User login
ORLANDO – Low-dose asenapine provided global improvement in symptoms and mood in older nondemented patients with bipolar disorder with prior suboptimal response to other therapies, according to a pilot study.
This atypical antipsychotic agent, which was used in the study at a mean daily dose of just 7.4 mg, is a novel therapy worth considering in selected geriatric patients with bipolar disorder, Dr. Martha N. Sajatovic said at the annual meeting of the American Association for Geriatric Psychiatry.
She presented a 12-week, prospective, open-label pilot study of asenapine (Saphris) in 15 nondemented patients over age 60 years with type 1 or 2 bipolar disorder who had a poor response to previous treatments. Patients started on asenapine at 5 mg/day and were titrated as indicated to a maximum of 20 mg/day, although the mean dose used was 7.4 mg/day.
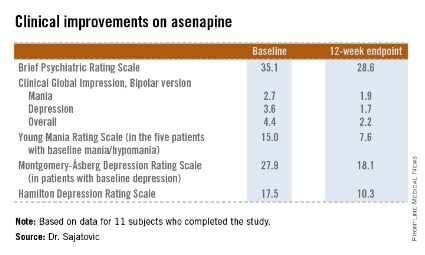
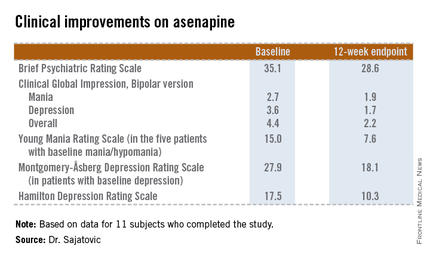
The 11 subjects who completed the study showed significant improvements on the Brief Psychiatric Rating Scale, on the Clinical Global Impression, Bipolar version, and on measures of mood symptoms. No changes were found from baseline in terms of mean body mass index, abnormal movement scores, blood glucose levels, lipid levels, or functional status as measured by the Short Form–12, or on the WHO-Disability Assessment Scale II.
Of the four study dropouts, one left because of elevated liver function test results, another because of the emergence of manic symptoms while on asenapine, and two others for reasons unrelated to the study medication, according to Dr. Sajatovic, professor of psychiatry and director of the geropsychiatry program at Case Western Reserve University, Cleveland.
The most common treatment-emergent adverse event was mild, transient GI discomfort, which affected one-third of participants.
The addition of asenapine to the armamentarium for bipolar disorder in later life is a welcome development, she said. Of the 6 million American adults with bipolar disorder, roughly 1 million are over the age of 60. Traditional mood stabilizers such as lithium often have limiting side effects in the older population, and treatment failures are common, she noted.
This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.
ORLANDO – Low-dose asenapine provided global improvement in symptoms and mood in older nondemented patients with bipolar disorder with prior suboptimal response to other therapies, according to a pilot study.
This atypical antipsychotic agent, which was used in the study at a mean daily dose of just 7.4 mg, is a novel therapy worth considering in selected geriatric patients with bipolar disorder, Dr. Martha N. Sajatovic said at the annual meeting of the American Association for Geriatric Psychiatry.

She presented a 12-week, prospective, open-label pilot study of asenapine (Saphris) in 15 nondemented patients over age 60 years with type 1 or 2 bipolar disorder who had a poor response to previous treatments. Patients started on asenapine at 5 mg/day and were titrated as indicated to a maximum of 20 mg/day, although the mean dose used was 7.4 mg/day.
The 11 subjects who completed the study showed significant improvements on the Brief Psychiatric Rating Scale, on the Clinical Global Impression, Bipolar version, and on measures of mood symptoms. No changes were found from baseline in terms of mean body mass index, abnormal movement scores, blood glucose levels, lipid levels, or functional status as measured by the Short Form–12, or on the WHO-Disability Assessment Scale II.
Of the four study dropouts, one left because of elevated liver function test results, another because of the emergence of manic symptoms while on asenapine, and two others for reasons unrelated to the study medication, according to Dr. Sajatovic, professor of psychiatry and director of the geropsychiatry program at Case Western Reserve University, Cleveland.
The most common treatment-emergent adverse event was mild, transient GI discomfort, which affected one-third of participants.
The addition of asenapine to the armamentarium for bipolar disorder in later life is a welcome development, she said. Of the 6 million American adults with bipolar disorder, roughly 1 million are over the age of 60. Traditional mood stabilizers such as lithium often have limiting side effects in the older population, and treatment failures are common, she noted.
This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.
ORLANDO – Low-dose asenapine provided global improvement in symptoms and mood in older nondemented patients with bipolar disorder with prior suboptimal response to other therapies, according to a pilot study.
This atypical antipsychotic agent, which was used in the study at a mean daily dose of just 7.4 mg, is a novel therapy worth considering in selected geriatric patients with bipolar disorder, Dr. Martha N. Sajatovic said at the annual meeting of the American Association for Geriatric Psychiatry.

She presented a 12-week, prospective, open-label pilot study of asenapine (Saphris) in 15 nondemented patients over age 60 years with type 1 or 2 bipolar disorder who had a poor response to previous treatments. Patients started on asenapine at 5 mg/day and were titrated as indicated to a maximum of 20 mg/day, although the mean dose used was 7.4 mg/day.
The 11 subjects who completed the study showed significant improvements on the Brief Psychiatric Rating Scale, on the Clinical Global Impression, Bipolar version, and on measures of mood symptoms. No changes were found from baseline in terms of mean body mass index, abnormal movement scores, blood glucose levels, lipid levels, or functional status as measured by the Short Form–12, or on the WHO-Disability Assessment Scale II.
Of the four study dropouts, one left because of elevated liver function test results, another because of the emergence of manic symptoms while on asenapine, and two others for reasons unrelated to the study medication, according to Dr. Sajatovic, professor of psychiatry and director of the geropsychiatry program at Case Western Reserve University, Cleveland.
The most common treatment-emergent adverse event was mild, transient GI discomfort, which affected one-third of participants.
The addition of asenapine to the armamentarium for bipolar disorder in later life is a welcome development, she said. Of the 6 million American adults with bipolar disorder, roughly 1 million are over the age of 60. Traditional mood stabilizers such as lithium often have limiting side effects in the older population, and treatment failures are common, she noted.
This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.
AT THE AAGP ANNUAL MEETING
Major finding: The atypical antipsychotic asenapine produced significant improvement in symptoms and mood in patients with later-life bipolar disorder that previously had shown a poor response to other therapies.
Data source: A prospective, 12-week, open-label pilot study in which 15 nondemented patients over age 60 with bipolar disorder suboptimally responsive to other therapies were treated with asenapine starting at 5 mg/day.
Disclosures: This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.