User login
• Evaluate for chronic kidney disease (CKD) anemia when a patient has a serum creatinine ≥2 mg/dL and hemoglobin <12 g/dL (adult males and postmenopausal females) or <11 g/dL (premenopausal females). A
• Before you treat CKD anemia, correct any underlying iron deficiency. A
• Start anemia therapy with erythropoietin-stimulating agents when hemoglobin is ≤10 g/dL, and maintain target hemoglobin levels between 11 and 12 g/dL, in accordance with National Kidney Foundation guidelines. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary J, a 65-year-old woman with stage 3 chronic kidney disease (CKD), is in your office for a follow-up appointment. Over the past 6 months, she has noticed a decrease in her energy level. On her routine blood work, you see that her hemoglobin has been slowly declining over the past year. It is now 9 g/dL and her estimated glomerular filtration rate (GFR) is 40 mL/min.
How would you evaluate Mary’s anemia, and would you suspect that it was related to her CKD?
Most physicians are aware that CKD—which affects approximately 10% of the US population1—has a deleterious effect on cardiovascular disease, but many fail to recognize the impact it has on the hematopoietic system. Managing the anemia that accompanies CKD in patients like Mary requires a finely tuned diagnostic approach and treatment strategy. This article will help toward that end.
Anemia of CKD: A common problem
Anemia of CKD is one of the first signs of kidney dysfunction, yet it often goes undetected because of its insidious onset. Anemia develops gradually as kidney function declines and the GFR drops to 70 mL/min in male patients and 50 mL/min in females.2 Epidemiologic data indicate that two-thirds of patients in the early stages of kidney failure are also anemic, with a hemoglobin level of less than 11 g/dL, yet only one-third of these patients have ever received erythropoietin-stimulating agents (ESAs) to treat their anemia.1 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that the evaluation of anemia of CKD begin in patients with a serum creatinine ≥2 mg/dL when the hemoglobin is <12 g/dL in adult males and postmenopausal females and <11 g/dL in premenopausal females.3
How kidney failure leads to anemia
Patients like Mary develop anemia of CKD because failing kidneys produce less erythropoietin (EPO) than the body requires for the production of red blood cells. EPO is an endogenous hormone produced by peritubular fibroblasts in the renal cortex.4 Most of this hormone (90%) is produced in the kidney, with the remainder manufactured by hepatocytes.
Erythropoiesis is stimulated by blood loss, decreased oxygen tension, and an increase in oxygen affinity, which leads to an increase in EPO production via upregulation of the EPO gene. In healthy individuals, detection of hypoxia by the kidney can result in a 1000-fold increase in EPO production.5 Patients with CKD don’t have that kind of robust response, and their EPO levels remain normal or below normal even when challenged by lack of oxygen. Anemia in CKD can also be caused by nutritional deficiencies, decreased red blood cell survival because of uremic toxins, oxidative stress, inflammation, and the use of angiotensin-converting enzyme (ACE) inhibitors.
Chronic anemia, CKD, and CV disease: A deadly triad
The leading cause of death in patients with CKD is cardiovascular disease. Patients with cardiorenal anemia syndrome develop a self-perpetuating triad that increases the risk of death when all 3 conditions are present. Anemic patients double their relative risk of death when CKD is present and triple their risk if they have anemia, CKD, and cardiovascular disease.6
Epidemiologic studies suggest an association among anemia, left ventricular hypertrophy (LVH), mortality, and cardiovascular outcomes. One study evaluated 2423 stage 3 and 4 CKD patients with anemia, defined as hemoglobin <13 g/dL in males and <12 g/dL in females. The results showed an increase in composite outcomes of myocardial infarction, stroke, and death.7 A prospective study evaluating 246 people with stages 2 to 4 CKD reported anemia to be an independent risk factor for the development of LVH.8 The stages of CKD are shown in the TABLE.
Suspected mechanisms of cardiovascular disease progression due to chronic anemia include tissue hypoxia, free radical formation, endothelial dysfunction, and vascular damage. Compensatory neurohumeral adaptations result in an increased sympathetic response and upregulation of the reninangiotensin-aldosterone system.9
TABLE
Stages of chronic kidney disease
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mildly decreased GFR | 60-89 |
| 3 | Moderately decreased GFR | 30-59 |
| 4 | Severely decreased GFR | 15-29 |
| 5 | Kidney failure | <15 or dialysis |
| GFR, glomerular filtration rate. | ||
| Source: KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007.3 | ||
Anemia of CKD: A diagnosis of exclusion
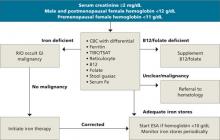
Because anemia can have many causes, other possibilities must be ruled out before a diagnosis of CKD anemia can be made. Testing should be tailored to each individual situation, determined by a thorough history and physical. Steps in the diagnosis are shown in the FLOW CHART. A basic work-up should include complete blood count with differential, iron studies (ferritin, serum Fe, and total iron binding capacity), reticulocyte count, and a guaiac test. Other blood tests, such as thyroid-stimulating hormone (TSH), B12, and folate levels, and a hemolysis panel (lactate dehydrogenase, haptoglobin), should be obtained if the history suggests these disorders. A peripheral blood smear showing normocytic red blood cells with a normochromic pattern would favor the diagnosis of anemia of CKD.
