
Dr. Hanke and his colleagues recently wrote a detailed how-to guide to the tower technique (J. Drugs Dermatol. 2011;10:1277-80).
He reported having no relevant financial interests.
LISBON – The tower technique is a novel method of injecting hyaluronic acid fillers that is particularly well suited for anatomic locations where underlying structural support for soft tissue has been lost because of aging.
The tower technique creates a deep base of scaffolding that extends through the entire subcutis. It reintroduces lost structural support for the overlying facial soft tissue rather than simply filling lines, Dr. C. William Hanke explained in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
"The Big Three with facial aging are skin quality, facial volume, and laxity. Volume trumps the other two. ... You can improve skin quality and laxity with volume alone," said Dr. Hanke of the Laser and Skin Surgery Center of Indiana, Carmel.
Areas particularly amenable to the tower technique include the nasolabial folds, marionette lines, the brow region, the chin compartment, and the prejowl sulcus. The best areas in which to employ the tower technique have underlying bony structural support, a thick subcutis, or an overlying thick dermis.
The technique is easily mastered, he said. "You take your syringe of hyaluronic acid and point it straight down at the bone, perpendicular, and then you inject as you withdraw. You deposit decreasing amounts of the filler as you withdraw the needle, creating a pyramid-like tower," he explained. "This is really an amazing thing in that it props up the skin right before your eyes. It’s minimally painful, there are a minimum number of injections, and the complications – such as bruising – are very minimal as well."
Dr. Hanke generally utilizes 30-gauge half-inch needles for the injections. For a nasolabial fold or superior marionette line he creates two or three towers, injecting 0.1-0.2 mL of filler into each. Inferior marionette lines get one or two towers, with 0.1-0.3 mL of product placed in each. The lateral brow lift entails two or three towers, each with 0.1 mL of filler.
Restoring support of the mental area requires one to three towers, each constructed of 0.2-0.3 mL of filler placed from the periosteum to the deep dermis. The prejowl sulcus can be shored up with one or two towers, each containing 0.2-0.3 mL of filler.
The tower technique is for use with hyaluronic acid fillers. It’s not appropriate for poly-L-lactic acid or calcium hydroxyapatite fillers, which need to be placed deeper or in a single plane.
Dr. Hanke noted that he didn’t come up with the tower technique.
"I first heard about it in Germany. I think other people were using it, too. I’ve [also] heard about this technique from the Canadians. They call it the tent pole technique," he said.
Dr. Hanke and his colleagues recently wrote a detailed how-to guide to the tower technique (J. Drugs Dermatol. 2011;10:1277-80).
He reported having no relevant financial interests.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Instant Glue Thwarts Onychotillomania
LISBON – Applying a fast-drying cyanoacrylate glue to the proximal nail fold once or twice weekly is an inexpensive and effective treatment for the habit-tic condition of onychotillomania.
Onychotillomania is often categorized as a compulsive psychiatric disorder. The same psychiatric medications employed in cases of obsessive-compulsive disorder, including selective serotonin reuptake inhibitors, are sometimes prescribed to good effect.
Gluing the problem nail – it is most often a thumbnail – can work, and at negligible cost, with low risk, and no risk of systemic side effects stemming from psychiatric medications, Dr. Veronique Blatiere said at the annual congress of the European Academy of Dermatology and Venereology.
The likely mechanisms of benefit from the cyanoacrylate glue, such as Super Glue or Krazy Glue, are twofold: the built-up layer of glue creates a physical barrier that helps protect the proximal nail fold against the patient’s mindless repetitive picking, and, at the same time, the artificial layer promotes self-awareness of the tic habit, said Dr. Blatiere, a dermatologist at the University of Montpellier (France).
She credited the instant nail-gluing therapy to Dr. Daniel S. Ring, a Chesterfield, Mo., dermatologist who presented the approach in the Archives of Dermatology (2010;146:1222-3).
Dr. Ring described onychotillomania as a common condition given little attention in most dermatologic textbooks. Onset is typically in adulthood, and patients frequently bring up the problem as an "oh, by the way" afterthought during an office visit scheduled for another reason.
Patients typically display parallel transverse ridges running from the proximal nail fold to the distal nail plate along with a lack of cuticle. These changes result from years of picking at the cuticle or pushing it back.
In his report, Dr. Ring described two cases in detail and mentioned 10 others successfully treated with glue. The affected nails typically normalized after 3-6 months of weekly gluing.
Dr. Blatiere said that instant-drying cyanoacrylate glues may also be worth investigating for the treatment of chronic paronychia.
However, she noted that a potential hazard of applying this therapy for months at a time is development of an allergic contact dermatitis to the acrylate.
Neither Dr. Ring nor Dr. Blatiere reported having any financial conflicts.
