User login
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
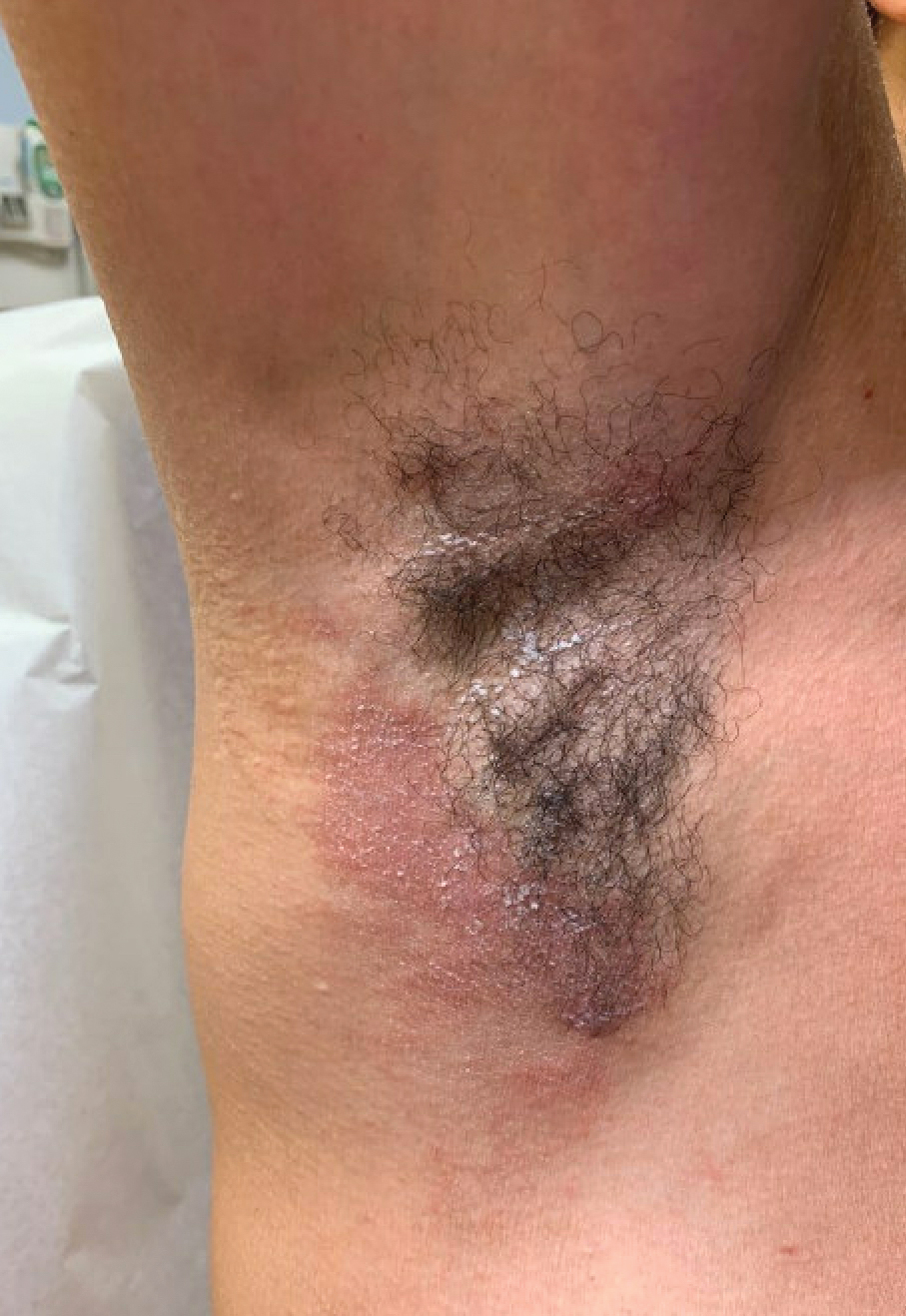
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34
Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58
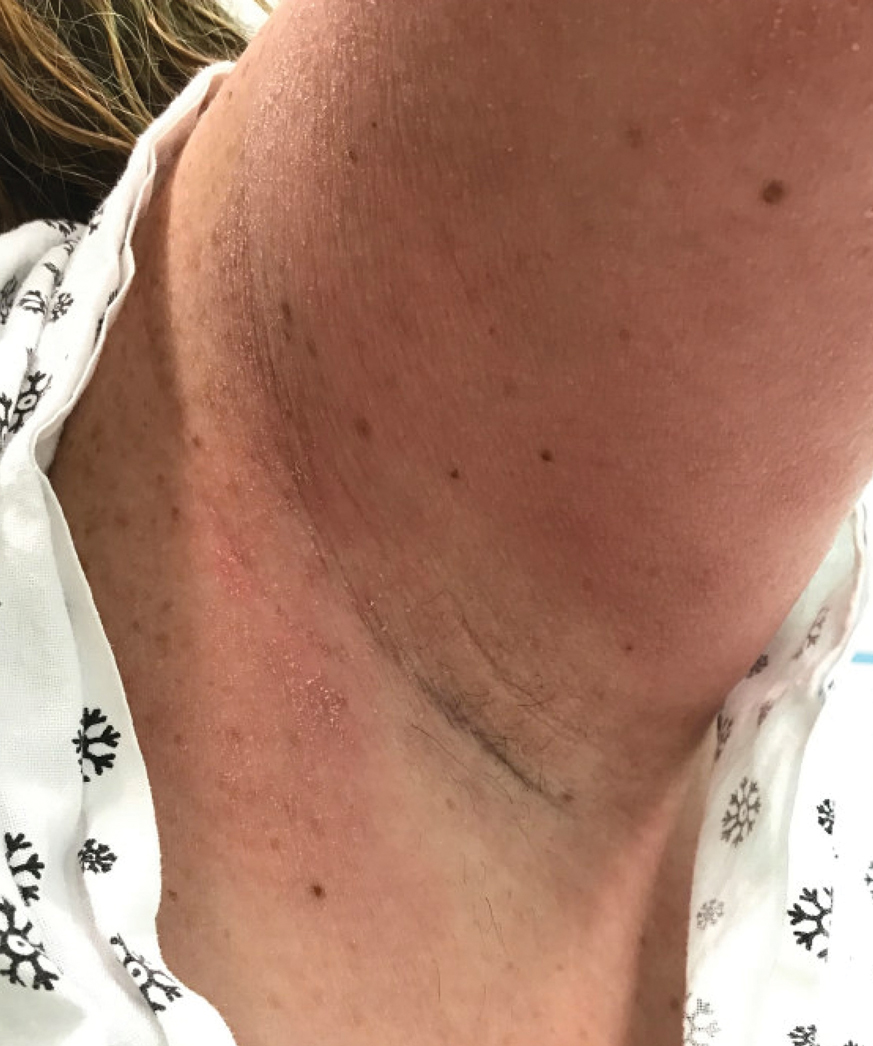
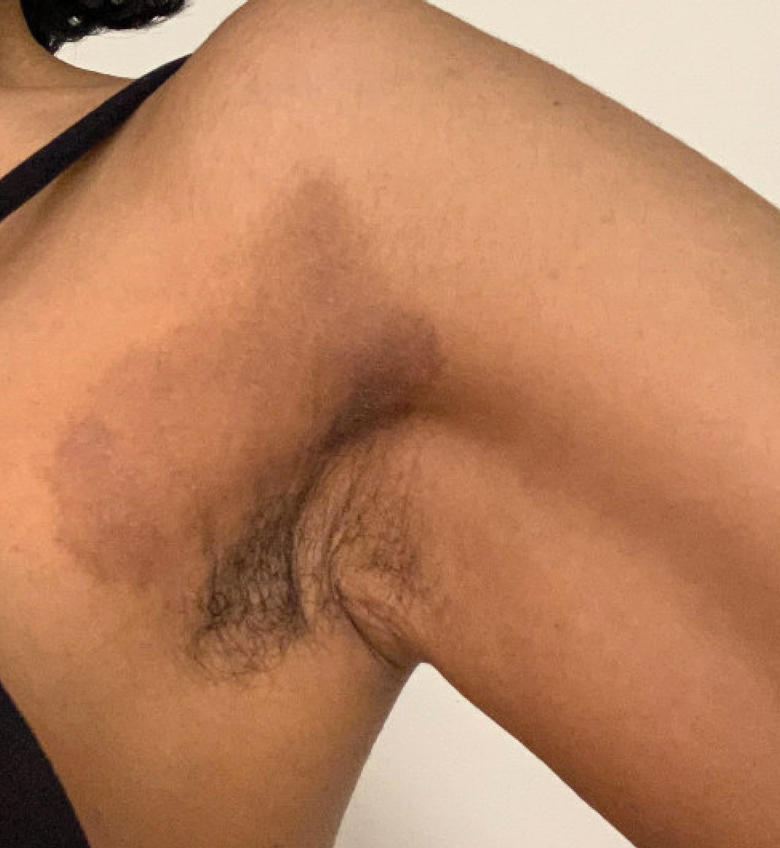
Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65
Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
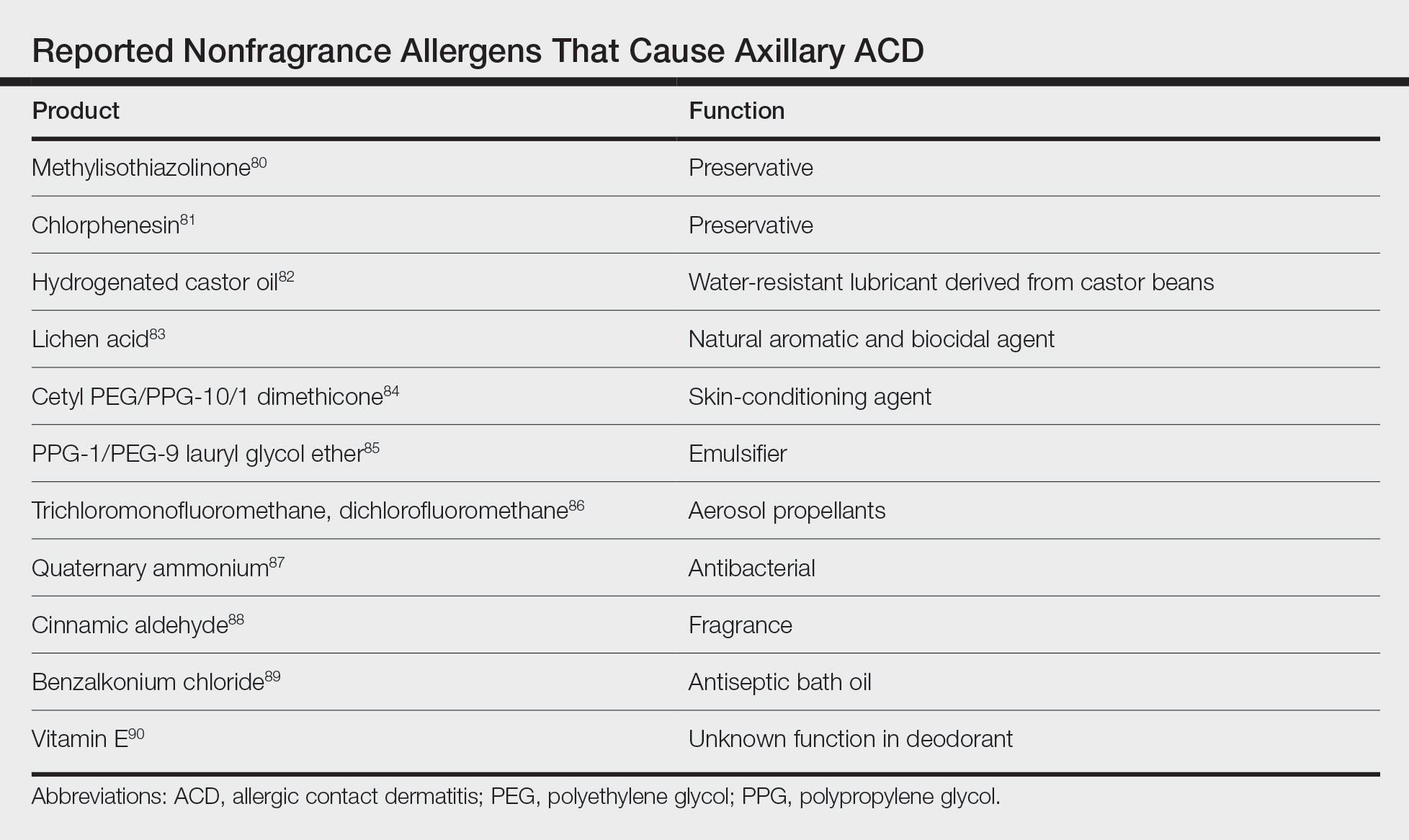
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92
Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Practice Points
- The differential diagnosis of axillary dermatitis is broad. Contact dermatitis—both irritant and allergic—represents common etiologies.
- Understanding the clinical features and range of potential sources in axillary contact dermatitis allows for efficient recognition and elimination of causative exposure.
- For cases of suspected allergic contact dermatitis, patch testing and subsequent allergen avoidance are paramount in the management of axillary eruptions.