FLOW CHART
A step-by-step guide to CKD anemia diagnosis and treatment
CBC, complete blood count; CKD, chronic kidney disease; ESA, erythropoietin-stimulating agents; R/O, rule out; TIBC/TSAT, total iron-binding capacity/transferrin saturation.
A look at the iron connection
Many patients with CKD anemia have iron deficiency and are unable to produce adequate numbers of red blood cells. Iron deficiency can have many causes: not enough iron-rich food in the diet, chronic bleeding, malabsorption, or an occult gastrointestinal malignancy. Once iron deficiency anemia is diagnosed, a colonoscopy is warranted to rule out occult malignancy. Ferritin, a protein found mostly in macrophages and hepatocytes, stores iron and serves as a marker for total iron stores. Using stored iron requires transferrin, a transporting protein, to shuttle iron from the reticuloendothelial system and gut to the bone marrow. CKD is a pro-inflammatory state that results in a limited ability to use iron stores. For this reason, patients with CKD require higher levels of iron.
Absolute iron deficiency. Iron deficiency in CKD patients with serum ferritin <100 ng/mL and transferrin saturation (TSAT) <20% is characterized as absolute iron deficiency. The TSAT represents the percent of iron bound to transferrin and is a good indicator of the body’s functional capacity to use stored iron.
Relative iron deficiency and iron block. Patients who do not respond to ESA therapy even though they have adequate iron stores are said to have a functional or relative iron deficiency. Iron block is a condition that results in anemia from a chronic inflammatory state such as infection, autoimmune disorders, or malignancies. It resolves once the inflammatory process abates. Both conditions have similar anemia profiles, with a serum ferritin >100 ng/mL and a TSAT <20%. Differentiating between these conditions requires dynamic testing using serial iron studies and observing responses to ESAs and iron supplementation.
Options for correcting iron deficiency
After a thorough history and physical with appropriate screening, you find that Mary has an iron deficiency that must be corrected before her anemia can be treated effectively. Treatment for iron deficiency is usually initiated with oral therapy, at the recommended dose of 200 mg oral elemental iron a day in 3 divided doses.
If the oral therapy does not correct iron deficiency within 3 months, or a patient cannot tolerate the constipation that is often a side effect of this therapy, IV iron administration can be considered. Because CKD patients do not have the ongoing iron losses seen in patients with end-stage renal disease (ESRD), a conservative approach using a single IV dose followed by repeat testing is warranted. The goal is to achieve ferritin levels >100 ng/dL and TSAT >20%. A number of products for IV iron administration are available. The most widely used are iron dextran (INFeD), ferric gluconate (Ferrlecit), and iron sucrose (Venofer).
Iron stores are replenished? Time to treat the anemia
When ferritin levels and TSAT show that iron deficiency has been corrected, ESA treatment for anemia can begin. Two major brands of ESAs currently in use in the United States are a recombinant human erythropoietin (rHuEPO) known as epoetin alfa (Procrit, Epogen), and darbepoetin alpha (Aranesp). Both medications are effective and can be given intravenously or subcutaneously. Subcutaneous darbepoetin alpha has a longer half-life compared with epoetin alpha (70 vs 24 hours), so dosing intervals can be longer.10,11 ESAs should not be started in patients with uncontrolled hypertension until the blood pressure is controlled, or in patients with an active malignancy unless the treatment is directly supervised by an oncologist.
Aim for complete anemia resolution? That’s controversial
Treatment of CKD anemia with ESAs is widely practiced, but controversy over whether it is beneficial to aim for complete resolution of anemia is ongoing. The CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment) and CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trials published in 2006 failed to resolve the issue.12,13
In the CREATE trial, patients targeted to achieve normal hemoglobin levels did no better in avoiding cardiovascular events than patients targeted for lower levels. The CHOIR trial was stopped early because of an increased trend toward death and hospitalization for congestive heart failure in the group with therapy targeted to achieve normal hemoglobin levels.
