User login
The incidence of cutaneous melanoma, the deadliest form of skin cancer, has been steadily increasing over the last several decades.1 Currently, there are 73,870 new diagnoses of melanoma anticipated in the United States in 2015 alone.2 Many cases of melanoma are caught at early, actionable, and curable stages thanks in part to patient education and screening by dermatologists.3 However, until recently, few options existed for the treatment of locally advanced and metastatic melanomas, with a median survival rate of less than 1 year.4
Clinical trials represent the most reliable method for advancing treatment and improving outcomes for patients with disease; however, patient accrual and access to clinical trials remain formidable barriers. Studies have suggested that patients in rural areas perceive both an increased distance to clinical trial sites and a lack of awareness of available trials compared to their urban counterparts. Additionally, studies have shown that provider awareness of actively enrolling clinical trials in their respective fields is a key determinate in patient enrollment.5 Finally, insufficient funding and lack of collaboration has resulted in many small phase 1 or phase 2 single-center trials, which are less likely to quickly impact clinical care.6 Increased awareness of the ClinicalTrials.gov registry, a publicly available and easily accessible database, can facilitate referral, enrollment, and collaboration among physicians, patients, and researchers alike.
Using the ClinicalTrials.gov database, we sought to analyze the clinical trial landscape for cutaneous melanoma to understand the current state of melanoma research, future direction, and potential barriers that may impede success.
Methods
The primary objective was to provide a snapshot of the melanoma clinical research landscape from 2005 to 2013, including the number of registered trials, phase distribution, recruitment status, location of trials, type of intervention, and disease state being studied. Secondary objectives included describing patterns of clinical trial distribution within the United States in the context of melanoma mortality and examining changing trends in interventions studied in trials over time.
ClinicalTrials.gov is a comprehensive online registry of clinical trials conducted in the United States and abroad that is maintained by the National Library of Medicine.7 Although the initiative was launched in 2000, the registry became effectively comprehensive in September 2005 when the International Committee of Medical Journal Editors declared prospective registration of clinical trials as a prerequisite for publication. The US Food and Drug Administration followed suit in September 2007, expanding the requirements for registration and declaring penalties for parties who did not comply.8 Each registered trial can be found through searchable keywords, and each study page contains details of study design, principal investigators, and inclusion and exclusion criteria, as well as contact information for enrollment.
Study Selection
Clinical trials registered between September 15, 2005, and December 31, 2013, were evaluated; a total of 138,312 trials were found to be registered on ClinicalTrials.gov during that time period. We limited our study selection to interventional studies, which were filtered by topic to yield only those pertaining to melanoma patients. To minimize reporting bias, trials registered prior to the implementation of the International Committee of Medical Journal Editors’ reporting requirements were excluded. To focus specifically on the landscape of trials in cutaneous melanoma, trials investigating multiple advanced malignancies, uveal or ocular melanoma, and mucosal melanoma were manually excluded.
Study Variables
Information on each clinical trial was extracted from ClinicalTrials.gov. Each trial was manually reviewed by an investigator to determine the disease state and type of intervention being studied. Studies investigating multiple modalities concurrently were classified as “other.”
Data Analysis
Study variables were first analyzed among the entire cohort as a whole. Using each trial location and a python script based on open-source code, the number of actively recruiting melanoma trials in each US county was identified and mapped. County-level, melanoma-specific mortality data from 2001 to 2010 was extracted from the Centers for Disease Control and Prevention’s WONDER (Wide-ranging Online Data for Epidemiologic Research) mortality database (wonder.cdc.gov). Finally, to analyze changing trends in cutaneous melanoma investigation, trials were grouped into 3 categories based on the date they were received on ClinicalTrials.gov: (1) 2005-2007, (2) 2008-2010, and (3) 2011-2013. Disease state and type of intervention were analyzed and compared among each group using the χ2 statistic.
Results
Of the 138,312 trials registered on ClinicalTrials.gov between September 15, 2005, and December 31, 2013, only 931 were identified as interventional studies pertaining to melanoma patients. Of these, 154 were excluded because of a focus on uveal, ocular, or mucosal melanoma or because of the inclusion of participants with multiple types of advanced malignancies. The final analysis included 777 trials specifically focusing on cutaneous melanoma.
Characteristics of these 777 trials were varied. Many interventions were in the early stages of development, with 339 (44%) trials classified as phase 0, phase 1, or phase 1/phase 2; 306 (39%) as phase 2; and 71 (9%) as nonpharmacologic (nonphase) trials. Only 58 trials (8%) were classified as phase 3 or phase 4. The majority of the trials were actively recruiting (225 [29%]), active but not yet recruiting (172 [22%]), or completed (255 [33%]); however, 98 trials (13%) had been suspended, terminated, or withdrawn. Additionally, 22 trials (3%) were not yet recruiting and 5 (<1%) were classified as “other” because they did not have a recruitment status listed.