LISBON – Applying a fast-drying cyanoacrylate glue to the proximal nail fold once or twice weekly is an inexpensive and effective treatment for the habit-tic condition of onychotillomania.
Onychotillomania is often categorized as a compulsive psychiatric disorder. The same psychiatric medications employed in cases of obsessive-compulsive disorder, including selective serotonin reuptake inhibitors, are sometimes prescribed to good effect.
Gluing the problem nail – it is most often a thumbnail – can work, and at negligible cost, with low risk, and no risk of systemic side effects stemming from psychiatric medications, Dr. Veronique Blatiere said at the annual congress of the European Academy of Dermatology and Venereology.
The likely mechanisms of benefit from the cyanoacrylate glue, such as Super Glue or Krazy Glue, are twofold: the built-up layer of glue creates a physical barrier that helps protect the proximal nail fold against the patient’s mindless repetitive picking, and, at the same time, the artificial layer promotes self-awareness of the tic habit, said Dr. Blatiere, a dermatologist at the University of Montpellier (France).
She credited the instant nail-gluing therapy to Dr. Daniel S. Ring, a Chesterfield, Mo., dermatologist who presented the approach in the Archives of Dermatology (2010;146:1222-3).
Dr. Ring described onychotillomania as a common condition given little attention in most dermatologic textbooks. Onset is typically in adulthood, and patients frequently bring up the problem as an "oh, by the way" afterthought during an office visit scheduled for another reason.
Patients typically display parallel transverse ridges running from the proximal nail fold to the distal nail plate along with a lack of cuticle. These changes result from years of picking at the cuticle or pushing it back.
In his report, Dr. Ring described two cases in detail and mentioned 10 others successfully treated with glue. The affected nails typically normalized after 3-6 months of weekly gluing.
Dr. Blatiere said that instant-drying cyanoacrylate glues may also be worth investigating for the treatment of chronic paronychia.
However, she noted that a potential hazard of applying this therapy for months at a time is development of an allergic contact dermatitis to the acrylate.
Neither Dr. Ring nor Dr. Blatiere reported having any financial conflicts.
LISBON – Applying a fast-drying cyanoacrylate glue to the proximal nail fold once or twice weekly is an inexpensive and effective treatment for the habit-tic condition of onychotillomania.
Onychotillomania is often categorized as a compulsive psychiatric disorder. The same psychiatric medications employed in cases of obsessive-compulsive disorder, including selective serotonin reuptake inhibitors, are sometimes prescribed to good effect.
Gluing the problem nail – it is most often a thumbnail – can work, and at negligible cost, with low risk, and no risk of systemic side effects stemming from psychiatric medications, Dr. Veronique Blatiere said at the annual congress of the European Academy of Dermatology and Venereology.
The likely mechanisms of benefit from the cyanoacrylate glue, such as Super Glue or Krazy Glue, are twofold: the built-up layer of glue creates a physical barrier that helps protect the proximal nail fold against the patient’s mindless repetitive picking, and, at the same time, the artificial layer promotes self-awareness of the tic habit, said Dr. Blatiere, a dermatologist at the University of Montpellier (France).
She credited the instant nail-gluing therapy to Dr. Daniel S. Ring, a Chesterfield, Mo., dermatologist who presented the approach in the Archives of Dermatology (2010;146:1222-3).
Dr. Ring described onychotillomania as a common condition given little attention in most dermatologic textbooks. Onset is typically in adulthood, and patients frequently bring up the problem as an "oh, by the way" afterthought during an office visit scheduled for another reason.
Patients typically display parallel transverse ridges running from the proximal nail fold to the distal nail plate along with a lack of cuticle. These changes result from years of picking at the cuticle or pushing it back.
In his report, Dr. Ring described two cases in detail and mentioned 10 others successfully treated with glue. The affected nails typically normalized after 3-6 months of weekly gluing.
Dr. Blatiere said that instant-drying cyanoacrylate glues may also be worth investigating for the treatment of chronic paronychia.
However, she noted that a potential hazard of applying this therapy for months at a time is development of an allergic contact dermatitis to the acrylate.
Neither Dr. Ring nor Dr. Blatiere reported having any financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Neck Liposuction: A 'Home Run' Dermatologic Surgery Procedure
LISBON – Neck liposuction using tumescent local anesthesia is the cosmetic dermatology procedure having the optimal combination of high patient satisfaction, minimal complications, and a short learning curve.
"What I consider to be the No. 1, home-run procedure in all of dermatosurgery is liposuction of the neck," Dr. C. William Hanke, a dermatologic surgeon, said during a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
"A long, youthful neck is felt to be a sign of beauty. When patients lose that – when their neck fills in with fat, and when the face and the neck become one – it’s not felt to be a sign of beauty. And that’s when liposuction of the neck comes in," explained Dr. Hanke of the Laser and Skin Surgery Center of Indiana, Carmel.
