User login
Although many developments have occurred in the last decade for managing atrial fibrillation, challenges remain. New and emerging alternatives to warfarin (Coumadin) for anticoagulation therapy prevent stroke marginally better and pose slightly less risk of hemorrhage, but they have important drawbacks.
The antiarrhythmic drug dronedarone (Multaq) has been found to offer only temporary benefit for persistent atrial fibrillation, and significant risks have emerged.
Radiofrequency ablation is gaining prominence, but repeat procedures are sometimes necessary.
An investigational device can be implanted via percutaneous catheter in the left atrial appendage to prevent embolization. It is too soon to know its eventual role in clinical practice.
This article reviews the results of clinical trials of these new treatments and discusses their role in clinical practice.
CHALLENGES OF ANTICOAGULATION
The main focus of managing atrial fibrillation is on alleviating symptoms, by either rate control or rhythm control. The other focus is on preventing stroke—a devastating outcome—with anticoagulation therapy.
For deciding whether to give warfarin to patients with atrial fibrillation, the six-point CHADS2 score is a crude but effective way of assessing the risk of stroke based on the following risk factors: congestive heart failure, hypertension, age 75 years or older, and diabetes (1 point each); or a history of stroke or transient ischemic attack (2 points).1 Warfarin is given if patients have a score of at least 2 points.
Warfarin has a narrow therapeutic window, with a higher risk of ischemic stroke if the international normalized ratio (INR) is less than 2.0,2 and a higher risk of intracranial hemorrhage if the INR is more than 3.0.3 Keeping the INR in the therapeutic range is difficult because of variations in diet, concurrent medications, and other factors.
The percent of time that the INR is within the therapeutic range predicts the risk of adverse events. Connolly et al4 showed that the cumulative risk of stroke, myocardial infarction, systemic embolism, or vascular death was no better with warfarin than with clopidogrel (Plavix) plus aspirin if the INR was in the therapeutic range less than 65% of the time, but the risk was significantly less if the INR was in the therapeutic range more than 65% of the time.
Also, comparing warfarin with the combination of aspirin and clopidogrel, Verheugt5 found that the rates of stroke of any kind, of disabling and fatal stroke, and of stroke per major bleed were lower in patients taking warfarin. Although many physicians prefer aspirin plus clopidogrel because of concerns about bleeding with warfarin, the rates of major bleeding were about the same in the two groups.
In a trial in patients for whom warfarin was “unsuitable,”6 the combination of aspirin plus clopidogrel was associated with a lower rate of stroke than aspirin alone (2.4% per year vs 3.3% per year, relative risk 0.762) but a higher rate of major bleeding events (2.0% per year vs 1.3% per year, relative risk 1.57).
NEW ALTERNATIVES TO WARFARIN
Because of the problems with warfarin, alternatives have been sought for many years. Several new oral anticoagulants are available or are being developed,7 including the factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis) and the direct factor II (thrombin) inhibitor dabigatran (Pradaxa) (Table 1).
Dabigatran’s advantages and drawbacks
Dabigatran has been on the market for more than a year and has gained rapid acceptance. The dosage is 150 mg twice a day, or 75 mg twice a day if renal function is impaired. Cleared by the kidneys, it has a half-life of 12 to 17 hours; 75% is cleared within 24 hours. For a patient who needs surgery that poses a low risk of bleeding, the general recommendation is to stop dabigatran the night before the surgical procedure. For operations with a greater risk of bleeding, many surgeons recommend stopping the drug 3 or 4 days before.
Advantages of dabigatran include that it is not influenced by diet and that the onset of therapeutic benefit is within 1 hour. Although some drugs affect dabigatran, drug interactions are more troublesome with warfarin.
A serious concern about dabigatran and the other new agents is that if a bleeding problem arises, the effects of these drugs are not reversible by administration of fresh frozen plasma. Dabigatran is reversible by dialysis; however, if a patient is also hypotensive, dialysis is not an option, and simply waiting for the drug to clear is the only choice.
Another drawback is that therapeutic levels cannot be monitored. If a patient taking warfarin requires cardioversion, the INR is carefully monitored for several weeks beforehand to reduce the risk of stroke. With dabigatran, there is no way to know if a patient is actually taking the drug as prescribed.
Clinical trials show that alternatives are marginally better than warfarin
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,8 dabigatran was associated with a significantly lower incidence of intracranial hemorrhage, combined strokes, and systemic embolization than warfarin. The incidence of major bleeds was slightly lower with dabigatran. Although dabigatran performed better, the differences were small and would not require patients to change from warfarin if they are already doing well.
Apixaban and rivaroxaban are other alternatives to warfarin, with different mechanisms of action and metabolism. Although rivaroxaban’s half-life is similar to that of apixaban and dabigatran, it is being marketed as allowing once-daily dosing instead of twice-daily.
Recent randomized controlled clinical trials of the new drugs include:
- The Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,9 which compared apixaban and aspirin
- The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin10
- The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),11 comparing rivaroxaban and warfarin
- RE-LY,8 comparing dabigatran and warfarin.
In the ARISTOTLE,10 ROCKET AF,11 and RE-LY trials,8 the time that the warfarin patients’ INRs were in the therapeutic range varied from 55% to 68%. This seems low and is a problem when trying to compare therapies, but is probably about as high as one can expect in the real world.
In AVERROES,9 the combined rate of stroke and embolism was higher with aspirin than with apixaban. In the other trials, the rates were slightly better with the new drugs than with warfarin, and the rates of major hemorrhage and hemorrhagic stroke were only slightly higher with warfarin than with the new drugs. Because the differences in benefits and risks are so small, the main advantage of the newer drugs will probably be for patients who have difficulty staying in the therapeutic INR range on warfarin.
RATE CONTROL VS RESTORATION OF SINUS RHYTHM
Evidence is insufficient to determine the risk of very-long-term asymptomatic atrial fibrillation in patients on appropriate anticoagulation. Rate control is an option for asymptomatic patients but provides no change in quality of life and no definitive reduction in the risk of stroke. The main argument for restoring normal sinus rhythm in patients with mild to moderate symptoms is that it improves exercise capacity. The need for anticoagulation persists when patients are converted to sinus rhythm because the risk of recurrent atrial fibrillation remains high.
For patients with symptomatic atrial fibrillation, rate control is sometimes achieved with beta-blockers or calcium channel blockers. Rate control may be augmented with the addition of digoxin, but when used alone digoxin generally does not control the rate of atrial fibrillation. However, in many cases of atrial fibrillation, symptoms are not rate-related, and cardioversion to normal sinus rhythm should be attempted. In such cases, the symptoms may be attributable to a loss of atrial transport function.
Patients with the following risk factors should be admitted to the hospital to start antiarrhythmic drugs:
- Borderline or a long QTc interval at baseline (> 450 msec)
- Treatment with dofetilide (Tikosyn) because of its effects on the QT interval
- Heart failure or poor left-ventricular function
- Sinus node dysfunction
- Significant atrioventricular conduction disease.
Selecting an antiarrhythmic drug
Any of the antiarrhythmic drugs listed in Table 2 can be used for a patient with lone atrial fibrillation (ie, not caused by underlying heart disease). The choice of drug should be determined by whether coronary artery disease or renal failure is present as well. Liver disease or chronic obstructive pulmonary disease also may affect this decision.
Benefits of dronedarone are mixed
In a randomized trial of dronedarone vs placebo in patients with atrial fibrillation, the rate of death and the rate of first hospitalization due to a cardiovascular event at 21 months were significantly lower with dronedarone.12 No difference was found between the two groups in the rate of death from all causes, but fewer people died of cardiovascular causes in the dronedarone group. More patients taking dronedarone developed bradycardia, QT-interval prolongation, nausea, diarrhea, rash, or a higher serum creatinine level. Gastrointestinal side effects are often a problem with dronedarone: 20% to 30% of patients cannot tolerate the drug.
Dronedarone may cause a small rise in creatinine, and although this effect should be monitored, by itself it should not be interpreted as impairment of renal function. In a study in healthy people,13 dronedarone caused a 10% to 15% increase in serum creatinine, but the glomerular filtration rate was unchanged, as were renal plasma flow and anion secretion.
Another trial, in patients with severe heart failure, found that patients taking dronedarone had higher rates of hospitalization and overall mortality, raising serious concern about the safety of this drug in patients with advanced heart failure.14
Singh et al15 pooled the data from two multicenter, randomized trials that compared dronedarone with placebo for maintaining sinus rhythm in patients with atrial fibrillation or flutter. The mean time to the recurrence of atrial fibrillation was 116 days with dronedarone and 53 days with placebo. Other trials also showed longer times to recurrence and lower recurrence rates with dronedarone. Although the differences were statistically significant, they may not be clinically meaningful for patients.
Dronedarone is structurally similar to amiodarone (Cordarone), but the two drugs work differently. A meta-analysis of clinical trials16 found that amiodarone recipients had a lower rate of recurrence of atrial fibrillation than did those receiving dronedarone.
Two safety warnings for dronedarone
In January 2011, the US Food and Drug Administration (FDA) issued an alert about cases of rare but severe liver injury in patients treated with dronedarone, including two cases of acute liver failure leading to liver transplantation.17
The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)18 compared dronedarone and placebo in patients with permanent atrial fibrillation. More people died or had serious cardiovascular adverse events in the dronedarone group. The study was stopped early after data monitoring showed that rates of death, stroke, and hospitalization for heart failure were two times higher in patients receiving dronedarone. This prompted the FDA to issue another safety alert in July 2011.
Interestingly, the PALLAS study did not set out to determine whether dronedarone controls atrial fibrillation, as the study patients had long-standing, persistent atrial fibrillation. The study was designed only to determine if the drug reduces the rate of adverse events; it clearly does not, and the study shows that dronedarone should not be used to control the heart rate in patients with persistent atrial fibrillation. Instead, its use is best restricted to patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
ABLATION OF ATRIAL FIBRILLATION
Another way to try to restore sinus rhythm is to destroy or isolate the area that is generating the abnormal beats via a catheter-based procedure.
Radiofrequency ablation is generally tried in patients in whom one or two drugs have failed to control atrial fibrillation. Direct comparisons show that ablation is superior to drug therapy and is effective in about 75% of patients with paroxysmal atrial fibrillation vs 20% to 40% of patients on drug therapy. Ablation plus drug therapy is often more effective than either treatment alone.
Mechanisms of atrial fibrillation and ablation
In many cases, atrial fibrillation is stimulated by vagal and sympathetic inputs to the atrium that enter around the pulmonary veins and trigger electrical activations in the area, generating spiraling, reentering circuits. Focal atrial fibrillation also originates predominantly in the pulmonary veins. Ablation of tissue widely circumscribing the mouth of the pulmonary veins prevents the electrical signal from exiting into the atrium.
In about 11% to 37% of cases, atrial fibrillation originates elsewhere, eg, in the left atrium, in the superior vena cava, or in the vein of Marshall. Techniques have evolved to also ablate these regions.
Anticoagulation therapy is recommended before the procedure, and patients at low risk should continue it for a minimum of 2 months afterward. Patients with a higher CHADS2 score should receive anticoagulation therapy for at least 1 year. The consensus statement by the Heart Rhythm Society19 recommends that patients remain on warfarin or one of the newer anticoagulants if their CHADS2 score is 2 or higher. This is because patients have a significant risk of recurrence of atrial fibrillation after radiofrequency ablation, so if their stroke risk is high they should remain on anticoagulant therapy.
Ablation is usually effective, but it carries rare but serious risks
The efficacy of a single radiofrequency ablation procedure is in the range of 60% to 80% for paroxysmal atrial fibrillation and 40% to 60% for persistent atrial fibrillation. The Second International Ablation Registry20 shows a success rate of about 75% in patients with paroxysmal atrial fibrillation and about 65% in patients with persistent and permanent atrial fibrillation. Registry data are often more favorable because reporting is optional, but these results are consistent with those from experienced medical centers. Rates of suppression of atrial fibrillation are higher in patients who also take antiarrhythmic drugs, making a “hybrid” approach useful when ablation alone fails.
According to a worldwide survey, the risk of serious complications is 4.5%. These include stroke (0.23%), tamponade (1.3%), and pulmonary vein stenosis (< 0.29%). The esophagus lies just behind the right atrium, and burning through and creating a fistula between them occurs in about 0.04% of cases and is almost uniformly fatal.20
A second ablation procedure is sometimes indicated for the recurrence of atrial fibrillation, which is almost always caused by recovery of the pulmonary veins. Bhargava et al21 found that the success rate at Cleveland Clinic for a single procedure for paroxysmal atrial fibrillation was 77%, and that it was 92% after a repeat procedure. For persistent atrial fibrillation, success rates were 76% after the first procedure and 90% after the second. Even for long-standing persistent atrial fibrillation (ie, lasting more than 1 year), 80% success was achieved after two procedures. Patients who are less likely to have a successful ablation procedure are those with long-standing atrial fibrillation and coexisting heart disease, including severe valvular disease, although mitral regurgitation sometimes improves if sinus rhythm can be maintained.
The need for a second procedure
After ablation, patients should be closely monitored for a recurrence of atrial fibrillation. Continuous monitoring with implantable cardiac monitor loop recorders can detect unrecognized episodes of arrhythmia. Long-term follow-up is also required to track outcomes and quality of life.
According to the Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation,19 atrial fibrillation recurs after ablation in about 35% to 60% of patients in the first 3 months, with recurrence rates after 1 year ranging from 5% to 16%. The rate of success is determined by the skill of the surgeon, underlying heart disease, attention to follow-up, and how success is defined.
Freedom from recurrence early on is a good predictor that late recurrence is unlikely. Patients who only have a very early recurrence (within the first 4 weeks) are more likely to have long-term freedom from atrial fibrillation tha those who have recurrences after that time.22
In a study of 831 patients, Hussein et al23 found recurrence rates of 24% between months 3 to 13 following ablation and 9% after 12 months. At 55 months, 79% were free from atrial fibrillation without drugs, 11% were free of atrial fibrillation with medications, and 5% had refractory atrial fibrillation.
Recurrence—whether early or late—was more likely to occur in people with persistent vs paroxysmal atrial fibrillation. Other risk factors for late recurrence included older age and larger left atrial size (which is also a risk factor for recurrence on drug therapy). Although recurrent arrhythmia was most often atrial fibrillation, atrial flutter also occurred frequently (in 27% of patients with late recurrence). Three patients (4% of patients with late recurrence) developed atrial tachycardia.23
In patients with early recurrence, 81% underwent repeat ablation, all of whom had recovery of one or more pulmonary veins. After the second ablation, 21% had recurrence, 65% of whom were controlled by medications.23
Whether a patient should undergo subsequent ablation procedures depends on the severity of symptoms, the likelihood of success (based on an educated guess), and the patient’s willingness to undergo another procedure.
ATRIAL APPENDAGE OCCLUSION DEVICE UNDER INVESTIGATION
New devices are being investigated that occlude the left atrial appendage to try to prevent embolization.
The Watchman device, resembling an umbrella, is implanted via a percutaneous catheter in the left atrial appendage, closing it off to preclude a thrombus from forming in the appendage and embolizing to the body. Clinical trials showed that patients who received a device had a slightly lower risk of stroke than otherwise seen in clinical practice.24 Safety and efficacy are still being determined.
The device cannot be deployed in a patient with an existing thrombus because of the danger of dislodging the thrombus, allowing it to embolize.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Connolly SJ, Pogue J, Eikelboom J, et al; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008; 118:2029–2037.
- Verheugt FWA. Who is ineligible for warfarin in atrial fibrillation? Lancet 2009; 374:510–511.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Harenberg J. New anticoagulants in atrial fibrillation. Semin Thromb Hemost 2009; 35:574–585.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Connolly SJ, Eikelboom J, Joyner C, et al; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJV, et al; for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med August 28, 2011; 10.1056/nejmoa1107039.
- Patel MR, Mahaffey KW, Garg J, et al; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Hohnloser SH, Crijns HJ, van Eickels M, et al; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:688–678.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Kóber L, Torp-Pederson C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol 2009; 54:1089–1095.
- US Food and Drug Administration. FDA drug safety communication: severe liver injury associated with the use of dronedarone (marketed as Multaq). http://www.fda.gov/drugs/drugsafety/ucm240011.htm. Accessed July 5, 2012.
- Connolly SJ, Camm AJ, Halperin JL, et al; for the PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011; 365:2268–2276.
- HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm 2007; 4:1–46.
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3:32–38.
- Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm 2009; 6:1403–1412.
- Themistoclakis S, Schweikert RA, Sliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008; 5:679–685.
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4:271–278.
- Holmes DR, Reddy VY, Turi ZG, et al; for the PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
Although many developments have occurred in the last decade for managing atrial fibrillation, challenges remain. New and emerging alternatives to warfarin (Coumadin) for anticoagulation therapy prevent stroke marginally better and pose slightly less risk of hemorrhage, but they have important drawbacks.
The antiarrhythmic drug dronedarone (Multaq) has been found to offer only temporary benefit for persistent atrial fibrillation, and significant risks have emerged.
Radiofrequency ablation is gaining prominence, but repeat procedures are sometimes necessary.
An investigational device can be implanted via percutaneous catheter in the left atrial appendage to prevent embolization. It is too soon to know its eventual role in clinical practice.
This article reviews the results of clinical trials of these new treatments and discusses their role in clinical practice.
CHALLENGES OF ANTICOAGULATION
The main focus of managing atrial fibrillation is on alleviating symptoms, by either rate control or rhythm control. The other focus is on preventing stroke—a devastating outcome—with anticoagulation therapy.
For deciding whether to give warfarin to patients with atrial fibrillation, the six-point CHADS2 score is a crude but effective way of assessing the risk of stroke based on the following risk factors: congestive heart failure, hypertension, age 75 years or older, and diabetes (1 point each); or a history of stroke or transient ischemic attack (2 points).1 Warfarin is given if patients have a score of at least 2 points.
Warfarin has a narrow therapeutic window, with a higher risk of ischemic stroke if the international normalized ratio (INR) is less than 2.0,2 and a higher risk of intracranial hemorrhage if the INR is more than 3.0.3 Keeping the INR in the therapeutic range is difficult because of variations in diet, concurrent medications, and other factors.
The percent of time that the INR is within the therapeutic range predicts the risk of adverse events. Connolly et al4 showed that the cumulative risk of stroke, myocardial infarction, systemic embolism, or vascular death was no better with warfarin than with clopidogrel (Plavix) plus aspirin if the INR was in the therapeutic range less than 65% of the time, but the risk was significantly less if the INR was in the therapeutic range more than 65% of the time.
Also, comparing warfarin with the combination of aspirin and clopidogrel, Verheugt5 found that the rates of stroke of any kind, of disabling and fatal stroke, and of stroke per major bleed were lower in patients taking warfarin. Although many physicians prefer aspirin plus clopidogrel because of concerns about bleeding with warfarin, the rates of major bleeding were about the same in the two groups.
In a trial in patients for whom warfarin was “unsuitable,”6 the combination of aspirin plus clopidogrel was associated with a lower rate of stroke than aspirin alone (2.4% per year vs 3.3% per year, relative risk 0.762) but a higher rate of major bleeding events (2.0% per year vs 1.3% per year, relative risk 1.57).
NEW ALTERNATIVES TO WARFARIN
Because of the problems with warfarin, alternatives have been sought for many years. Several new oral anticoagulants are available or are being developed,7 including the factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis) and the direct factor II (thrombin) inhibitor dabigatran (Pradaxa) (Table 1).
Dabigatran’s advantages and drawbacks
Dabigatran has been on the market for more than a year and has gained rapid acceptance. The dosage is 150 mg twice a day, or 75 mg twice a day if renal function is impaired. Cleared by the kidneys, it has a half-life of 12 to 17 hours; 75% is cleared within 24 hours. For a patient who needs surgery that poses a low risk of bleeding, the general recommendation is to stop dabigatran the night before the surgical procedure. For operations with a greater risk of bleeding, many surgeons recommend stopping the drug 3 or 4 days before.
Advantages of dabigatran include that it is not influenced by diet and that the onset of therapeutic benefit is within 1 hour. Although some drugs affect dabigatran, drug interactions are more troublesome with warfarin.
A serious concern about dabigatran and the other new agents is that if a bleeding problem arises, the effects of these drugs are not reversible by administration of fresh frozen plasma. Dabigatran is reversible by dialysis; however, if a patient is also hypotensive, dialysis is not an option, and simply waiting for the drug to clear is the only choice.
Another drawback is that therapeutic levels cannot be monitored. If a patient taking warfarin requires cardioversion, the INR is carefully monitored for several weeks beforehand to reduce the risk of stroke. With dabigatran, there is no way to know if a patient is actually taking the drug as prescribed.
Clinical trials show that alternatives are marginally better than warfarin
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,8 dabigatran was associated with a significantly lower incidence of intracranial hemorrhage, combined strokes, and systemic embolization than warfarin. The incidence of major bleeds was slightly lower with dabigatran. Although dabigatran performed better, the differences were small and would not require patients to change from warfarin if they are already doing well.
Apixaban and rivaroxaban are other alternatives to warfarin, with different mechanisms of action and metabolism. Although rivaroxaban’s half-life is similar to that of apixaban and dabigatran, it is being marketed as allowing once-daily dosing instead of twice-daily.
Recent randomized controlled clinical trials of the new drugs include:
- The Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,9 which compared apixaban and aspirin
- The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin10
- The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),11 comparing rivaroxaban and warfarin
- RE-LY,8 comparing dabigatran and warfarin.
In the ARISTOTLE,10 ROCKET AF,11 and RE-LY trials,8 the time that the warfarin patients’ INRs were in the therapeutic range varied from 55% to 68%. This seems low and is a problem when trying to compare therapies, but is probably about as high as one can expect in the real world.
In AVERROES,9 the combined rate of stroke and embolism was higher with aspirin than with apixaban. In the other trials, the rates were slightly better with the new drugs than with warfarin, and the rates of major hemorrhage and hemorrhagic stroke were only slightly higher with warfarin than with the new drugs. Because the differences in benefits and risks are so small, the main advantage of the newer drugs will probably be for patients who have difficulty staying in the therapeutic INR range on warfarin.
RATE CONTROL VS RESTORATION OF SINUS RHYTHM
Evidence is insufficient to determine the risk of very-long-term asymptomatic atrial fibrillation in patients on appropriate anticoagulation. Rate control is an option for asymptomatic patients but provides no change in quality of life and no definitive reduction in the risk of stroke. The main argument for restoring normal sinus rhythm in patients with mild to moderate symptoms is that it improves exercise capacity. The need for anticoagulation persists when patients are converted to sinus rhythm because the risk of recurrent atrial fibrillation remains high.
For patients with symptomatic atrial fibrillation, rate control is sometimes achieved with beta-blockers or calcium channel blockers. Rate control may be augmented with the addition of digoxin, but when used alone digoxin generally does not control the rate of atrial fibrillation. However, in many cases of atrial fibrillation, symptoms are not rate-related, and cardioversion to normal sinus rhythm should be attempted. In such cases, the symptoms may be attributable to a loss of atrial transport function.
Patients with the following risk factors should be admitted to the hospital to start antiarrhythmic drugs:
- Borderline or a long QTc interval at baseline (> 450 msec)
- Treatment with dofetilide (Tikosyn) because of its effects on the QT interval
- Heart failure or poor left-ventricular function
- Sinus node dysfunction
- Significant atrioventricular conduction disease.
Selecting an antiarrhythmic drug
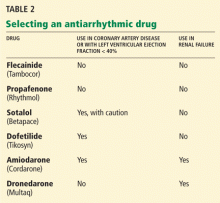
Any of the antiarrhythmic drugs listed in Table 2 can be used for a patient with lone atrial fibrillation (ie, not caused by underlying heart disease). The choice of drug should be determined by whether coronary artery disease or renal failure is present as well. Liver disease or chronic obstructive pulmonary disease also may affect this decision.
Benefits of dronedarone are mixed
In a randomized trial of dronedarone vs placebo in patients with atrial fibrillation, the rate of death and the rate of first hospitalization due to a cardiovascular event at 21 months were significantly lower with dronedarone.12 No difference was found between the two groups in the rate of death from all causes, but fewer people died of cardiovascular causes in the dronedarone group. More patients taking dronedarone developed bradycardia, QT-interval prolongation, nausea, diarrhea, rash, or a higher serum creatinine level. Gastrointestinal side effects are often a problem with dronedarone: 20% to 30% of patients cannot tolerate the drug.
Dronedarone may cause a small rise in creatinine, and although this effect should be monitored, by itself it should not be interpreted as impairment of renal function. In a study in healthy people,13 dronedarone caused a 10% to 15% increase in serum creatinine, but the glomerular filtration rate was unchanged, as were renal plasma flow and anion secretion.
Another trial, in patients with severe heart failure, found that patients taking dronedarone had higher rates of hospitalization and overall mortality, raising serious concern about the safety of this drug in patients with advanced heart failure.14
Singh et al15 pooled the data from two multicenter, randomized trials that compared dronedarone with placebo for maintaining sinus rhythm in patients with atrial fibrillation or flutter. The mean time to the recurrence of atrial fibrillation was 116 days with dronedarone and 53 days with placebo. Other trials also showed longer times to recurrence and lower recurrence rates with dronedarone. Although the differences were statistically significant, they may not be clinically meaningful for patients.
Dronedarone is structurally similar to amiodarone (Cordarone), but the two drugs work differently. A meta-analysis of clinical trials16 found that amiodarone recipients had a lower rate of recurrence of atrial fibrillation than did those receiving dronedarone.
Two safety warnings for dronedarone
In January 2011, the US Food and Drug Administration (FDA) issued an alert about cases of rare but severe liver injury in patients treated with dronedarone, including two cases of acute liver failure leading to liver transplantation.17
The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)18 compared dronedarone and placebo in patients with permanent atrial fibrillation. More people died or had serious cardiovascular adverse events in the dronedarone group. The study was stopped early after data monitoring showed that rates of death, stroke, and hospitalization for heart failure were two times higher in patients receiving dronedarone. This prompted the FDA to issue another safety alert in July 2011.
Interestingly, the PALLAS study did not set out to determine whether dronedarone controls atrial fibrillation, as the study patients had long-standing, persistent atrial fibrillation. The study was designed only to determine if the drug reduces the rate of adverse events; it clearly does not, and the study shows that dronedarone should not be used to control the heart rate in patients with persistent atrial fibrillation. Instead, its use is best restricted to patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
ABLATION OF ATRIAL FIBRILLATION
Another way to try to restore sinus rhythm is to destroy or isolate the area that is generating the abnormal beats via a catheter-based procedure.
Radiofrequency ablation is generally tried in patients in whom one or two drugs have failed to control atrial fibrillation. Direct comparisons show that ablation is superior to drug therapy and is effective in about 75% of patients with paroxysmal atrial fibrillation vs 20% to 40% of patients on drug therapy. Ablation plus drug therapy is often more effective than either treatment alone.
Mechanisms of atrial fibrillation and ablation
In many cases, atrial fibrillation is stimulated by vagal and sympathetic inputs to the atrium that enter around the pulmonary veins and trigger electrical activations in the area, generating spiraling, reentering circuits. Focal atrial fibrillation also originates predominantly in the pulmonary veins. Ablation of tissue widely circumscribing the mouth of the pulmonary veins prevents the electrical signal from exiting into the atrium.
In about 11% to 37% of cases, atrial fibrillation originates elsewhere, eg, in the left atrium, in the superior vena cava, or in the vein of Marshall. Techniques have evolved to also ablate these regions.
Anticoagulation therapy is recommended before the procedure, and patients at low risk should continue it for a minimum of 2 months afterward. Patients with a higher CHADS2 score should receive anticoagulation therapy for at least 1 year. The consensus statement by the Heart Rhythm Society19 recommends that patients remain on warfarin or one of the newer anticoagulants if their CHADS2 score is 2 or higher. This is because patients have a significant risk of recurrence of atrial fibrillation after radiofrequency ablation, so if their stroke risk is high they should remain on anticoagulant therapy.
Ablation is usually effective, but it carries rare but serious risks
The efficacy of a single radiofrequency ablation procedure is in the range of 60% to 80% for paroxysmal atrial fibrillation and 40% to 60% for persistent atrial fibrillation. The Second International Ablation Registry20 shows a success rate of about 75% in patients with paroxysmal atrial fibrillation and about 65% in patients with persistent and permanent atrial fibrillation. Registry data are often more favorable because reporting is optional, but these results are consistent with those from experienced medical centers. Rates of suppression of atrial fibrillation are higher in patients who also take antiarrhythmic drugs, making a “hybrid” approach useful when ablation alone fails.
According to a worldwide survey, the risk of serious complications is 4.5%. These include stroke (0.23%), tamponade (1.3%), and pulmonary vein stenosis (< 0.29%). The esophagus lies just behind the right atrium, and burning through and creating a fistula between them occurs in about 0.04% of cases and is almost uniformly fatal.20
A second ablation procedure is sometimes indicated for the recurrence of atrial fibrillation, which is almost always caused by recovery of the pulmonary veins. Bhargava et al21 found that the success rate at Cleveland Clinic for a single procedure for paroxysmal atrial fibrillation was 77%, and that it was 92% after a repeat procedure. For persistent atrial fibrillation, success rates were 76% after the first procedure and 90% after the second. Even for long-standing persistent atrial fibrillation (ie, lasting more than 1 year), 80% success was achieved after two procedures. Patients who are less likely to have a successful ablation procedure are those with long-standing atrial fibrillation and coexisting heart disease, including severe valvular disease, although mitral regurgitation sometimes improves if sinus rhythm can be maintained.
The need for a second procedure
After ablation, patients should be closely monitored for a recurrence of atrial fibrillation. Continuous monitoring with implantable cardiac monitor loop recorders can detect unrecognized episodes of arrhythmia. Long-term follow-up is also required to track outcomes and quality of life.
According to the Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation,19 atrial fibrillation recurs after ablation in about 35% to 60% of patients in the first 3 months, with recurrence rates after 1 year ranging from 5% to 16%. The rate of success is determined by the skill of the surgeon, underlying heart disease, attention to follow-up, and how success is defined.
Freedom from recurrence early on is a good predictor that late recurrence is unlikely. Patients who only have a very early recurrence (within the first 4 weeks) are more likely to have long-term freedom from atrial fibrillation tha those who have recurrences after that time.22
In a study of 831 patients, Hussein et al23 found recurrence rates of 24% between months 3 to 13 following ablation and 9% after 12 months. At 55 months, 79% were free from atrial fibrillation without drugs, 11% were free of atrial fibrillation with medications, and 5% had refractory atrial fibrillation.
Recurrence—whether early or late—was more likely to occur in people with persistent vs paroxysmal atrial fibrillation. Other risk factors for late recurrence included older age and larger left atrial size (which is also a risk factor for recurrence on drug therapy). Although recurrent arrhythmia was most often atrial fibrillation, atrial flutter also occurred frequently (in 27% of patients with late recurrence). Three patients (4% of patients with late recurrence) developed atrial tachycardia.23
In patients with early recurrence, 81% underwent repeat ablation, all of whom had recovery of one or more pulmonary veins. After the second ablation, 21% had recurrence, 65% of whom were controlled by medications.23
Whether a patient should undergo subsequent ablation procedures depends on the severity of symptoms, the likelihood of success (based on an educated guess), and the patient’s willingness to undergo another procedure.
ATRIAL APPENDAGE OCCLUSION DEVICE UNDER INVESTIGATION
New devices are being investigated that occlude the left atrial appendage to try to prevent embolization.
The Watchman device, resembling an umbrella, is implanted via a percutaneous catheter in the left atrial appendage, closing it off to preclude a thrombus from forming in the appendage and embolizing to the body. Clinical trials showed that patients who received a device had a slightly lower risk of stroke than otherwise seen in clinical practice.24 Safety and efficacy are still being determined.
The device cannot be deployed in a patient with an existing thrombus because of the danger of dislodging the thrombus, allowing it to embolize.
Although many developments have occurred in the last decade for managing atrial fibrillation, challenges remain. New and emerging alternatives to warfarin (Coumadin) for anticoagulation therapy prevent stroke marginally better and pose slightly less risk of hemorrhage, but they have important drawbacks.
The antiarrhythmic drug dronedarone (Multaq) has been found to offer only temporary benefit for persistent atrial fibrillation, and significant risks have emerged.
Radiofrequency ablation is gaining prominence, but repeat procedures are sometimes necessary.
An investigational device can be implanted via percutaneous catheter in the left atrial appendage to prevent embolization. It is too soon to know its eventual role in clinical practice.
This article reviews the results of clinical trials of these new treatments and discusses their role in clinical practice.
CHALLENGES OF ANTICOAGULATION
The main focus of managing atrial fibrillation is on alleviating symptoms, by either rate control or rhythm control. The other focus is on preventing stroke—a devastating outcome—with anticoagulation therapy.
For deciding whether to give warfarin to patients with atrial fibrillation, the six-point CHADS2 score is a crude but effective way of assessing the risk of stroke based on the following risk factors: congestive heart failure, hypertension, age 75 years or older, and diabetes (1 point each); or a history of stroke or transient ischemic attack (2 points).1 Warfarin is given if patients have a score of at least 2 points.
Warfarin has a narrow therapeutic window, with a higher risk of ischemic stroke if the international normalized ratio (INR) is less than 2.0,2 and a higher risk of intracranial hemorrhage if the INR is more than 3.0.3 Keeping the INR in the therapeutic range is difficult because of variations in diet, concurrent medications, and other factors.
The percent of time that the INR is within the therapeutic range predicts the risk of adverse events. Connolly et al4 showed that the cumulative risk of stroke, myocardial infarction, systemic embolism, or vascular death was no better with warfarin than with clopidogrel (Plavix) plus aspirin if the INR was in the therapeutic range less than 65% of the time, but the risk was significantly less if the INR was in the therapeutic range more than 65% of the time.
Also, comparing warfarin with the combination of aspirin and clopidogrel, Verheugt5 found that the rates of stroke of any kind, of disabling and fatal stroke, and of stroke per major bleed were lower in patients taking warfarin. Although many physicians prefer aspirin plus clopidogrel because of concerns about bleeding with warfarin, the rates of major bleeding were about the same in the two groups.
In a trial in patients for whom warfarin was “unsuitable,”6 the combination of aspirin plus clopidogrel was associated with a lower rate of stroke than aspirin alone (2.4% per year vs 3.3% per year, relative risk 0.762) but a higher rate of major bleeding events (2.0% per year vs 1.3% per year, relative risk 1.57).
NEW ALTERNATIVES TO WARFARIN
Because of the problems with warfarin, alternatives have been sought for many years. Several new oral anticoagulants are available or are being developed,7 including the factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis) and the direct factor II (thrombin) inhibitor dabigatran (Pradaxa) (Table 1).
Dabigatran’s advantages and drawbacks
Dabigatran has been on the market for more than a year and has gained rapid acceptance. The dosage is 150 mg twice a day, or 75 mg twice a day if renal function is impaired. Cleared by the kidneys, it has a half-life of 12 to 17 hours; 75% is cleared within 24 hours. For a patient who needs surgery that poses a low risk of bleeding, the general recommendation is to stop dabigatran the night before the surgical procedure. For operations with a greater risk of bleeding, many surgeons recommend stopping the drug 3 or 4 days before.
Advantages of dabigatran include that it is not influenced by diet and that the onset of therapeutic benefit is within 1 hour. Although some drugs affect dabigatran, drug interactions are more troublesome with warfarin.
A serious concern about dabigatran and the other new agents is that if a bleeding problem arises, the effects of these drugs are not reversible by administration of fresh frozen plasma. Dabigatran is reversible by dialysis; however, if a patient is also hypotensive, dialysis is not an option, and simply waiting for the drug to clear is the only choice.
Another drawback is that therapeutic levels cannot be monitored. If a patient taking warfarin requires cardioversion, the INR is carefully monitored for several weeks beforehand to reduce the risk of stroke. With dabigatran, there is no way to know if a patient is actually taking the drug as prescribed.
Clinical trials show that alternatives are marginally better than warfarin
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,8 dabigatran was associated with a significantly lower incidence of intracranial hemorrhage, combined strokes, and systemic embolization than warfarin. The incidence of major bleeds was slightly lower with dabigatran. Although dabigatran performed better, the differences were small and would not require patients to change from warfarin if they are already doing well.
Apixaban and rivaroxaban are other alternatives to warfarin, with different mechanisms of action and metabolism. Although rivaroxaban’s half-life is similar to that of apixaban and dabigatran, it is being marketed as allowing once-daily dosing instead of twice-daily.
Recent randomized controlled clinical trials of the new drugs include:
- The Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,9 which compared apixaban and aspirin
- The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin10
- The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),11 comparing rivaroxaban and warfarin
- RE-LY,8 comparing dabigatran and warfarin.
In the ARISTOTLE,10 ROCKET AF,11 and RE-LY trials,8 the time that the warfarin patients’ INRs were in the therapeutic range varied from 55% to 68%. This seems low and is a problem when trying to compare therapies, but is probably about as high as one can expect in the real world.
In AVERROES,9 the combined rate of stroke and embolism was higher with aspirin than with apixaban. In the other trials, the rates were slightly better with the new drugs than with warfarin, and the rates of major hemorrhage and hemorrhagic stroke were only slightly higher with warfarin than with the new drugs. Because the differences in benefits and risks are so small, the main advantage of the newer drugs will probably be for patients who have difficulty staying in the therapeutic INR range on warfarin.
RATE CONTROL VS RESTORATION OF SINUS RHYTHM
Evidence is insufficient to determine the risk of very-long-term asymptomatic atrial fibrillation in patients on appropriate anticoagulation. Rate control is an option for asymptomatic patients but provides no change in quality of life and no definitive reduction in the risk of stroke. The main argument for restoring normal sinus rhythm in patients with mild to moderate symptoms is that it improves exercise capacity. The need for anticoagulation persists when patients are converted to sinus rhythm because the risk of recurrent atrial fibrillation remains high.
For patients with symptomatic atrial fibrillation, rate control is sometimes achieved with beta-blockers or calcium channel blockers. Rate control may be augmented with the addition of digoxin, but when used alone digoxin generally does not control the rate of atrial fibrillation. However, in many cases of atrial fibrillation, symptoms are not rate-related, and cardioversion to normal sinus rhythm should be attempted. In such cases, the symptoms may be attributable to a loss of atrial transport function.
Patients with the following risk factors should be admitted to the hospital to start antiarrhythmic drugs:
- Borderline or a long QTc interval at baseline (> 450 msec)
- Treatment with dofetilide (Tikosyn) because of its effects on the QT interval
- Heart failure or poor left-ventricular function
- Sinus node dysfunction
- Significant atrioventricular conduction disease.
Selecting an antiarrhythmic drug
Any of the antiarrhythmic drugs listed in Table 2 can be used for a patient with lone atrial fibrillation (ie, not caused by underlying heart disease). The choice of drug should be determined by whether coronary artery disease or renal failure is present as well. Liver disease or chronic obstructive pulmonary disease also may affect this decision.
Benefits of dronedarone are mixed
In a randomized trial of dronedarone vs placebo in patients with atrial fibrillation, the rate of death and the rate of first hospitalization due to a cardiovascular event at 21 months were significantly lower with dronedarone.12 No difference was found between the two groups in the rate of death from all causes, but fewer people died of cardiovascular causes in the dronedarone group. More patients taking dronedarone developed bradycardia, QT-interval prolongation, nausea, diarrhea, rash, or a higher serum creatinine level. Gastrointestinal side effects are often a problem with dronedarone: 20% to 30% of patients cannot tolerate the drug.
Dronedarone may cause a small rise in creatinine, and although this effect should be monitored, by itself it should not be interpreted as impairment of renal function. In a study in healthy people,13 dronedarone caused a 10% to 15% increase in serum creatinine, but the glomerular filtration rate was unchanged, as were renal plasma flow and anion secretion.
Another trial, in patients with severe heart failure, found that patients taking dronedarone had higher rates of hospitalization and overall mortality, raising serious concern about the safety of this drug in patients with advanced heart failure.14
Singh et al15 pooled the data from two multicenter, randomized trials that compared dronedarone with placebo for maintaining sinus rhythm in patients with atrial fibrillation or flutter. The mean time to the recurrence of atrial fibrillation was 116 days with dronedarone and 53 days with placebo. Other trials also showed longer times to recurrence and lower recurrence rates with dronedarone. Although the differences were statistically significant, they may not be clinically meaningful for patients.
Dronedarone is structurally similar to amiodarone (Cordarone), but the two drugs work differently. A meta-analysis of clinical trials16 found that amiodarone recipients had a lower rate of recurrence of atrial fibrillation than did those receiving dronedarone.
Two safety warnings for dronedarone
In January 2011, the US Food and Drug Administration (FDA) issued an alert about cases of rare but severe liver injury in patients treated with dronedarone, including two cases of acute liver failure leading to liver transplantation.17
The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)18 compared dronedarone and placebo in patients with permanent atrial fibrillation. More people died or had serious cardiovascular adverse events in the dronedarone group. The study was stopped early after data monitoring showed that rates of death, stroke, and hospitalization for heart failure were two times higher in patients receiving dronedarone. This prompted the FDA to issue another safety alert in July 2011.
Interestingly, the PALLAS study did not set out to determine whether dronedarone controls atrial fibrillation, as the study patients had long-standing, persistent atrial fibrillation. The study was designed only to determine if the drug reduces the rate of adverse events; it clearly does not, and the study shows that dronedarone should not be used to control the heart rate in patients with persistent atrial fibrillation. Instead, its use is best restricted to patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
ABLATION OF ATRIAL FIBRILLATION
Another way to try to restore sinus rhythm is to destroy or isolate the area that is generating the abnormal beats via a catheter-based procedure.
Radiofrequency ablation is generally tried in patients in whom one or two drugs have failed to control atrial fibrillation. Direct comparisons show that ablation is superior to drug therapy and is effective in about 75% of patients with paroxysmal atrial fibrillation vs 20% to 40% of patients on drug therapy. Ablation plus drug therapy is often more effective than either treatment alone.
Mechanisms of atrial fibrillation and ablation
In many cases, atrial fibrillation is stimulated by vagal and sympathetic inputs to the atrium that enter around the pulmonary veins and trigger electrical activations in the area, generating spiraling, reentering circuits. Focal atrial fibrillation also originates predominantly in the pulmonary veins. Ablation of tissue widely circumscribing the mouth of the pulmonary veins prevents the electrical signal from exiting into the atrium.
In about 11% to 37% of cases, atrial fibrillation originates elsewhere, eg, in the left atrium, in the superior vena cava, or in the vein of Marshall. Techniques have evolved to also ablate these regions.
Anticoagulation therapy is recommended before the procedure, and patients at low risk should continue it for a minimum of 2 months afterward. Patients with a higher CHADS2 score should receive anticoagulation therapy for at least 1 year. The consensus statement by the Heart Rhythm Society19 recommends that patients remain on warfarin or one of the newer anticoagulants if their CHADS2 score is 2 or higher. This is because patients have a significant risk of recurrence of atrial fibrillation after radiofrequency ablation, so if their stroke risk is high they should remain on anticoagulant therapy.
Ablation is usually effective, but it carries rare but serious risks
The efficacy of a single radiofrequency ablation procedure is in the range of 60% to 80% for paroxysmal atrial fibrillation and 40% to 60% for persistent atrial fibrillation. The Second International Ablation Registry20 shows a success rate of about 75% in patients with paroxysmal atrial fibrillation and about 65% in patients with persistent and permanent atrial fibrillation. Registry data are often more favorable because reporting is optional, but these results are consistent with those from experienced medical centers. Rates of suppression of atrial fibrillation are higher in patients who also take antiarrhythmic drugs, making a “hybrid” approach useful when ablation alone fails.
According to a worldwide survey, the risk of serious complications is 4.5%. These include stroke (0.23%), tamponade (1.3%), and pulmonary vein stenosis (< 0.29%). The esophagus lies just behind the right atrium, and burning through and creating a fistula between them occurs in about 0.04% of cases and is almost uniformly fatal.20
A second ablation procedure is sometimes indicated for the recurrence of atrial fibrillation, which is almost always caused by recovery of the pulmonary veins. Bhargava et al21 found that the success rate at Cleveland Clinic for a single procedure for paroxysmal atrial fibrillation was 77%, and that it was 92% after a repeat procedure. For persistent atrial fibrillation, success rates were 76% after the first procedure and 90% after the second. Even for long-standing persistent atrial fibrillation (ie, lasting more than 1 year), 80% success was achieved after two procedures. Patients who are less likely to have a successful ablation procedure are those with long-standing atrial fibrillation and coexisting heart disease, including severe valvular disease, although mitral regurgitation sometimes improves if sinus rhythm can be maintained.
The need for a second procedure
After ablation, patients should be closely monitored for a recurrence of atrial fibrillation. Continuous monitoring with implantable cardiac monitor loop recorders can detect unrecognized episodes of arrhythmia. Long-term follow-up is also required to track outcomes and quality of life.
According to the Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation,19 atrial fibrillation recurs after ablation in about 35% to 60% of patients in the first 3 months, with recurrence rates after 1 year ranging from 5% to 16%. The rate of success is determined by the skill of the surgeon, underlying heart disease, attention to follow-up, and how success is defined.
Freedom from recurrence early on is a good predictor that late recurrence is unlikely. Patients who only have a very early recurrence (within the first 4 weeks) are more likely to have long-term freedom from atrial fibrillation tha those who have recurrences after that time.22
In a study of 831 patients, Hussein et al23 found recurrence rates of 24% between months 3 to 13 following ablation and 9% after 12 months. At 55 months, 79% were free from atrial fibrillation without drugs, 11% were free of atrial fibrillation with medications, and 5% had refractory atrial fibrillation.
Recurrence—whether early or late—was more likely to occur in people with persistent vs paroxysmal atrial fibrillation. Other risk factors for late recurrence included older age and larger left atrial size (which is also a risk factor for recurrence on drug therapy). Although recurrent arrhythmia was most often atrial fibrillation, atrial flutter also occurred frequently (in 27% of patients with late recurrence). Three patients (4% of patients with late recurrence) developed atrial tachycardia.23
In patients with early recurrence, 81% underwent repeat ablation, all of whom had recovery of one or more pulmonary veins. After the second ablation, 21% had recurrence, 65% of whom were controlled by medications.23
Whether a patient should undergo subsequent ablation procedures depends on the severity of symptoms, the likelihood of success (based on an educated guess), and the patient’s willingness to undergo another procedure.
ATRIAL APPENDAGE OCCLUSION DEVICE UNDER INVESTIGATION
New devices are being investigated that occlude the left atrial appendage to try to prevent embolization.
The Watchman device, resembling an umbrella, is implanted via a percutaneous catheter in the left atrial appendage, closing it off to preclude a thrombus from forming in the appendage and embolizing to the body. Clinical trials showed that patients who received a device had a slightly lower risk of stroke than otherwise seen in clinical practice.24 Safety and efficacy are still being determined.
The device cannot be deployed in a patient with an existing thrombus because of the danger of dislodging the thrombus, allowing it to embolize.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Connolly SJ, Pogue J, Eikelboom J, et al; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008; 118:2029–2037.
- Verheugt FWA. Who is ineligible for warfarin in atrial fibrillation? Lancet 2009; 374:510–511.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Harenberg J. New anticoagulants in atrial fibrillation. Semin Thromb Hemost 2009; 35:574–585.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Connolly SJ, Eikelboom J, Joyner C, et al; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJV, et al; for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med August 28, 2011; 10.1056/nejmoa1107039.
- Patel MR, Mahaffey KW, Garg J, et al; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Hohnloser SH, Crijns HJ, van Eickels M, et al; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:688–678.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Kóber L, Torp-Pederson C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol 2009; 54:1089–1095.
- US Food and Drug Administration. FDA drug safety communication: severe liver injury associated with the use of dronedarone (marketed as Multaq). http://www.fda.gov/drugs/drugsafety/ucm240011.htm. Accessed July 5, 2012.
- Connolly SJ, Camm AJ, Halperin JL, et al; for the PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011; 365:2268–2276.
- HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm 2007; 4:1–46.
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3:32–38.
- Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm 2009; 6:1403–1412.
- Themistoclakis S, Schweikert RA, Sliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008; 5:679–685.
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4:271–278.
- Holmes DR, Reddy VY, Turi ZG, et al; for the PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Connolly SJ, Pogue J, Eikelboom J, et al; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008; 118:2029–2037.
- Verheugt FWA. Who is ineligible for warfarin in atrial fibrillation? Lancet 2009; 374:510–511.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Harenberg J. New anticoagulants in atrial fibrillation. Semin Thromb Hemost 2009; 35:574–585.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Connolly SJ, Eikelboom J, Joyner C, et al; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJV, et al; for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med August 28, 2011; 10.1056/nejmoa1107039.
- Patel MR, Mahaffey KW, Garg J, et al; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Hohnloser SH, Crijns HJ, van Eickels M, et al; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:688–678.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Kóber L, Torp-Pederson C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol 2009; 54:1089–1095.
- US Food and Drug Administration. FDA drug safety communication: severe liver injury associated with the use of dronedarone (marketed as Multaq). http://www.fda.gov/drugs/drugsafety/ucm240011.htm. Accessed July 5, 2012.
- Connolly SJ, Camm AJ, Halperin JL, et al; for the PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011; 365:2268–2276.
- HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm 2007; 4:1–46.
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3:32–38.
- Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm 2009; 6:1403–1412.
- Themistoclakis S, Schweikert RA, Sliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008; 5:679–685.
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4:271–278.
- Holmes DR, Reddy VY, Turi ZG, et al; for the PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
KEY POINTS
- Warfarin is as safe as—and more effective than—the combination of aspirin and clopidogrel (Plavix) if the international normalized ratio is in the therapeutic range 65% of the time or more.
- New anticoagulants are promising alternatives to warfarin, but they also pose risks. Patients who are doing well on warfarin need not change.
- Several antiarrhythmic drugs are available to control symptomatic atrial fibrillation. Dronedarone (Multaq) should only be considered for patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
- Ablation is often effective in controlling atrial fibrillation, but recurrence is common. Early recurrence often subsides, but late recurrence often requires a repeat procedure.