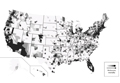
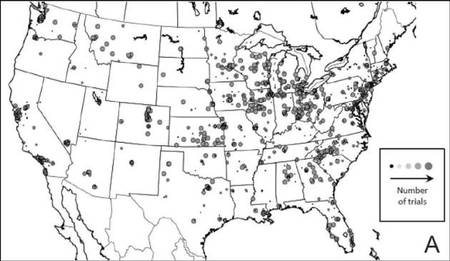
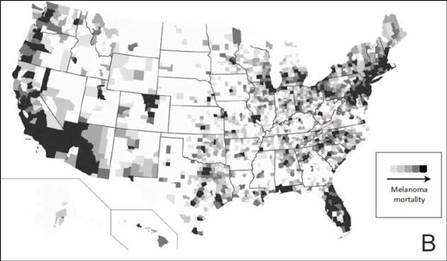
The distribution of actively enrolling clinical trials corresponds to major metropolitan areas within the Northeast, Upper Midwest, and Coastal California (Figure 1A). Figure 1B demonstrates the melanoma-specific mortality across the United States. Areas in the Southwest and Florida shared some of the greatest disease burden.
|
| Figure 1. Geographical representation of US clinical trial enrollment with the number of actively recruiting trials for each unique US zip code presented. The circle size corresponds to the number of trials. The largest circles indicate more than 5 trials within a given zip code (A). County-level melanoma-specific mortality data are presented for 2001 to 2010 (B). Darkest areas represent the highest numbers of melanoma deaths.
|
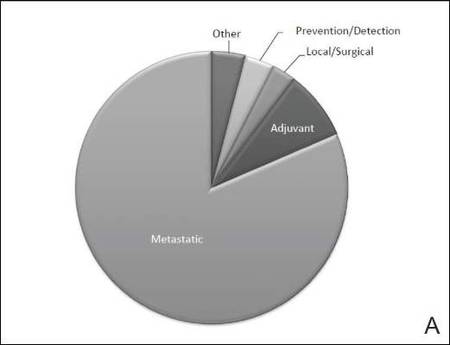
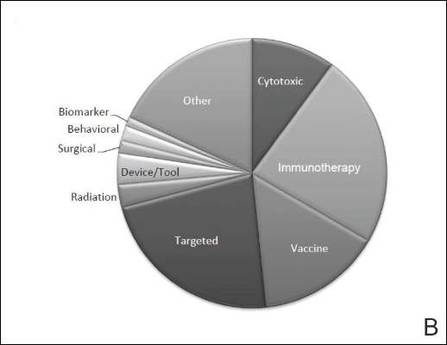
The disease state and type of intervention for all the included trials are summarized in Figure 2. The vast majority of trials (633/777 [82%]) enrolled participants with metastatic melanoma. Unlike many other tumor types, only 64 (8%) trials enrolled patients specifically in the adjuvant setting. Most trials focused on targeted (175 [23%]), immune (180 [23%]), and vaccine (117 [15%]) therapy.
|
| Figure 2. Trial distribution stratified by disease state (A) and type of intervention (B). Trial distribution is shown for 777 interventional clinical trials including melanoma patients. The majority of clinical trials involved patients with metastatic melanoma. The majority of trials investigated targeted therapy, immunotherapy, and vaccine therapy. |
We subsequently analyzed changes in trial characteristics over time. We noted a decrease in the number of trials investigating cytotoxic and vaccine-based therapies, and increasing numbers of trials investigating immunotherapy (P=.041). Between 2005 and 2007, 14% (27/201) of all trials investigated cytotoxic therapies compared to just 7% (20/294) of trials between 2011 and 2013. With the approval of ipilimumab, 29% (85/294) of all clinical trials between 2011 and 2013 investigated immunotherapies, which comprised only 18% (37/201) of clinical trials between 2005 and 2007. The majority of trials continued to enroll patients in the metastatic setting where outcomes remain poor. Importantly, only 6% (49/777) of all clinical trials have focused on prevention, early detection, and local management of melanoma, which has remained constant over time.
Comment
Cutaneous melanoma remains an area of active investigation, interdisciplinary collaboration, and great promise. The ClinicalTrials.gov registry serves not only to increase transparency among interested parties but also as a rich resource to study the clinical research landscape as demonstrated in this study.
Greater understanding of the underlying genetic and immunogenic properties of melanoma tissues has led to the US Food and Drug Administration approval of several novel agents to treat metastatic disease. BRAF inhibitors such as vemurafenib and dabrafenib target more than 50% of all melanoma tumors harboring mutations in the BRAF gene and have shown unparalleled efficacy in clinical trials; however, durability of response and adverse effects still remain a concern.4,9-11 Ipilimumab, a CTLA-4 inhibitor, enhances antitumor immunity and demonstrated improved survival in clinical trials.12,13 Nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitor antibody, also demonstrated improved overall and progression-free survival.14 Finally, trametinib, a MEK inhibitor, used in combination with BRAF inhibitors has demonstrated improved response over BRAF inhibitors alone.15
Although traditional cytotoxic chemotherapy was one of the few available treatment options before 2011, response was infrequent.16 Our data indicate that the melanoma research landscape has shifted to follow advances in targeted therapy and immunotherapy. We noted a decrease in the study of cytotoxic chemotherapy in metastatic melanoma, with a compensatory increase in immunotherapy trials and a continued commitment to targeted therapy. Further, with the approval of BRAF inhibitors, CTLA-4 inhibitors, and PD-1 inhibitors for metastatic disease, some have pushed to move these agents into the adjuvant setting to prevent micrometastases from evolving into clinically significant disease.17 Early results from EORTC (European Organisation for Research and Treatment of Cancer) 18071 comparing adjuvant ipilimumab to placebo demonstrated a 26.1-month versus 17.1-month improvement in relapse-free survival, respectively.18 However, this finding has important implications for clinical dermatologists. Patients treated with BRAF inhibitors are at increased risk for keratoacanthomas, invasive squamous cell carcinomas, and secondary primary melanomas.19,20 Caring for these patients requires increased vigilance and collaboration between dermatologists and oncologists.