It is important to understand, however, that liposuction is no substitute for weight loss. The ideal candidate for neck liposuction is a young patient with good skin elasticity who has excess submandibular fat resulting in lost definition of the mandibular border.
Using liposuction to remove adipose tissue lying superficial to the platysmal muscle in such a patient, and redraping the skin to reduce the cervicomental angle to a well-defined 105-120 degrees with sharp mandibular demarcation, brings stellar cosmetic results.
In contrast, an elderly patient who is overweight or obese and has poor skin quality and redundant neck skin may need additional procedures, such as platysmal plication and laser resurfacing, he said.
Basically, neck liposuction entails filling the neck with tumescent anesthesia. "Any dermatologist can learn this," Dr. Hanke said. The fat is then suctioned using a combination of 1- to 3-mm cannulas with spatula tips placed through a primary entry point located in the mental crease. Additional holes can be placed for suctioning the lateral neck. The patient is entirely awake for the procedure, which can be performed in an office surgical suite, an ambulatory surgery center, or a hospital operating room.
"We sweep across the neck, suctioning the fat and transecting the septae that run through the fat. We can march into the jowls as well," he said.
Neuropraxia of the marginal mandibular nerve occurs in roughly 5% of cases of neck liposuction. The interruption of motor function typically lasts 4-6 weeks and is never permanent. The marginal mandibular nerve lies deep to the platysma, and the muscle would actually have to be punctured by the cannula to cause permanent nerve injury, Dr. Hanke said.
Immediately after the procedure he has patients wear a compression garment 24 hours a day for the first 1-2 days except while showering, then for 2-4 hours daily for 1-2 weeks. The compression garment helps in redraping the skin, prevents hematomas, speeds absorption of tumescent fluid, and prevents skin wrinkling.
Serious complications are exceedingly rare. In fact, in an early national survey of more than 15,000 patients treated with liposuction using tumescent local anesthesia, Dr. Hanke and coworkers reported no deaths or complications requiring hospital transfer (Dermatol. Surg. 1995;21:459-62).
The finding was confirmed in another national survey, this one involving nearly 67,000 liposuction procedures performed by dermatologic surgeons from 1994 to 2000 (Dermatol. Surg. 2002;28:971-8).
Occasionally, the platysmal bands are unmasked by neck liposuction. This is an unwelcome event, but marked improvement can be achieved through injection of small doses of botulinum toxin.
Dr. Hanke noted that he and Dr. William G. Stebbins recently performed an in-depth review of neck rejuvenation via liposuction, including proper patient selection, technical aspects of the procedure, postop care, and ancillary techniques (Dermatol. Ther. 2011;24:28-40).
Dr. Hanke reported having no financial conflicts of interest.
LISBON – Neck liposuction using tumescent local anesthesia is the cosmetic dermatology procedure having the optimal combination of high patient satisfaction, minimal complications, and a short learning curve.
"What I consider to be the No. 1, home-run procedure in all of dermatosurgery is liposuction of the neck," Dr. C. William Hanke, a dermatologic surgeon, said during a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
"A long, youthful neck is felt to be a sign of beauty. When patients lose that – when their neck fills in with fat, and when the face and the neck become one – it’s not felt to be a sign of beauty. And that’s when liposuction of the neck comes in," explained Dr. Hanke of the Laser and Skin Surgery Center of Indiana, Carmel.
It is important to understand, however, that liposuction is no substitute for weight loss. The ideal candidate for neck liposuction is a young patient with good skin elasticity who has excess submandibular fat resulting in lost definition of the mandibular border.
Using liposuction to remove adipose tissue lying superficial to the platysmal muscle in such a patient, and redraping the skin to reduce the cervicomental angle to a well-defined 105-120 degrees with sharp mandibular demarcation, brings stellar cosmetic results.
In contrast, an elderly patient who is overweight or obese and has poor skin quality and redundant neck skin may need additional procedures, such as platysmal plication and laser resurfacing, he said.
Basically, neck liposuction entails filling the neck with tumescent anesthesia. "Any dermatologist can learn this," Dr. Hanke said. The fat is then suctioned using a combination of 1- to 3-mm cannulas with spatula tips placed through a primary entry point located in the mental crease. Additional holes can be placed for suctioning the lateral neck. The patient is entirely awake for the procedure, which can be performed in an office surgical suite, an ambulatory surgery center, or a hospital operating room.
"We sweep across the neck, suctioning the fat and transecting the septae that run through the fat. We can march into the jowls as well," he said.
