User login
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
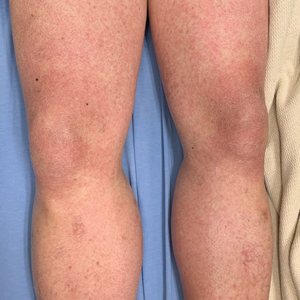
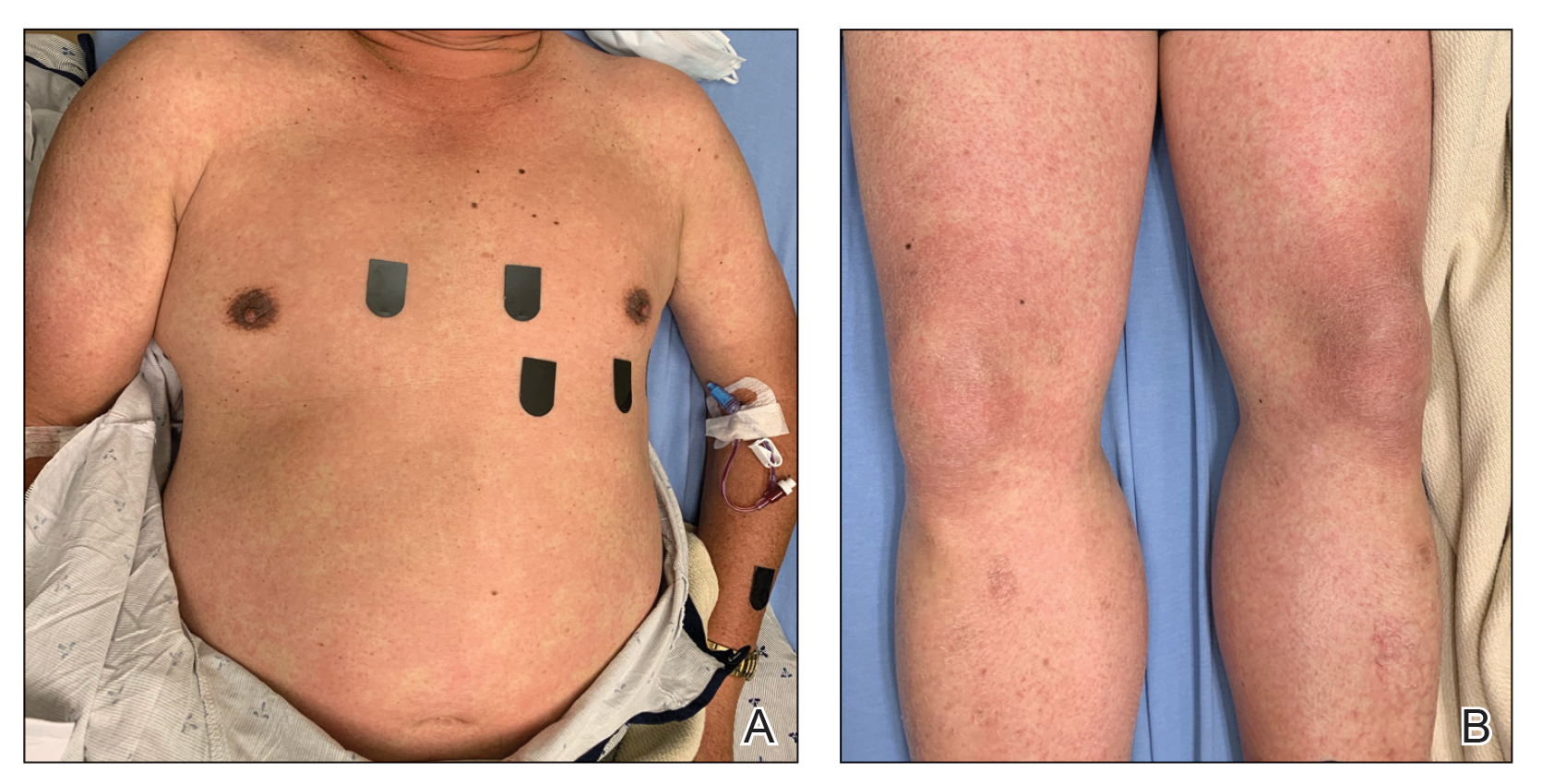
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Practice Points
- It is important to maintain a high index of suspicion for angioimmunoblastic T-cell lymphoma in older patients with a longstanding rash and no clear culprit for drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
- Consider performing a lymph node biopsy early in the course of disease in patients with presumed DRESS syndrome who do not improve with drug withdrawal and steroid therapy.