Our study also highlights the dynamic nature of the field. For example, novel vaccine therapies have demonstrated promise in the metastatic/ unresectable tumor setting, with some herpes simplex virus–based vaccines generating durable antitumor immune responses in patients with melanoma.21 Combination therapy with CTLA-4 and PD-1 inhibitors has demonstrated improved objective response rates and progression-free survival over monotherapy.22 As the status of actively recruiting trials changes on a regular basis, we encourage physicians to access ClinicalTrials.gov to find details and contact information for actively recruiting trials and results on completed trials.
Early detection and management, however, still remain our primary option for cure, and the role of community dermatologists cannot be overstated.23 Patients with stage I and stage II disease have excellent long-term survival rates, yet only 6% of all clinical trials in cutaneous melanoma have focused on patient education, disease prevention, early detection, and local management. With an increasing incidence of melanoma among an aging population, the disease burden remains of substantial concern.24 Optimizing disease prevention, appropriate screening, and early detection are critical roles for dermatologists.
Finally, our data offer some insight into accrual barriers often faced by clinical trials. Actively enrolling clinical trials cluster within major metropolitan areas, presumably with large academic medical centers; however, areas in the southwestern United States and Florida, for example, have some of the highest burden of disease, likely secondary to sun exposure and aging populations.25 Integration of community dermatologists and oncologists may decrease both actual and patient-perceived barriers to care, which requires further exploration.6
Conclusion
Melanoma incidence and disease burden is increasing, and the field of melanoma research is incredibly dynamic. Going forward, we believe dermatologists will continue to play a critical role both in primary disease prevention and detection as well as in detection of secondary treatment-related skin toxicities. ClinicalTrials.gov is an invaluable resource to keep interested parties informed, foster collaboration, identify potential barriers to success, and suggest future directions.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271.
2. American Cancer Society. Cancer Facts and Figures 2015. American Cancer Society Web site. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed August 26, 2015.
3. Cheng MY, Moreau JF, McGuire ST, et al. Melanoma depth in patients with an established dermatologist. J Am Acad Dermatol. 2014;70:841-846.
4. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60-e69.
5. Kim SH, Tanner A, Friedman DB, et al. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health. 2014;39:562-571.
6. Gregg JR, Horn L, Davidson MA, et al. Patient enrollment onto clinical trials: the role of physician knowledge. J Cancer Educ. 2014;29:74-79.
7. Galsky MD, Hendricks R, Svatek R, et al. Critical analysis of contemporary clinical research in muscle-invasive and metastatic urothelial cancer: a report from the
Bladder Cancer Advocacy Network Clinical Trials Working Group. Cancer. 2013;119:1994-1998.
8. Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364:852-860.
9. Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18:717-725.
10. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
11. Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775-787.
12. Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
14. Robert C, Long G, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
15. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
16. Espinosa E, Berrocal A, López Martin JA, et al. Advances in cutaneous melanoma. Clin Transl Oncol. 2012;14:325-332.
17. Chapman PB. Treating metastatic melanoma in 2014: what just happened and what is next? Am Soc Clin Oncol Educ Book. 2014:16-19.
18. Eggermont A, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
19. Curry JL, Tetzlaff MT, Nicholson K, et al. Histological features associated with vemurafenib-induced skin toxicities: examination of 141 cutaneous lesions biopsied during therapy. Am J Dermatopathol. 2014; 36:557-561.
20. Perier-Muzet M, Thomas L, Poulalhon N, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-1358.
21. Ross MI, Andtbacka RI, Puzanov I, et al. Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): results from a phase III trial in patients with stage IIIb-IV melanoma. Paper presented at: ASCO Annual Meeting; June 2, 2014; Boston, MA.
22. Postow MA, Chesney J, Pavlick AC, et al. Novolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
23. Gorantla VC, Kirkwood JM. State of melanoma: an historic overview of a field in transition. Hematol Oncol Clin North Am. 2014;28:415-435.
24. Coit DG, Olszanski AJ. Progress in the management of melanoma in 2013. J Natl Compr Canc Netw. 2013; 11(5 suppl):645-648.
25. Watson M, Johnson CJ, Chen VW, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65(5, suppl 1):S6-S16.
The incidence of cutaneous melanoma, the deadliest form of skin cancer, has been steadily increasing over the last several decades.1 Currently, there are 73,870 new diagnoses of melanoma anticipated in the United States in 2015 alone.2 Many cases of melanoma are caught at early, actionable, and curable stages thanks in part to patient education and screening by dermatologists.3 However, until recently, few options existed for the treatment of locally advanced and metastatic melanomas, with a median survival rate of less than 1 year.4
Clinical trials represent the most reliable method for advancing treatment and improving outcomes for patients with disease; however, patient accrual and access to clinical trials remain formidable barriers. Studies have suggested that patients in rural areas perceive both an increased distance to clinical trial sites and a lack of awareness of available trials compared to their urban counterparts. Additionally, studies have shown that provider awareness of actively enrolling clinical trials in their respective fields is a key determinate in patient enrollment.5 Finally, insufficient funding and lack of collaboration has resulted in many small phase 1 or phase 2 single-center trials, which are less likely to quickly impact clinical care.6 Increased awareness of the ClinicalTrials.gov registry, a publicly available and easily accessible database, can facilitate referral, enrollment, and collaboration among physicians, patients, and researchers alike.