Neuropraxia of the marginal mandibular nerve occurs in roughly 5% of cases of neck liposuction. The interruption of motor function typically lasts 4-6 weeks and is never permanent. The marginal mandibular nerve lies deep to the platysma, and the muscle would actually have to be punctured by the cannula to cause permanent nerve injury, Dr. Hanke said.
Immediately after the procedure he has patients wear a compression garment 24 hours a day for the first 1-2 days except while showering, then for 2-4 hours daily for 1-2 weeks. The compression garment helps in redraping the skin, prevents hematomas, speeds absorption of tumescent fluid, and prevents skin wrinkling.
Serious complications are exceedingly rare. In fact, in an early national survey of more than 15,000 patients treated with liposuction using tumescent local anesthesia, Dr. Hanke and coworkers reported no deaths or complications requiring hospital transfer (Dermatol. Surg. 1995;21:459-62).
The finding was confirmed in another national survey, this one involving nearly 67,000 liposuction procedures performed by dermatologic surgeons from 1994 to 2000 (Dermatol. Surg. 2002;28:971-8).
Occasionally, the platysmal bands are unmasked by neck liposuction. This is an unwelcome event, but marked improvement can be achieved through injection of small doses of botulinum toxin.
Dr. Hanke noted that he and Dr. William G. Stebbins recently performed an in-depth review of neck rejuvenation via liposuction, including proper patient selection, technical aspects of the procedure, postop care, and ancillary techniques (Dermatol. Ther. 2011;24:28-40).
Dr. Hanke reported having no financial conflicts of interest.
LISBON – Neck liposuction using tumescent local anesthesia is the cosmetic dermatology procedure having the optimal combination of high patient satisfaction, minimal complications, and a short learning curve.
"What I consider to be the No. 1, home-run procedure in all of dermatosurgery is liposuction of the neck," Dr. C. William Hanke, a dermatologic surgeon, said during a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
"A long, youthful neck is felt to be a sign of beauty. When patients lose that – when their neck fills in with fat, and when the face and the neck become one – it’s not felt to be a sign of beauty. And that’s when liposuction of the neck comes in," explained Dr. Hanke of the Laser and Skin Surgery Center of Indiana, Carmel.
It is important to understand, however, that liposuction is no substitute for weight loss. The ideal candidate for neck liposuction is a young patient with good skin elasticity who has excess submandibular fat resulting in lost definition of the mandibular border.
Using liposuction to remove adipose tissue lying superficial to the platysmal muscle in such a patient, and redraping the skin to reduce the cervicomental angle to a well-defined 105-120 degrees with sharp mandibular demarcation, brings stellar cosmetic results.
In contrast, an elderly patient who is overweight or obese and has poor skin quality and redundant neck skin may need additional procedures, such as platysmal plication and laser resurfacing, he said.
Basically, neck liposuction entails filling the neck with tumescent anesthesia. "Any dermatologist can learn this," Dr. Hanke said. The fat is then suctioned using a combination of 1- to 3-mm cannulas with spatula tips placed through a primary entry point located in the mental crease. Additional holes can be placed for suctioning the lateral neck. The patient is entirely awake for the procedure, which can be performed in an office surgical suite, an ambulatory surgery center, or a hospital operating room.
"We sweep across the neck, suctioning the fat and transecting the septae that run through the fat. We can march into the jowls as well," he said.
Neuropraxia of the marginal mandibular nerve occurs in roughly 5% of cases of neck liposuction. The interruption of motor function typically lasts 4-6 weeks and is never permanent. The marginal mandibular nerve lies deep to the platysma, and the muscle would actually have to be punctured by the cannula to cause permanent nerve injury, Dr. Hanke said.
Immediately after the procedure he has patients wear a compression garment 24 hours a day for the first 1-2 days except while showering, then for 2-4 hours daily for 1-2 weeks. The compression garment helps in redraping the skin, prevents hematomas, speeds absorption of tumescent fluid, and prevents skin wrinkling.
Serious complications are exceedingly rare. In fact, in an early national survey of more than 15,000 patients treated with liposuction using tumescent local anesthesia, Dr. Hanke and coworkers reported no deaths or complications requiring hospital transfer (Dermatol. Surg. 1995;21:459-62).
The finding was confirmed in another national survey, this one involving nearly 67,000 liposuction procedures performed by dermatologic surgeons from 1994 to 2000 (Dermatol. Surg. 2002;28:971-8).
Occasionally, the platysmal bands are unmasked by neck liposuction. This is an unwelcome event, but marked improvement can be achieved through injection of small doses of botulinum toxin.
Dr. Hanke noted that he and Dr. William G. Stebbins recently performed an in-depth review of neck rejuvenation via liposuction, including proper patient selection, technical aspects of the procedure, postop care, and ancillary techniques (Dermatol. Ther. 2011;24:28-40).
Dr. Hanke reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY