User login
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
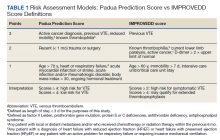
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
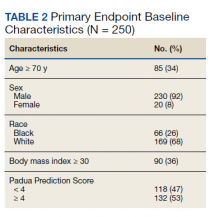
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
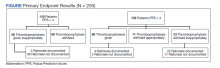
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404