Using the ClinicalTrials.gov database, we sought to analyze the clinical trial landscape for cutaneous melanoma to understand the current state of melanoma research, future direction, and potential barriers that may impede success.
Methods
The primary objective was to provide a snapshot of the melanoma clinical research landscape from 2005 to 2013, including the number of registered trials, phase distribution, recruitment status, location of trials, type of intervention, and disease state being studied. Secondary objectives included describing patterns of clinical trial distribution within the United States in the context of melanoma mortality and examining changing trends in interventions studied in trials over time.
ClinicalTrials.gov is a comprehensive online registry of clinical trials conducted in the United States and abroad that is maintained by the National Library of Medicine.7 Although the initiative was launched in 2000, the registry became effectively comprehensive in September 2005 when the International Committee of Medical Journal Editors declared prospective registration of clinical trials as a prerequisite for publication. The US Food and Drug Administration followed suit in September 2007, expanding the requirements for registration and declaring penalties for parties who did not comply.8 Each registered trial can be found through searchable keywords, and each study page contains details of study design, principal investigators, and inclusion and exclusion criteria, as well as contact information for enrollment.
Study Selection
Clinical trials registered between September 15, 2005, and December 31, 2013, were evaluated; a total of 138,312 trials were found to be registered on ClinicalTrials.gov during that time period. We limited our study selection to interventional studies, which were filtered by topic to yield only those pertaining to melanoma patients. To minimize reporting bias, trials registered prior to the implementation of the International Committee of Medical Journal Editors’ reporting requirements were excluded. To focus specifically on the landscape of trials in cutaneous melanoma, trials investigating multiple advanced malignancies, uveal or ocular melanoma, and mucosal melanoma were manually excluded.
Study Variables
Information on each clinical trial was extracted from ClinicalTrials.gov. Each trial was manually reviewed by an investigator to determine the disease state and type of intervention being studied. Studies investigating multiple modalities concurrently were classified as “other.”
Data Analysis
Study variables were first analyzed among the entire cohort as a whole. Using each trial location and a python script based on open-source code, the number of actively recruiting melanoma trials in each US county was identified and mapped. County-level, melanoma-specific mortality data from 2001 to 2010 was extracted from the Centers for Disease Control and Prevention’s WONDER (Wide-ranging Online Data for Epidemiologic Research) mortality database (wonder.cdc.gov). Finally, to analyze changing trends in cutaneous melanoma investigation, trials were grouped into 3 categories based on the date they were received on ClinicalTrials.gov: (1) 2005-2007, (2) 2008-2010, and (3) 2011-2013. Disease state and type of intervention were analyzed and compared among each group using the χ2 statistic.
Results
Of the 138,312 trials registered on ClinicalTrials.gov between September 15, 2005, and December 31, 2013, only 931 were identified as interventional studies pertaining to melanoma patients. Of these, 154 were excluded because of a focus on uveal, ocular, or mucosal melanoma or because of the inclusion of participants with multiple types of advanced malignancies. The final analysis included 777 trials specifically focusing on cutaneous melanoma.
Characteristics of these 777 trials were varied. Many interventions were in the early stages of development, with 339 (44%) trials classified as phase 0, phase 1, or phase 1/phase 2; 306 (39%) as phase 2; and 71 (9%) as nonpharmacologic (nonphase) trials. Only 58 trials (8%) were classified as phase 3 or phase 4. The majority of the trials were actively recruiting (225 [29%]), active but not yet recruiting (172 [22%]), or completed (255 [33%]); however, 98 trials (13%) had been suspended, terminated, or withdrawn. Additionally, 22 trials (3%) were not yet recruiting and 5 (<1%) were classified as “other” because they did not have a recruitment status listed.
The distribution of actively enrolling clinical trials corresponds to major metropolitan areas within the Northeast, Upper Midwest, and Coastal California (Figure 1A). Figure 1B demonstrates the melanoma-specific mortality across the United States. Areas in the Southwest and Florida shared some of the greatest disease burden.


|
| Figure 1. Geographical representation of US clinical trial enrollment with the number of actively recruiting trials for each unique US zip code presented. The circle size corresponds to the number of trials. The largest circles indicate more than 5 trials within a given zip code (A). County-level melanoma-specific mortality data are presented for 2001 to 2010 (B). Darkest areas represent the highest numbers of melanoma deaths.
|
The disease state and type of intervention for all the included trials are summarized in Figure 2. The vast majority of trials (633/777 [82%]) enrolled participants with metastatic melanoma. Unlike many other tumor types, only 64 (8%) trials enrolled patients specifically in the adjuvant setting. Most trials focused on targeted (175 [23%]), immune (180 [23%]), and vaccine (117 [15%]) therapy.


|
| Figure 2. Trial distribution stratified by disease state (A) and type of intervention (B). Trial distribution is shown for 777 interventional clinical trials including melanoma patients. The majority of clinical trials involved patients with metastatic melanoma. The majority of trials investigated targeted therapy, immunotherapy, and vaccine therapy. |
We subsequently analyzed changes in trial characteristics over time. We noted a decrease in the number of trials investigating cytotoxic and vaccine-based therapies, and increasing numbers of trials investigating immunotherapy (P=.041). Between 2005 and 2007, 14% (27/201) of all trials investigated cytotoxic therapies compared to just 7% (20/294) of trials between 2011 and 2013. With the approval of ipilimumab, 29% (85/294) of all clinical trials between 2011 and 2013 investigated immunotherapies, which comprised only 18% (37/201) of clinical trials between 2005 and 2007. The majority of trials continued to enroll patients in the metastatic setting where outcomes remain poor. Importantly, only 6% (49/777) of all clinical trials have focused on prevention, early detection, and local management of melanoma, which has remained constant over time.
Comment
Cutaneous melanoma remains an area of active investigation, interdisciplinary collaboration, and great promise. The ClinicalTrials.gov registry serves not only to increase transparency among interested parties but also as a rich resource to study the clinical research landscape as demonstrated in this study.
Greater understanding of the underlying genetic and immunogenic properties of melanoma tissues has led to the US Food and Drug Administration approval of several novel agents to treat metastatic disease. BRAF inhibitors such as vemurafenib and dabrafenib target more than 50% of all melanoma tumors harboring mutations in the BRAF gene and have shown unparalleled efficacy in clinical trials; however, durability of response and adverse effects still remain a concern.4,9-11 Ipilimumab, a CTLA-4 inhibitor, enhances antitumor immunity and demonstrated improved survival in clinical trials.12,13 Nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitor antibody, also demonstrated improved overall and progression-free survival.14 Finally, trametinib, a MEK inhibitor, used in combination with BRAF inhibitors has demonstrated improved response over BRAF inhibitors alone.15
Although traditional cytotoxic chemotherapy was one of the few available treatment options before 2011, response was infrequent.16 Our data indicate that the melanoma research landscape has shifted to follow advances in targeted therapy and immunotherapy. We noted a decrease in the study of cytotoxic chemotherapy in metastatic melanoma, with a compensatory increase in immunotherapy trials and a continued commitment to targeted therapy. Further, with the approval of BRAF inhibitors, CTLA-4 inhibitors, and PD-1 inhibitors for metastatic disease, some have pushed to move these agents into the adjuvant setting to prevent micrometastases from evolving into clinically significant disease.17 Early results from EORTC (European Organisation for Research and Treatment of Cancer) 18071 comparing adjuvant ipilimumab to placebo demonstrated a 26.1-month versus 17.1-month improvement in relapse-free survival, respectively.18 However, this finding has important implications for clinical dermatologists. Patients treated with BRAF inhibitors are at increased risk for keratoacanthomas, invasive squamous cell carcinomas, and secondary primary melanomas.19,20 Caring for these patients requires increased vigilance and collaboration between dermatologists and oncologists.
Our study also highlights the dynamic nature of the field. For example, novel vaccine therapies have demonstrated promise in the metastatic/ unresectable tumor setting, with some herpes simplex virus–based vaccines generating durable antitumor immune responses in patients with melanoma.21 Combination therapy with CTLA-4 and PD-1 inhibitors has demonstrated improved objective response rates and progression-free survival over monotherapy.22 As the status of actively recruiting trials changes on a regular basis, we encourage physicians to access ClinicalTrials.gov to find details and contact information for actively recruiting trials and results on completed trials.
Early detection and management, however, still remain our primary option for cure, and the role of community dermatologists cannot be overstated.23 Patients with stage I and stage II disease have excellent long-term survival rates, yet only 6% of all clinical trials in cutaneous melanoma have focused on patient education, disease prevention, early detection, and local management. With an increasing incidence of melanoma among an aging population, the disease burden remains of substantial concern.24 Optimizing disease prevention, appropriate screening, and early detection are critical roles for dermatologists.
Finally, our data offer some insight into accrual barriers often faced by clinical trials. Actively enrolling clinical trials cluster within major metropolitan areas, presumably with large academic medical centers; however, areas in the southwestern United States and Florida, for example, have some of the highest burden of disease, likely secondary to sun exposure and aging populations.25 Integration of community dermatologists and oncologists may decrease both actual and patient-perceived barriers to care, which requires further exploration.6
Conclusion
Melanoma incidence and disease burden is increasing, and the field of melanoma research is incredibly dynamic. Going forward, we believe dermatologists will continue to play a critical role both in primary disease prevention and detection as well as in detection of secondary treatment-related skin toxicities. ClinicalTrials.gov is an invaluable resource to keep interested parties informed, foster collaboration, identify potential barriers to success, and suggest future directions.
