User login
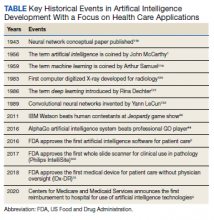
Artificial Intelligence (AI) was first described in 1956 and refers to machines having the ability to learn as they receive and process information, resulting in the ability to “think” like humans.1 AI’s impact in medicine is increasing; currently, at least 29 AI medical devices and algorithms are approved by the US Food and Drug Administration (FDA) in a variety of areas, including radiograph interpretation, managing glucose levels in patients with diabetes mellitus, analyzing electrocardiograms (ECGs), and diagnosing sleep disorders among others.2 Significantly, in 2020, the Centers for Medicare and Medicaid Services (CMS) announced the first reimbursement to hospitals for an AI platform, a model for early detection of strokes.3 AI is rapidly becoming an integral part of health care, and its role will only increase in the future (Table).
As knowledge in medicine is expanding exponentially, AI has great potential to assist with handling complex patient care data. The concept of exponential growth is not a natural one. As Bini described, with exponential growth the volume of knowledge amassed over the past 10 years will now occur in perhaps only 1 year.1 Likewise, equivalent advances over the past year may take just a few months. This phenomenon is partly due to the law of accelerating returns, which states that advances feed on themselves, continually increasing the rate of further advances.4 The volume of medical data doubles every 2 to 5 years.5 Fortunately, the field of AI is growing exponentially as well and can help health care practitioners (HCPs) keep pace, allowing the continued delivery of effective health care.
In this report, we review common terminology, principles, and general applications of AI, followed by current and potential applications of AI for selected medical specialties. Finally, we discuss AI’s future in health care, along with potential risks and pitfalls.
AI Overview
AI refers to machine programs that can “learn” or think based on past experiences. This functionality contrasts with simple rules-based programming available to health care for years. An example of rules-based programming is the warfarindosing.org website developed by Barnes-Jewish Hospital at Washington University Medical Center, which guides initial warfarin dosing.6,7 The prescriber inputs detailed patient information, including age, sex, height, weight, tobacco history, medications, laboratory results, and genotype if available. The application then calculates recommended warfarin dosing regimens to avoid over- or underanticoagulation. While the dosing algorithm may be complex, it depends entirely on preprogrammed rules. The program does not learn to reach its conclusions and recommendations from patient data.
In contrast, one of the most common subsets of AI is machine learning (ML). ML describes a program that “learns from experience and improves its performance as it learns.”1 With ML, the computer is initially provided with a training data set—data with known outcomes or labels. Because the initial data are input from known samples, this type of AI is known as supervised learning.8-10 As an example, we recently reported using ML to diagnose various types of cancer from pathology slides.11 In one experiment, we captured images of colon adenocarcinoma and normal colon (these 2 groups represent the training data set). Unlike traditional programming, we did not define characteristics that would differentiate colon cancer from normal; rather, the machine learned these characteristics independently by assessing the labeled images provided. A second data set (the validation data set) was used to evaluate the program and fine-tune the ML training model’s parameters. Finally, the program was presented with new images of cancer and normal cases for final assessment of accuracy (test data set). Our program learned to recognize differences from the images provided and was able to differentiate normal and cancer images with > 95% accuracy.
Advances in computer processing have allowed for the development of artificial neural networks (ANNs). While there are several types of ANNs, the most common types used for image classification and segmentation are known as convolutional neural networks (CNNs).9,12-14 The programs are designed to work similar to the human brain, specifically the visual cortex.15,16 As data are acquired, they are processed by various layers in the program. Much like neurons in the brain, one layer decides whether to advance information to the next.13,14 CNNs can be many layers deep, leading to the term deep learning: “computational models that are composed of multiple processing layers to learn representations of data with multiple levels of abstraction.”1,13,17
ANNs can process larger volumes of data. This advance has led to the development of unstructured or unsupervised learning. With this type of learning, imputing defined features (ie, predetermined answers) of the training data set described above is no longer required.1,8,10,14 The advantage of unsupervised learning is that the program can be presented raw data and extract meaningful interpretation without human input, often with less bias than may exist with supervised learning.1,18 If shown enough data, the program can extract relevant features to make conclusions independently without predefined definitions, potentially uncovering markers not previously known. For example, several studies have used unsupervised learning to search patient data to assess readmission risks of patients with congestive heart failure.10,19,20 AI compiled features independently and not previously defined, predicting patients at greater risk for readmission superior to traditional methods.
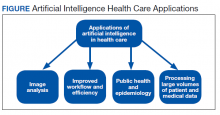
A more detailed description of the various terminologies and techniques of AI is beyond the scope of this review.9,10,17,21 However, in this basic overview, we describe 4 general areas that AI impacts health care (Figure).
Health Care Applications
Image analysis has seen the most AI health care applications.8,15 AI has shown potential in interpreting many types of medical images, including pathology slides, radiographs of various types, retina and other eye scans, and photographs of skin lesions. Many studies have demonstrated that AI can interpret these images as accurately as or even better than experienced clinicians.9,13,22-29 Studies have suggested AI interpretation of radiographs may better distinguish patients infected with COVID-19 from other causes of pneumonia, and AI interpretation of pathology slides may detect specific genetic mutations not previously identified without additional molecular tests.11,14,23,24,30-32
The second area in which AI can impact health care is improving workflow and efficiency. AI has improved surgery scheduling, saving significant revenue, and decreased patient wait times for appointments.1 AI can screen and triage radiographs, allowing attention to be directed to critical patients. This use would be valuable in many busy clinical settings, such as the recent COVID-19 pandemic.8,23 Similarly, AI can screen retina images to prioritize urgent conditions.25 AI has improved pathologists’ efficiency when used to detect breast metastases.33 Finally, AI may reduce medical errors, thereby ensuring patient safety.8,9,34
A third health care benefit of AI is in public health and epidemiology. AI can assist with clinical decision-making and diagnoses in low-income countries and areas with limited health care resources and personnel.25,29 AI can improve identification of infectious outbreaks, such as tuberculosis, malaria, dengue fever, and influenza.29,35-40 AI has been used to predict transmission patterns of the Zika virus and the current COVID-19 pandemic.41,42 Applications can stratify the risk of outbreaks based on multiple factors, including age, income, race, atypical geographic clusters, and seasonal factors like rainfall and temperature.35,36,38,43 AI has been used to assess morbidity and mortality, such as predicting disease severity with malaria and identifying treatment failures in tuberculosis.29
Finally, AI can dramatically impact health care due to processing large data sets or disconnected volumes of patient information—so-called big data.44-46 An example is the widespread use of electronic health records (EHRs) such as the Computerized Patient Record System used in Veteran Affairs medical centers (VAMCs). Much of patient information exists in written text: HCP notes, laboratory and radiology reports, medication records, etc. Natural language processing (NLP) allows platforms to sort through extensive volumes of data on complex patients at rates much faster than human capability, which has great potential to assist with diagnosis and treatment decisions.9
Medical literature is being produced at rates that exceed our ability to digest. More than 200,000 cancer-related articles were published in 2019 alone.14 NLP capabilities of AI have the potential to rapidly sort through this extensive medical literature and relate specific verbiage in patient records guiding therapy.46 IBM Watson, a supercomputer based on ML and NLP, demonstrates this concept with many potential applications, only some of which relate to health care.1,9 Watson has an oncology component to assimilate multiple aspects of patient care, including clinical notes, pathology results, radiograph findings, staging, and a tumor’s genetic profile. It coordinates these inputs from the EHR and mines medical literature and research databases to recommend treatment options.1,46 AI can assess and compile far greater patient data and therapeutic options than would be feasible by individual clinicians, thus providing customized patient care.47 Watson has partnered with numerous medical centers, including MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center, with variable success.44,47-49 While the full potential of Watson appears not yet realized, these AI-driven approaches will likely play an important role in leveraging the hidden value in the expanding volume of health care information.
Medical Specialty Applications
Radiology
Currently > 70% of FDA-approved AI medical devices are in the field of radiology.2 Most radiology departments have used AI-friendly digital imaging for years, such as the picture archiving and communication systems used by numerous health care systems, including VAMCs.2,15 Gray-scale images common in radiology lend themselves to standardization, although AI is not limited to black-and- white image interpretation.15
An abundance of literature describes plain radiograph interpretation using AI. One FDA-approved platform improved X-ray diagnosis of wrist fractures when used by emergency medicine clinicians.2,50 AI has been applied to chest X-ray (CXR) interpretation of many conditions, including pneumonia, tuberculosis, malignant lung lesions, and COVID-19.23,25,28,44,51-53 For example, Nam and colleagues suggested AI is better at diagnosing malignant pulmonary nodules from CXRs than are trained radiologists.28
In addition to plain radiographs, AI has been applied to many other imaging technologies, including ultrasounds, positron emission tomography, mammograms, computed tomography (CT), and magnetic resonance imaging (MRI).15,26,44,48,54-56 A large study demonstrated that ML platforms significantly reduced the time to diagnose intracranial hemorrhages on CT and identified subtle hemorrhages missed by radiologists.55 Other studies have claimed that AI programs may be better than radiologists in detecting cancer in screening mammograms, and 3 FDA-approved devices focus on mammogram interpretation.2,15,54,57 There is also great interest in MRI applications to detect and predict prognosis for breast cancer based on imaging findings.21,56
Aside from providing accurate diagnoses, other studies focus on AI radiograph interpretation to assist with patient screening, triage, improving time to final diagnosis, providing a rapid “second opinion,” and even monitoring disease progression and offering insights into prognosis.8,21,23,52,55,56,58 These features help in busy urban centers but may play an even greater role in areas with limited access to health care or trained specialists such as radiologists.52
Cardiology
Cardiology has the second highest number of FDA-approved AI applications.2 Many cardiology AI platforms involve image analysis, as described in several recent reviews.45,59,60 AI has been applied to echocardiography to measure ejection fractions, detect valvular disease, and assess heart failure from hypertrophic and restrictive cardiomyopathy and amyloidosis.45,48,59 Applications for cardiac CT scans and CT angiography have successfully quantified both calcified and noncalcified coronary artery plaques and lumen assessments, assessed myocardial perfusion, and performed coronary artery calcium scoring.45,59,60 Likewise, AI applications for cardiac MRI have been used to quantitate ejection fraction, large vessel flow assessment, and cardiac scar burden.45,59
For years ECG devices have provided interpretation with limited accuracy using preprogrammed parameters.48 However, the application of AI allows ECG interpretation on par with trained cardiologists. Numerous such AI applications exist, and 2 FDA-approved devices perform ECG interpretation.2,61-64 One of these devices incorporates an AI-powered stethoscope to detect atrial fibrillation and heart murmurs.65
Pathology
The advancement of whole slide imaging, wherein entire slides can be scanned and digitized at high speed and resolution, creates great potential for AI applications in pathology.12,24,32,33,66 A landmark study demonstrating the potential of AI for assessing whole slide imaging examined sentinel lymph node metastases in patients with breast cancer.22 Multiple algorithms in the study demonstrated that AI was equivalent or better than pathologists in detecting metastases, especially when the pathologists were time-constrained consistent with a normal working environment. Significantly, the most accurate and efficient diagnoses were achieved when the pathologist and AI interpretations were used together.22,33
AI has shown promise in diagnosing many other entities, including cancers of the prostate (including Gleason scoring), lung, colon, breast, and skin.11,12,24,27,32,67 In addition, AI has shown great potential in scoring biomarkers important for prognosis and treatment, such as immunohistochemistry (IHC) labeling of Ki-67 and PD-L1.32 Pathologists can have difficulty classifying certain tumors or determining the site of origin for metastases, often having to rely on IHC with limited success. The unique features of image analysis with AI have the potential to assist in classifying difficult tumors and identifying sites of origin for metastatic disease based on morphology alone.11
Oncology depends heavily on molecular pathology testing to dictate treatment options and determine prognosis. Preliminary studies suggest that AI interpretation alone has the potential to delineate whether certain molecular mutations are present in tumors from various sites.11,14,24,32 One study combined histology and genomic results for AI interpretation that improved prognostic predictions.68 In addition, AI analysis may have potential in predicting tumor recurrence or prognosis based on cellular features, as demonstrated for lung cancer and melanoma.67,69,70
Ophthalmology
AI applications for ophthalmology have focused on diabetic retinopathy, age-related macular degeneration, glaucoma, retinopathy of prematurity, age-related and congenital cataracts, and retinal vein occlusion.71-73 Diabetic retinopathy is a leading cause of blindness and has been studied by numerous platforms with good success, most having used color fundus photography.71,72 One study showed AI could diagnose diabetic retinopathy and diabetic macular edema with specificities similar to ophthalmologists.74 In 2018, the FDA approved the AI platform IDx-DR. This diagnostic system classifies retinal images and recommends referral for patients determined to have “more than mild diabetic retinopathy” and reexamination within a year for other patients.8,75 Significantly, the platform recommendations do not require confirmation by a clinician.8
AI has been applied to other modalities in ophthalmology such as optical coherence tomography (OCT) to diagnose retinal disease and to predict appropriate management of congenital cataracts.25,73,76 For example, an AI application using OCT has been demonstrated to match or exceed the accuracy of retinal experts in diagnosing and triaging patients with a variety of retinal pathologies, including patients needing urgent referrals.77
Dermatology
Multiple studies demonstrate AI performs at least equal to experienced dermatologists in differentiating selected skin lesions.78-81 For example, Esteva and colleagues demonstrated AI could differentiate keratinocyte carcinomas from benign seborrheic keratoses and malignant melanomas from benign nevi with accuracy equal to 21 board-certified dermatologists.78
AI is applicable to various imaging procedures common to dermatology, such as dermoscopy, very high-frequency ultrasound, and reflectance confocal microscopy.82 Several studies have demonstrated that AI interpretation compared favorably to dermatologists evaluating dermoscopy to assess melanocytic lesions.78-81,83
A limitation in these studies is that they differentiate only a few diagnoses.82 Furthermore, dermatologists have sensory input such as touch and visual examination under various conditions, something AI has yet to replicate.15,34,84 Also, most AI devices use no or limited clinical information.81 Dermatologists can recognize rarer conditions for which AI models may have had limited or no training.34 Nevertheless, a recent study assessed AI for the diagnosis of 134 separate skin disorders with promising results, including providing diagnoses with accuracy comparable to that of dermatologists and providing accurate treatment strategies.84 As Topol points out, most skin lesions are diagnosed in the primary care setting where AI can have a greater impact when used in conjunction with the clinical impression, especially where specialists are in limited supply.48,78
Finally, dermatology lends itself to using portable or smartphone applications (apps) wherein the user can photograph a lesion for analysis by AI algorithms to assess the need for further evaluation or make treatment recommendations.34,84,85 Although results from currently available apps are not encouraging, they may play a greater role as the technology advances.34,85
Oncology
Applications of AI in oncology include predicting prognosis for patients with cancer based on histologic and/or genetic information.14,68,86 Programs can predict the risk of complications before and recurrence risks after surgery for malignancies.44,87-89 AI can also assist in treatment planning and predict treatment failure with radiation therapy.90,91
AI has great potential in processing the large volumes of patient data in cancer genomics. Next-generation sequencing has allowed for the identification of millions of DNA sequences in a single tumor to detect genetic anomalies.92 Thousands of mutations can be found in individual tumor samples, and processing this information and determining its significance can be beyond human capability.14 We know little about the effects of various mutation combinations, and most tumors have a heterogeneous molecular profile among different cell populations.14,93 The presence or absence of various mutations can have diagnostic, prognostic, and therapeutic implications.93 AI has great potential to sort through these complex data and identify actionable findings.
More than 200,000 cancer-related articles were published in 2019, and publications in the field of cancer genomics are increasing exponentially.14,92,93 Patel and colleagues assessed the utility of IBM Watson for Genomics against results from a molecular tumor board.93 Watson for Genomics identified potentially significant mutations not identified by the tumor board in 32% of patients. Most mutations were related to new clinical trials not yet added to the tumor board watch list, demonstrating the role AI will have in processing the large volume of genetic data required to deliver personalized medicine moving forward.
Gastroenterology
AI has shown promise in predicting risk or outcomes based on clinical parameters in various common gastroenterology problems, including gastric reflux, acute pancreatitis, gastrointestinal bleeding, celiac disease, and inflammatory bowel disease.94,95 AI endoscopic analysis has demonstrated potential in assessing Barrett’s esophagus, gastric Helicobacter pylori infections, gastric atrophy, and gastric intestinal metaplasia.95 Applications have been used to assess esophageal, gastric, and colonic malignancies, including depth of invasion based on endoscopic images.95 Finally, studies have evaluated AI to assess small colon polyps during colonoscopy, including differentiating benign and premalignant polyps with success comparable to gastroenterologists.94,95 AI has been shown to increase the speed and accuracy of gastroenterologists in detecting small polyps during colonoscopy.48 In a prospective randomized study, colonoscopies performed using an AI device identified significantly more small adenomatous polyps than colonoscopies without AI.96
Neurology
It has been suggested that AI technologies are well suited for application in neurology due to the subtle presentation of many neurologic diseases.16 Viz LVO, the first CMS-approved AI reimbursement for the diagnosis of strokes, analyzes CTs to detect early ischemic strokes and alerts the medical team, thus shortening time to treatment.3,97 Many other AI platforms are in use or development that use CT and MRI for the early detection of strokes as well as for treatment and prognosis.9,97
AI technologies have been applied to neurodegenerative diseases, such as Alzheimer and Parkinson diseases.16,98 For example, several studies have evaluated patient movements in Parkinson disease for both early diagnosis and to assess response to treatment.98 These evaluations included assessment with both external cameras as well as wearable devices and smartphone apps.
AI has also been applied to seizure disorders, attempting to determine seizure type, localize the area of seizure onset, and address the challenges of identifying seizures in neonates.99,100 Other potential applications range from early detection and prognosis predictions for cases of multiple sclerosis to restoring movement in paralysis from a variety of conditions such as spinal cord injury.9,101,102
Mental Health
Due to the interactive nature of mental health care, the field has been slower to develop AI applications.18 With heavy reliance on textual information (eg, clinic notes, mood rating scales, and documentation of conversations), successful AI applications in this field will likely rely heavily on NLP.18 However, studies investigating the application of AI to mental health have also incorporated data such as brain imaging, smartphone monitoring, and social media platforms, such as Facebook and Twitter.18,103,104
The risk of suicide is higher in veteran patients, and ML algorithms have had limited success in predicting suicide risk in both veteran and nonveteran populations.104-106 While early models have low positive predictive values and low sensitivities, they still promise to be a useful tool in conjunction with traditional risk assessments.106 Kessler and colleagues suggest that combining multiple rather than single ML algorithms might lead to greater success.105,106
AI may assist in diagnosing other mental health disorders, including major depressive disorder, attention deficit hyperactivity disorder (ADHD), schizophrenia, posttraumatic stress disorder, and Alzheimer disease.103,104,107 These investigations are in the early stages with limited clinical applicability. However, 2 AI applications awaiting FDA approval relate to ADHD and opioid use.2 Furthermore, potential exists for AI to not only assist with prevention and diagnosis of ADHD, but also to identify optimal treatment options.2,103
General and Personalized Medicine
Additional AI applications include diagnosing patients with suspected sepsis, measuring liver iron concentrations, predicting hospital mortality at the time of admission, and more.2,108,109 AI can guide end-of-life decisions such as resuscitation status or whether to initiate mechanical ventilation.48
AI-driven smartphone apps can be beneficial to both patients and clinicians. Examples include predicting nonadherence to anticoagulation therapy, monitoring heart rhythms for atrial fibrillation or signs of hyperkalemia in patients with renal failure, and improving outcomes for patients with diabetes mellitus by decreasing glycemic variability and reducing hypoglycemia.8,48,110,111 The potential for AI applications to health care and personalized medicine are almost limitless.
Discussion
With ever-increasing expectations for all health care sectors to deliver timely, fiscally-responsible, high-quality health care, AI has the potential to have numerous impacts. AI can improve diagnostic accuracy while limiting errors and impact patient safety such as assisting with prescription delivery.8,9,34 It can screen and triage patients, alerting clinicians to those needing more urgent evaluation.8,23,77,97 AI also may increase a clinician’s efficiency and speed to render a diagnosis.12,13,55,97 AI can provide a rapid second opinion, an ability especially beneficial in underserved areas with shortages of specialists.23,25,26,29,34 Similarly, AI may decrease the inter- and intraobserver variability common in many medical specialties.12,27,45 AI applications can also monitor disease progression, identifying patients at greatest risk, and provide information for prognosis.21,23,56,58 Finally, as described with applications using IBM Watson, AI can allow for an integrated approach to health care that is currently lacking.
We have described many reports suggesting AI can render diagnoses as well as or better than experienced clinicians, and speculation exists that AI will replace many roles currently performed by health care practitioners.9,26 However, most studies demonstrate that AI’s diagnostic benefits are best realized when used to supplement a clinician’s impression.8,22,30,33,52,54,56,69,84 AI is not likely to replace humans in health care in the foreseeable future. The technology can be likened to the impact of CT scans developed in the 1970s in neurology. Prior to such detailed imaging, neurologists spent extensive time performing detailed physicals to render diagnoses and locate lesions before surgery. There was mistrust of this new technology and concern that CT scans would eliminate the need for neurologists.112 On the contrary, neurology is alive and well, frequently being augmented by the technologies once speculated to replace it.
Commercial AI health care platforms represented a $2 billion industry in 2018 and are growing rapidly each year.13,32 Many AI products are offered ready for implementation for various tasks, including diagnostics, patient management, and improved efficiency. Others will likely be provided as templates suitable for modification to meet the specific needs of the facility, practice, or specialty for its patient population.
AI Risks and Limitations
AI has several risks and limitations. Although there is progress in explainable AI, at times we still struggle to understand how the output provided by machine learning algorithms was created.44,48 The many layers associated with deep learning self-determine the criteria to reach its conclusion, and these criteria can continually evolve. The parameters of deep learning are not preprogrammed, and there are too many individual data points to be extrapolated or deconvoluted for evaluation at our current level of knowledge.26,51 These apparent lack of constraints cause concern for patient safety and suggest that greater validation and continued scrutiny of validity is required.8,48 Efforts are underway to create explainable AI programs to make their processes more transparent, but such clarification is limited presently.14,26,48,77
Another challenge of AI is determining the amount of training data required to function optimally. Also, if the output describes multiple variables or diagnoses, are each equally valid?113 Furthermore, many AI applications look for a specific process, such as cancer diagnoses on CXRs. However, how coexisting conditions like cardiomegaly, emphysema, pneumonia, etc, seen on CXRs will affect the diagnosis needs to be considered.51,52 Zech and colleagues provide the example that diagnoses for pneumothorax are frequently rendered on CXRs with chest tubes in place.51 They suggest that CNNs may develop a bias toward diagnosing pneumothorax when chest tubes are present. Many current studies approach an issue in isolation, a situation not realistic in real-world clinical practice.26
Most studies on AI have been retrospective, and frequently data used to train the program are preselected.13,26 The data are typically validated on available databases rather than actual patients in the clinical setting, limiting confidence in the validity of the AI output when applied to real-world situations. Currently, fewer than 12 prospective trials had been published comparing AI with traditional clinical care.13,114 Randomized prospective clinical trials are even fewer, with none currently reported from the United States.13,114 The results from several studies have been shown to diminish when repeated prospectively.114
The FDA has created a new category known as Software as a Medical Device and has a Digital Health Innovation Action Plan to regulate AI platforms. Still, the process of AI regulation is of necessity different from traditional approval processes and is continually evolving.8 The FDA approval process cannot account for the fact that the program’s parameters may continually evolve or adapt.2
Guidelines for investigating and reporting AI research with its unique attributes are being developed. Examples include the TRIPOD-ML statement and others.49,115 In September 2020, 2 publications addressed the paucity of gold-standard randomized clinical trials in clinical AI applications.116,117 The SPIRIT-AI statement expands on the original SPIRIT statement published in 2013 to guide minimal reporting standards for AI clinical trial protocols to promote transparency of design and methodology.116 Similarly, the CONSORT-AI extension, stemming from the original CONSORT statement in 1996, aims to ensure quality reporting of randomized controlled trials in AI.117
Another risk with AI is that while an individual physician making a mistake may adversely affect 1 patient, a single mistake in an AI algorithm could potentially affect thousands of patients.48 Also, AI programs developed for patient populations at a facility may not translate to another. Referred to as overfitting, this phenomenon relates to selection bias in training data sets.15,34,49,51,52 Studies have shown that programs that underrepresent certain group characteristics such as age, sex, or race may be less effective when applied to a population in which these characteristics have differing representations.8,48,49 This problem of underrepresentation has been demonstrated in programs interpreting pathology slides, radiographs, and skin lesions.15,32,51
Admittedly, most of these challenges are not specific to AI and existed in health care previously. Physicians make mistakes, treatments are sometimes used without adequate prospective studies, and medications are given without understanding their mechanism of action, much like AI-facilitated processes reach a conclusion that cannot be fully explained.48
Conclusions
The view that AI will dramatically impact health care in the coming years will likely prove true. However, much work is needed, especially because of the paucity of prospective clinical trials as has been historically required in medical research. Any concern that AI will replace HCPs seems unwarranted. Early studies suggest that even AI programs that appear to exceed human interpretation perform best when working in cooperation with and oversight from clinicians. AI’s greatest potential appears to be its ability to augment care from health professionals, improving efficiency and accuracy, and should be anticipated with enthusiasm as the field moves forward at an exponential rate.
Acknowledgments
The authors thank Makenna G. Thomas for proofreading and review of the manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital. This research has been approved by the James A. Haley Veteran’s Hospital Office of Communications and Media.
1. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358-2361. doi:10.1016/j.arth.2018.02.067
2. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
3. Viz. AI powered synchronized stroke care. Accessed September 15, 2021. https://www.viz.ai/ischemic-stroke
4. Buchanan M. The law of accelerating returns. Nat Phys. 2008;4(7):507. doi:10.1038/nphys1010
5. IBM Watson Health computes a pair of new solutions to improve healthcare data and security. Published September 10, 2015. Accessed October 21, 2020. https://www.techrepublic.com/article/ibm-watson-health-computes-a-pair-of-new-solutions-to-improve-healthcare-data-and-security
6. Borkowski AA, Kardani A, Mastorides SM, Thomas LB. Warfarin pharmacogenomics: recommendations with available patented clinical technologies. Recent Pat Biotechnol. 2014;8(2):110-115. doi:10.2174/1872208309666140904112003
7. Washington University in St. Louis. Warfarin dosing. Accessed September 15, 2021. http://www.warfarindosing.org/Source/Home.aspx
8. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
9. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. Published 2017 Jun 21. doi:10.1136/svn-2017-000101
10. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668-2679. doi:10.1016/j.jacc.2018.03.521
11. Borkowski AA, Wilson CP, Borkowski SA, et al. Comparing artificial intelligence platforms for histopathologic cancer diagnosis. Fed Pract. 2019;36(10):456-463.
12. Cruz-Roa A, Gilmore H, Basavanhally A, et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: application to invasive breast cancer detection. PLoS One. 2018;13(5):e0196828. Published 2018 May 24. doi:10.1371/journal.pone.0196828
13. Nagendran M, Chen Y, Lovejoy CA, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689. Published 2020 Mar 25. doi:10.1136/bmj.m689
14. Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452-1460. doi:10.1111/cas.14377
15. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists?. Cutis. 2020;105(1):28-31.
16. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
17. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444. doi:10.1038/nature14539
18. Graham S, Depp C, Lee EE, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21(11):116. Published 2019 Nov 7. doi:10.1007/s11920-019-1094-0
19. Golas SB, Shibahara T, Agboola S, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18(1):44. Published 2018 Jun 22. doi:10.1186/s12911-018-0620-z
20. Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629-640. doi:10.1161/CIRCOUTCOMES.116.003039
21. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. Published 2020 Aug 28. doi:10.1155/2020/6805710
22. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210. doi:10.1001/jama.2017.14585
23. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
24. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567. doi:10.1038/s41591-018-0177-5
25. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
26. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
27. Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer [published correction appears in NPJ Digit Med. 2019 Nov 19;2:113]. NPJ Digit Med. 2019;2:48. Published 2019 Jun 7. doi:10.1038/s41746-019-0112-2
28. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
29. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395(10236):1579-1586. doi:10.1016/S0140-6736(20)30226-9
30. Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT [published correction appears in Radiology. 2021 Apr;299(1):E225]. Radiology. 2020;296(3):E156-E165. doi:10.1148/radiol.2020201491
31. Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65-E71. doi:10.1148/radiol.2020200905
32. Serag A, Ion-Margineanu A, Qureshi H, et al. Translational AI and deep learning in diagnostic pathology. Front Med (Lausanne). 2019;6:185. Published 2019 Oct 1. doi:10.3389/fmed.2019.00185

33. Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep learning for identifying metastatic breast cancer. ArXiv. 2016 June 18:arXiv:1606.05718v1. Published online June 18, 2016. Accessed September 15, 2021. http://arxiv.org/abs/1606.05718
34. Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatology Dermatol Surg. 2019;23(2):57-60. doi:10.4103/jdds.jdds_19_19
35. Mollalo A, Mao L, Rashidi P, Glass GE. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. Int J Environ Res Public Health. 2019;16(1):157. Published 2019 Jan 8. doi:10.3390/ijerph16010157
36. Haddawy P, Hasan AHMI, Kasantikul R, et al. Spatiotemporal Bayesian networks for malaria prediction. Artif Intell Med. 2018;84:127-138. doi:10.1016/j.artmed.2017.12.002
37. Laureano-Rosario AE, Duncan AP, Mendez-Lazaro PA, et al. Application of artificial neural networks for dengue fever outbreak predictions in the northwest coast of Yucatan, Mexico and San Juan, Puerto Rico. Trop Med Infect Dis. 2018;3(1):5. Published 2018 Jan 5. doi:10.3390/tropicalmed3010005
38. Buczak AL, Koshute PT, Babin SM, Feighner BH, Lewis SH. A data-driven epidemiological prediction method for dengue outbreaks using local and remote sensing data. BMC Med Inform Decis Mak. 2012;12:124. Published 2012 Nov 5. doi:10.1186/1472-6947-12-124
39. Scavuzzo JM, Trucco F, Espinosa M, et al. Modeling dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018;185:167-175. doi:10.1016/j.actatropica.2018.05.003
40. Xue H, Bai Y, Hu H, Liang H. Influenza activity surveillance based on multiple regression model and artificial neural network. IEEE Access. 2018;6:563-575. doi:10.1109/ACCESS.2017.2771798
41. Jiang D, Hao M, Ding F, Fu J, Li M. Mapping the transmission risk of Zika virus using machine learning models. Acta Trop. 2018;185:391-399. doi:10.1016/j.actatropica.2018.06.021
42. Bragazzi NL, Dai H, Damiani G, Behzadifar M, Martini M, Wu J. How big data and artificial intelligence can help better manage the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(9):3176. Published 2020 May 2. doi:10.3390/ijerph17093176
43. Lake IR, Colón-González FJ, Barker GC, Morbey RA, Smith GE, Elliot AJ. Machine learning to refine decision making within a syndromic surveillance service. BMC Public Health. 2019;19(1):559. Published 2019 May 14. doi:10.1186/s12889-019-6916-9
44. Khan OF, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential-and its limitations. Oncol Exch. 2017;16(4):8-13. Accessed September 1, 2021. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
45. Badano LP, Keller DM, Muraru D, Torlasco C, Parati G. Artificial intelligence and cardiovascular imaging: A win-win combination. Anatol J Cardiol. 2020;24(4):214-223. doi:10.14744/AnatolJCardiol.2020.94491
46. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309(13):1351-1352. doi:10.1001/jama.2013.393
47. Greatbatch O, Garrett A, Snape K. The impact of artificial intelligence on the current and future practice of clinical cancer genomics. Genet Res (Camb). 2019;101:e9. Published 2019 Oct 31. doi:10.1017/S0016672319000089
48. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
49. Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness [published correction appears in BMJ. 2020 Apr 1;369:m1312]. BMJ. 2020;368:l6927. Published 2020 Mar 20. doi:10.1136/bmj.l6927
50. Lindsey R, Daluiski A, Chopra S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A. 2018;115(45):11591-11596. doi:10.1073/pnas.1806905115
51. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
52. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
53. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest x-ray algorithms to the clinical setting. ArXiv. 2020 Feb 26:arXiv:2002.11379v2. Revised March 11, 2020. Accessed September 15, 2021. http://arxiv.org/abs/2002.11379
54. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
55. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1:9. doi:10.1038/s41746-017-0015-z
56. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
57. McKinney SM, Sieniek M, Godbole V, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94. doi:10.1038/s41586-019-1799-6
58. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2021;34(3):522-531. doi:10.1038/s41379-020-00700-x
59. Xu B, Kocyigit D, Grimm R, Griffin BP, Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog Cardiovasc Dis. 2020;63(3):367-376. doi:10.1016/j.pcad.2020.03.003
60. Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(11):1317-1335. doi:10.1016/j.jacc.2018.12.054
61. Carewell Health. AI powered ECG diagnosis solutions. Accessed November 2, 2020. https://www.carewellhealth.com/products_aiecg.html
62. Strodthoff N, Strodthoff C. Detecting and interpreting myocardial infarction using fully convolutional neural networks. Physiol Meas. 2019;40(1):015001. doi:10.1088/1361-6579/aaf34d
63. Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25(1):65-69. doi:10.1038/s41591-018-0268-3
64. Kwon JM, Jeon KH, Kim HM, et al. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace. 2020;22(3):412-419. doi:10.1093/europace/euz324

65. Eko. FDA clears Eko’s AFib and heart murmur detection algorithms, making it the first AI-powered stethoscope to screen for serious heart conditions [press release]. Published January 28, 2020. Accessed September 15, 2021. https://www.businesswire.com/news/home/20200128005232/en/FDA-Clears-Eko’s-AFib-and-Heart-Murmur-Detection-Algorithms-Making-It-the-First-AI-Powered-Stethoscope-to-Screen-for-Serious-Heart-Conditions
66. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450. doi:10.1038/srep46450
67. Acs B, Rantalainen M, Hartman J. Artificial intelligence as the next step towards precision pathology. J Intern Med. 2020;288(1):62-81. doi:10.1111/joim.13030
68. Mobadersany P, Yousefi S, Amgad M, et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci U S A. 2018;115(13):E2970-E2979. doi:10.1073/pnas.1717139115
69. Wang X, Janowczyk A, Zhou Y, et al. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci Rep. 2017;7:13543. doi:10.1038/s41598-017-13773-7
70. Kulkarni PM, Robinson EJ, Pradhan JS, et al. Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res. 2020;26(5):1126-1134. doi:10.1158/1078-0432.CCR-19-1495
71. Du XL, Li WB, Hu BJ. Application of artificial intelligence in ophthalmology. Int J Ophthalmol. 2018;11(9):1555-1561. doi:10.18240/ijo.2018.09.21
72. Gunasekeran DV, Wong TY. Artificial intelligence in ophthalmology in 2020: a technology on the cusp for translation and implementation. Asia Pac J Ophthalmol (Phila). 2020;9(2):61-66. doi:10.1097/01.APO.0000656984.56467.2c
73. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-175. doi:10.1136/bjophthalmol-2018-313173
74. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. doi:10.1001/jama.2016.17216
75. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [press release]. Published April 11, 2018. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye
76. Long E, Chen J, Wu X, et al. Artificial intelligence manages congenital cataract with individualized prediction and telehealth computing. NPJ Digit Med. 2020;3:112. doi:10.1038/s41746-020-00319-x
77. De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24(9):1342-1350. doi:10.1038/s41591-018-0107-6
78. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118. doi:10.1038/nature21056
79. Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
80. Brinker TJ, Hekler A, Enk AH, et al. A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task. Eur J Cancer. 2019;111:148-154. doi:10.1016/j.ejca.2019.02.005
81. Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8):1836-1842. doi:10.1093/annonc/mdy166
82. Li CX, Shen CB, Xue K, et al. Artificial intelligence in dermatology: past, present, and future. Chin Med J (Engl). 2019;132(17):2017-2020. doi:10.1097/CM9.0000000000000372
83. Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20(7):938-947. doi:10.1016/S1470-2045(19)30333-X
84. Han SS, Park I, Eun Chang SE, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140(9):1753-1761. doi:10.1016/j.jid.2020.01.019
85. Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies [published correction appears in BMJ. 2020 Feb 25;368:m645]. BMJ. 2020;368:m127. Published 2020 Feb 10. doi:10.1136/bmj.m127
86. Chen YC, Ke WC, Chiu HW. Risk classification of cancer survival using ANN with gene expression data from multiple laboratories. Comput Biol Med. 2014;48:1-7. doi:10.1016/j.compbiomed.2014.02.006
87. Kim W, Kim KS, Lee JE, et al. Development of novel breast cancer recurrence prediction model using support vector machine. J Breast Cancer. 2012;15(2):230-238. doi:10.4048/jbc.2012.15.2.230
88. Merath K, Hyer JM, Mehta R, et al. Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. 2020;24(8):1843-1851. doi:10.1007/s11605-019-04338-2
89. Santos-García G, Varela G, Novoa N, Jiménez MF. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Artif Intell Med. 2004;30(1):61-69. doi:10.1016/S0933-3657(03)00059-9
90. Ibragimov B, Xing L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med Phys. 2017;44(2):547-557. doi:10.1002/mp.12045
91. Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136-e147. doi:10.1016/S2589-7500(19)30058-5
92. Xu J, Yang P, Xue S, et al. Translating cancer genomics into precision medicine with artificial intelligence: applications, challenges and future perspectives. Hum Genet. 2019;138(2):109-124. doi:10.1007/s00439-019-01970-5
93. Patel NM, Michelini VV, Snell JM, et al. Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist. 2018;23(2):179-185. doi:10.1634/theoncologist.2017-0170
94. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76-94.e2. doi:10.1053/j.gastro.2019.08.058
95. Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25(14):1666-1683. doi:10.3748/wjg.v25.i14.1666
96. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68(10):1813-1819. doi:10.1136/gutjnl-2018-317500

97. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An East Coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
98. Beli M, Bobi V, Badža M, Šolaja N, Duri-Jovii M, Kosti VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—a review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
99. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
100. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
101. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging [published online ahead of print, 2020 Oct 28]. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
102. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
103. Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24(11):1583-1598. doi:10.1038/s41380-019-0365-9
104. Fonseka TM, Bhat V, Kennedy SH. The utility of artificial intelligence in suicide risk prediction and the management of suicidal behaviors. Aust N Z J Psychiatry. 2019;53(10):954-964. doi:10.1177/0004867419864428
105. Kessler RC, Hwang I, Hoffmire CA, et al. Developing a practical suicide risk prediction model for targeting high-risk patients in the Veterans Health Administration. Int J Methods Psychiatr Res. 2017;26(3):e1575. doi:10.1002/mpr.1575
106. Kessler RC, Bauer MS, Bishop TM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the Veterans Health Administration System. Front Psychiatry. 2020;11:390. doi:10.3389/fpsyt.2020.00390
107. Kessler RC, van Loo HM, Wardenaar KJ, et al. Testing a machine-learning algorithm to predict the persistence and severity of major depressive disorder from baseline self-reports. Mol Psychiatry. 2016;21(10):1366-1371. doi:10.1038/mp.2015.198
108. Horng S, Sontag DA, Halpern Y, Jernite Y, Shapiro NI, Nathanson LA. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS One. 2017;12(4):e0174708. doi:10.1371/journal.pone.0174708
109. Soffer S, Klang E, Barash Y, Grossman E, Zimlichman E. Predicting in-hospital mortality at admission to the medical ward: a big-data machine learning model. Am J Med. 2021;134(2):227-234.e4. doi:10.1016/j.amjmed.2020.07.014
110. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416-1419. doi:10.1161/STROKEAHA.116.016281
111. Forlenza GP. Use of artificial intelligence to improve diabetes outcomes in patients using multiple daily injections therapy. Diabetes Technol Ther. 2019;21(S2):S24-S28. doi:10.1089/dia.2019.0077
112. Poser CM. CT scan and the practice of neurology. Arch Neurol. 1977;34(2):132. doi:10.1001/archneur.1977.00500140086023
113. Angus DC. Randomized clinical trials of artificial intelligence. JAMA. 2020;323(11):1043-1045. doi:10.1001/jama.2020.1039
114. Topol EJ. Welcoming new guidelines for AI clinical research. Nat Med. 2020;26(9):1318-1320. doi:10.1038/s41591-020-1042-x
115. Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577-1579. doi:10.1016/S0140-6736(19)30037-6
116. Cruz Rivera S, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351-1363. doi:10.1038/s41591-020-1037-7
117. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364-1374. doi:10.1038/s41591-020-1034-x
118. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5(4):115-133. doi:10.1007/BF02478259
119. Samuel AL. Some studies in machine learning using the game of Checkers. IBM J Res Dev. 1959;3(3):535-554. Accessed September 15, 2021. https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.368.2254
120. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology. 1983;148(3):833-838. doi:10.1148/radiology.148.3.6878707
121. Dechter R. Learning while searching in constraint-satisfaction-problems. AAAI’86: proceedings of the fifth AAAI national conference on artificial intelligence. Published 1986. Accessed September 15, 2021. https://www.aaai.org/Papers/AAAI/1986/AAAI86-029.pdf
122. Le Cun Y, Jackel LD, Boser B, et al. Handwritten digit recognition: applications of neural network chips and automatic learning. IEEE Commun Mag. 1989;27(11):41-46. doi:10.1109/35.41400
123. US Food and Drug Administration. FDA allows marketing of first whole slide imaging system for digital pathology [press release]. Published April 12, 2017. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology
Artificial Intelligence (AI) was first described in 1956 and refers to machines having the ability to learn as they receive and process information, resulting in the ability to “think” like humans.1 AI’s impact in medicine is increasing; currently, at least 29 AI medical devices and algorithms are approved by the US Food and Drug Administration (FDA) in a variety of areas, including radiograph interpretation, managing glucose levels in patients with diabetes mellitus, analyzing electrocardiograms (ECGs), and diagnosing sleep disorders among others.2 Significantly, in 2020, the Centers for Medicare and Medicaid Services (CMS) announced the first reimbursement to hospitals for an AI platform, a model for early detection of strokes.3 AI is rapidly becoming an integral part of health care, and its role will only increase in the future (Table).
As knowledge in medicine is expanding exponentially, AI has great potential to assist with handling complex patient care data. The concept of exponential growth is not a natural one. As Bini described, with exponential growth the volume of knowledge amassed over the past 10 years will now occur in perhaps only 1 year.1 Likewise, equivalent advances over the past year may take just a few months. This phenomenon is partly due to the law of accelerating returns, which states that advances feed on themselves, continually increasing the rate of further advances.4 The volume of medical data doubles every 2 to 5 years.5 Fortunately, the field of AI is growing exponentially as well and can help health care practitioners (HCPs) keep pace, allowing the continued delivery of effective health care.
In this report, we review common terminology, principles, and general applications of AI, followed by current and potential applications of AI for selected medical specialties. Finally, we discuss AI’s future in health care, along with potential risks and pitfalls.
AI Overview
AI refers to machine programs that can “learn” or think based on past experiences. This functionality contrasts with simple rules-based programming available to health care for years. An example of rules-based programming is the warfarindosing.org website developed by Barnes-Jewish Hospital at Washington University Medical Center, which guides initial warfarin dosing.6,7 The prescriber inputs detailed patient information, including age, sex, height, weight, tobacco history, medications, laboratory results, and genotype if available. The application then calculates recommended warfarin dosing regimens to avoid over- or underanticoagulation. While the dosing algorithm may be complex, it depends entirely on preprogrammed rules. The program does not learn to reach its conclusions and recommendations from patient data.
In contrast, one of the most common subsets of AI is machine learning (ML). ML describes a program that “learns from experience and improves its performance as it learns.”1 With ML, the computer is initially provided with a training data set—data with known outcomes or labels. Because the initial data are input from known samples, this type of AI is known as supervised learning.8-10 As an example, we recently reported using ML to diagnose various types of cancer from pathology slides.11 In one experiment, we captured images of colon adenocarcinoma and normal colon (these 2 groups represent the training data set). Unlike traditional programming, we did not define characteristics that would differentiate colon cancer from normal; rather, the machine learned these characteristics independently by assessing the labeled images provided. A second data set (the validation data set) was used to evaluate the program and fine-tune the ML training model’s parameters. Finally, the program was presented with new images of cancer and normal cases for final assessment of accuracy (test data set). Our program learned to recognize differences from the images provided and was able to differentiate normal and cancer images with > 95% accuracy.
Advances in computer processing have allowed for the development of artificial neural networks (ANNs). While there are several types of ANNs, the most common types used for image classification and segmentation are known as convolutional neural networks (CNNs).9,12-14 The programs are designed to work similar to the human brain, specifically the visual cortex.15,16 As data are acquired, they are processed by various layers in the program. Much like neurons in the brain, one layer decides whether to advance information to the next.13,14 CNNs can be many layers deep, leading to the term deep learning: “computational models that are composed of multiple processing layers to learn representations of data with multiple levels of abstraction.”1,13,17
ANNs can process larger volumes of data. This advance has led to the development of unstructured or unsupervised learning. With this type of learning, imputing defined features (ie, predetermined answers) of the training data set described above is no longer required.1,8,10,14 The advantage of unsupervised learning is that the program can be presented raw data and extract meaningful interpretation without human input, often with less bias than may exist with supervised learning.1,18 If shown enough data, the program can extract relevant features to make conclusions independently without predefined definitions, potentially uncovering markers not previously known. For example, several studies have used unsupervised learning to search patient data to assess readmission risks of patients with congestive heart failure.10,19,20 AI compiled features independently and not previously defined, predicting patients at greater risk for readmission superior to traditional methods.
A more detailed description of the various terminologies and techniques of AI is beyond the scope of this review.9,10,17,21 However, in this basic overview, we describe 4 general areas that AI impacts health care (Figure).
Health Care Applications
Image analysis has seen the most AI health care applications.8,15 AI has shown potential in interpreting many types of medical images, including pathology slides, radiographs of various types, retina and other eye scans, and photographs of skin lesions. Many studies have demonstrated that AI can interpret these images as accurately as or even better than experienced clinicians.9,13,22-29 Studies have suggested AI interpretation of radiographs may better distinguish patients infected with COVID-19 from other causes of pneumonia, and AI interpretation of pathology slides may detect specific genetic mutations not previously identified without additional molecular tests.11,14,23,24,30-32
The second area in which AI can impact health care is improving workflow and efficiency. AI has improved surgery scheduling, saving significant revenue, and decreased patient wait times for appointments.1 AI can screen and triage radiographs, allowing attention to be directed to critical patients. This use would be valuable in many busy clinical settings, such as the recent COVID-19 pandemic.8,23 Similarly, AI can screen retina images to prioritize urgent conditions.25 AI has improved pathologists’ efficiency when used to detect breast metastases.33 Finally, AI may reduce medical errors, thereby ensuring patient safety.8,9,34
A third health care benefit of AI is in public health and epidemiology. AI can assist with clinical decision-making and diagnoses in low-income countries and areas with limited health care resources and personnel.25,29 AI can improve identification of infectious outbreaks, such as tuberculosis, malaria, dengue fever, and influenza.29,35-40 AI has been used to predict transmission patterns of the Zika virus and the current COVID-19 pandemic.41,42 Applications can stratify the risk of outbreaks based on multiple factors, including age, income, race, atypical geographic clusters, and seasonal factors like rainfall and temperature.35,36,38,43 AI has been used to assess morbidity and mortality, such as predicting disease severity with malaria and identifying treatment failures in tuberculosis.29
Finally, AI can dramatically impact health care due to processing large data sets or disconnected volumes of patient information—so-called big data.44-46 An example is the widespread use of electronic health records (EHRs) such as the Computerized Patient Record System used in Veteran Affairs medical centers (VAMCs). Much of patient information exists in written text: HCP notes, laboratory and radiology reports, medication records, etc. Natural language processing (NLP) allows platforms to sort through extensive volumes of data on complex patients at rates much faster than human capability, which has great potential to assist with diagnosis and treatment decisions.9
Medical literature is being produced at rates that exceed our ability to digest. More than 200,000 cancer-related articles were published in 2019 alone.14 NLP capabilities of AI have the potential to rapidly sort through this extensive medical literature and relate specific verbiage in patient records guiding therapy.46 IBM Watson, a supercomputer based on ML and NLP, demonstrates this concept with many potential applications, only some of which relate to health care.1,9 Watson has an oncology component to assimilate multiple aspects of patient care, including clinical notes, pathology results, radiograph findings, staging, and a tumor’s genetic profile. It coordinates these inputs from the EHR and mines medical literature and research databases to recommend treatment options.1,46 AI can assess and compile far greater patient data and therapeutic options than would be feasible by individual clinicians, thus providing customized patient care.47 Watson has partnered with numerous medical centers, including MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center, with variable success.44,47-49 While the full potential of Watson appears not yet realized, these AI-driven approaches will likely play an important role in leveraging the hidden value in the expanding volume of health care information.
Medical Specialty Applications
Radiology
Currently > 70% of FDA-approved AI medical devices are in the field of radiology.2 Most radiology departments have used AI-friendly digital imaging for years, such as the picture archiving and communication systems used by numerous health care systems, including VAMCs.2,15 Gray-scale images common in radiology lend themselves to standardization, although AI is not limited to black-and- white image interpretation.15
An abundance of literature describes plain radiograph interpretation using AI. One FDA-approved platform improved X-ray diagnosis of wrist fractures when used by emergency medicine clinicians.2,50 AI has been applied to chest X-ray (CXR) interpretation of many conditions, including pneumonia, tuberculosis, malignant lung lesions, and COVID-19.23,25,28,44,51-53 For example, Nam and colleagues suggested AI is better at diagnosing malignant pulmonary nodules from CXRs than are trained radiologists.28
In addition to plain radiographs, AI has been applied to many other imaging technologies, including ultrasounds, positron emission tomography, mammograms, computed tomography (CT), and magnetic resonance imaging (MRI).15,26,44,48,54-56 A large study demonstrated that ML platforms significantly reduced the time to diagnose intracranial hemorrhages on CT and identified subtle hemorrhages missed by radiologists.55 Other studies have claimed that AI programs may be better than radiologists in detecting cancer in screening mammograms, and 3 FDA-approved devices focus on mammogram interpretation.2,15,54,57 There is also great interest in MRI applications to detect and predict prognosis for breast cancer based on imaging findings.21,56
Aside from providing accurate diagnoses, other studies focus on AI radiograph interpretation to assist with patient screening, triage, improving time to final diagnosis, providing a rapid “second opinion,” and even monitoring disease progression and offering insights into prognosis.8,21,23,52,55,56,58 These features help in busy urban centers but may play an even greater role in areas with limited access to health care or trained specialists such as radiologists.52
Cardiology
Cardiology has the second highest number of FDA-approved AI applications.2 Many cardiology AI platforms involve image analysis, as described in several recent reviews.45,59,60 AI has been applied to echocardiography to measure ejection fractions, detect valvular disease, and assess heart failure from hypertrophic and restrictive cardiomyopathy and amyloidosis.45,48,59 Applications for cardiac CT scans and CT angiography have successfully quantified both calcified and noncalcified coronary artery plaques and lumen assessments, assessed myocardial perfusion, and performed coronary artery calcium scoring.45,59,60 Likewise, AI applications for cardiac MRI have been used to quantitate ejection fraction, large vessel flow assessment, and cardiac scar burden.45,59
For years ECG devices have provided interpretation with limited accuracy using preprogrammed parameters.48 However, the application of AI allows ECG interpretation on par with trained cardiologists. Numerous such AI applications exist, and 2 FDA-approved devices perform ECG interpretation.2,61-64 One of these devices incorporates an AI-powered stethoscope to detect atrial fibrillation and heart murmurs.65
Pathology
The advancement of whole slide imaging, wherein entire slides can be scanned and digitized at high speed and resolution, creates great potential for AI applications in pathology.12,24,32,33,66 A landmark study demonstrating the potential of AI for assessing whole slide imaging examined sentinel lymph node metastases in patients with breast cancer.22 Multiple algorithms in the study demonstrated that AI was equivalent or better than pathologists in detecting metastases, especially when the pathologists were time-constrained consistent with a normal working environment. Significantly, the most accurate and efficient diagnoses were achieved when the pathologist and AI interpretations were used together.22,33
AI has shown promise in diagnosing many other entities, including cancers of the prostate (including Gleason scoring), lung, colon, breast, and skin.11,12,24,27,32,67 In addition, AI has shown great potential in scoring biomarkers important for prognosis and treatment, such as immunohistochemistry (IHC) labeling of Ki-67 and PD-L1.32 Pathologists can have difficulty classifying certain tumors or determining the site of origin for metastases, often having to rely on IHC with limited success. The unique features of image analysis with AI have the potential to assist in classifying difficult tumors and identifying sites of origin for metastatic disease based on morphology alone.11
Oncology depends heavily on molecular pathology testing to dictate treatment options and determine prognosis. Preliminary studies suggest that AI interpretation alone has the potential to delineate whether certain molecular mutations are present in tumors from various sites.11,14,24,32 One study combined histology and genomic results for AI interpretation that improved prognostic predictions.68 In addition, AI analysis may have potential in predicting tumor recurrence or prognosis based on cellular features, as demonstrated for lung cancer and melanoma.67,69,70
Ophthalmology
AI applications for ophthalmology have focused on diabetic retinopathy, age-related macular degeneration, glaucoma, retinopathy of prematurity, age-related and congenital cataracts, and retinal vein occlusion.71-73 Diabetic retinopathy is a leading cause of blindness and has been studied by numerous platforms with good success, most having used color fundus photography.71,72 One study showed AI could diagnose diabetic retinopathy and diabetic macular edema with specificities similar to ophthalmologists.74 In 2018, the FDA approved the AI platform IDx-DR. This diagnostic system classifies retinal images and recommends referral for patients determined to have “more than mild diabetic retinopathy” and reexamination within a year for other patients.8,75 Significantly, the platform recommendations do not require confirmation by a clinician.8
AI has been applied to other modalities in ophthalmology such as optical coherence tomography (OCT) to diagnose retinal disease and to predict appropriate management of congenital cataracts.25,73,76 For example, an AI application using OCT has been demonstrated to match or exceed the accuracy of retinal experts in diagnosing and triaging patients with a variety of retinal pathologies, including patients needing urgent referrals.77
Dermatology
Multiple studies demonstrate AI performs at least equal to experienced dermatologists in differentiating selected skin lesions.78-81 For example, Esteva and colleagues demonstrated AI could differentiate keratinocyte carcinomas from benign seborrheic keratoses and malignant melanomas from benign nevi with accuracy equal to 21 board-certified dermatologists.78
AI is applicable to various imaging procedures common to dermatology, such as dermoscopy, very high-frequency ultrasound, and reflectance confocal microscopy.82 Several studies have demonstrated that AI interpretation compared favorably to dermatologists evaluating dermoscopy to assess melanocytic lesions.78-81,83
A limitation in these studies is that they differentiate only a few diagnoses.82 Furthermore, dermatologists have sensory input such as touch and visual examination under various conditions, something AI has yet to replicate.15,34,84 Also, most AI devices use no or limited clinical information.81 Dermatologists can recognize rarer conditions for which AI models may have had limited or no training.34 Nevertheless, a recent study assessed AI for the diagnosis of 134 separate skin disorders with promising results, including providing diagnoses with accuracy comparable to that of dermatologists and providing accurate treatment strategies.84 As Topol points out, most skin lesions are diagnosed in the primary care setting where AI can have a greater impact when used in conjunction with the clinical impression, especially where specialists are in limited supply.48,78
Finally, dermatology lends itself to using portable or smartphone applications (apps) wherein the user can photograph a lesion for analysis by AI algorithms to assess the need for further evaluation or make treatment recommendations.34,84,85 Although results from currently available apps are not encouraging, they may play a greater role as the technology advances.34,85
Oncology
Applications of AI in oncology include predicting prognosis for patients with cancer based on histologic and/or genetic information.14,68,86 Programs can predict the risk of complications before and recurrence risks after surgery for malignancies.44,87-89 AI can also assist in treatment planning and predict treatment failure with radiation therapy.90,91
AI has great potential in processing the large volumes of patient data in cancer genomics. Next-generation sequencing has allowed for the identification of millions of DNA sequences in a single tumor to detect genetic anomalies.92 Thousands of mutations can be found in individual tumor samples, and processing this information and determining its significance can be beyond human capability.14 We know little about the effects of various mutation combinations, and most tumors have a heterogeneous molecular profile among different cell populations.14,93 The presence or absence of various mutations can have diagnostic, prognostic, and therapeutic implications.93 AI has great potential to sort through these complex data and identify actionable findings.
More than 200,000 cancer-related articles were published in 2019, and publications in the field of cancer genomics are increasing exponentially.14,92,93 Patel and colleagues assessed the utility of IBM Watson for Genomics against results from a molecular tumor board.93 Watson for Genomics identified potentially significant mutations not identified by the tumor board in 32% of patients. Most mutations were related to new clinical trials not yet added to the tumor board watch list, demonstrating the role AI will have in processing the large volume of genetic data required to deliver personalized medicine moving forward.
Gastroenterology
AI has shown promise in predicting risk or outcomes based on clinical parameters in various common gastroenterology problems, including gastric reflux, acute pancreatitis, gastrointestinal bleeding, celiac disease, and inflammatory bowel disease.94,95 AI endoscopic analysis has demonstrated potential in assessing Barrett’s esophagus, gastric Helicobacter pylori infections, gastric atrophy, and gastric intestinal metaplasia.95 Applications have been used to assess esophageal, gastric, and colonic malignancies, including depth of invasion based on endoscopic images.95 Finally, studies have evaluated AI to assess small colon polyps during colonoscopy, including differentiating benign and premalignant polyps with success comparable to gastroenterologists.94,95 AI has been shown to increase the speed and accuracy of gastroenterologists in detecting small polyps during colonoscopy.48 In a prospective randomized study, colonoscopies performed using an AI device identified significantly more small adenomatous polyps than colonoscopies without AI.96
Neurology
It has been suggested that AI technologies are well suited for application in neurology due to the subtle presentation of many neurologic diseases.16 Viz LVO, the first CMS-approved AI reimbursement for the diagnosis of strokes, analyzes CTs to detect early ischemic strokes and alerts the medical team, thus shortening time to treatment.3,97 Many other AI platforms are in use or development that use CT and MRI for the early detection of strokes as well as for treatment and prognosis.9,97
AI technologies have been applied to neurodegenerative diseases, such as Alzheimer and Parkinson diseases.16,98 For example, several studies have evaluated patient movements in Parkinson disease for both early diagnosis and to assess response to treatment.98 These evaluations included assessment with both external cameras as well as wearable devices and smartphone apps.
AI has also been applied to seizure disorders, attempting to determine seizure type, localize the area of seizure onset, and address the challenges of identifying seizures in neonates.99,100 Other potential applications range from early detection and prognosis predictions for cases of multiple sclerosis to restoring movement in paralysis from a variety of conditions such as spinal cord injury.9,101,102
Mental Health
Due to the interactive nature of mental health care, the field has been slower to develop AI applications.18 With heavy reliance on textual information (eg, clinic notes, mood rating scales, and documentation of conversations), successful AI applications in this field will likely rely heavily on NLP.18 However, studies investigating the application of AI to mental health have also incorporated data such as brain imaging, smartphone monitoring, and social media platforms, such as Facebook and Twitter.18,103,104
The risk of suicide is higher in veteran patients, and ML algorithms have had limited success in predicting suicide risk in both veteran and nonveteran populations.104-106 While early models have low positive predictive values and low sensitivities, they still promise to be a useful tool in conjunction with traditional risk assessments.106 Kessler and colleagues suggest that combining multiple rather than single ML algorithms might lead to greater success.105,106
AI may assist in diagnosing other mental health disorders, including major depressive disorder, attention deficit hyperactivity disorder (ADHD), schizophrenia, posttraumatic stress disorder, and Alzheimer disease.103,104,107 These investigations are in the early stages with limited clinical applicability. However, 2 AI applications awaiting FDA approval relate to ADHD and opioid use.2 Furthermore, potential exists for AI to not only assist with prevention and diagnosis of ADHD, but also to identify optimal treatment options.2,103
General and Personalized Medicine
Additional AI applications include diagnosing patients with suspected sepsis, measuring liver iron concentrations, predicting hospital mortality at the time of admission, and more.2,108,109 AI can guide end-of-life decisions such as resuscitation status or whether to initiate mechanical ventilation.48
AI-driven smartphone apps can be beneficial to both patients and clinicians. Examples include predicting nonadherence to anticoagulation therapy, monitoring heart rhythms for atrial fibrillation or signs of hyperkalemia in patients with renal failure, and improving outcomes for patients with diabetes mellitus by decreasing glycemic variability and reducing hypoglycemia.8,48,110,111 The potential for AI applications to health care and personalized medicine are almost limitless.
Discussion
With ever-increasing expectations for all health care sectors to deliver timely, fiscally-responsible, high-quality health care, AI has the potential to have numerous impacts. AI can improve diagnostic accuracy while limiting errors and impact patient safety such as assisting with prescription delivery.8,9,34 It can screen and triage patients, alerting clinicians to those needing more urgent evaluation.8,23,77,97 AI also may increase a clinician’s efficiency and speed to render a diagnosis.12,13,55,97 AI can provide a rapid second opinion, an ability especially beneficial in underserved areas with shortages of specialists.23,25,26,29,34 Similarly, AI may decrease the inter- and intraobserver variability common in many medical specialties.12,27,45 AI applications can also monitor disease progression, identifying patients at greatest risk, and provide information for prognosis.21,23,56,58 Finally, as described with applications using IBM Watson, AI can allow for an integrated approach to health care that is currently lacking.