The recently published TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of patients with type 2 diabetes and CKD showed no reduction in all-cause mortality, cardiovascular morbidity, or ESRD in patients receiving Aranesp targeted to achieve a hemoglobin level of approximately 13 g/dL, compared with placebo.14 The study did demonstrate, however, that patients receiving Aranesp were about twice as likely to have a stroke than the placebo subjects (101 vs 53)—which might lead clinicians to ponder whether the gains, if any, were worth the risk.
Revised labeling. Late last year, the US Food and Drug Administration approved a label change for Procrit and Aranesp, warning that patients with renal failure “experienced greater risks for death and serious cardiovascular events when administered ESAs to target higher vs lower hemoglobin levels” and advising physicians to “individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.”10,11 The 2007 NKF KDOQI guidelines suggest maintaining a hemoglobin level between 11 and 12 g/dL and have not incorporated the results of the TREAT trial.
Some patients don’t respond to ESAs
Inadequate response to ESAs is most commonly caused by underdosing or inadequate iron stores. NKF KDOQI guidelines recommend checking TSAT and ferritin prior to initiating therapy and monitoring these levels every 3 months.3 True nonresponders are individuals with good iron stores who are unable to achieve target hemoglobin within 4 to 6 months despite receiving subcutaneous epoetin 300 IU/kg per week. Inadequate response to ESAs can be caused by ongoing occult blood loss, infection, inflammation, nutritional deficiencies, hemolysis, hemoglobinemias, aluminum toxicity, anti-EPO antibody, hyperparathyroidism, multiple myeloma, and bone marrow dysfunction.10,11 If patients do not respond to ESA therapy, the NKF KDOQI guidelines recommend referral to a nephrologist or hematologist.3
How did Mary fare?
Mary did well taking oral iron supplementation. Once her iron deficiency was corrected, you were able to begin treating her anemia. After appropriate titration of her ESA, she was able to maintain a hemoglobin level between 11 and 12 g/dL 4 months into therapy. On a follow-up visit, she had no side effects from the medication and reported an increase in her energy level.
CORRESPONDENCE
Jonathan Taliercio, DO, Cleveland Clinic, Department of Nephrology and Hypertension, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. United States Renal Data System, USRDS. 2009 Annual Data Report. Atlas of Chronic Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. Hsu CJ, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504-510.
3. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
4. Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415-425.
5. Ebert B, Franklin H. Regulation of the erythropoietin gene. Blood. 1999;94:1864-1877.
6. Silverberg D, Wexler D, Blum M, et al. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii 7-viii 12.
7. Weiner D, Tighiouart H, Vlagopoulos P, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803-1810.
8. Levin A, Thompson C, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134.
9. Rao M, Pereira B. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005;68:1432-1438.
10. Amgen. Aranesp (Darbepoetin Alpha) package insert. Available at www.aranesp.com/professional/crf/full_prescribing_info/pi.jsp. Accessed November 16, 2009.
11. Amgen. Procrit (Epoetin Alpha) package insert. Available at www.procrit.com/sites/default/files/shared/OBI/PI/ProcritBooklet.pdf#page=1. Accessed November 16, 2009.
12. Drueke T, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with CKD and anemia. N Engl J Med. 2006;355:2071-2084.
13. Singh A, Szczech L, Tang K. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098.
14. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032.
• Evaluate for chronic kidney disease (CKD) anemia when a patient has a serum creatinine ≥2 mg/dL and hemoglobin <12 g/dL (adult males and postmenopausal females) or <11 g/dL (premenopausal females). A
• Before you treat CKD anemia, correct any underlying iron deficiency. A
• Start anemia therapy with erythropoietin-stimulating agents when hemoglobin is ≤10 g/dL, and maintain target hemoglobin levels between 11 and 12 g/dL, in accordance with National Kidney Foundation guidelines. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary J, a 65-year-old woman with stage 3 chronic kidney disease (CKD), is in your office for a follow-up appointment. Over the past 6 months, she has noticed a decrease in her energy level. On her routine blood work, you see that her hemoglobin has been slowly declining over the past year. It is now 9 g/dL and her estimated glomerular filtration rate (GFR) is 40 mL/min.
How would you evaluate Mary’s anemia, and would you suspect that it was related to her CKD?
Most physicians are aware that CKD—which affects approximately 10% of the US population1—has a deleterious effect on cardiovascular disease, but many fail to recognize the impact it has on the hematopoietic system. Managing the anemia that accompanies CKD in patients like Mary requires a finely tuned diagnostic approach and treatment strategy. This article will help toward that end.
Anemia of CKD: A common problem
Anemia of CKD is one of the first signs of kidney dysfunction, yet it often goes undetected because of its insidious onset. Anemia develops gradually as kidney function declines and the GFR drops to 70 mL/min in male patients and 50 mL/min in females.2 Epidemiologic data indicate that two-thirds of patients in the early stages of kidney failure are also anemic, with a hemoglobin level of less than 11 g/dL, yet only one-third of these patients have ever received erythropoietin-stimulating agents (ESAs) to treat their anemia.1 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that the evaluation of anemia of CKD begin in patients with a serum creatinine ≥2 mg/dL when the hemoglobin is <12 g/dL in adult males and postmenopausal females and <11 g/dL in premenopausal females.3
How kidney failure leads to anemia
Patients like Mary develop anemia of CKD because failing kidneys produce less erythropoietin (EPO) than the body requires for the production of red blood cells. EPO is an endogenous hormone produced by peritubular fibroblasts in the renal cortex.4 Most of this hormone (90%) is produced in the kidney, with the remainder manufactured by hepatocytes.
Erythropoiesis is stimulated by blood loss, decreased oxygen tension, and an increase in oxygen affinity, which leads to an increase in EPO production via upregulation of the EPO gene. In healthy individuals, detection of hypoxia by the kidney can result in a 1000-fold increase in EPO production.5 Patients with CKD don’t have that kind of robust response, and their EPO levels remain normal or below normal even when challenged by lack of oxygen. Anemia in CKD can also be caused by nutritional deficiencies, decreased red blood cell survival because of uremic toxins, oxidative stress, inflammation, and the use of angiotensin-converting enzyme (ACE) inhibitors.
Chronic anemia, CKD, and CV disease: A deadly triad
The leading cause of death in patients with CKD is cardiovascular disease. Patients with cardiorenal anemia syndrome develop a self-perpetuating triad that increases the risk of death when all 3 conditions are present. Anemic patients double their relative risk of death when CKD is present and triple their risk if they have anemia, CKD, and cardiovascular disease.6
Epidemiologic studies suggest an association among anemia, left ventricular hypertrophy (LVH), mortality, and cardiovascular outcomes. One study evaluated 2423 stage 3 and 4 CKD patients with anemia, defined as hemoglobin <13 g/dL in males and <12 g/dL in females. The results showed an increase in composite outcomes of myocardial infarction, stroke, and death.7 A prospective study evaluating 246 people with stages 2 to 4 CKD reported anemia to be an independent risk factor for the development of LVH.8 The stages of CKD are shown in the TABLE.
Suspected mechanisms of cardiovascular disease progression due to chronic anemia include tissue hypoxia, free radical formation, endothelial dysfunction, and vascular damage. Compensatory neurohumeral adaptations result in an increased sympathetic response and upregulation of the reninangiotensin-aldosterone system.9
TABLE
Stages of chronic kidney disease
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mildly decreased GFR | 60-89 |
| 3 | Moderately decreased GFR | 30-59 |
| 4 | Severely decreased GFR | 15-29 |
| 5 | Kidney failure | <15 or dialysis |
| GFR, glomerular filtration rate. | ||
| Source: KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007.3 | ||
Anemia of CKD: A diagnosis of exclusion
Because anemia can have many causes, other possibilities must be ruled out before a diagnosis of CKD anemia can be made. Testing should be tailored to each individual situation, determined by a thorough history and physical. Steps in the diagnosis are shown in the FLOW CHART. A basic work-up should include complete blood count with differential, iron studies (ferritin, serum Fe, and total iron binding capacity), reticulocyte count, and a guaiac test. Other blood tests, such as thyroid-stimulating hormone (TSH), B12, and folate levels, and a hemolysis panel (lactate dehydrogenase, haptoglobin), should be obtained if the history suggests these disorders. A peripheral blood smear showing normocytic red blood cells with a normochromic pattern would favor the diagnosis of anemia of CKD.
FLOW CHART
A step-by-step guide to CKD anemia diagnosis and treatment
CBC, complete blood count; CKD, chronic kidney disease; ESA, erythropoietin-stimulating agents; R/O, rule out; TIBC/TSAT, total iron-binding capacity/transferrin saturation.
A look at the iron connection
Many patients with CKD anemia have iron deficiency and are unable to produce adequate numbers of red blood cells. Iron deficiency can have many causes: not enough iron-rich food in the diet, chronic bleeding, malabsorption, or an occult gastrointestinal malignancy. Once iron deficiency anemia is diagnosed, a colonoscopy is warranted to rule out occult malignancy. Ferritin, a protein found mostly in macrophages and hepatocytes, stores iron and serves as a marker for total iron stores. Using stored iron requires transferrin, a transporting protein, to shuttle iron from the reticuloendothelial system and gut to the bone marrow. CKD is a pro-inflammatory state that results in a limited ability to use iron stores. For this reason, patients with CKD require higher levels of iron.
Absolute iron deficiency. Iron deficiency in CKD patients with serum ferritin <100 ng/mL and transferrin saturation (TSAT) <20% is characterized as absolute iron deficiency. The TSAT represents the percent of iron bound to transferrin and is a good indicator of the body’s functional capacity to use stored iron.
Relative iron deficiency and iron block. Patients who do not respond to ESA therapy even though they have adequate iron stores are said to have a functional or relative iron deficiency. Iron block is a condition that results in anemia from a chronic inflammatory state such as infection, autoimmune disorders, or malignancies. It resolves once the inflammatory process abates. Both conditions have similar anemia profiles, with a serum ferritin >100 ng/mL and a TSAT <20%. Differentiating between these conditions requires dynamic testing using serial iron studies and observing responses to ESAs and iron supplementation.
Options for correcting iron deficiency
After a thorough history and physical with appropriate screening, you find that Mary has an iron deficiency that must be corrected before her anemia can be treated effectively. Treatment for iron deficiency is usually initiated with oral therapy, at the recommended dose of 200 mg oral elemental iron a day in 3 divided doses.
If the oral therapy does not correct iron deficiency within 3 months, or a patient cannot tolerate the constipation that is often a side effect of this therapy, IV iron administration can be considered. Because CKD patients do not have the ongoing iron losses seen in patients with end-stage renal disease (ESRD), a conservative approach using a single IV dose followed by repeat testing is warranted. The goal is to achieve ferritin levels >100 ng/dL and TSAT >20%. A number of products for IV iron administration are available. The most widely used are iron dextran (INFeD), ferric gluconate (Ferrlecit), and iron sucrose (Venofer).
Iron stores are replenished? Time to treat the anemia
When ferritin levels and TSAT show that iron deficiency has been corrected, ESA treatment for anemia can begin. Two major brands of ESAs currently in use in the United States are a recombinant human erythropoietin (rHuEPO) known as epoetin alfa (Procrit, Epogen), and darbepoetin alpha (Aranesp). Both medications are effective and can be given intravenously or subcutaneously. Subcutaneous darbepoetin alpha has a longer half-life compared with epoetin alpha (70 vs 24 hours), so dosing intervals can be longer.10,11 ESAs should not be started in patients with uncontrolled hypertension until the blood pressure is controlled, or in patients with an active malignancy unless the treatment is directly supervised by an oncologist.
Aim for complete anemia resolution? That’s controversial
Treatment of CKD anemia with ESAs is widely practiced, but controversy over whether it is beneficial to aim for complete resolution of anemia is ongoing. The CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment) and CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trials published in 2006 failed to resolve the issue.12,13
In the CREATE trial, patients targeted to achieve normal hemoglobin levels did no better in avoiding cardiovascular events than patients targeted for lower levels. The CHOIR trial was stopped early because of an increased trend toward death and hospitalization for congestive heart failure in the group with therapy targeted to achieve normal hemoglobin levels.
The recently published TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of patients with type 2 diabetes and CKD showed no reduction in all-cause mortality, cardiovascular morbidity, or ESRD in patients receiving Aranesp targeted to achieve a hemoglobin level of approximately 13 g/dL, compared with placebo.14 The study did demonstrate, however, that patients receiving Aranesp were about twice as likely to have a stroke than the placebo subjects (101 vs 53)—which might lead clinicians to ponder whether the gains, if any, were worth the risk.
Revised labeling. Late last year, the US Food and Drug Administration approved a label change for Procrit and Aranesp, warning that patients with renal failure “experienced greater risks for death and serious cardiovascular events when administered ESAs to target higher vs lower hemoglobin levels” and advising physicians to “individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.”10,11 The 2007 NKF KDOQI guidelines suggest maintaining a hemoglobin level between 11 and 12 g/dL and have not incorporated the results of the TREAT trial.
Some patients don’t respond to ESAs
Inadequate response to ESAs is most commonly caused by underdosing or inadequate iron stores. NKF KDOQI guidelines recommend checking TSAT and ferritin prior to initiating therapy and monitoring these levels every 3 months.3 True nonresponders are individuals with good iron stores who are unable to achieve target hemoglobin within 4 to 6 months despite receiving subcutaneous epoetin 300 IU/kg per week. Inadequate response to ESAs can be caused by ongoing occult blood loss, infection, inflammation, nutritional deficiencies, hemolysis, hemoglobinemias, aluminum toxicity, anti-EPO antibody, hyperparathyroidism, multiple myeloma, and bone marrow dysfunction.10,11 If patients do not respond to ESA therapy, the NKF KDOQI guidelines recommend referral to a nephrologist or hematologist.3
How did Mary fare?
Mary did well taking oral iron supplementation. Once her iron deficiency was corrected, you were able to begin treating her anemia. After appropriate titration of her ESA, she was able to maintain a hemoglobin level between 11 and 12 g/dL 4 months into therapy. On a follow-up visit, she had no side effects from the medication and reported an increase in her energy level.
CORRESPONDENCE
Jonathan Taliercio, DO, Cleveland Clinic, Department of Nephrology and Hypertension, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
• Evaluate for chronic kidney disease (CKD) anemia when a patient has a serum creatinine ≥2 mg/dL and hemoglobin <12 g/dL (adult males and postmenopausal females) or <11 g/dL (premenopausal females). A
• Before you treat CKD anemia, correct any underlying iron deficiency. A
• Start anemia therapy with erythropoietin-stimulating agents when hemoglobin is ≤10 g/dL, and maintain target hemoglobin levels between 11 and 12 g/dL, in accordance with National Kidney Foundation guidelines. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary J, a 65-year-old woman with stage 3 chronic kidney disease (CKD), is in your office for a follow-up appointment. Over the past 6 months, she has noticed a decrease in her energy level. On her routine blood work, you see that her hemoglobin has been slowly declining over the past year. It is now 9 g/dL and her estimated glomerular filtration rate (GFR) is 40 mL/min.
How would you evaluate Mary’s anemia, and would you suspect that it was related to her CKD?
Most physicians are aware that CKD—which affects approximately 10% of the US population1—has a deleterious effect on cardiovascular disease, but many fail to recognize the impact it has on the hematopoietic system. Managing the anemia that accompanies CKD in patients like Mary requires a finely tuned diagnostic approach and treatment strategy. This article will help toward that end.
Anemia of CKD: A common problem
Anemia of CKD is one of the first signs of kidney dysfunction, yet it often goes undetected because of its insidious onset. Anemia develops gradually as kidney function declines and the GFR drops to 70 mL/min in male patients and 50 mL/min in females.2 Epidemiologic data indicate that two-thirds of patients in the early stages of kidney failure are also anemic, with a hemoglobin level of less than 11 g/dL, yet only one-third of these patients have ever received erythropoietin-stimulating agents (ESAs) to treat their anemia.1 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that the evaluation of anemia of CKD begin in patients with a serum creatinine ≥2 mg/dL when the hemoglobin is <12 g/dL in adult males and postmenopausal females and <11 g/dL in premenopausal females.3
How kidney failure leads to anemia
Patients like Mary develop anemia of CKD because failing kidneys produce less erythropoietin (EPO) than the body requires for the production of red blood cells. EPO is an endogenous hormone produced by peritubular fibroblasts in the renal cortex.4 Most of this hormone (90%) is produced in the kidney, with the remainder manufactured by hepatocytes.
Erythropoiesis is stimulated by blood loss, decreased oxygen tension, and an increase in oxygen affinity, which leads to an increase in EPO production via upregulation of the EPO gene. In healthy individuals, detection of hypoxia by the kidney can result in a 1000-fold increase in EPO production.5 Patients with CKD don’t have that kind of robust response, and their EPO levels remain normal or below normal even when challenged by lack of oxygen. Anemia in CKD can also be caused by nutritional deficiencies, decreased red blood cell survival because of uremic toxins, oxidative stress, inflammation, and the use of angiotensin-converting enzyme (ACE) inhibitors.
Chronic anemia, CKD, and CV disease: A deadly triad
The leading cause of death in patients with CKD is cardiovascular disease. Patients with cardiorenal anemia syndrome develop a self-perpetuating triad that increases the risk of death when all 3 conditions are present. Anemic patients double their relative risk of death when CKD is present and triple their risk if they have anemia, CKD, and cardiovascular disease.6
Epidemiologic studies suggest an association among anemia, left ventricular hypertrophy (LVH), mortality, and cardiovascular outcomes. One study evaluated 2423 stage 3 and 4 CKD patients with anemia, defined as hemoglobin <13 g/dL in males and <12 g/dL in females. The results showed an increase in composite outcomes of myocardial infarction, stroke, and death.7 A prospective study evaluating 246 people with stages 2 to 4 CKD reported anemia to be an independent risk factor for the development of LVH.8 The stages of CKD are shown in the TABLE.
Suspected mechanisms of cardiovascular disease progression due to chronic anemia include tissue hypoxia, free radical formation, endothelial dysfunction, and vascular damage. Compensatory neurohumeral adaptations result in an increased sympathetic response and upregulation of the reninangiotensin-aldosterone system.9
TABLE
Stages of chronic kidney disease
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mildly decreased GFR | 60-89 |
| 3 | Moderately decreased GFR | 30-59 |
| 4 | Severely decreased GFR | 15-29 |
| 5 | Kidney failure | <15 or dialysis |
| GFR, glomerular filtration rate. | ||
| Source: KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007.3 | ||
Anemia of CKD: A diagnosis of exclusion
Because anemia can have many causes, other possibilities must be ruled out before a diagnosis of CKD anemia can be made. Testing should be tailored to each individual situation, determined by a thorough history and physical. Steps in the diagnosis are shown in the FLOW CHART. A basic work-up should include complete blood count with differential, iron studies (ferritin, serum Fe, and total iron binding capacity), reticulocyte count, and a guaiac test. Other blood tests, such as thyroid-stimulating hormone (TSH), B12, and folate levels, and a hemolysis panel (lactate dehydrogenase, haptoglobin), should be obtained if the history suggests these disorders. A peripheral blood smear showing normocytic red blood cells with a normochromic pattern would favor the diagnosis of anemia of CKD.
FLOW CHART
A step-by-step guide to CKD anemia diagnosis and treatment
CBC, complete blood count; CKD, chronic kidney disease; ESA, erythropoietin-stimulating agents; R/O, rule out; TIBC/TSAT, total iron-binding capacity/transferrin saturation.
A look at the iron connection
Many patients with CKD anemia have iron deficiency and are unable to produce adequate numbers of red blood cells. Iron deficiency can have many causes: not enough iron-rich food in the diet, chronic bleeding, malabsorption, or an occult gastrointestinal malignancy. Once iron deficiency anemia is diagnosed, a colonoscopy is warranted to rule out occult malignancy. Ferritin, a protein found mostly in macrophages and hepatocytes, stores iron and serves as a marker for total iron stores. Using stored iron requires transferrin, a transporting protein, to shuttle iron from the reticuloendothelial system and gut to the bone marrow. CKD is a pro-inflammatory state that results in a limited ability to use iron stores. For this reason, patients with CKD require higher levels of iron.
Absolute iron deficiency. Iron deficiency in CKD patients with serum ferritin <100 ng/mL and transferrin saturation (TSAT) <20% is characterized as absolute iron deficiency. The TSAT represents the percent of iron bound to transferrin and is a good indicator of the body’s functional capacity to use stored iron.
Relative iron deficiency and iron block. Patients who do not respond to ESA therapy even though they have adequate iron stores are said to have a functional or relative iron deficiency. Iron block is a condition that results in anemia from a chronic inflammatory state such as infection, autoimmune disorders, or malignancies. It resolves once the inflammatory process abates. Both conditions have similar anemia profiles, with a serum ferritin >100 ng/mL and a TSAT <20%. Differentiating between these conditions requires dynamic testing using serial iron studies and observing responses to ESAs and iron supplementation.
Options for correcting iron deficiency
After a thorough history and physical with appropriate screening, you find that Mary has an iron deficiency that must be corrected before her anemia can be treated effectively. Treatment for iron deficiency is usually initiated with oral therapy, at the recommended dose of 200 mg oral elemental iron a day in 3 divided doses.
If the oral therapy does not correct iron deficiency within 3 months, or a patient cannot tolerate the constipation that is often a side effect of this therapy, IV iron administration can be considered. Because CKD patients do not have the ongoing iron losses seen in patients with end-stage renal disease (ESRD), a conservative approach using a single IV dose followed by repeat testing is warranted. The goal is to achieve ferritin levels >100 ng/dL and TSAT >20%. A number of products for IV iron administration are available. The most widely used are iron dextran (INFeD), ferric gluconate (Ferrlecit), and iron sucrose (Venofer).
Iron stores are replenished? Time to treat the anemia
When ferritin levels and TSAT show that iron deficiency has been corrected, ESA treatment for anemia can begin. Two major brands of ESAs currently in use in the United States are a recombinant human erythropoietin (rHuEPO) known as epoetin alfa (Procrit, Epogen), and darbepoetin alpha (Aranesp). Both medications are effective and can be given intravenously or subcutaneously. Subcutaneous darbepoetin alpha has a longer half-life compared with epoetin alpha (70 vs 24 hours), so dosing intervals can be longer.10,11 ESAs should not be started in patients with uncontrolled hypertension until the blood pressure is controlled, or in patients with an active malignancy unless the treatment is directly supervised by an oncologist.
Aim for complete anemia resolution? That’s controversial
Treatment of CKD anemia with ESAs is widely practiced, but controversy over whether it is beneficial to aim for complete resolution of anemia is ongoing. The CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment) and CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trials published in 2006 failed to resolve the issue.12,13
In the CREATE trial, patients targeted to achieve normal hemoglobin levels did no better in avoiding cardiovascular events than patients targeted for lower levels. The CHOIR trial was stopped early because of an increased trend toward death and hospitalization for congestive heart failure in the group with therapy targeted to achieve normal hemoglobin levels.
The recently published TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of patients with type 2 diabetes and CKD showed no reduction in all-cause mortality, cardiovascular morbidity, or ESRD in patients receiving Aranesp targeted to achieve a hemoglobin level of approximately 13 g/dL, compared with placebo.14 The study did demonstrate, however, that patients receiving Aranesp were about twice as likely to have a stroke than the placebo subjects (101 vs 53)—which might lead clinicians to ponder whether the gains, if any, were worth the risk.
Revised labeling. Late last year, the US Food and Drug Administration approved a label change for Procrit and Aranesp, warning that patients with renal failure “experienced greater risks for death and serious cardiovascular events when administered ESAs to target higher vs lower hemoglobin levels” and advising physicians to “individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.”10,11 The 2007 NKF KDOQI guidelines suggest maintaining a hemoglobin level between 11 and 12 g/dL and have not incorporated the results of the TREAT trial.
Some patients don’t respond to ESAs
Inadequate response to ESAs is most commonly caused by underdosing or inadequate iron stores. NKF KDOQI guidelines recommend checking TSAT and ferritin prior to initiating therapy and monitoring these levels every 3 months.3 True nonresponders are individuals with good iron stores who are unable to achieve target hemoglobin within 4 to 6 months despite receiving subcutaneous epoetin 300 IU/kg per week. Inadequate response to ESAs can be caused by ongoing occult blood loss, infection, inflammation, nutritional deficiencies, hemolysis, hemoglobinemias, aluminum toxicity, anti-EPO antibody, hyperparathyroidism, multiple myeloma, and bone marrow dysfunction.10,11 If patients do not respond to ESA therapy, the NKF KDOQI guidelines recommend referral to a nephrologist or hematologist.3
How did Mary fare?
Mary did well taking oral iron supplementation. Once her iron deficiency was corrected, you were able to begin treating her anemia. After appropriate titration of her ESA, she was able to maintain a hemoglobin level between 11 and 12 g/dL 4 months into therapy. On a follow-up visit, she had no side effects from the medication and reported an increase in her energy level.
CORRESPONDENCE
Jonathan Taliercio, DO, Cleveland Clinic, Department of Nephrology and Hypertension, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. United States Renal Data System, USRDS. 2009 Annual Data Report. Atlas of Chronic Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. Hsu CJ, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504-510.
3. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
4. Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415-425.
5. Ebert B, Franklin H. Regulation of the erythropoietin gene. Blood. 1999;94:1864-1877.
6. Silverberg D, Wexler D, Blum M, et al. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii 7-viii 12.
7. Weiner D, Tighiouart H, Vlagopoulos P, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803-1810.
8. Levin A, Thompson C, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134.
9. Rao M, Pereira B. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005;68:1432-1438.
10. Amgen. Aranesp (Darbepoetin Alpha) package insert. Available at www.aranesp.com/professional/crf/full_prescribing_info/pi.jsp. Accessed November 16, 2009.
11. Amgen. Procrit (Epoetin Alpha) package insert. Available at www.procrit.com/sites/default/files/shared/OBI/PI/ProcritBooklet.pdf#page=1. Accessed November 16, 2009.
12. Drueke T, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with CKD and anemia. N Engl J Med. 2006;355:2071-2084.
13. Singh A, Szczech L, Tang K. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098.
14. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032.
1. United States Renal Data System, USRDS. 2009 Annual Data Report. Atlas of Chronic Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. Hsu CJ, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504-510.
3. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
4. Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415-425.
5. Ebert B, Franklin H. Regulation of the erythropoietin gene. Blood. 1999;94:1864-1877.
6. Silverberg D, Wexler D, Blum M, et al. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii 7-viii 12.
7. Weiner D, Tighiouart H, Vlagopoulos P, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803-1810.
8. Levin A, Thompson C, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134.
9. Rao M, Pereira B. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005;68:1432-1438.
10. Amgen. Aranesp (Darbepoetin Alpha) package insert. Available at www.aranesp.com/professional/crf/full_prescribing_info/pi.jsp. Accessed November 16, 2009.
11. Amgen. Procrit (Epoetin Alpha) package insert. Available at www.procrit.com/sites/default/files/shared/OBI/PI/ProcritBooklet.pdf#page=1. Accessed November 16, 2009.
12. Drueke T, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with CKD and anemia. N Engl J Med. 2006;355:2071-2084.
13. Singh A, Szczech L, Tang K. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098.
14. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032.