The incidence of cutaneous melanoma, the deadliest form of skin cancer, has been steadily increasing over the last several decades.1 Currently, there are 73,870 new diagnoses of melanoma anticipated in the United States in 2015 alone.2 Many cases of melanoma are caught at early, actionable, and curable stages thanks in part to patient education and screening by dermatologists.3 However, until recently, few options existed for the treatment of locally advanced and metastatic melanomas, with a median survival rate of less than 1 year.4
Clinical trials represent the most reliable method for advancing treatment and improving outcomes for patients with disease; however, patient accrual and access to clinical trials remain formidable barriers. Studies have suggested that patients in rural areas perceive both an increased distance to clinical trial sites and a lack of awareness of available trials compared to their urban counterparts. Additionally, studies have shown that provider awareness of actively enrolling clinical trials in their respective fields is a key determinate in patient enrollment.5 Finally, insufficient funding and lack of collaboration has resulted in many small phase 1 or phase 2 single-center trials, which are less likely to quickly impact clinical care.6 Increased awareness of the ClinicalTrials.gov registry, a publicly available and easily accessible database, can facilitate referral, enrollment, and collaboration among physicians, patients, and researchers alike.
Using the ClinicalTrials.gov database, we sought to analyze the clinical trial landscape for cutaneous melanoma to understand the current state of melanoma research, future direction, and potential barriers that may impede success.
Methods
The primary objective was to provide a snapshot of the melanoma clinical research landscape from 2005 to 2013, including the number of registered trials, phase distribution, recruitment status, location of trials, type of intervention, and disease state being studied. Secondary objectives included describing patterns of clinical trial distribution within the United States in the context of melanoma mortality and examining changing trends in interventions studied in trials over time.
ClinicalTrials.gov is a comprehensive online registry of clinical trials conducted in the United States and abroad that is maintained by the National Library of Medicine.7 Although the initiative was launched in 2000, the registry became effectively comprehensive in September 2005 when the International Committee of Medical Journal Editors declared prospective registration of clinical trials as a prerequisite for publication. The US Food and Drug Administration followed suit in September 2007, expanding the requirements for registration and declaring penalties for parties who did not comply.8 Each registered trial can be found through searchable keywords, and each study page contains details of study design, principal investigators, and inclusion and exclusion criteria, as well as contact information for enrollment.
Study Selection
Clinical trials registered between September 15, 2005, and December 31, 2013, were evaluated; a total of 138,312 trials were found to be registered on ClinicalTrials.gov during that time period. We limited our study selection to interventional studies, which were filtered by topic to yield only those pertaining to melanoma patients. To minimize reporting bias, trials registered prior to the implementation of the International Committee of Medical Journal Editors’ reporting requirements were excluded. To focus specifically on the landscape of trials in cutaneous melanoma, trials investigating multiple advanced malignancies, uveal or ocular melanoma, and mucosal melanoma were manually excluded.
Study Variables
Information on each clinical trial was extracted from ClinicalTrials.gov. Each trial was manually reviewed by an investigator to determine the disease state and type of intervention being studied. Studies investigating multiple modalities concurrently were classified as “other.”
Data Analysis
Study variables were first analyzed among the entire cohort as a whole. Using each trial location and a python script based on open-source code, the number of actively recruiting melanoma trials in each US county was identified and mapped. County-level, melanoma-specific mortality data from 2001 to 2010 was extracted from the Centers for Disease Control and Prevention’s WONDER (Wide-ranging Online Data for Epidemiologic Research) mortality database (wonder.cdc.gov). Finally, to analyze changing trends in cutaneous melanoma investigation, trials were grouped into 3 categories based on the date they were received on ClinicalTrials.gov: (1) 2005-2007, (2) 2008-2010, and (3) 2011-2013. Disease state and type of intervention were analyzed and compared among each group using the χ2 statistic.
Results
Of the 138,312 trials registered on ClinicalTrials.gov between September 15, 2005, and December 31, 2013, only 931 were identified as interventional studies pertaining to melanoma patients. Of these, 154 were excluded because of a focus on uveal, ocular, or mucosal melanoma or because of the inclusion of participants with multiple types of advanced malignancies. The final analysis included 777 trials specifically focusing on cutaneous melanoma.
Characteristics of these 777 trials were varied. Many interventions were in the early stages of development, with 339 (44%) trials classified as phase 0, phase 1, or phase 1/phase 2; 306 (39%) as phase 2; and 71 (9%) as nonpharmacologic (nonphase) trials. Only 58 trials (8%) were classified as phase 3 or phase 4. The majority of the trials were actively recruiting (225 [29%]), active but not yet recruiting (172 [22%]), or completed (255 [33%]); however, 98 trials (13%) had been suspended, terminated, or withdrawn. Additionally, 22 trials (3%) were not yet recruiting and 5 (<1%) were classified as “other” because they did not have a recruitment status listed.
The distribution of actively enrolling clinical trials corresponds to major metropolitan areas within the Northeast, Upper Midwest, and Coastal California (Figure 1A). Figure 1B demonstrates the melanoma-specific mortality across the United States. Areas in the Southwest and Florida shared some of the greatest disease burden.


|
| Figure 1. Geographical representation of US clinical trial enrollment with the number of actively recruiting trials for each unique US zip code presented. The circle size corresponds to the number of trials. The largest circles indicate more than 5 trials within a given zip code (A). County-level melanoma-specific mortality data are presented for 2001 to 2010 (B). Darkest areas represent the highest numbers of melanoma deaths.
|
The disease state and type of intervention for all the included trials are summarized in Figure 2. The vast majority of trials (633/777 [82%]) enrolled participants with metastatic melanoma. Unlike many other tumor types, only 64 (8%) trials enrolled patients specifically in the adjuvant setting. Most trials focused on targeted (175 [23%]), immune (180 [23%]), and vaccine (117 [15%]) therapy.


|
| Figure 2. Trial distribution stratified by disease state (A) and type of intervention (B). Trial distribution is shown for 777 interventional clinical trials including melanoma patients. The majority of clinical trials involved patients with metastatic melanoma. The majority of trials investigated targeted therapy, immunotherapy, and vaccine therapy. |
We subsequently analyzed changes in trial characteristics over time. We noted a decrease in the number of trials investigating cytotoxic and vaccine-based therapies, and increasing numbers of trials investigating immunotherapy (P=.041). Between 2005 and 2007, 14% (27/201) of all trials investigated cytotoxic therapies compared to just 7% (20/294) of trials between 2011 and 2013. With the approval of ipilimumab, 29% (85/294) of all clinical trials between 2011 and 2013 investigated immunotherapies, which comprised only 18% (37/201) of clinical trials between 2005 and 2007. The majority of trials continued to enroll patients in the metastatic setting where outcomes remain poor. Importantly, only 6% (49/777) of all clinical trials have focused on prevention, early detection, and local management of melanoma, which has remained constant over time.
Comment
Cutaneous melanoma remains an area of active investigation, interdisciplinary collaboration, and great promise. The ClinicalTrials.gov registry serves not only to increase transparency among interested parties but also as a rich resource to study the clinical research landscape as demonstrated in this study.
Greater understanding of the underlying genetic and immunogenic properties of melanoma tissues has led to the US Food and Drug Administration approval of several novel agents to treat metastatic disease. BRAF inhibitors such as vemurafenib and dabrafenib target more than 50% of all melanoma tumors harboring mutations in the BRAF gene and have shown unparalleled efficacy in clinical trials; however, durability of response and adverse effects still remain a concern.4,9-11 Ipilimumab, a CTLA-4 inhibitor, enhances antitumor immunity and demonstrated improved survival in clinical trials.12,13 Nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitor antibody, also demonstrated improved overall and progression-free survival.14 Finally, trametinib, a MEK inhibitor, used in combination with BRAF inhibitors has demonstrated improved response over BRAF inhibitors alone.15
Although traditional cytotoxic chemotherapy was one of the few available treatment options before 2011, response was infrequent.16 Our data indicate that the melanoma research landscape has shifted to follow advances in targeted therapy and immunotherapy. We noted a decrease in the study of cytotoxic chemotherapy in metastatic melanoma, with a compensatory increase in immunotherapy trials and a continued commitment to targeted therapy. Further, with the approval of BRAF inhibitors, CTLA-4 inhibitors, and PD-1 inhibitors for metastatic disease, some have pushed to move these agents into the adjuvant setting to prevent micrometastases from evolving into clinically significant disease.17 Early results from EORTC (European Organisation for Research and Treatment of Cancer) 18071 comparing adjuvant ipilimumab to placebo demonstrated a 26.1-month versus 17.1-month improvement in relapse-free survival, respectively.18 However, this finding has important implications for clinical dermatologists. Patients treated with BRAF inhibitors are at increased risk for keratoacanthomas, invasive squamous cell carcinomas, and secondary primary melanomas.19,20 Caring for these patients requires increased vigilance and collaboration between dermatologists and oncologists.
Our study also highlights the dynamic nature of the field. For example, novel vaccine therapies have demonstrated promise in the metastatic/ unresectable tumor setting, with some herpes simplex virus–based vaccines generating durable antitumor immune responses in patients with melanoma.21 Combination therapy with CTLA-4 and PD-1 inhibitors has demonstrated improved objective response rates and progression-free survival over monotherapy.22 As the status of actively recruiting trials changes on a regular basis, we encourage physicians to access ClinicalTrials.gov to find details and contact information for actively recruiting trials and results on completed trials.
Early detection and management, however, still remain our primary option for cure, and the role of community dermatologists cannot be overstated.23 Patients with stage I and stage II disease have excellent long-term survival rates, yet only 6% of all clinical trials in cutaneous melanoma have focused on patient education, disease prevention, early detection, and local management. With an increasing incidence of melanoma among an aging population, the disease burden remains of substantial concern.24 Optimizing disease prevention, appropriate screening, and early detection are critical roles for dermatologists.
Finally, our data offer some insight into accrual barriers often faced by clinical trials. Actively enrolling clinical trials cluster within major metropolitan areas, presumably with large academic medical centers; however, areas in the southwestern United States and Florida, for example, have some of the highest burden of disease, likely secondary to sun exposure and aging populations.25 Integration of community dermatologists and oncologists may decrease both actual and patient-perceived barriers to care, which requires further exploration.6
Conclusion
Melanoma incidence and disease burden is increasing, and the field of melanoma research is incredibly dynamic. Going forward, we believe dermatologists will continue to play a critical role both in primary disease prevention and detection as well as in detection of secondary treatment-related skin toxicities. ClinicalTrials.gov is an invaluable resource to keep interested parties informed, foster collaboration, identify potential barriers to success, and suggest future directions.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271.
2. American Cancer Society. Cancer Facts and Figures 2015. American Cancer Society Web site. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed August 26, 2015.
3. Cheng MY, Moreau JF, McGuire ST, et al. Melanoma depth in patients with an established dermatologist. J Am Acad Dermatol. 2014;70:841-846.
4. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60-e69.
5. Kim SH, Tanner A, Friedman DB, et al. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health. 2014;39:562-571.
6. Gregg JR, Horn L, Davidson MA, et al. Patient enrollment onto clinical trials: the role of physician knowledge. J Cancer Educ. 2014;29:74-79.
7. Galsky MD, Hendricks R, Svatek R, et al. Critical analysis of contemporary clinical research in muscle-invasive and metastatic urothelial cancer: a report from the
Bladder Cancer Advocacy Network Clinical Trials Working Group. Cancer. 2013;119:1994-1998.
8. Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364:852-860.
9. Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18:717-725.
10. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
11. Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775-787.
12. Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
14. Robert C, Long G, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
15. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
16. Espinosa E, Berrocal A, López Martin JA, et al. Advances in cutaneous melanoma. Clin Transl Oncol. 2012;14:325-332.
17. Chapman PB. Treating metastatic melanoma in 2014: what just happened and what is next? Am Soc Clin Oncol Educ Book. 2014:16-19.
18. Eggermont A, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
19. Curry JL, Tetzlaff MT, Nicholson K, et al. Histological features associated with vemurafenib-induced skin toxicities: examination of 141 cutaneous lesions biopsied during therapy. Am J Dermatopathol. 2014; 36:557-561.
20. Perier-Muzet M, Thomas L, Poulalhon N, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-1358.
21. Ross MI, Andtbacka RI, Puzanov I, et al. Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): results from a phase III trial in patients with stage IIIb-IV melanoma. Paper presented at: ASCO Annual Meeting; June 2, 2014; Boston, MA.
22. Postow MA, Chesney J, Pavlick AC, et al. Novolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
23. Gorantla VC, Kirkwood JM. State of melanoma: an historic overview of a field in transition. Hematol Oncol Clin North Am. 2014;28:415-435.
24. Coit DG, Olszanski AJ. Progress in the management of melanoma in 2013. J Natl Compr Canc Netw. 2013; 11(5 suppl):645-648.
25. Watson M, Johnson CJ, Chen VW, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65(5, suppl 1):S6-S16.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271.
2. American Cancer Society. Cancer Facts and Figures 2015. American Cancer Society Web site. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed August 26, 2015.
3. Cheng MY, Moreau JF, McGuire ST, et al. Melanoma depth in patients with an established dermatologist. J Am Acad Dermatol. 2014;70:841-846.
4. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60-e69.
5. Kim SH, Tanner A, Friedman DB, et al. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health. 2014;39:562-571.
6. Gregg JR, Horn L, Davidson MA, et al. Patient enrollment onto clinical trials: the role of physician knowledge. J Cancer Educ. 2014;29:74-79.
7. Galsky MD, Hendricks R, Svatek R, et al. Critical analysis of contemporary clinical research in muscle-invasive and metastatic urothelial cancer: a report from the
Bladder Cancer Advocacy Network Clinical Trials Working Group. Cancer. 2013;119:1994-1998.
8. Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364:852-860.
9. Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18:717-725.
10. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
11. Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775-787.
12. Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
14. Robert C, Long G, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
15. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
16. Espinosa E, Berrocal A, López Martin JA, et al. Advances in cutaneous melanoma. Clin Transl Oncol. 2012;14:325-332.
17. Chapman PB. Treating metastatic melanoma in 2014: what just happened and what is next? Am Soc Clin Oncol Educ Book. 2014:16-19.
18. Eggermont A, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
19. Curry JL, Tetzlaff MT, Nicholson K, et al. Histological features associated with vemurafenib-induced skin toxicities: examination of 141 cutaneous lesions biopsied during therapy. Am J Dermatopathol. 2014; 36:557-561.
20. Perier-Muzet M, Thomas L, Poulalhon N, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-1358.
21. Ross MI, Andtbacka RI, Puzanov I, et al. Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): results from a phase III trial in patients with stage IIIb-IV melanoma. Paper presented at: ASCO Annual Meeting; June 2, 2014; Boston, MA.
22. Postow MA, Chesney J, Pavlick AC, et al. Novolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
23. Gorantla VC, Kirkwood JM. State of melanoma: an historic overview of a field in transition. Hematol Oncol Clin North Am. 2014;28:415-435.
24. Coit DG, Olszanski AJ. Progress in the management of melanoma in 2013. J Natl Compr Canc Netw. 2013; 11(5 suppl):645-648.
25. Watson M, Johnson CJ, Chen VW, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65(5, suppl 1):S6-S16.
Practice Points
- The landscape of melanoma clinical trial research has shifted to follow advances in targeted therapy and immunotherapy.
- With these new treatments there is an increased risk for nonmelanoma skin toxicities requiring increased vigilance and collaboration between dermatologists and oncologists.
- Physicians are encouraged to use ClinicalTrials.gov to find details and contact information for actively recruiting clinical trials and results on completed trials.