We have described many reports suggesting AI can render diagnoses as well as or better than experienced clinicians, and speculation exists that AI will replace many roles currently performed by health care practitioners.9,26 However, most studies demonstrate that AI’s diagnostic benefits are best realized when used to supplement a clinician’s impression.8,22,30,33,52,54,56,69,84 AI is not likely to replace humans in health care in the foreseeable future. The technology can be likened to the impact of CT scans developed in the 1970s in neurology. Prior to such detailed imaging, neurologists spent extensive time performing detailed physicals to render diagnoses and locate lesions before surgery. There was mistrust of this new technology and concern that CT scans would eliminate the need for neurologists.112 On the contrary, neurology is alive and well, frequently being augmented by the technologies once speculated to replace it.
Commercial AI health care platforms represented a $2 billion industry in 2018 and are growing rapidly each year.13,32 Many AI products are offered ready for implementation for various tasks, including diagnostics, patient management, and improved efficiency. Others will likely be provided as templates suitable for modification to meet the specific needs of the facility, practice, or specialty for its patient population.
AI Risks and Limitations
AI has several risks and limitations. Although there is progress in explainable AI, at times we still struggle to understand how the output provided by machine learning algorithms was created.44,48 The many layers associated with deep learning self-determine the criteria to reach its conclusion, and these criteria can continually evolve. The parameters of deep learning are not preprogrammed, and there are too many individual data points to be extrapolated or deconvoluted for evaluation at our current level of knowledge.26,51 These apparent lack of constraints cause concern for patient safety and suggest that greater validation and continued scrutiny of validity is required.8,48 Efforts are underway to create explainable AI programs to make their processes more transparent, but such clarification is limited presently.14,26,48,77
Another challenge of AI is determining the amount of training data required to function optimally. Also, if the output describes multiple variables or diagnoses, are each equally valid?113 Furthermore, many AI applications look for a specific process, such as cancer diagnoses on CXRs. However, how coexisting conditions like cardiomegaly, emphysema, pneumonia, etc, seen on CXRs will affect the diagnosis needs to be considered.51,52 Zech and colleagues provide the example that diagnoses for pneumothorax are frequently rendered on CXRs with chest tubes in place.51 They suggest that CNNs may develop a bias toward diagnosing pneumothorax when chest tubes are present. Many current studies approach an issue in isolation, a situation not realistic in real-world clinical practice.26
Most studies on AI have been retrospective, and frequently data used to train the program are preselected.13,26 The data are typically validated on available databases rather than actual patients in the clinical setting, limiting confidence in the validity of the AI output when applied to real-world situations. Currently, fewer than 12 prospective trials had been published comparing AI with traditional clinical care.13,114 Randomized prospective clinical trials are even fewer, with none currently reported from the United States.13,114 The results from several studies have been shown to diminish when repeated prospectively.114
The FDA has created a new category known as Software as a Medical Device and has a Digital Health Innovation Action Plan to regulate AI platforms. Still, the process of AI regulation is of necessity different from traditional approval processes and is continually evolving.8 The FDA approval process cannot account for the fact that the program’s parameters may continually evolve or adapt.2
Guidelines for investigating and reporting AI research with its unique attributes are being developed. Examples include the TRIPOD-ML statement and others.49,115 In September 2020, 2 publications addressed the paucity of gold-standard randomized clinical trials in clinical AI applications.116,117 The SPIRIT-AI statement expands on the original SPIRIT statement published in 2013 to guide minimal reporting standards for AI clinical trial protocols to promote transparency of design and methodology.116 Similarly, the CONSORT-AI extension, stemming from the original CONSORT statement in 1996, aims to ensure quality reporting of randomized controlled trials in AI.117
Another risk with AI is that while an individual physician making a mistake may adversely affect 1 patient, a single mistake in an AI algorithm could potentially affect thousands of patients.48 Also, AI programs developed for patient populations at a facility may not translate to another. Referred to as overfitting, this phenomenon relates to selection bias in training data sets.15,34,49,51,52 Studies have shown that programs that underrepresent certain group characteristics such as age, sex, or race may be less effective when applied to a population in which these characteristics have differing representations.8,48,49 This problem of underrepresentation has been demonstrated in programs interpreting pathology slides, radiographs, and skin lesions.15,32,51
Admittedly, most of these challenges are not specific to AI and existed in health care previously. Physicians make mistakes, treatments are sometimes used without adequate prospective studies, and medications are given without understanding their mechanism of action, much like AI-facilitated processes reach a conclusion that cannot be fully explained.48
Conclusions
The view that AI will dramatically impact health care in the coming years will likely prove true. However, much work is needed, especially because of the paucity of prospective clinical trials as has been historically required in medical research. Any concern that AI will replace HCPs seems unwarranted. Early studies suggest that even AI programs that appear to exceed human interpretation perform best when working in cooperation with and oversight from clinicians. AI’s greatest potential appears to be its ability to augment care from health professionals, improving efficiency and accuracy, and should be anticipated with enthusiasm as the field moves forward at an exponential rate.
Acknowledgments
The authors thank Makenna G. Thomas for proofreading and review of the manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital. This research has been approved by the James A. Haley Veteran’s Hospital Office of Communications and Media.
Artificial Intelligence (AI) was first described in 1956 and refers to machines having the ability to learn as they receive and process information, resulting in the ability to “think” like humans.1 AI’s impact in medicine is increasing; currently, at least 29 AI medical devices and algorithms are approved by the US Food and Drug Administration (FDA) in a variety of areas, including radiograph interpretation, managing glucose levels in patients with diabetes mellitus, analyzing electrocardiograms (ECGs), and diagnosing sleep disorders among others.2 Significantly, in 2020, the Centers for Medicare and Medicaid Services (CMS) announced the first reimbursement to hospitals for an AI platform, a model for early detection of strokes.3 AI is rapidly becoming an integral part of health care, and its role will only increase in the future (Table).
As knowledge in medicine is expanding exponentially, AI has great potential to assist with handling complex patient care data. The concept of exponential growth is not a natural one. As Bini described, with exponential growth the volume of knowledge amassed over the past 10 years will now occur in perhaps only 1 year.1 Likewise, equivalent advances over the past year may take just a few months. This phenomenon is partly due to the law of accelerating returns, which states that advances feed on themselves, continually increasing the rate of further advances.4 The volume of medical data doubles every 2 to 5 years.5 Fortunately, the field of AI is growing exponentially as well and can help health care practitioners (HCPs) keep pace, allowing the continued delivery of effective health care.
In this report, we review common terminology, principles, and general applications of AI, followed by current and potential applications of AI for selected medical specialties. Finally, we discuss AI’s future in health care, along with potential risks and pitfalls.
AI Overview
AI refers to machine programs that can “learn” or think based on past experiences. This functionality contrasts with simple rules-based programming available to health care for years. An example of rules-based programming is the warfarindosing.org website developed by Barnes-Jewish Hospital at Washington University Medical Center, which guides initial warfarin dosing.6,7 The prescriber inputs detailed patient information, including age, sex, height, weight, tobacco history, medications, laboratory results, and genotype if available. The application then calculates recommended warfarin dosing regimens to avoid over- or underanticoagulation. While the dosing algorithm may be complex, it depends entirely on preprogrammed rules. The program does not learn to reach its conclusions and recommendations from patient data.
In contrast, one of the most common subsets of AI is machine learning (ML). ML describes a program that “learns from experience and improves its performance as it learns.”1 With ML, the computer is initially provided with a training data set—data with known outcomes or labels. Because the initial data are input from known samples, this type of AI is known as supervised learning.8-10 As an example, we recently reported using ML to diagnose various types of cancer from pathology slides.11 In one experiment, we captured images of colon adenocarcinoma and normal colon (these 2 groups represent the training data set). Unlike traditional programming, we did not define characteristics that would differentiate colon cancer from normal; rather, the machine learned these characteristics independently by assessing the labeled images provided. A second data set (the validation data set) was used to evaluate the program and fine-tune the ML training model’s parameters. Finally, the program was presented with new images of cancer and normal cases for final assessment of accuracy (test data set). Our program learned to recognize differences from the images provided and was able to differentiate normal and cancer images with > 95% accuracy.
Advances in computer processing have allowed for the development of artificial neural networks (ANNs). While there are several types of ANNs, the most common types used for image classification and segmentation are known as convolutional neural networks (CNNs).9,12-14 The programs are designed to work similar to the human brain, specifically the visual cortex.15,16 As data are acquired, they are processed by various layers in the program. Much like neurons in the brain, one layer decides whether to advance information to the next.13,14 CNNs can be many layers deep, leading to the term deep learning: “computational models that are composed of multiple processing layers to learn representations of data with multiple levels of abstraction.”1,13,17
ANNs can process larger volumes of data. This advance has led to the development of unstructured or unsupervised learning. With this type of learning, imputing defined features (ie, predetermined answers) of the training data set described above is no longer required.1,8,10,14 The advantage of unsupervised learning is that the program can be presented raw data and extract meaningful interpretation without human input, often with less bias than may exist with supervised learning.1,18 If shown enough data, the program can extract relevant features to make conclusions independently without predefined definitions, potentially uncovering markers not previously known. For example, several studies have used unsupervised learning to search patient data to assess readmission risks of patients with congestive heart failure.10,19,20 AI compiled features independently and not previously defined, predicting patients at greater risk for readmission superior to traditional methods.
A more detailed description of the various terminologies and techniques of AI is beyond the scope of this review.9,10,17,21 However, in this basic overview, we describe 4 general areas that AI impacts health care (Figure).
Health Care Applications
Image analysis has seen the most AI health care applications.8,15 AI has shown potential in interpreting many types of medical images, including pathology slides, radiographs of various types, retina and other eye scans, and photographs of skin lesions. Many studies have demonstrated that AI can interpret these images as accurately as or even better than experienced clinicians.9,13,22-29 Studies have suggested AI interpretation of radiographs may better distinguish patients infected with COVID-19 from other causes of pneumonia, and AI interpretation of pathology slides may detect specific genetic mutations not previously identified without additional molecular tests.11,14,23,24,30-32
The second area in which AI can impact health care is improving workflow and efficiency. AI has improved surgery scheduling, saving significant revenue, and decreased patient wait times for appointments.1 AI can screen and triage radiographs, allowing attention to be directed to critical patients. This use would be valuable in many busy clinical settings, such as the recent COVID-19 pandemic.8,23 Similarly, AI can screen retina images to prioritize urgent conditions.25 AI has improved pathologists’ efficiency when used to detect breast metastases.33 Finally, AI may reduce medical errors, thereby ensuring patient safety.8,9,34
A third health care benefit of AI is in public health and epidemiology. AI can assist with clinical decision-making and diagnoses in low-income countries and areas with limited health care resources and personnel.25,29 AI can improve identification of infectious outbreaks, such as tuberculosis, malaria, dengue fever, and influenza.29,35-40 AI has been used to predict transmission patterns of the Zika virus and the current COVID-19 pandemic.41,42 Applications can stratify the risk of outbreaks based on multiple factors, including age, income, race, atypical geographic clusters, and seasonal factors like rainfall and temperature.35,36,38,43 AI has been used to assess morbidity and mortality, such as predicting disease severity with malaria and identifying treatment failures in tuberculosis.29
Finally, AI can dramatically impact health care due to processing large data sets or disconnected volumes of patient information—so-called big data.44-46 An example is the widespread use of electronic health records (EHRs) such as the Computerized Patient Record System used in Veteran Affairs medical centers (VAMCs). Much of patient information exists in written text: HCP notes, laboratory and radiology reports, medication records, etc. Natural language processing (NLP) allows platforms to sort through extensive volumes of data on complex patients at rates much faster than human capability, which has great potential to assist with diagnosis and treatment decisions.9
Medical literature is being produced at rates that exceed our ability to digest. More than 200,000 cancer-related articles were published in 2019 alone.14 NLP capabilities of AI have the potential to rapidly sort through this extensive medical literature and relate specific verbiage in patient records guiding therapy.46 IBM Watson, a supercomputer based on ML and NLP, demonstrates this concept with many potential applications, only some of which relate to health care.1,9 Watson has an oncology component to assimilate multiple aspects of patient care, including clinical notes, pathology results, radiograph findings, staging, and a tumor’s genetic profile. It coordinates these inputs from the EHR and mines medical literature and research databases to recommend treatment options.1,46 AI can assess and compile far greater patient data and therapeutic options than would be feasible by individual clinicians, thus providing customized patient care.47 Watson has partnered with numerous medical centers, including MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center, with variable success.44,47-49 While the full potential of Watson appears not yet realized, these AI-driven approaches will likely play an important role in leveraging the hidden value in the expanding volume of health care information.
