User login
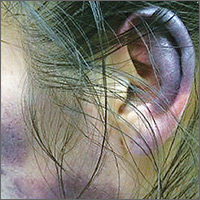
Over the course of a month, this 34-year-old woman had sought care at our facility—and another—on 3 separate occasions for painful bruises (visits #1 and #3) and deep vein thrombosis (DVT; visit #2). The bruises first appeared acutely on her arms (FIGURE 1A), prompting her first visit to our ED and leading to a hospital stay. Several weeks later, the patient developed new bruise-like lesions on her earlobes (FIGURE 1B), face, trunk, and lower extremities. In between these 2 visits, the patient was seen in another ED (and admitted) for right upper extremity DVT and was started on enoxaparin, followed by warfarin.
The patient had no history of trauma, but did have a 7-year history of cocaine abuse. The initial bruises appeared one week after using cocaine from a different dealer.
On her most recent visit, her vitals and physical examination were unremarkable, apart from the skin findings. Her complete blood count, complete metabolic panel, and urinalysis were unremarkable. On her previous admissions, the patient’s urine drug test had been positive for cocaine. She’d also tested positive for cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anticardiolipin IgM.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Levamisole-induced cutaneous vasculopathy
The patient was given a diagnosis of levamisole-induced cutaneous vasculopathy based on her history of cocaine use; the typical, painful palpable purpura with angulated borders and a necrotic center (retiform purpura); and positive immunologic markers.1-5 DVT has also been reported in association with levamisole-induced vasculopathy.6
Intended for livestock. Levamisole is a pharmaceutical agent typically used as an anthelmintic in livestock, but, since 2007, it has increasingly been found as an adulterant in cocaine.7 According to the Drug Enforcement Administration, 71% of cocaine tested in 2009 contained levamisole.7
Experts speculate that levamisole is used in the production process of cocaine to increase its volume and enhance its psychoactive effects.1-4 In humans, levamisole’s immunomodulatory properties were once used to treat various cancers and immunologic conditions, but it was withdrawn from the US market in 2000 due to adverse effects.1,2,4 Severe adverse reactions associated with levamisole include agranulocytosis, vascular occlusive disease, and thrombotic vasculopathy with or without vasculitis.1,2
The incidence of levamisole-induced cutaneous vasculopathy is unknown. That said, it’s important to suspect the condition in cocaine users who present with retiform purpura. Earlobe lesions are characteristic, while involvement of other areas is variable.2-4
Differential includes other causes of purpura
A number of conditions make up the differential. While each of these presents with areas of skin necrosis or purpura, a thorough history can be revealing. Factors such as substance abuse, recent treatment with a vitamin K antagonist, or even a recent infection can be the key to making the diagnosis.
Warfarin-induced skin necrosis presents with large, irregular bullae that eventually become necrotic. It appears one to 10 days after treatment with a vitamin K antagonist and typically affects areas of the body with greater subcutaneous adipose tissue, including the breasts, thighs, buttocks, and penis. Microscopic examination reveals bland thrombi with no inflammation of the vessel wall.5
ANCA-associated small-vessel vasculitis includes microscopic polyangiitis, Wegner’s granulomatosis, and Churg-Strauss syndrome. With these conditions, palpable purpuras are more commonly seen in areas that are dependent on, or affected by, venous stasis. Microscopic evaluation reveals leukocytoclastic vasculitis. Perinuclear ANCA is more often positive than c-ANCA.5
Purpura fulminans is a medical emergency characterized by skin necrosis and disseminated intravascular coagulation. It can rapidly lead to multi-organ failure. Microscopic evaluation reveals thrombotic occlusion of small- and medium-sized vessels. It affects neonates and children, and is associated with severe sepsis or an autoimmune response to an otherwise benign childhood infection. It may also be a symptom of hereditary protein C or protein S deficiency.8
Cholesterol emboli are more common in men ages 50 and older with atherosclerotic disease, hypertension, or tobacco use. Abrupt onset of livedo reticularis may be followed by retiform purpura, ulcers, nodules, and gangrene. Lesions most often appear on the distal lower extremities and buttocks. Systemic symptoms may include fever, weight loss, myalgia, and altered mental status. Multiple organ systems may be involved. Frozen sections reveal needle-shaped clefts and doubly-refractile crystals.5
Evolving skin lesions, relevant lab findings
Initially, patients with levamisole-induced cutaneous vasculopathy will develop painful, palpable purpura with angulated borders and a necrotic center. The lesions can progress, though, to bullae, necrosis, or eschar formation.1,3,4
Patients with this condition frequently test positive for ANCA and, even more frequently, for c-ANCA, ANA, antiphospholipid antibodies, leukopenia, and neutropenia. Other immunologic markers for which patients may test positive include anti-cardiolipin antibodies, lupus anticoagulant, anti-dsDNA, myeloperoxidase, and anti-Sjögren’s-syndrome-related antigen A (also known as anti-Ro) antibodies.1-4
Natural progression of levamisole-induced cutaneous vasculopathy is generally benign. Most clinical signs and symptoms—as well as serologic manifestations—resolve without intervention after cessation of cocaine use. However, signs and symptoms may recur with subsequent exposure.
There is no specific therapy for levamisole-induced cutaneous vasculopathy, but prednisone and other immunosuppressive agents can be used in patients with severe or systemic symptoms.1-4 Necrotic lesions and eschar formation may be complicated by infection and require debridement and/or skin grafts.
Our patient was discharged after a brief hospital stay, as she had no indication of systemic involvement and no new or worsening skin lesions. She was given wound care instructions and advised to stop using cocaine. The patient was counseled at bedside by a physician and given information on community resources (outpatient treatment, support groups, etc) by social services. However, the patient continued to use the substance and had several readmissions with worsening skin lesions complicated by secondary bacterial infection. She did not have systemic complications, but required antibiotics, multiple wound debridement sessions, and subsequent skin grafts.
CORRESPONDENCE
Yu Wah, MD, ABIHM, University of Texas Health Science Center at Houston, 6431 Fannin Street, Suite JJL 308, Houston, TX 77030; [email protected].
1. Gaertner EM, Switlyk SA. Dermatologic complications from levamisole-contaminated cocaine: a case report and review of the literature. Cutis. 2014;93:102-106.
2. Strazzula L, Brown KK, Brieva JC, et al. Levamisole toxicity mimicking autoimmune disease. J Am Acad Dermatol. 2013;69:954-959.
3. Espinoza LR, Perez Alamino R. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rheumatol Rep. 2012;14:532-538.
4. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia–a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
5. Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172.
6. Wilson L, Hull C, Petersen M, et al. End organ damage in levamisole adulterated cocaine: More than just purpura and agranulocytosis. J Am Acad Dermatol. 2013;68:AB9.
7. US Department of Justice. National Drug Threat Assessment 2010. Impact of drugs on society. Available at: http://www.justice.gov/archive/ndic/pubs38/38661/drugImpact.htm. Accessed July 26, 2017.
8. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071.
Over the course of a month, this 34-year-old woman had sought care at our facility—and another—on 3 separate occasions for painful bruises (visits #1 and #3) and deep vein thrombosis (DVT; visit #2). The bruises first appeared acutely on her arms (FIGURE 1A), prompting her first visit to our ED and leading to a hospital stay. Several weeks later, the patient developed new bruise-like lesions on her earlobes (FIGURE 1B), face, trunk, and lower extremities. In between these 2 visits, the patient was seen in another ED (and admitted) for right upper extremity DVT and was started on enoxaparin, followed by warfarin.
The patient had no history of trauma, but did have a 7-year history of cocaine abuse. The initial bruises appeared one week after using cocaine from a different dealer.
On her most recent visit, her vitals and physical examination were unremarkable, apart from the skin findings. Her complete blood count, complete metabolic panel, and urinalysis were unremarkable. On her previous admissions, the patient’s urine drug test had been positive for cocaine. She’d also tested positive for cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anticardiolipin IgM.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Levamisole-induced cutaneous vasculopathy
The patient was given a diagnosis of levamisole-induced cutaneous vasculopathy based on her history of cocaine use; the typical, painful palpable purpura with angulated borders and a necrotic center (retiform purpura); and positive immunologic markers.1-5 DVT has also been reported in association with levamisole-induced vasculopathy.6
Intended for livestock. Levamisole is a pharmaceutical agent typically used as an anthelmintic in livestock, but, since 2007, it has increasingly been found as an adulterant in cocaine.7 According to the Drug Enforcement Administration, 71% of cocaine tested in 2009 contained levamisole.7
Experts speculate that levamisole is used in the production process of cocaine to increase its volume and enhance its psychoactive effects.1-4 In humans, levamisole’s immunomodulatory properties were once used to treat various cancers and immunologic conditions, but it was withdrawn from the US market in 2000 due to adverse effects.1,2,4 Severe adverse reactions associated with levamisole include agranulocytosis, vascular occlusive disease, and thrombotic vasculopathy with or without vasculitis.1,2
The incidence of levamisole-induced cutaneous vasculopathy is unknown. That said, it’s important to suspect the condition in cocaine users who present with retiform purpura. Earlobe lesions are characteristic, while involvement of other areas is variable.2-4
Differential includes other causes of purpura
A number of conditions make up the differential. While each of these presents with areas of skin necrosis or purpura, a thorough history can be revealing. Factors such as substance abuse, recent treatment with a vitamin K antagonist, or even a recent infection can be the key to making the diagnosis.
Warfarin-induced skin necrosis presents with large, irregular bullae that eventually become necrotic. It appears one to 10 days after treatment with a vitamin K antagonist and typically affects areas of the body with greater subcutaneous adipose tissue, including the breasts, thighs, buttocks, and penis. Microscopic examination reveals bland thrombi with no inflammation of the vessel wall.5
ANCA-associated small-vessel vasculitis includes microscopic polyangiitis, Wegner’s granulomatosis, and Churg-Strauss syndrome. With these conditions, palpable purpuras are more commonly seen in areas that are dependent on, or affected by, venous stasis. Microscopic evaluation reveals leukocytoclastic vasculitis. Perinuclear ANCA is more often positive than c-ANCA.5
Purpura fulminans is a medical emergency characterized by skin necrosis and disseminated intravascular coagulation. It can rapidly lead to multi-organ failure. Microscopic evaluation reveals thrombotic occlusion of small- and medium-sized vessels. It affects neonates and children, and is associated with severe sepsis or an autoimmune response to an otherwise benign childhood infection. It may also be a symptom of hereditary protein C or protein S deficiency.8
Cholesterol emboli are more common in men ages 50 and older with atherosclerotic disease, hypertension, or tobacco use. Abrupt onset of livedo reticularis may be followed by retiform purpura, ulcers, nodules, and gangrene. Lesions most often appear on the distal lower extremities and buttocks. Systemic symptoms may include fever, weight loss, myalgia, and altered mental status. Multiple organ systems may be involved. Frozen sections reveal needle-shaped clefts and doubly-refractile crystals.5
Evolving skin lesions, relevant lab findings
Initially, patients with levamisole-induced cutaneous vasculopathy will develop painful, palpable purpura with angulated borders and a necrotic center. The lesions can progress, though, to bullae, necrosis, or eschar formation.1,3,4
Patients with this condition frequently test positive for ANCA and, even more frequently, for c-ANCA, ANA, antiphospholipid antibodies, leukopenia, and neutropenia. Other immunologic markers for which patients may test positive include anti-cardiolipin antibodies, lupus anticoagulant, anti-dsDNA, myeloperoxidase, and anti-Sjögren’s-syndrome-related antigen A (also known as anti-Ro) antibodies.1-4
Natural progression of levamisole-induced cutaneous vasculopathy is generally benign. Most clinical signs and symptoms—as well as serologic manifestations—resolve without intervention after cessation of cocaine use. However, signs and symptoms may recur with subsequent exposure.
There is no specific therapy for levamisole-induced cutaneous vasculopathy, but prednisone and other immunosuppressive agents can be used in patients with severe or systemic symptoms.1-4 Necrotic lesions and eschar formation may be complicated by infection and require debridement and/or skin grafts.
Our patient was discharged after a brief hospital stay, as she had no indication of systemic involvement and no new or worsening skin lesions. She was given wound care instructions and advised to stop using cocaine. The patient was counseled at bedside by a physician and given information on community resources (outpatient treatment, support groups, etc) by social services. However, the patient continued to use the substance and had several readmissions with worsening skin lesions complicated by secondary bacterial infection. She did not have systemic complications, but required antibiotics, multiple wound debridement sessions, and subsequent skin grafts.
CORRESPONDENCE
Yu Wah, MD, ABIHM, University of Texas Health Science Center at Houston, 6431 Fannin Street, Suite JJL 308, Houston, TX 77030; [email protected].
Over the course of a month, this 34-year-old woman had sought care at our facility—and another—on 3 separate occasions for painful bruises (visits #1 and #3) and deep vein thrombosis (DVT; visit #2). The bruises first appeared acutely on her arms (FIGURE 1A), prompting her first visit to our ED and leading to a hospital stay. Several weeks later, the patient developed new bruise-like lesions on her earlobes (FIGURE 1B), face, trunk, and lower extremities. In between these 2 visits, the patient was seen in another ED (and admitted) for right upper extremity DVT and was started on enoxaparin, followed by warfarin.
The patient had no history of trauma, but did have a 7-year history of cocaine abuse. The initial bruises appeared one week after using cocaine from a different dealer.
On her most recent visit, her vitals and physical examination were unremarkable, apart from the skin findings. Her complete blood count, complete metabolic panel, and urinalysis were unremarkable. On her previous admissions, the patient’s urine drug test had been positive for cocaine. She’d also tested positive for cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anticardiolipin IgM.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Levamisole-induced cutaneous vasculopathy
The patient was given a diagnosis of levamisole-induced cutaneous vasculopathy based on her history of cocaine use; the typical, painful palpable purpura with angulated borders and a necrotic center (retiform purpura); and positive immunologic markers.1-5 DVT has also been reported in association with levamisole-induced vasculopathy.6
Intended for livestock. Levamisole is a pharmaceutical agent typically used as an anthelmintic in livestock, but, since 2007, it has increasingly been found as an adulterant in cocaine.7 According to the Drug Enforcement Administration, 71% of cocaine tested in 2009 contained levamisole.7
Experts speculate that levamisole is used in the production process of cocaine to increase its volume and enhance its psychoactive effects.1-4 In humans, levamisole’s immunomodulatory properties were once used to treat various cancers and immunologic conditions, but it was withdrawn from the US market in 2000 due to adverse effects.1,2,4 Severe adverse reactions associated with levamisole include agranulocytosis, vascular occlusive disease, and thrombotic vasculopathy with or without vasculitis.1,2
The incidence of levamisole-induced cutaneous vasculopathy is unknown. That said, it’s important to suspect the condition in cocaine users who present with retiform purpura. Earlobe lesions are characteristic, while involvement of other areas is variable.2-4
Differential includes other causes of purpura
A number of conditions make up the differential. While each of these presents with areas of skin necrosis or purpura, a thorough history can be revealing. Factors such as substance abuse, recent treatment with a vitamin K antagonist, or even a recent infection can be the key to making the diagnosis.
Warfarin-induced skin necrosis presents with large, irregular bullae that eventually become necrotic. It appears one to 10 days after treatment with a vitamin K antagonist and typically affects areas of the body with greater subcutaneous adipose tissue, including the breasts, thighs, buttocks, and penis. Microscopic examination reveals bland thrombi with no inflammation of the vessel wall.5
ANCA-associated small-vessel vasculitis includes microscopic polyangiitis, Wegner’s granulomatosis, and Churg-Strauss syndrome. With these conditions, palpable purpuras are more commonly seen in areas that are dependent on, or affected by, venous stasis. Microscopic evaluation reveals leukocytoclastic vasculitis. Perinuclear ANCA is more often positive than c-ANCA.5
Purpura fulminans is a medical emergency characterized by skin necrosis and disseminated intravascular coagulation. It can rapidly lead to multi-organ failure. Microscopic evaluation reveals thrombotic occlusion of small- and medium-sized vessels. It affects neonates and children, and is associated with severe sepsis or an autoimmune response to an otherwise benign childhood infection. It may also be a symptom of hereditary protein C or protein S deficiency.8
Cholesterol emboli are more common in men ages 50 and older with atherosclerotic disease, hypertension, or tobacco use. Abrupt onset of livedo reticularis may be followed by retiform purpura, ulcers, nodules, and gangrene. Lesions most often appear on the distal lower extremities and buttocks. Systemic symptoms may include fever, weight loss, myalgia, and altered mental status. Multiple organ systems may be involved. Frozen sections reveal needle-shaped clefts and doubly-refractile crystals.5
Evolving skin lesions, relevant lab findings
Initially, patients with levamisole-induced cutaneous vasculopathy will develop painful, palpable purpura with angulated borders and a necrotic center. The lesions can progress, though, to bullae, necrosis, or eschar formation.1,3,4
Patients with this condition frequently test positive for ANCA and, even more frequently, for c-ANCA, ANA, antiphospholipid antibodies, leukopenia, and neutropenia. Other immunologic markers for which patients may test positive include anti-cardiolipin antibodies, lupus anticoagulant, anti-dsDNA, myeloperoxidase, and anti-Sjögren’s-syndrome-related antigen A (also known as anti-Ro) antibodies.1-4
Natural progression of levamisole-induced cutaneous vasculopathy is generally benign. Most clinical signs and symptoms—as well as serologic manifestations—resolve without intervention after cessation of cocaine use. However, signs and symptoms may recur with subsequent exposure.
There is no specific therapy for levamisole-induced cutaneous vasculopathy, but prednisone and other immunosuppressive agents can be used in patients with severe or systemic symptoms.1-4 Necrotic lesions and eschar formation may be complicated by infection and require debridement and/or skin grafts.
Our patient was discharged after a brief hospital stay, as she had no indication of systemic involvement and no new or worsening skin lesions. She was given wound care instructions and advised to stop using cocaine. The patient was counseled at bedside by a physician and given information on community resources (outpatient treatment, support groups, etc) by social services. However, the patient continued to use the substance and had several readmissions with worsening skin lesions complicated by secondary bacterial infection. She did not have systemic complications, but required antibiotics, multiple wound debridement sessions, and subsequent skin grafts.
CORRESPONDENCE
Yu Wah, MD, ABIHM, University of Texas Health Science Center at Houston, 6431 Fannin Street, Suite JJL 308, Houston, TX 77030; [email protected].
1. Gaertner EM, Switlyk SA. Dermatologic complications from levamisole-contaminated cocaine: a case report and review of the literature. Cutis. 2014;93:102-106.
2. Strazzula L, Brown KK, Brieva JC, et al. Levamisole toxicity mimicking autoimmune disease. J Am Acad Dermatol. 2013;69:954-959.
3. Espinoza LR, Perez Alamino R. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rheumatol Rep. 2012;14:532-538.
4. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia–a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
5. Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172.
6. Wilson L, Hull C, Petersen M, et al. End organ damage in levamisole adulterated cocaine: More than just purpura and agranulocytosis. J Am Acad Dermatol. 2013;68:AB9.
7. US Department of Justice. National Drug Threat Assessment 2010. Impact of drugs on society. Available at: http://www.justice.gov/archive/ndic/pubs38/38661/drugImpact.htm. Accessed July 26, 2017.
8. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071.
1. Gaertner EM, Switlyk SA. Dermatologic complications from levamisole-contaminated cocaine: a case report and review of the literature. Cutis. 2014;93:102-106.
2. Strazzula L, Brown KK, Brieva JC, et al. Levamisole toxicity mimicking autoimmune disease. J Am Acad Dermatol. 2013;69:954-959.
3. Espinoza LR, Perez Alamino R. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rheumatol Rep. 2012;14:532-538.
4. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia–a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
5. Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172.
6. Wilson L, Hull C, Petersen M, et al. End organ damage in levamisole adulterated cocaine: More than just purpura and agranulocytosis. J Am Acad Dermatol. 2013;68:AB9.
7. US Department of Justice. National Drug Threat Assessment 2010. Impact of drugs on society. Available at: http://www.justice.gov/archive/ndic/pubs38/38661/drugImpact.htm. Accessed July 26, 2017.
8. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071.