Medical Specialty Applications
Radiology
Currently > 70% of FDA-approved AI medical devices are in the field of radiology.2 Most radiology departments have used AI-friendly digital imaging for years, such as the picture archiving and communication systems used by numerous health care systems, including VAMCs.2,15 Gray-scale images common in radiology lend themselves to standardization, although AI is not limited to black-and- white image interpretation.15
An abundance of literature describes plain radiograph interpretation using AI. One FDA-approved platform improved X-ray diagnosis of wrist fractures when used by emergency medicine clinicians.2,50 AI has been applied to chest X-ray (CXR) interpretation of many conditions, including pneumonia, tuberculosis, malignant lung lesions, and COVID-19.23,25,28,44,51-53 For example, Nam and colleagues suggested AI is better at diagnosing malignant pulmonary nodules from CXRs than are trained radiologists.28
In addition to plain radiographs, AI has been applied to many other imaging technologies, including ultrasounds, positron emission tomography, mammograms, computed tomography (CT), and magnetic resonance imaging (MRI).15,26,44,48,54-56 A large study demonstrated that ML platforms significantly reduced the time to diagnose intracranial hemorrhages on CT and identified subtle hemorrhages missed by radiologists.55 Other studies have claimed that AI programs may be better than radiologists in detecting cancer in screening mammograms, and 3 FDA-approved devices focus on mammogram interpretation.2,15,54,57 There is also great interest in MRI applications to detect and predict prognosis for breast cancer based on imaging findings.21,56
Aside from providing accurate diagnoses, other studies focus on AI radiograph interpretation to assist with patient screening, triage, improving time to final diagnosis, providing a rapid “second opinion,” and even monitoring disease progression and offering insights into prognosis.8,21,23,52,55,56,58 These features help in busy urban centers but may play an even greater role in areas with limited access to health care or trained specialists such as radiologists.52
Cardiology
Cardiology has the second highest number of FDA-approved AI applications.2 Many cardiology AI platforms involve image analysis, as described in several recent reviews.45,59,60 AI has been applied to echocardiography to measure ejection fractions, detect valvular disease, and assess heart failure from hypertrophic and restrictive cardiomyopathy and amyloidosis.45,48,59 Applications for cardiac CT scans and CT angiography have successfully quantified both calcified and noncalcified coronary artery plaques and lumen assessments, assessed myocardial perfusion, and performed coronary artery calcium scoring.45,59,60 Likewise, AI applications for cardiac MRI have been used to quantitate ejection fraction, large vessel flow assessment, and cardiac scar burden.45,59
For years ECG devices have provided interpretation with limited accuracy using preprogrammed parameters.48 However, the application of AI allows ECG interpretation on par with trained cardiologists. Numerous such AI applications exist, and 2 FDA-approved devices perform ECG interpretation.2,61-64 One of these devices incorporates an AI-powered stethoscope to detect atrial fibrillation and heart murmurs.65
Pathology
The advancement of whole slide imaging, wherein entire slides can be scanned and digitized at high speed and resolution, creates great potential for AI applications in pathology.12,24,32,33,66 A landmark study demonstrating the potential of AI for assessing whole slide imaging examined sentinel lymph node metastases in patients with breast cancer.22 Multiple algorithms in the study demonstrated that AI was equivalent or better than pathologists in detecting metastases, especially when the pathologists were time-constrained consistent with a normal working environment. Significantly, the most accurate and efficient diagnoses were achieved when the pathologist and AI interpretations were used together.22,33
AI has shown promise in diagnosing many other entities, including cancers of the prostate (including Gleason scoring), lung, colon, breast, and skin.11,12,24,27,32,67 In addition, AI has shown great potential in scoring biomarkers important for prognosis and treatment, such as immunohistochemistry (IHC) labeling of Ki-67 and PD-L1.32 Pathologists can have difficulty classifying certain tumors or determining the site of origin for metastases, often having to rely on IHC with limited success. The unique features of image analysis with AI have the potential to assist in classifying difficult tumors and identifying sites of origin for metastatic disease based on morphology alone.11
Oncology depends heavily on molecular pathology testing to dictate treatment options and determine prognosis. Preliminary studies suggest that AI interpretation alone has the potential to delineate whether certain molecular mutations are present in tumors from various sites.11,14,24,32 One study combined histology and genomic results for AI interpretation that improved prognostic predictions.68 In addition, AI analysis may have potential in predicting tumor recurrence or prognosis based on cellular features, as demonstrated for lung cancer and melanoma.67,69,70
Ophthalmology
AI applications for ophthalmology have focused on diabetic retinopathy, age-related macular degeneration, glaucoma, retinopathy of prematurity, age-related and congenital cataracts, and retinal vein occlusion.71-73 Diabetic retinopathy is a leading cause of blindness and has been studied by numerous platforms with good success, most having used color fundus photography.71,72 One study showed AI could diagnose diabetic retinopathy and diabetic macular edema with specificities similar to ophthalmologists.74 In 2018, the FDA approved the AI platform IDx-DR. This diagnostic system classifies retinal images and recommends referral for patients determined to have “more than mild diabetic retinopathy” and reexamination within a year for other patients.8,75 Significantly, the platform recommendations do not require confirmation by a clinician.8
AI has been applied to other modalities in ophthalmology such as optical coherence tomography (OCT) to diagnose retinal disease and to predict appropriate management of congenital cataracts.25,73,76 For example, an AI application using OCT has been demonstrated to match or exceed the accuracy of retinal experts in diagnosing and triaging patients with a variety of retinal pathologies, including patients needing urgent referrals.77
Dermatology
Multiple studies demonstrate AI performs at least equal to experienced dermatologists in differentiating selected skin lesions.78-81 For example, Esteva and colleagues demonstrated AI could differentiate keratinocyte carcinomas from benign seborrheic keratoses and malignant melanomas from benign nevi with accuracy equal to 21 board-certified dermatologists.78
AI is applicable to various imaging procedures common to dermatology, such as dermoscopy, very high-frequency ultrasound, and reflectance confocal microscopy.82 Several studies have demonstrated that AI interpretation compared favorably to dermatologists evaluating dermoscopy to assess melanocytic lesions.78-81,83
A limitation in these studies is that they differentiate only a few diagnoses.82 Furthermore, dermatologists have sensory input such as touch and visual examination under various conditions, something AI has yet to replicate.15,34,84 Also, most AI devices use no or limited clinical information.81 Dermatologists can recognize rarer conditions for which AI models may have had limited or no training.34 Nevertheless, a recent study assessed AI for the diagnosis of 134 separate skin disorders with promising results, including providing diagnoses with accuracy comparable to that of dermatologists and providing accurate treatment strategies.84 As Topol points out, most skin lesions are diagnosed in the primary care setting where AI can have a greater impact when used in conjunction with the clinical impression, especially where specialists are in limited supply.48,78
Finally, dermatology lends itself to using portable or smartphone applications (apps) wherein the user can photograph a lesion for analysis by AI algorithms to assess the need for further evaluation or make treatment recommendations.34,84,85 Although results from currently available apps are not encouraging, they may play a greater role as the technology advances.34,85
Oncology
Applications of AI in oncology include predicting prognosis for patients with cancer based on histologic and/or genetic information.14,68,86 Programs can predict the risk of complications before and recurrence risks after surgery for malignancies.44,87-89 AI can also assist in treatment planning and predict treatment failure with radiation therapy.90,91
AI has great potential in processing the large volumes of patient data in cancer genomics. Next-generation sequencing has allowed for the identification of millions of DNA sequences in a single tumor to detect genetic anomalies.92 Thousands of mutations can be found in individual tumor samples, and processing this information and determining its significance can be beyond human capability.14 We know little about the effects of various mutation combinations, and most tumors have a heterogeneous molecular profile among different cell populations.14,93 The presence or absence of various mutations can have diagnostic, prognostic, and therapeutic implications.93 AI has great potential to sort through these complex data and identify actionable findings.
More than 200,000 cancer-related articles were published in 2019, and publications in the field of cancer genomics are increasing exponentially.14,92,93 Patel and colleagues assessed the utility of IBM Watson for Genomics against results from a molecular tumor board.93 Watson for Genomics identified potentially significant mutations not identified by the tumor board in 32% of patients. Most mutations were related to new clinical trials not yet added to the tumor board watch list, demonstrating the role AI will have in processing the large volume of genetic data required to deliver personalized medicine moving forward.
Gastroenterology
AI has shown promise in predicting risk or outcomes based on clinical parameters in various common gastroenterology problems, including gastric reflux, acute pancreatitis, gastrointestinal bleeding, celiac disease, and inflammatory bowel disease.94,95 AI endoscopic analysis has demonstrated potential in assessing Barrett’s esophagus, gastric Helicobacter pylori infections, gastric atrophy, and gastric intestinal metaplasia.95 Applications have been used to assess esophageal, gastric, and colonic malignancies, including depth of invasion based on endoscopic images.95 Finally, studies have evaluated AI to assess small colon polyps during colonoscopy, including differentiating benign and premalignant polyps with success comparable to gastroenterologists.94,95 AI has been shown to increase the speed and accuracy of gastroenterologists in detecting small polyps during colonoscopy.48 In a prospective randomized study, colonoscopies performed using an AI device identified significantly more small adenomatous polyps than colonoscopies without AI.96
Neurology
It has been suggested that AI technologies are well suited for application in neurology due to the subtle presentation of many neurologic diseases.16 Viz LVO, the first CMS-approved AI reimbursement for the diagnosis of strokes, analyzes CTs to detect early ischemic strokes and alerts the medical team, thus shortening time to treatment.3,97 Many other AI platforms are in use or development that use CT and MRI for the early detection of strokes as well as for treatment and prognosis.9,97
AI technologies have been applied to neurodegenerative diseases, such as Alzheimer and Parkinson diseases.16,98 For example, several studies have evaluated patient movements in Parkinson disease for both early diagnosis and to assess response to treatment.98 These evaluations included assessment with both external cameras as well as wearable devices and smartphone apps.
AI has also been applied to seizure disorders, attempting to determine seizure type, localize the area of seizure onset, and address the challenges of identifying seizures in neonates.99,100 Other potential applications range from early detection and prognosis predictions for cases of multiple sclerosis to restoring movement in paralysis from a variety of conditions such as spinal cord injury.9,101,102
Mental Health
Due to the interactive nature of mental health care, the field has been slower to develop AI applications.18 With heavy reliance on textual information (eg, clinic notes, mood rating scales, and documentation of conversations), successful AI applications in this field will likely rely heavily on NLP.18 However, studies investigating the application of AI to mental health have also incorporated data such as brain imaging, smartphone monitoring, and social media platforms, such as Facebook and Twitter.18,103,104
The risk of suicide is higher in veteran patients, and ML algorithms have had limited success in predicting suicide risk in both veteran and nonveteran populations.104-106 While early models have low positive predictive values and low sensitivities, they still promise to be a useful tool in conjunction with traditional risk assessments.106 Kessler and colleagues suggest that combining multiple rather than single ML algorithms might lead to greater success.105,106
AI may assist in diagnosing other mental health disorders, including major depressive disorder, attention deficit hyperactivity disorder (ADHD), schizophrenia, posttraumatic stress disorder, and Alzheimer disease.103,104,107 These investigations are in the early stages with limited clinical applicability. However, 2 AI applications awaiting FDA approval relate to ADHD and opioid use.2 Furthermore, potential exists for AI to not only assist with prevention and diagnosis of ADHD, but also to identify optimal treatment options.2,103
General and Personalized Medicine
Additional AI applications include diagnosing patients with suspected sepsis, measuring liver iron concentrations, predicting hospital mortality at the time of admission, and more.2,108,109 AI can guide end-of-life decisions such as resuscitation status or whether to initiate mechanical ventilation.48
AI-driven smartphone apps can be beneficial to both patients and clinicians. Examples include predicting nonadherence to anticoagulation therapy, monitoring heart rhythms for atrial fibrillation or signs of hyperkalemia in patients with renal failure, and improving outcomes for patients with diabetes mellitus by decreasing glycemic variability and reducing hypoglycemia.8,48,110,111 The potential for AI applications to health care and personalized medicine are almost limitless.
Discussion
With ever-increasing expectations for all health care sectors to deliver timely, fiscally-responsible, high-quality health care, AI has the potential to have numerous impacts. AI can improve diagnostic accuracy while limiting errors and impact patient safety such as assisting with prescription delivery.8,9,34 It can screen and triage patients, alerting clinicians to those needing more urgent evaluation.8,23,77,97 AI also may increase a clinician’s efficiency and speed to render a diagnosis.12,13,55,97 AI can provide a rapid second opinion, an ability especially beneficial in underserved areas with shortages of specialists.23,25,26,29,34 Similarly, AI may decrease the inter- and intraobserver variability common in many medical specialties.12,27,45 AI applications can also monitor disease progression, identifying patients at greatest risk, and provide information for prognosis.21,23,56,58 Finally, as described with applications using IBM Watson, AI can allow for an integrated approach to health care that is currently lacking.
We have described many reports suggesting AI can render diagnoses as well as or better than experienced clinicians, and speculation exists that AI will replace many roles currently performed by health care practitioners.9,26 However, most studies demonstrate that AI’s diagnostic benefits are best realized when used to supplement a clinician’s impression.8,22,30,33,52,54,56,69,84 AI is not likely to replace humans in health care in the foreseeable future. The technology can be likened to the impact of CT scans developed in the 1970s in neurology. Prior to such detailed imaging, neurologists spent extensive time performing detailed physicals to render diagnoses and locate lesions before surgery. There was mistrust of this new technology and concern that CT scans would eliminate the need for neurologists.112 On the contrary, neurology is alive and well, frequently being augmented by the technologies once speculated to replace it.
Commercial AI health care platforms represented a $2 billion industry in 2018 and are growing rapidly each year.13,32 Many AI products are offered ready for implementation for various tasks, including diagnostics, patient management, and improved efficiency. Others will likely be provided as templates suitable for modification to meet the specific needs of the facility, practice, or specialty for its patient population.
AI Risks and Limitations
AI has several risks and limitations. Although there is progress in explainable AI, at times we still struggle to understand how the output provided by machine learning algorithms was created.44,48 The many layers associated with deep learning self-determine the criteria to reach its conclusion, and these criteria can continually evolve. The parameters of deep learning are not preprogrammed, and there are too many individual data points to be extrapolated or deconvoluted for evaluation at our current level of knowledge.26,51 These apparent lack of constraints cause concern for patient safety and suggest that greater validation and continued scrutiny of validity is required.8,48 Efforts are underway to create explainable AI programs to make their processes more transparent, but such clarification is limited presently.14,26,48,77
Another challenge of AI is determining the amount of training data required to function optimally. Also, if the output describes multiple variables or diagnoses, are each equally valid?113 Furthermore, many AI applications look for a specific process, such as cancer diagnoses on CXRs. However, how coexisting conditions like cardiomegaly, emphysema, pneumonia, etc, seen on CXRs will affect the diagnosis needs to be considered.51,52 Zech and colleagues provide the example that diagnoses for pneumothorax are frequently rendered on CXRs with chest tubes in place.51 They suggest that CNNs may develop a bias toward diagnosing pneumothorax when chest tubes are present. Many current studies approach an issue in isolation, a situation not realistic in real-world clinical practice.26
Most studies on AI have been retrospective, and frequently data used to train the program are preselected.13,26 The data are typically validated on available databases rather than actual patients in the clinical setting, limiting confidence in the validity of the AI output when applied to real-world situations. Currently, fewer than 12 prospective trials had been published comparing AI with traditional clinical care.13,114 Randomized prospective clinical trials are even fewer, with none currently reported from the United States.13,114 The results from several studies have been shown to diminish when repeated prospectively.114
The FDA has created a new category known as Software as a Medical Device and has a Digital Health Innovation Action Plan to regulate AI platforms. Still, the process of AI regulation is of necessity different from traditional approval processes and is continually evolving.8 The FDA approval process cannot account for the fact that the program’s parameters may continually evolve or adapt.2
Guidelines for investigating and reporting AI research with its unique attributes are being developed. Examples include the TRIPOD-ML statement and others.49,115 In September 2020, 2 publications addressed the paucity of gold-standard randomized clinical trials in clinical AI applications.116,117 The SPIRIT-AI statement expands on the original SPIRIT statement published in 2013 to guide minimal reporting standards for AI clinical trial protocols to promote transparency of design and methodology.116 Similarly, the CONSORT-AI extension, stemming from the original CONSORT statement in 1996, aims to ensure quality reporting of randomized controlled trials in AI.117
Another risk with AI is that while an individual physician making a mistake may adversely affect 1 patient, a single mistake in an AI algorithm could potentially affect thousands of patients.48 Also, AI programs developed for patient populations at a facility may not translate to another. Referred to as overfitting, this phenomenon relates to selection bias in training data sets.15,34,49,51,52 Studies have shown that programs that underrepresent certain group characteristics such as age, sex, or race may be less effective when applied to a population in which these characteristics have differing representations.8,48,49 This problem of underrepresentation has been demonstrated in programs interpreting pathology slides, radiographs, and skin lesions.15,32,51
Admittedly, most of these challenges are not specific to AI and existed in health care previously. Physicians make mistakes, treatments are sometimes used without adequate prospective studies, and medications are given without understanding their mechanism of action, much like AI-facilitated processes reach a conclusion that cannot be fully explained.48
Conclusions
The view that AI will dramatically impact health care in the coming years will likely prove true. However, much work is needed, especially because of the paucity of prospective clinical trials as has been historically required in medical research. Any concern that AI will replace HCPs seems unwarranted. Early studies suggest that even AI programs that appear to exceed human interpretation perform best when working in cooperation with and oversight from clinicians. AI’s greatest potential appears to be its ability to augment care from health professionals, improving efficiency and accuracy, and should be anticipated with enthusiasm as the field moves forward at an exponential rate.
Acknowledgments
The authors thank Makenna G. Thomas for proofreading and review of the manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital. This research has been approved by the James A. Haley Veteran’s Hospital Office of Communications and Media.
1. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358-2361. doi:10.1016/j.arth.2018.02.067
2. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
3. Viz. AI powered synchronized stroke care. Accessed September 15, 2021. https://www.viz.ai/ischemic-stroke
4. Buchanan M. The law of accelerating returns. Nat Phys. 2008;4(7):507. doi:10.1038/nphys1010
5. IBM Watson Health computes a pair of new solutions to improve healthcare data and security. Published September 10, 2015. Accessed October 21, 2020. https://www.techrepublic.com/article/ibm-watson-health-computes-a-pair-of-new-solutions-to-improve-healthcare-data-and-security
6. Borkowski AA, Kardani A, Mastorides SM, Thomas LB. Warfarin pharmacogenomics: recommendations with available patented clinical technologies. Recent Pat Biotechnol. 2014;8(2):110-115. doi:10.2174/1872208309666140904112003
7. Washington University in St. Louis. Warfarin dosing. Accessed September 15, 2021. http://www.warfarindosing.org/Source/Home.aspx
8. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
9. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. Published 2017 Jun 21. doi:10.1136/svn-2017-000101
10. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668-2679. doi:10.1016/j.jacc.2018.03.521
11. Borkowski AA, Wilson CP, Borkowski SA, et al. Comparing artificial intelligence platforms for histopathologic cancer diagnosis. Fed Pract. 2019;36(10):456-463.
12. Cruz-Roa A, Gilmore H, Basavanhally A, et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: application to invasive breast cancer detection. PLoS One. 2018;13(5):e0196828. Published 2018 May 24. doi:10.1371/journal.pone.0196828
13. Nagendran M, Chen Y, Lovejoy CA, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689. Published 2020 Mar 25. doi:10.1136/bmj.m689
14. Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452-1460. doi:10.1111/cas.14377
15. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists?. Cutis. 2020;105(1):28-31.
16. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
17. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444. doi:10.1038/nature14539
18. Graham S, Depp C, Lee EE, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21(11):116. Published 2019 Nov 7. doi:10.1007/s11920-019-1094-0
19. Golas SB, Shibahara T, Agboola S, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18(1):44. Published 2018 Jun 22. doi:10.1186/s12911-018-0620-z
20. Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629-640. doi:10.1161/CIRCOUTCOMES.116.003039
21. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. Published 2020 Aug 28. doi:10.1155/2020/6805710
22. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210. doi:10.1001/jama.2017.14585
23. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
24. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567. doi:10.1038/s41591-018-0177-5
25. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
26. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
27. Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer [published correction appears in NPJ Digit Med. 2019 Nov 19;2:113]. NPJ Digit Med. 2019;2:48. Published 2019 Jun 7. doi:10.1038/s41746-019-0112-2
28. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
29. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395(10236):1579-1586. doi:10.1016/S0140-6736(20)30226-9
30. Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT [published correction appears in Radiology. 2021 Apr;299(1):E225]. Radiology. 2020;296(3):E156-E165. doi:10.1148/radiol.2020201491
31. Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65-E71. doi:10.1148/radiol.2020200905
32. Serag A, Ion-Margineanu A, Qureshi H, et al. Translational AI and deep learning in diagnostic pathology. Front Med (Lausanne). 2019;6:185. Published 2019 Oct 1. doi:10.3389/fmed.2019.00185

33. Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep learning for identifying metastatic breast cancer. ArXiv. 2016 June 18:arXiv:1606.05718v1. Published online June 18, 2016. Accessed September 15, 2021. http://arxiv.org/abs/1606.05718
34. Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatology Dermatol Surg. 2019;23(2):57-60. doi:10.4103/jdds.jdds_19_19
35. Mollalo A, Mao L, Rashidi P, Glass GE. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. Int J Environ Res Public Health. 2019;16(1):157. Published 2019 Jan 8. doi:10.3390/ijerph16010157
36. Haddawy P, Hasan AHMI, Kasantikul R, et al. Spatiotemporal Bayesian networks for malaria prediction. Artif Intell Med. 2018;84:127-138. doi:10.1016/j.artmed.2017.12.002
37. Laureano-Rosario AE, Duncan AP, Mendez-Lazaro PA, et al. Application of artificial neural networks for dengue fever outbreak predictions in the northwest coast of Yucatan, Mexico and San Juan, Puerto Rico. Trop Med Infect Dis. 2018;3(1):5. Published 2018 Jan 5. doi:10.3390/tropicalmed3010005
38. Buczak AL, Koshute PT, Babin SM, Feighner BH, Lewis SH. A data-driven epidemiological prediction method for dengue outbreaks using local and remote sensing data. BMC Med Inform Decis Mak. 2012;12:124. Published 2012 Nov 5. doi:10.1186/1472-6947-12-124
39. Scavuzzo JM, Trucco F, Espinosa M, et al. Modeling dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018;185:167-175. doi:10.1016/j.actatropica.2018.05.003
40. Xue H, Bai Y, Hu H, Liang H. Influenza activity surveillance based on multiple regression model and artificial neural network. IEEE Access. 2018;6:563-575. doi:10.1109/ACCESS.2017.2771798
41. Jiang D, Hao M, Ding F, Fu J, Li M. Mapping the transmission risk of Zika virus using machine learning models. Acta Trop. 2018;185:391-399. doi:10.1016/j.actatropica.2018.06.021
42. Bragazzi NL, Dai H, Damiani G, Behzadifar M, Martini M, Wu J. How big data and artificial intelligence can help better manage the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(9):3176. Published 2020 May 2. doi:10.3390/ijerph17093176
43. Lake IR, Colón-González FJ, Barker GC, Morbey RA, Smith GE, Elliot AJ. Machine learning to refine decision making within a syndromic surveillance service. BMC Public Health. 2019;19(1):559. Published 2019 May 14. doi:10.1186/s12889-019-6916-9
44. Khan OF, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential-and its limitations. Oncol Exch. 2017;16(4):8-13. Accessed September 1, 2021. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
45. Badano LP, Keller DM, Muraru D, Torlasco C, Parati G. Artificial intelligence and cardiovascular imaging: A win-win combination. Anatol J Cardiol. 2020;24(4):214-223. doi:10.14744/AnatolJCardiol.2020.94491
46. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309(13):1351-1352. doi:10.1001/jama.2013.393
47. Greatbatch O, Garrett A, Snape K. The impact of artificial intelligence on the current and future practice of clinical cancer genomics. Genet Res (Camb). 2019;101:e9. Published 2019 Oct 31. doi:10.1017/S0016672319000089
48. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
49. Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness [published correction appears in BMJ. 2020 Apr 1;369:m1312]. BMJ. 2020;368:l6927. Published 2020 Mar 20. doi:10.1136/bmj.l6927
50. Lindsey R, Daluiski A, Chopra S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A. 2018;115(45):11591-11596. doi:10.1073/pnas.1806905115
51. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
52. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
53. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest x-ray algorithms to the clinical setting. ArXiv. 2020 Feb 26:arXiv:2002.11379v2. Revised March 11, 2020. Accessed September 15, 2021. http://arxiv.org/abs/2002.11379
54. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
55. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1:9. doi:10.1038/s41746-017-0015-z
56. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
57. McKinney SM, Sieniek M, Godbole V, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94. doi:10.1038/s41586-019-1799-6
58. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2021;34(3):522-531. doi:10.1038/s41379-020-00700-x
59. Xu B, Kocyigit D, Grimm R, Griffin BP, Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog Cardiovasc Dis. 2020;63(3):367-376. doi:10.1016/j.pcad.2020.03.003
60. Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(11):1317-1335. doi:10.1016/j.jacc.2018.12.054
61. Carewell Health. AI powered ECG diagnosis solutions. Accessed November 2, 2020. https://www.carewellhealth.com/products_aiecg.html
62. Strodthoff N, Strodthoff C. Detecting and interpreting myocardial infarction using fully convolutional neural networks. Physiol Meas. 2019;40(1):015001. doi:10.1088/1361-6579/aaf34d
63. Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25(1):65-69. doi:10.1038/s41591-018-0268-3
64. Kwon JM, Jeon KH, Kim HM, et al. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace. 2020;22(3):412-419. doi:10.1093/europace/euz324

65. Eko. FDA clears Eko’s AFib and heart murmur detection algorithms, making it the first AI-powered stethoscope to screen for serious heart conditions [press release]. Published January 28, 2020. Accessed September 15, 2021. https://www.businesswire.com/news/home/20200128005232/en/FDA-Clears-Eko’s-AFib-and-Heart-Murmur-Detection-Algorithms-Making-It-the-First-AI-Powered-Stethoscope-to-Screen-for-Serious-Heart-Conditions
66. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450. doi:10.1038/srep46450
67. Acs B, Rantalainen M, Hartman J. Artificial intelligence as the next step towards precision pathology. J Intern Med. 2020;288(1):62-81. doi:10.1111/joim.13030
68. Mobadersany P, Yousefi S, Amgad M, et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci U S A. 2018;115(13):E2970-E2979. doi:10.1073/pnas.1717139115
69. Wang X, Janowczyk A, Zhou Y, et al. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci Rep. 2017;7:13543. doi:10.1038/s41598-017-13773-7
70. Kulkarni PM, Robinson EJ, Pradhan JS, et al. Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res. 2020;26(5):1126-1134. doi:10.1158/1078-0432.CCR-19-1495
71. Du XL, Li WB, Hu BJ. Application of artificial intelligence in ophthalmology. Int J Ophthalmol. 2018;11(9):1555-1561. doi:10.18240/ijo.2018.09.21
72. Gunasekeran DV, Wong TY. Artificial intelligence in ophthalmology in 2020: a technology on the cusp for translation and implementation. Asia Pac J Ophthalmol (Phila). 2020;9(2):61-66. doi:10.1097/01.APO.0000656984.56467.2c
73. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-175. doi:10.1136/bjophthalmol-2018-313173
74. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. doi:10.1001/jama.2016.17216
75. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [press release]. Published April 11, 2018. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye
76. Long E, Chen J, Wu X, et al. Artificial intelligence manages congenital cataract with individualized prediction and telehealth computing. NPJ Digit Med. 2020;3:112. doi:10.1038/s41746-020-00319-x
77. De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24(9):1342-1350. doi:10.1038/s41591-018-0107-6
78. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118. doi:10.1038/nature21056
79. Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
80. Brinker TJ, Hekler A, Enk AH, et al. A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task. Eur J Cancer. 2019;111:148-154. doi:10.1016/j.ejca.2019.02.005
81. Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8):1836-1842. doi:10.1093/annonc/mdy166
82. Li CX, Shen CB, Xue K, et al. Artificial intelligence in dermatology: past, present, and future. Chin Med J (Engl). 2019;132(17):2017-2020. doi:10.1097/CM9.0000000000000372
83. Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20(7):938-947. doi:10.1016/S1470-2045(19)30333-X
84. Han SS, Park I, Eun Chang SE, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140(9):1753-1761. doi:10.1016/j.jid.2020.01.019
85. Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies [published correction appears in BMJ. 2020 Feb 25;368:m645]. BMJ. 2020;368:m127. Published 2020 Feb 10. doi:10.1136/bmj.m127
86. Chen YC, Ke WC, Chiu HW. Risk classification of cancer survival using ANN with gene expression data from multiple laboratories. Comput Biol Med. 2014;48:1-7. doi:10.1016/j.compbiomed.2014.02.006
87. Kim W, Kim KS, Lee JE, et al. Development of novel breast cancer recurrence prediction model using support vector machine. J Breast Cancer. 2012;15(2):230-238. doi:10.4048/jbc.2012.15.2.230
88. Merath K, Hyer JM, Mehta R, et al. Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. 2020;24(8):1843-1851. doi:10.1007/s11605-019-04338-2
89. Santos-García G, Varela G, Novoa N, Jiménez MF. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Artif Intell Med. 2004;30(1):61-69. doi:10.1016/S0933-3657(03)00059-9
90. Ibragimov B, Xing L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med Phys. 2017;44(2):547-557. doi:10.1002/mp.12045
91. Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136-e147. doi:10.1016/S2589-7500(19)30058-5
92. Xu J, Yang P, Xue S, et al. Translating cancer genomics into precision medicine with artificial intelligence: applications, challenges and future perspectives. Hum Genet. 2019;138(2):109-124. doi:10.1007/s00439-019-01970-5
93. Patel NM, Michelini VV, Snell JM, et al. Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist. 2018;23(2):179-185. doi:10.1634/theoncologist.2017-0170
94. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76-94.e2. doi:10.1053/j.gastro.2019.08.058
95. Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25(14):1666-1683. doi:10.3748/wjg.v25.i14.1666
96. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68(10):1813-1819. doi:10.1136/gutjnl-2018-317500

97. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An East Coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
98. Beli M, Bobi V, Badža M, Šolaja N, Duri-Jovii M, Kosti VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—a review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
99. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
100. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
101. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging [published online ahead of print, 2020 Oct 28]. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
102. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
103. Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24(11):1583-1598. doi:10.1038/s41380-019-0365-9
104. Fonseka TM, Bhat V, Kennedy SH. The utility of artificial intelligence in suicide risk prediction and the management of suicidal behaviors. Aust N Z J Psychiatry. 2019;53(10):954-964. doi:10.1177/0004867419864428
105. Kessler RC, Hwang I, Hoffmire CA, et al. Developing a practical suicide risk prediction model for targeting high-risk patients in the Veterans Health Administration. Int J Methods Psychiatr Res. 2017;26(3):e1575. doi:10.1002/mpr.1575
106. Kessler RC, Bauer MS, Bishop TM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the Veterans Health Administration System. Front Psychiatry. 2020;11:390. doi:10.3389/fpsyt.2020.00390
107. Kessler RC, van Loo HM, Wardenaar KJ, et al. Testing a machine-learning algorithm to predict the persistence and severity of major depressive disorder from baseline self-reports. Mol Psychiatry. 2016;21(10):1366-1371. doi:10.1038/mp.2015.198
108. Horng S, Sontag DA, Halpern Y, Jernite Y, Shapiro NI, Nathanson LA. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS One. 2017;12(4):e0174708. doi:10.1371/journal.pone.0174708
109. Soffer S, Klang E, Barash Y, Grossman E, Zimlichman E. Predicting in-hospital mortality at admission to the medical ward: a big-data machine learning model. Am J Med. 2021;134(2):227-234.e4. doi:10.1016/j.amjmed.2020.07.014
110. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416-1419. doi:10.1161/STROKEAHA.116.016281
111. Forlenza GP. Use of artificial intelligence to improve diabetes outcomes in patients using multiple daily injections therapy. Diabetes Technol Ther. 2019;21(S2):S24-S28. doi:10.1089/dia.2019.0077
112. Poser CM. CT scan and the practice of neurology. Arch Neurol. 1977;34(2):132. doi:10.1001/archneur.1977.00500140086023
113. Angus DC. Randomized clinical trials of artificial intelligence. JAMA. 2020;323(11):1043-1045. doi:10.1001/jama.2020.1039
114. Topol EJ. Welcoming new guidelines for AI clinical research. Nat Med. 2020;26(9):1318-1320. doi:10.1038/s41591-020-1042-x
115. Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577-1579. doi:10.1016/S0140-6736(19)30037-6
116. Cruz Rivera S, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351-1363. doi:10.1038/s41591-020-1037-7
117. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364-1374. doi:10.1038/s41591-020-1034-x
118. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5(4):115-133. doi:10.1007/BF02478259
119. Samuel AL. Some studies in machine learning using the game of Checkers. IBM J Res Dev. 1959;3(3):535-554. Accessed September 15, 2021. https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.368.2254
120. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology. 1983;148(3):833-838. doi:10.1148/radiology.148.3.6878707
121. Dechter R. Learning while searching in constraint-satisfaction-problems. AAAI’86: proceedings of the fifth AAAI national conference on artificial intelligence. Published 1986. Accessed September 15, 2021. https://www.aaai.org/Papers/AAAI/1986/AAAI86-029.pdf
122. Le Cun Y, Jackel LD, Boser B, et al. Handwritten digit recognition: applications of neural network chips and automatic learning. IEEE Commun Mag. 1989;27(11):41-46. doi:10.1109/35.41400
123. US Food and Drug Administration. FDA allows marketing of first whole slide imaging system for digital pathology [press release]. Published April 12, 2017. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology
1. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358-2361. doi:10.1016/j.arth.2018.02.067
2. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
3. Viz. AI powered synchronized stroke care. Accessed September 15, 2021. https://www.viz.ai/ischemic-stroke
4. Buchanan M. The law of accelerating returns. Nat Phys. 2008;4(7):507. doi:10.1038/nphys1010
5. IBM Watson Health computes a pair of new solutions to improve healthcare data and security. Published September 10, 2015. Accessed October 21, 2020. https://www.techrepublic.com/article/ibm-watson-health-computes-a-pair-of-new-solutions-to-improve-healthcare-data-and-security
6. Borkowski AA, Kardani A, Mastorides SM, Thomas LB. Warfarin pharmacogenomics: recommendations with available patented clinical technologies. Recent Pat Biotechnol. 2014;8(2):110-115. doi:10.2174/1872208309666140904112003
7. Washington University in St. Louis. Warfarin dosing. Accessed September 15, 2021. http://www.warfarindosing.org/Source/Home.aspx
8. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
9. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. Published 2017 Jun 21. doi:10.1136/svn-2017-000101
10. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668-2679. doi:10.1016/j.jacc.2018.03.521
11. Borkowski AA, Wilson CP, Borkowski SA, et al. Comparing artificial intelligence platforms for histopathologic cancer diagnosis. Fed Pract. 2019;36(10):456-463.
12. Cruz-Roa A, Gilmore H, Basavanhally A, et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: application to invasive breast cancer detection. PLoS One. 2018;13(5):e0196828. Published 2018 May 24. doi:10.1371/journal.pone.0196828
13. Nagendran M, Chen Y, Lovejoy CA, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689. Published 2020 Mar 25. doi:10.1136/bmj.m689
14. Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452-1460. doi:10.1111/cas.14377
15. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists?. Cutis. 2020;105(1):28-31.
16. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
17. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444. doi:10.1038/nature14539
18. Graham S, Depp C, Lee EE, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21(11):116. Published 2019 Nov 7. doi:10.1007/s11920-019-1094-0
19. Golas SB, Shibahara T, Agboola S, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18(1):44. Published 2018 Jun 22. doi:10.1186/s12911-018-0620-z
20. Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629-640. doi:10.1161/CIRCOUTCOMES.116.003039
21. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. Published 2020 Aug 28. doi:10.1155/2020/6805710
22. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210. doi:10.1001/jama.2017.14585
23. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
24. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567. doi:10.1038/s41591-018-0177-5
25. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
26. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
27. Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer [published correction appears in NPJ Digit Med. 2019 Nov 19;2:113]. NPJ Digit Med. 2019;2:48. Published 2019 Jun 7. doi:10.1038/s41746-019-0112-2
28. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
29. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395(10236):1579-1586. doi:10.1016/S0140-6736(20)30226-9
30. Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT [published correction appears in Radiology. 2021 Apr;299(1):E225]. Radiology. 2020;296(3):E156-E165. doi:10.1148/radiol.2020201491
31. Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65-E71. doi:10.1148/radiol.2020200905
32. Serag A, Ion-Margineanu A, Qureshi H, et al. Translational AI and deep learning in diagnostic pathology. Front Med (Lausanne). 2019;6:185. Published 2019 Oct 1. doi:10.3389/fmed.2019.00185

33. Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep learning for identifying metastatic breast cancer. ArXiv. 2016 June 18:arXiv:1606.05718v1. Published online June 18, 2016. Accessed September 15, 2021. http://arxiv.org/abs/1606.05718
34. Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatology Dermatol Surg. 2019;23(2):57-60. doi:10.4103/jdds.jdds_19_19
35. Mollalo A, Mao L, Rashidi P, Glass GE. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. Int J Environ Res Public Health. 2019;16(1):157. Published 2019 Jan 8. doi:10.3390/ijerph16010157
36. Haddawy P, Hasan AHMI, Kasantikul R, et al. Spatiotemporal Bayesian networks for malaria prediction. Artif Intell Med. 2018;84:127-138. doi:10.1016/j.artmed.2017.12.002
37. Laureano-Rosario AE, Duncan AP, Mendez-Lazaro PA, et al. Application of artificial neural networks for dengue fever outbreak predictions in the northwest coast of Yucatan, Mexico and San Juan, Puerto Rico. Trop Med Infect Dis. 2018;3(1):5. Published 2018 Jan 5. doi:10.3390/tropicalmed3010005
38. Buczak AL, Koshute PT, Babin SM, Feighner BH, Lewis SH. A data-driven epidemiological prediction method for dengue outbreaks using local and remote sensing data. BMC Med Inform Decis Mak. 2012;12:124. Published 2012 Nov 5. doi:10.1186/1472-6947-12-124
39. Scavuzzo JM, Trucco F, Espinosa M, et al. Modeling dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018;185:167-175. doi:10.1016/j.actatropica.2018.05.003
40. Xue H, Bai Y, Hu H, Liang H. Influenza activity surveillance based on multiple regression model and artificial neural network. IEEE Access. 2018;6:563-575. doi:10.1109/ACCESS.2017.2771798
41. Jiang D, Hao M, Ding F, Fu J, Li M. Mapping the transmission risk of Zika virus using machine learning models. Acta Trop. 2018;185:391-399. doi:10.1016/j.actatropica.2018.06.021
42. Bragazzi NL, Dai H, Damiani G, Behzadifar M, Martini M, Wu J. How big data and artificial intelligence can help better manage the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(9):3176. Published 2020 May 2. doi:10.3390/ijerph17093176
43. Lake IR, Colón-González FJ, Barker GC, Morbey RA, Smith GE, Elliot AJ. Machine learning to refine decision making within a syndromic surveillance service. BMC Public Health. 2019;19(1):559. Published 2019 May 14. doi:10.1186/s12889-019-6916-9
44. Khan OF, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential-and its limitations. Oncol Exch. 2017;16(4):8-13. Accessed September 1, 2021. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
45. Badano LP, Keller DM, Muraru D, Torlasco C, Parati G. Artificial intelligence and cardiovascular imaging: A win-win combination. Anatol J Cardiol. 2020;24(4):214-223. doi:10.14744/AnatolJCardiol.2020.94491
46. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309(13):1351-1352. doi:10.1001/jama.2013.393
47. Greatbatch O, Garrett A, Snape K. The impact of artificial intelligence on the current and future practice of clinical cancer genomics. Genet Res (Camb). 2019;101:e9. Published 2019 Oct 31. doi:10.1017/S0016672319000089
48. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
49. Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness [published correction appears in BMJ. 2020 Apr 1;369:m1312]. BMJ. 2020;368:l6927. Published 2020 Mar 20. doi:10.1136/bmj.l6927
50. Lindsey R, Daluiski A, Chopra S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A. 2018;115(45):11591-11596. doi:10.1073/pnas.1806905115
51. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
52. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
53. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest x-ray algorithms to the clinical setting. ArXiv. 2020 Feb 26:arXiv:2002.11379v2. Revised March 11, 2020. Accessed September 15, 2021. http://arxiv.org/abs/2002.11379
54. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
55. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1:9. doi:10.1038/s41746-017-0015-z
56. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
57. McKinney SM, Sieniek M, Godbole V, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94. doi:10.1038/s41586-019-1799-6
58. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2021;34(3):522-531. doi:10.1038/s41379-020-00700-x
59. Xu B, Kocyigit D, Grimm R, Griffin BP, Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog Cardiovasc Dis. 2020;63(3):367-376. doi:10.1016/j.pcad.2020.03.003
60. Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(11):1317-1335. doi:10.1016/j.jacc.2018.12.054
61. Carewell Health. AI powered ECG diagnosis solutions. Accessed November 2, 2020. https://www.carewellhealth.com/products_aiecg.html
62. Strodthoff N, Strodthoff C. Detecting and interpreting myocardial infarction using fully convolutional neural networks. Physiol Meas. 2019;40(1):015001. doi:10.1088/1361-6579/aaf34d
63. Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25(1):65-69. doi:10.1038/s41591-018-0268-3
64. Kwon JM, Jeon KH, Kim HM, et al. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace. 2020;22(3):412-419. doi:10.1093/europace/euz324

65. Eko. FDA clears Eko’s AFib and heart murmur detection algorithms, making it the first AI-powered stethoscope to screen for serious heart conditions [press release]. Published January 28, 2020. Accessed September 15, 2021. https://www.businesswire.com/news/home/20200128005232/en/FDA-Clears-Eko’s-AFib-and-Heart-Murmur-Detection-Algorithms-Making-It-the-First-AI-Powered-Stethoscope-to-Screen-for-Serious-Heart-Conditions
66. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450. doi:10.1038/srep46450
67. Acs B, Rantalainen M, Hartman J. Artificial intelligence as the next step towards precision pathology. J Intern Med. 2020;288(1):62-81. doi:10.1111/joim.13030
68. Mobadersany P, Yousefi S, Amgad M, et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci U S A. 2018;115(13):E2970-E2979. doi:10.1073/pnas.1717139115
69. Wang X, Janowczyk A, Zhou Y, et al. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci Rep. 2017;7:13543. doi:10.1038/s41598-017-13773-7
70. Kulkarni PM, Robinson EJ, Pradhan JS, et al. Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res. 2020;26(5):1126-1134. doi:10.1158/1078-0432.CCR-19-1495
71. Du XL, Li WB, Hu BJ. Application of artificial intelligence in ophthalmology. Int J Ophthalmol. 2018;11(9):1555-1561. doi:10.18240/ijo.2018.09.21
72. Gunasekeran DV, Wong TY. Artificial intelligence in ophthalmology in 2020: a technology on the cusp for translation and implementation. Asia Pac J Ophthalmol (Phila). 2020;9(2):61-66. doi:10.1097/01.APO.0000656984.56467.2c
73. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-175. doi:10.1136/bjophthalmol-2018-313173
74. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. doi:10.1001/jama.2016.17216
75. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [press release]. Published April 11, 2018. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye
76. Long E, Chen J, Wu X, et al. Artificial intelligence manages congenital cataract with individualized prediction and telehealth computing. NPJ Digit Med. 2020;3:112. doi:10.1038/s41746-020-00319-x
77. De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24(9):1342-1350. doi:10.1038/s41591-018-0107-6
78. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118. doi:10.1038/nature21056
79. Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
80. Brinker TJ, Hekler A, Enk AH, et al. A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task. Eur J Cancer. 2019;111:148-154. doi:10.1016/j.ejca.2019.02.005
81. Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8):1836-1842. doi:10.1093/annonc/mdy166
82. Li CX, Shen CB, Xue K, et al. Artificial intelligence in dermatology: past, present, and future. Chin Med J (Engl). 2019;132(17):2017-2020. doi:10.1097/CM9.0000000000000372
83. Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20(7):938-947. doi:10.1016/S1470-2045(19)30333-X
84. Han SS, Park I, Eun Chang SE, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140(9):1753-1761. doi:10.1016/j.jid.2020.01.019
85. Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies [published correction appears in BMJ. 2020 Feb 25;368:m645]. BMJ. 2020;368:m127. Published 2020 Feb 10. doi:10.1136/bmj.m127
86. Chen YC, Ke WC, Chiu HW. Risk classification of cancer survival using ANN with gene expression data from multiple laboratories. Comput Biol Med. 2014;48:1-7. doi:10.1016/j.compbiomed.2014.02.006
87. Kim W, Kim KS, Lee JE, et al. Development of novel breast cancer recurrence prediction model using support vector machine. J Breast Cancer. 2012;15(2):230-238. doi:10.4048/jbc.2012.15.2.230
88. Merath K, Hyer JM, Mehta R, et al. Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. 2020;24(8):1843-1851. doi:10.1007/s11605-019-04338-2
89. Santos-García G, Varela G, Novoa N, Jiménez MF. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Artif Intell Med. 2004;30(1):61-69. doi:10.1016/S0933-3657(03)00059-9
90. Ibragimov B, Xing L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med Phys. 2017;44(2):547-557. doi:10.1002/mp.12045
91. Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136-e147. doi:10.1016/S2589-7500(19)30058-5
92. Xu J, Yang P, Xue S, et al. Translating cancer genomics into precision medicine with artificial intelligence: applications, challenges and future perspectives. Hum Genet. 2019;138(2):109-124. doi:10.1007/s00439-019-01970-5
93. Patel NM, Michelini VV, Snell JM, et al. Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist. 2018;23(2):179-185. doi:10.1634/theoncologist.2017-0170
94. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76-94.e2. doi:10.1053/j.gastro.2019.08.058
95. Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25(14):1666-1683. doi:10.3748/wjg.v25.i14.1666
96. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68(10):1813-1819. doi:10.1136/gutjnl-2018-317500

97. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An East Coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
98. Beli M, Bobi V, Badža M, Šolaja N, Duri-Jovii M, Kosti VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—a review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
99. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
100. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
101. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging [published online ahead of print, 2020 Oct 28]. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
102. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
103. Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24(11):1583-1598. doi:10.1038/s41380-019-0365-9
104. Fonseka TM, Bhat V, Kennedy SH. The utility of artificial intelligence in suicide risk prediction and the management of suicidal behaviors. Aust N Z J Psychiatry. 2019;53(10):954-964. doi:10.1177/0004867419864428
105. Kessler RC, Hwang I, Hoffmire CA, et al. Developing a practical suicide risk prediction model for targeting high-risk patients in the Veterans Health Administration. Int J Methods Psychiatr Res. 2017;26(3):e1575. doi:10.1002/mpr.1575
106. Kessler RC, Bauer MS, Bishop TM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the Veterans Health Administration System. Front Psychiatry. 2020;11:390. doi:10.3389/fpsyt.2020.00390
107. Kessler RC, van Loo HM, Wardenaar KJ, et al. Testing a machine-learning algorithm to predict the persistence and severity of major depressive disorder from baseline self-reports. Mol Psychiatry. 2016;21(10):1366-1371. doi:10.1038/mp.2015.198
108. Horng S, Sontag DA, Halpern Y, Jernite Y, Shapiro NI, Nathanson LA. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS One. 2017;12(4):e0174708. doi:10.1371/journal.pone.0174708
109. Soffer S, Klang E, Barash Y, Grossman E, Zimlichman E. Predicting in-hospital mortality at admission to the medical ward: a big-data machine learning model. Am J Med. 2021;134(2):227-234.e4. doi:10.1016/j.amjmed.2020.07.014
110. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416-1419. doi:10.1161/STROKEAHA.116.016281
111. Forlenza GP. Use of artificial intelligence to improve diabetes outcomes in patients using multiple daily injections therapy. Diabetes Technol Ther. 2019;21(S2):S24-S28. doi:10.1089/dia.2019.0077
112. Poser CM. CT scan and the practice of neurology. Arch Neurol. 1977;34(2):132. doi:10.1001/archneur.1977.00500140086023
113. Angus DC. Randomized clinical trials of artificial intelligence. JAMA. 2020;323(11):1043-1045. doi:10.1001/jama.2020.1039
114. Topol EJ. Welcoming new guidelines for AI clinical research. Nat Med. 2020;26(9):1318-1320. doi:10.1038/s41591-020-1042-x
115. Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577-1579. doi:10.1016/S0140-6736(19)30037-6
116. Cruz Rivera S, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351-1363. doi:10.1038/s41591-020-1037-7
117. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364-1374. doi:10.1038/s41591-020-1034-x
118. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5(4):115-133. doi:10.1007/BF02478259
119. Samuel AL. Some studies in machine learning using the game of Checkers. IBM J Res Dev. 1959;3(3):535-554. Accessed September 15, 2021. https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.368.2254
120. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology. 1983;148(3):833-838. doi:10.1148/radiology.148.3.6878707
121. Dechter R. Learning while searching in constraint-satisfaction-problems. AAAI’86: proceedings of the fifth AAAI national conference on artificial intelligence. Published 1986. Accessed September 15, 2021. https://www.aaai.org/Papers/AAAI/1986/AAAI86-029.pdf
122. Le Cun Y, Jackel LD, Boser B, et al. Handwritten digit recognition: applications of neural network chips and automatic learning. IEEE Commun Mag. 1989;27(11):41-46. doi:10.1109/35.41400
123. US Food and Drug Administration. FDA allows marketing of first whole slide imaging system for digital pathology [press release]. Published April 12, 2017. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology