User login
Cardiac tamponade is a life-threatening condition that can be palliated or cured, depending on its cause and on the timeliness of treatment. Making a timely diagnosis and providing the appropriate treatment can be gratifying for both patient and physician.
Cardiac tamponade occurs when fluid in the pericardial space reaches a pressure exceeding central venous pressure. This leads to jugular venous distention, visceral organ engorgement, edema, and elevated pulmonary venous pressure that causes dyspnea. Despite compensatory tachycardia, the decrease in cardiac filling leads to a fall in cardiac output and to arterial hypoperfusion of vital organs.
PEARL 1: SLOW ACCUMULATION LEADS TO EDEMA
The rate at which pericardial fluid accumulates influences the clinical presentation of cardiac tamponade, in particular whether or not there is edema. Whereas rapid accumulation is characterized more by hypotension than by edema, the slow accumulation of pericardial fluid affords the patient time to drink enough liquid to keep the central venous pressure higher than the rising pericardial pressure. Thus, edema and dyspnea are more prominent features of cardiac tamponade when there is a slow rise in pericardial pressure.
PEARL 2: EDEMA IS NOT ALWAYS TREATED WITH A DIURETIC
Edema is not always treated with a diuretic. In a patient who has a pericardial effusion that has developed slowly and who has been drinking enough fluid to keep the central venous pressure higher than the pericardial pressure, a diuretic can remove enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure and thus convert a benign pericardial effusion to potentially lethal cardiac tamponade.
One must understand the cause of edema or low urine output before treating it. This underscores the importance of the history and the physical examination. All of the following must be assessed:
- Symptoms and time course of the illness
- Concurrent medical illnesses
- Neck veins
- Blood pressure and its response to inspiration
- Heart sounds
- Heart rate and rhythm
- Abdominal organ engorgement
- Edema (or its absence).
PEARL 3: UNDERSTANDING THE CAUSE IS ESSENTIAL
Understanding the cause of cardiac tamponade is essential.
A trauma patient first encountered in the emergency department may have an underlying disease, but the focus is squarely on the effects of trauma or violent injury. In a patient with multiple trauma, hypotension and tachycardia that do not respond to intravenous volume replacement when there is an obvious rise in central venous pressure should be clues to cardiac tamponade.1
If the patient has recently undergone a cardiac procedure (for example, cardiac surgery, myocardial biopsy, coronary intervention, electrophysiologic study with intracardiac electrodes, transvenous pacemaker placement, pacemaker lead extraction, or radiofrequency ablation), knowing about the procedure narrows the differential diagnosis when hypotension, tachycardia, and jugular venous distention develop.
PEARL 4: CARDIAC OR AORTIC RUPTURE REQUIRES SURGERY
When the etiology of cardiac tamponade is cardiac or aortic rupture, the treatment is surgical.
Painful sudden causes of cardiac tamponade include hemopericardium due to rupture of the free wall after myocardial infarction, and spontaneous or posttraumatic dissection and rupture of the ascending aorta. Prompt diagnosis is necessary, but since these lesions will not close and heal spontaneously, the definitive treatment should be surgery. Moreover, needle removal of intrapericardial blood that has been opposing further bleeding is sure to permit bleeding to recur, often with lethal consequences.2
Causes of cardiac tamponade that have a less-acute onset are likely to be complications of medical problems. Medical illnesses known to be associated with cardiac tamponade include:
- Infectious disease (idiopathic or viral, associated with smallpox vaccination, mycobacterial, purulent bacterial, fungal)
- Metastatic cancer (lung, breast, esophagus, lymphoma, pancreas, liver, leukemia, stomach, melanoma)3
- Connective tissue disease (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, scleroderma, Wegener granulomatosis, acute rheumatic fever)
- Endocrine disease (hypothyroidism)
- Drug side effects (procainamide, isoniazid, hydralazine, minoxidil, phenytoin, anticoagulants, methysergide)
- Inflammatory bowel disease (Crohn disease, ulcerative colitis)
- Congestive heart failure
- Uremia
- Radiation therapy
- Postmyocardial infarction syndrome (Dressler syndrome)
- Postpericardiotomy syndrome.
PEARL 5: REVIEW IMAGING BEFORE DIAGNOSING
What often brings a patient with cardiac tamponade to the attention of the physician is a finding on echocardiography, computed tomography, or magnetic resonance imaging of the chest.
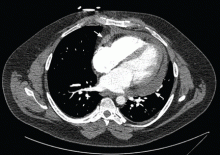
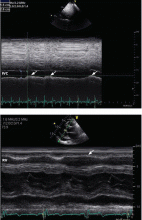
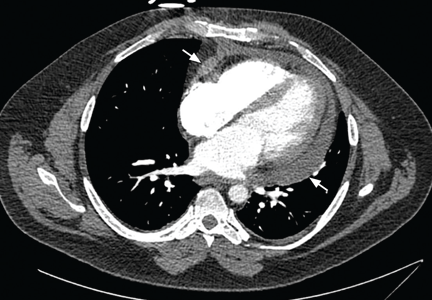
Always review the imaging studies before making the diagnosis of cardiac tamponade. These tests must be reviewed to assess the anatomy and the size and location of the effusion. Particularly, one must look for atrial and right ventricular collapse and inferior vena caval plethora, which are echocardiographic signs of cardiac tamponade.4 Figures 1, 2, and 3 show imaging studies in a patient who presented with worsening cough 2 weeks after undergoing a cardiac procedure and who was found to have cardiac tamponade.
When the history and these imaging studies place cardiac tamponade high in the differential diagnosis as the cause of edema or dyspnea, it is time to reexamine the patient. The best first step is to measure pulsus paradoxus.
HOW PULSUS PARADOXUS OCCURS
To fully appreciate the subtleties of the next pearls, it is necessary to understand the pathophysiology of cardiac tamponade.
When pericardial fluid accumulation raises the pericardial pressure above the central venous pressure and pulmonary venous pressure (intravascular pressure), blood will not passively return to the right side of the heart from the venae cavae nor to the left side of the heart from the pulmonary veins unless it is influenced by the effects of respiration on intrathoracic pressure. During respiration, the right and left sides of the heart are alternately filled and deprived of their respective venous return.
During inspiration, as the intrathoracic pressure decreases, blood in the venae cavae empties into the right side of the heart, while blood in the pulmonary veins preferentially remains in the pulmonary veins, underfilling the left side of the heart. Since the right ventricle is more filled than the left ventricle during inspiration, the ventricular septum shifts from right to left, further opposing pulmonary venous return. As a result, during cardiac tamponade, the systemic blood pressure falls with inspiration.
During expiration the opposite occurs. Expiration decreases the intrathoracic volume, so the intrathoracic pressure rises. This tends to oppose vena caval return to the right side of the heart and to favor pulmonary venous return to the left side of the heart. The ventricular septum shifts from left to right, further accommodating left ventricular filling, raising stroke volume, and increasing blood pressure. This exaggerated alternate filling of the right and left sides of the heart during cardiac tamponade is what accounts for pulsus paradoxus, an inspiratory fall in systolic blood pressure of greater than 10 mm Hg.
If intravascular pressure is low (due to hemorrhage, dehydration, or diuretic therapy), the pressure in the pericardial space needed to oppose venous return is much less. In this low-pressure scenario, the results are low cardiac output and hypotension, which are treated by giving intravenous fluids to maintain intravascular volume.
PEARL 6: MEASURE PULSUS PARADOXUS
When cardiac tamponade is considered, one must always measure the pulsus paradoxus.
The term pulsus paradoxus was coined by Adolph Kussmaul in 1873, before physicians could even measure blood pressure. All they could do at that time was palpate the pulse and listen to the heart. Kussmaul described his observation as a conspicuous discrepancy between the cardiac action and the arterial pulse.
Although not described by Kussmaul, another explanation for this finding might be more suited to the use of the word “paradoxical.” When the pulse is palpated in a normal patient, with inspiration the pulse rate will increase via the Bainbridge reflex, and with expiration it will decrease. But in a patient with cardiac tamponade, there is a paradoxical inspiratory slowing of the pulse (because the decreased magnitude of the pulse at times makes it imperceptible) and an expiratory increase in pulse rate as the magnitude of the pulse again makes it palpable.
The magnitude of the fall in systolic blood pressure during inspiration has been used to estimate the level of hemodynamic impairment resulting from pericardial effusion.5 A rapidly accumulating pericardial effusion can have more hemodynamic impact than a much larger one that accumulates slowly. Thus, the intrapericardial pressure must be considered more than the volume of pericardial fluid.
When there is severe cardiac tamponade and overt pulsus paradoxus, simple palpation of a proximal arterial pulse can detect a marked inspiratory decrease or loss of the pulse, which returns with expiration. Tachycardia is almost always present, unless the cause is hypothyroidism.6
How to measure pulsus paradoxus with a manual sphygmomanometer
A stethoscope and manual sphygmomanometer are all that is needed to measure pulsus paradoxus. A noninvasive blood pressure monitor that averages multiple measurements cannot detect or quantify pulsus paradoxus.
The patient should be supine with the head elevated 30° to 45°, and the examiner should be seated comfortably at the patient’s side. The manometer should be on the opposite side of the patient in plain view of the examiner. Position the cuff on the arm above the elbow and place your stethoscope on the antecubital fossa. Then:
- Inflate the cuff 20 mm Hg above the highest systolic pressure previously auscultated.
- Slowly decrease the manometer pressure by 5 mm Hg and hold it there through two or three respiratory cycles while listening for the first Korotkoff (systolic) sound. Repeat this until you can hear the systolic sound (but only during expiration) and mentally note the pressure.
- Continue to decrease the manometer pressure by 5-mm Hg increments while listening. When the Korotkoff sounds no longer disappear with inspiration, mentally note this second value as well. The pulsus paradoxus is the difference between these values.
- When the Korotkoff sounds disappear as the manometer pressure is decreased, note this final value. This is the diastolic blood pressure.
PEARL 7: THE PLETHYSMOGRAM WAVE-FORM PARALLELS PULSUS PARADOXUS
Manual measurement of blood pressure and pulsus paradoxus can be difficult, especially in an obese patient or one with a fat-distorted arm on which the cuff does not maintain its position. In such patients, increased girth of the neck and abdomen also make it difficult to assess the jugular venous distention and visceral organ engorgement that characterize cardiac tamponade.
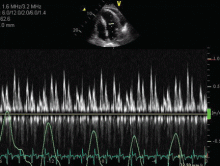
When the use of a sphygmomanometer is not possible, an arterial catheter can be inserted to demonstrate pulsus paradoxus. Simpler, however, is the novel use of another noninvasive instrument to detect and coarsely quantify pulsus paradoxus.7 The waveform on finger pulse oximetry can demonstrate pulsus paradoxus. The plethysmogram of the finger pulse oximeter can demonstrate the decrease in magnitude of the waveform with each inspiration (Figure 4).
Caution must be taken when interpreting this waveform, as with any measurement of pulsus paradoxus, to exclude a concomitant arrhythmia.
PEARL 8: PULSUS PARADOXUS WITHOUT CARDIAC TAMPONADE
Pulsus paradoxus can be present in the absence of cardiac tamponade. Once pulsus paradoxus of more than 10 mm Hg is measured, one must be sure the patient does not have a condition that can cause pulsus paradoxus without cardiac tamponade. Most of these are pulmonary conditions that necessitate an exaggerated inspiratory effort that can lower intrathoracic pressure sufficiently to oppose pulmonary venous return and cause a fall in systemic blood pressure:
- Chronic bronchitis
- Emphysema
- Mucus plug
- Pneumothorax
- Pulmonary embolism
- Stridor.
In these, there may be pulsus paradoxus, but not due to cardiac tamponade.
PEARL 9: CARDIAC TAMPONADE CAN BE PRESENT WITHOUT PULSUS PARADOXUS
Cardiac tamponade can be present without pulsus paradoxus. This occurs when certain conditions prevent inspiratory underfilling of the left ventricle relative to the filling of the right ventricle.8
How does this work? In cardiac tamponade, factors that drive the exaggerated fall in arterial pressure with inspiration (pulsus paradoxus) are the augmented right ventricular filling and the decreased left ventricular filling, both due to the lowering of the intrathoracic pressure. As the vena caval emptying is augmented, the right ventricular filling is increased, the ventricular septum shifts to the left, and pulmonary venous return to the heart is decreased.
Factors that can oppose pulsus paradoxus:
- Positive pressure ventilation prevents pulsus paradoxus by preventing the fall in intrathoracic pressure.
- Severe aortic regurgitation does not permit underfilling of the left ventricle during inspiration.
- An atrial septal defect will always equalize the right and left atrial pressures, preventing differential right ventricular and left ventricular filling with inspiration.
- Severe left ventricular hypertrophy does not permit the inspiratory shift of the ventricular septum from right to left that would otherwise lead to decreased left ventricular filling.
- Severe left ventricular dysfunction, with its low stroke volume and severe elevation of left ventricular end-diastolic pressure, never permits underfilling of the left ventricle, despite cardiac tamponade and an inspiratory decrease in intrathoracic pressure.
- Intravascular volume depletion due to hemorrhage, hemodialysis, or mistaken use of diuretics to treat edema can cause marked hypotension, making pulsus paradoxus impossible to detect.
Knowledge of underlying medical conditions, the likelihood of their causing cardiac tamponade, and the appearance of the echocardiogram prompt the physician to look further when the presence or absence of pulsus paradoxus does not fit with the working diagnosis.
The echocardiogram can give hints to the etiology of a pericardial effusion, such as clotted blood after trauma or a cardiac-perforating procedure, tumor studding of the epicardium,9 or fibrin strands indicating chronicity or an inflammatory process.10 Diastolic collapse of the right ventricle, more than collapse of the right atrium or left atrium, speaks for the severity of cardiac tamponade. With hemodynamically significant pericardial effusion and cardiac tamponade, the inferior vena cava is distended and does not decrease in size with inspiration unless there is severe intravascular volume depletion, at which time the inferior vena cava is underfilled throughout the respiratory cycle.
PEARL 10: PLAN HOW TO DRAIN
The size and location of the pericardial effusion and the patient’s hemodynamics must be integrated when deciding how to relieve cardiac tamponade. When cardiac tamponade is indeed severe and the patient and physician agree that it must be drained, the options are percutaneous needle aspiration (pericardiocentesis) and surgical pericardiostomy (creation of a pericardial window). Here again, as assessed by echocardiography, the access to the pericardial fluid should influence the choice.
Pericardiocentesis can be safely done if certain criteria are met. The patient must be able to lie still in the supine position, perhaps with the head of the bed elevated 30 degrees. Anticoagulation must be reversed or allowed time to resolve if drainage is not an emergency.
Pericardiocentesis can be risky or unsuccessful if there is not enough pericardial fluid to permit respiratory cardiac motion without perforating the heart with the needle; if the effusion is loculated (confined to a pocket) posteriorly; or if it is too far from the skin to permit precise control and placement of a spinal needle into the pericardial space. In cases of cardiac tamponade in which the anatomy indicates surgical pericardiostomy but severe hypotension prevents the induction of anesthesia and positive-pressure ventilation—which can result in profound, irreversible hypotension—percutaneous needle drainage (pericardiocentesis) should be performed in the operating room to relieve the tamponade before the induction of anesthesia and the surgical drainage.11
To reiterate, a suspected cardiac or aortic rupture that causes cardiac tamponade is usually large and not apt to self-seal. In such cases, the halt in the accumulation of pericardial blood is due to hypotension and not due to spontaneous resolution. Open surgical drainage is required from the outset because an initial success of pericardiocentesis yields to the recurrence of cardiac tamponade.
PEARL 11: ANTICIPATE WHAT THE FLUID SHOULD LOOK LIKE
Before performing pericardiocentesis, anticipate the appearance of the pericardial fluid on the basis of the presumed etiology, ie:
- Sanguinous—trauma, heart surgery, cardiac perforation from a procedure, anticoagulation, uremia, or malignancy
- Serous—congestive heart failure, acute radiation therapy
- Purulent—infections (natural or postoperative)
- Turbid (like gold paint)—mycobacterial infection, rheumatoid arthritis, myxedema
- Chylous—pericardium fistulized to the thoracic duct by a natural or postsurgical cause.
Sanguinous pericardial effusion encountered during a pericardiocentesis, if not anticipated, can be daunting and can cause the operator to question if it is the result of inadvertent needle placement in a cardiac chamber. If the needle is indeed in the heart, blood often surges out under pressure in pulses, which strongly suggests that the needle is not in the pericardial space and should be removed; but if confirmation of the location is needed before removing the needle, it can be done by injecting 2 mL of agitated sterile saline through the pericardiocentesis needle during echocardiographic imaging.12
Before inserting the needle, the ideal access location and needle angle must be determined by the operator with echocardiographic transducer in hand. The distance from skin to a point just through the parietal pericardium can also be measured at this time.
Once the needle is in the pericardial fluid (and you are confident of its placement), removal of 50 to 100 mL of the fluid with a large syringe can be enough to afford the patient easier breathing, higher blood pressure, and lower pulsus paradoxus—and even the physician will breathe easier. The same syringe can be filled and emptied multiple times. Less traumatic and more complete removal of pericardial fluid requires insertion of a multihole pigtail catheter over a J-tipped guidewire that is introduced through the needle.
PEARL 12: DRAIN SLOWLY TO AVOID PULMONARY EDEMA
Pulmonary edema is an uncommon complication of pericardiocentesis that might be avoidable. Heralded by sudden coughing and pink, frothy sputum, it can rapidly deteriorate into respiratory failure. The mechanism has been attributed to a sudden increase in right ventricular stroke volume and resultant left ventricular filling after the excess pericardial fluid has been removed, before the systemic arteries, which constrict to keep the systemic blood pressure up during cardiac tamponade, have had time to relax.13
To avoid this complication, if the volume of pericardial fluid responsible for cardiac tamponade is large, it should be removed slowly,14 stopping for a several-minute rest after each 250 mL. Catheter removal of pericardial fluid by gravity drainage over 24 hours has been suggested.15 A drawback to this approach is catheter clotting or sludging before all the fluid has been removed. It is helpful to keep the drainage catheter close to the patient’s body temperature to make the fluid less viscous. Output should be monitored hourly.
When the pericardial fluid has been completely drained, one must decide how long to leave the catheter in. One reason to remove the catheter at this time is that it causes pleuritic pain; another is to avoid introducing infection. A reason to leave the catheter in is to observe the effect of medical treatment on the hourly pericardial fluid output. Nonsteroidal anti-inflammatory drugs are the drugs of first choice when treating pericardial inflammation and suppressing production of pericardial fluid.16 In most cases the catheter should not be left in place for more than 3 days.
Laboratory analysis of the pericardial fluid should shed light on its suspected cause. Analysis usually involves chemistry testing, microscopic inspection of blood cell smears, cytology, microbiologic stains and cultures, and immunologic tests. Results often take days. Meyers and colleagues17 expound on this subject.
- Schiavone WA, Ghumrawi BK, Catalano DR, et al. The use of echocardiography in the emergency management of nonpenetrating traumatic cardiac rupture. Ann Emerg Med 1991; 20:1248–1250.
- Manuchehry A, Fontana GP, Gurudevan S, Marchevsky AM, Siegel RJ. Missed diagnosis of limited ascending aortic dissection by multiple imaging modalities leading to fatal cardiac tamponade and aortic rupture. Echocardiography 2011; 28:E187–E190.
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993; 117:1027–1031.
- Tsang TS, Oh JK, Seward JB, Tajik AJ. Diagnostic value of echocardiography in cardiac tamponade. Herz 2000; 25:734–740.
- Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J 1988; 115:391–398.
- Wang JL, Hsieh MJ, Lee CH, et al. Hypothyroid cardiac tamponade: clinical features, electrocardiography, pericardial fluid and management. Am J Med Sci 2010; 340:276–281.
- Tamburro RF, Ring JC, Womback K. Detection of pulsus paradoxus associated with large pericardial effusions in pediatric patients by analysis of the pulse-oximetry waveform. Pediatrics 2002; 109:673–677.
- Spodick DH. Pulsus paradoxus. In:Spodick DH, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:191–199.
- Burke A, Jeudy J, Virmani R. Cardiac tumors. In:Topol EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:710–720.
- Roberts WC. Pericardial heart disease: Its morphologic features and its causes. Proc (Bayl Univ Med Cent) 2005; 18:38–55.
- Stoelting RK, Miller RD, editors. Basics of Anesthesia. 4th ed. New York, NY: Churchill Livingstone; 2000:264–265.
- Ainsworth CD, Salehian O. Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 2011; 123:e210–e211.
- Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587–610.
- Vandyke WH, Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med 1983; 309:595–596.
- Bernal JM, Pradhan J, Li T, Tchokonte R, Afonso L. Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Can J Cardiol 2007; 23:1155–1156.
- Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol 2011; 3:135–143.
- Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997; 111:1213–1221.
Cardiac tamponade is a life-threatening condition that can be palliated or cured, depending on its cause and on the timeliness of treatment. Making a timely diagnosis and providing the appropriate treatment can be gratifying for both patient and physician.
Cardiac tamponade occurs when fluid in the pericardial space reaches a pressure exceeding central venous pressure. This leads to jugular venous distention, visceral organ engorgement, edema, and elevated pulmonary venous pressure that causes dyspnea. Despite compensatory tachycardia, the decrease in cardiac filling leads to a fall in cardiac output and to arterial hypoperfusion of vital organs.
PEARL 1: SLOW ACCUMULATION LEADS TO EDEMA
The rate at which pericardial fluid accumulates influences the clinical presentation of cardiac tamponade, in particular whether or not there is edema. Whereas rapid accumulation is characterized more by hypotension than by edema, the slow accumulation of pericardial fluid affords the patient time to drink enough liquid to keep the central venous pressure higher than the rising pericardial pressure. Thus, edema and dyspnea are more prominent features of cardiac tamponade when there is a slow rise in pericardial pressure.
PEARL 2: EDEMA IS NOT ALWAYS TREATED WITH A DIURETIC
Edema is not always treated with a diuretic. In a patient who has a pericardial effusion that has developed slowly and who has been drinking enough fluid to keep the central venous pressure higher than the pericardial pressure, a diuretic can remove enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure and thus convert a benign pericardial effusion to potentially lethal cardiac tamponade.
One must understand the cause of edema or low urine output before treating it. This underscores the importance of the history and the physical examination. All of the following must be assessed:
- Symptoms and time course of the illness
- Concurrent medical illnesses
- Neck veins
- Blood pressure and its response to inspiration
- Heart sounds
- Heart rate and rhythm
- Abdominal organ engorgement
- Edema (or its absence).
PEARL 3: UNDERSTANDING THE CAUSE IS ESSENTIAL
Understanding the cause of cardiac tamponade is essential.
A trauma patient first encountered in the emergency department may have an underlying disease, but the focus is squarely on the effects of trauma or violent injury. In a patient with multiple trauma, hypotension and tachycardia that do not respond to intravenous volume replacement when there is an obvious rise in central venous pressure should be clues to cardiac tamponade.1
If the patient has recently undergone a cardiac procedure (for example, cardiac surgery, myocardial biopsy, coronary intervention, electrophysiologic study with intracardiac electrodes, transvenous pacemaker placement, pacemaker lead extraction, or radiofrequency ablation), knowing about the procedure narrows the differential diagnosis when hypotension, tachycardia, and jugular venous distention develop.
PEARL 4: CARDIAC OR AORTIC RUPTURE REQUIRES SURGERY
When the etiology of cardiac tamponade is cardiac or aortic rupture, the treatment is surgical.
Painful sudden causes of cardiac tamponade include hemopericardium due to rupture of the free wall after myocardial infarction, and spontaneous or posttraumatic dissection and rupture of the ascending aorta. Prompt diagnosis is necessary, but since these lesions will not close and heal spontaneously, the definitive treatment should be surgery. Moreover, needle removal of intrapericardial blood that has been opposing further bleeding is sure to permit bleeding to recur, often with lethal consequences.2
Causes of cardiac tamponade that have a less-acute onset are likely to be complications of medical problems. Medical illnesses known to be associated with cardiac tamponade include:
- Infectious disease (idiopathic or viral, associated with smallpox vaccination, mycobacterial, purulent bacterial, fungal)
- Metastatic cancer (lung, breast, esophagus, lymphoma, pancreas, liver, leukemia, stomach, melanoma)3
- Connective tissue disease (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, scleroderma, Wegener granulomatosis, acute rheumatic fever)
- Endocrine disease (hypothyroidism)
- Drug side effects (procainamide, isoniazid, hydralazine, minoxidil, phenytoin, anticoagulants, methysergide)
- Inflammatory bowel disease (Crohn disease, ulcerative colitis)
- Congestive heart failure
- Uremia
- Radiation therapy
- Postmyocardial infarction syndrome (Dressler syndrome)
- Postpericardiotomy syndrome.
PEARL 5: REVIEW IMAGING BEFORE DIAGNOSING
What often brings a patient with cardiac tamponade to the attention of the physician is a finding on echocardiography, computed tomography, or magnetic resonance imaging of the chest.
Always review the imaging studies before making the diagnosis of cardiac tamponade. These tests must be reviewed to assess the anatomy and the size and location of the effusion. Particularly, one must look for atrial and right ventricular collapse and inferior vena caval plethora, which are echocardiographic signs of cardiac tamponade.4 Figures 1, 2, and 3 show imaging studies in a patient who presented with worsening cough 2 weeks after undergoing a cardiac procedure and who was found to have cardiac tamponade.
When the history and these imaging studies place cardiac tamponade high in the differential diagnosis as the cause of edema or dyspnea, it is time to reexamine the patient. The best first step is to measure pulsus paradoxus.
HOW PULSUS PARADOXUS OCCURS
To fully appreciate the subtleties of the next pearls, it is necessary to understand the pathophysiology of cardiac tamponade.
When pericardial fluid accumulation raises the pericardial pressure above the central venous pressure and pulmonary venous pressure (intravascular pressure), blood will not passively return to the right side of the heart from the venae cavae nor to the left side of the heart from the pulmonary veins unless it is influenced by the effects of respiration on intrathoracic pressure. During respiration, the right and left sides of the heart are alternately filled and deprived of their respective venous return.
During inspiration, as the intrathoracic pressure decreases, blood in the venae cavae empties into the right side of the heart, while blood in the pulmonary veins preferentially remains in the pulmonary veins, underfilling the left side of the heart. Since the right ventricle is more filled than the left ventricle during inspiration, the ventricular septum shifts from right to left, further opposing pulmonary venous return. As a result, during cardiac tamponade, the systemic blood pressure falls with inspiration.
During expiration the opposite occurs. Expiration decreases the intrathoracic volume, so the intrathoracic pressure rises. This tends to oppose vena caval return to the right side of the heart and to favor pulmonary venous return to the left side of the heart. The ventricular septum shifts from left to right, further accommodating left ventricular filling, raising stroke volume, and increasing blood pressure. This exaggerated alternate filling of the right and left sides of the heart during cardiac tamponade is what accounts for pulsus paradoxus, an inspiratory fall in systolic blood pressure of greater than 10 mm Hg.
If intravascular pressure is low (due to hemorrhage, dehydration, or diuretic therapy), the pressure in the pericardial space needed to oppose venous return is much less. In this low-pressure scenario, the results are low cardiac output and hypotension, which are treated by giving intravenous fluids to maintain intravascular volume.
PEARL 6: MEASURE PULSUS PARADOXUS
When cardiac tamponade is considered, one must always measure the pulsus paradoxus.
The term pulsus paradoxus was coined by Adolph Kussmaul in 1873, before physicians could even measure blood pressure. All they could do at that time was palpate the pulse and listen to the heart. Kussmaul described his observation as a conspicuous discrepancy between the cardiac action and the arterial pulse.
Although not described by Kussmaul, another explanation for this finding might be more suited to the use of the word “paradoxical.” When the pulse is palpated in a normal patient, with inspiration the pulse rate will increase via the Bainbridge reflex, and with expiration it will decrease. But in a patient with cardiac tamponade, there is a paradoxical inspiratory slowing of the pulse (because the decreased magnitude of the pulse at times makes it imperceptible) and an expiratory increase in pulse rate as the magnitude of the pulse again makes it palpable.
The magnitude of the fall in systolic blood pressure during inspiration has been used to estimate the level of hemodynamic impairment resulting from pericardial effusion.5 A rapidly accumulating pericardial effusion can have more hemodynamic impact than a much larger one that accumulates slowly. Thus, the intrapericardial pressure must be considered more than the volume of pericardial fluid.
When there is severe cardiac tamponade and overt pulsus paradoxus, simple palpation of a proximal arterial pulse can detect a marked inspiratory decrease or loss of the pulse, which returns with expiration. Tachycardia is almost always present, unless the cause is hypothyroidism.6
How to measure pulsus paradoxus with a manual sphygmomanometer
A stethoscope and manual sphygmomanometer are all that is needed to measure pulsus paradoxus. A noninvasive blood pressure monitor that averages multiple measurements cannot detect or quantify pulsus paradoxus.
The patient should be supine with the head elevated 30° to 45°, and the examiner should be seated comfortably at the patient’s side. The manometer should be on the opposite side of the patient in plain view of the examiner. Position the cuff on the arm above the elbow and place your stethoscope on the antecubital fossa. Then:
- Inflate the cuff 20 mm Hg above the highest systolic pressure previously auscultated.
- Slowly decrease the manometer pressure by 5 mm Hg and hold it there through two or three respiratory cycles while listening for the first Korotkoff (systolic) sound. Repeat this until you can hear the systolic sound (but only during expiration) and mentally note the pressure.
- Continue to decrease the manometer pressure by 5-mm Hg increments while listening. When the Korotkoff sounds no longer disappear with inspiration, mentally note this second value as well. The pulsus paradoxus is the difference between these values.
- When the Korotkoff sounds disappear as the manometer pressure is decreased, note this final value. This is the diastolic blood pressure.
PEARL 7: THE PLETHYSMOGRAM WAVE-FORM PARALLELS PULSUS PARADOXUS
Manual measurement of blood pressure and pulsus paradoxus can be difficult, especially in an obese patient or one with a fat-distorted arm on which the cuff does not maintain its position. In such patients, increased girth of the neck and abdomen also make it difficult to assess the jugular venous distention and visceral organ engorgement that characterize cardiac tamponade.
When the use of a sphygmomanometer is not possible, an arterial catheter can be inserted to demonstrate pulsus paradoxus. Simpler, however, is the novel use of another noninvasive instrument to detect and coarsely quantify pulsus paradoxus.7 The waveform on finger pulse oximetry can demonstrate pulsus paradoxus. The plethysmogram of the finger pulse oximeter can demonstrate the decrease in magnitude of the waveform with each inspiration (Figure 4).
Caution must be taken when interpreting this waveform, as with any measurement of pulsus paradoxus, to exclude a concomitant arrhythmia.
PEARL 8: PULSUS PARADOXUS WITHOUT CARDIAC TAMPONADE
Pulsus paradoxus can be present in the absence of cardiac tamponade. Once pulsus paradoxus of more than 10 mm Hg is measured, one must be sure the patient does not have a condition that can cause pulsus paradoxus without cardiac tamponade. Most of these are pulmonary conditions that necessitate an exaggerated inspiratory effort that can lower intrathoracic pressure sufficiently to oppose pulmonary venous return and cause a fall in systemic blood pressure:
- Chronic bronchitis
- Emphysema
- Mucus plug
- Pneumothorax
- Pulmonary embolism
- Stridor.
In these, there may be pulsus paradoxus, but not due to cardiac tamponade.
PEARL 9: CARDIAC TAMPONADE CAN BE PRESENT WITHOUT PULSUS PARADOXUS
Cardiac tamponade can be present without pulsus paradoxus. This occurs when certain conditions prevent inspiratory underfilling of the left ventricle relative to the filling of the right ventricle.8
How does this work? In cardiac tamponade, factors that drive the exaggerated fall in arterial pressure with inspiration (pulsus paradoxus) are the augmented right ventricular filling and the decreased left ventricular filling, both due to the lowering of the intrathoracic pressure. As the vena caval emptying is augmented, the right ventricular filling is increased, the ventricular septum shifts to the left, and pulmonary venous return to the heart is decreased.
Factors that can oppose pulsus paradoxus:
- Positive pressure ventilation prevents pulsus paradoxus by preventing the fall in intrathoracic pressure.
- Severe aortic regurgitation does not permit underfilling of the left ventricle during inspiration.
- An atrial septal defect will always equalize the right and left atrial pressures, preventing differential right ventricular and left ventricular filling with inspiration.
- Severe left ventricular hypertrophy does not permit the inspiratory shift of the ventricular septum from right to left that would otherwise lead to decreased left ventricular filling.
- Severe left ventricular dysfunction, with its low stroke volume and severe elevation of left ventricular end-diastolic pressure, never permits underfilling of the left ventricle, despite cardiac tamponade and an inspiratory decrease in intrathoracic pressure.
- Intravascular volume depletion due to hemorrhage, hemodialysis, or mistaken use of diuretics to treat edema can cause marked hypotension, making pulsus paradoxus impossible to detect.
Knowledge of underlying medical conditions, the likelihood of their causing cardiac tamponade, and the appearance of the echocardiogram prompt the physician to look further when the presence or absence of pulsus paradoxus does not fit with the working diagnosis.
The echocardiogram can give hints to the etiology of a pericardial effusion, such as clotted blood after trauma or a cardiac-perforating procedure, tumor studding of the epicardium,9 or fibrin strands indicating chronicity or an inflammatory process.10 Diastolic collapse of the right ventricle, more than collapse of the right atrium or left atrium, speaks for the severity of cardiac tamponade. With hemodynamically significant pericardial effusion and cardiac tamponade, the inferior vena cava is distended and does not decrease in size with inspiration unless there is severe intravascular volume depletion, at which time the inferior vena cava is underfilled throughout the respiratory cycle.
PEARL 10: PLAN HOW TO DRAIN
The size and location of the pericardial effusion and the patient’s hemodynamics must be integrated when deciding how to relieve cardiac tamponade. When cardiac tamponade is indeed severe and the patient and physician agree that it must be drained, the options are percutaneous needle aspiration (pericardiocentesis) and surgical pericardiostomy (creation of a pericardial window). Here again, as assessed by echocardiography, the access to the pericardial fluid should influence the choice.
Pericardiocentesis can be safely done if certain criteria are met. The patient must be able to lie still in the supine position, perhaps with the head of the bed elevated 30 degrees. Anticoagulation must be reversed or allowed time to resolve if drainage is not an emergency.
Pericardiocentesis can be risky or unsuccessful if there is not enough pericardial fluid to permit respiratory cardiac motion without perforating the heart with the needle; if the effusion is loculated (confined to a pocket) posteriorly; or if it is too far from the skin to permit precise control and placement of a spinal needle into the pericardial space. In cases of cardiac tamponade in which the anatomy indicates surgical pericardiostomy but severe hypotension prevents the induction of anesthesia and positive-pressure ventilation—which can result in profound, irreversible hypotension—percutaneous needle drainage (pericardiocentesis) should be performed in the operating room to relieve the tamponade before the induction of anesthesia and the surgical drainage.11
To reiterate, a suspected cardiac or aortic rupture that causes cardiac tamponade is usually large and not apt to self-seal. In such cases, the halt in the accumulation of pericardial blood is due to hypotension and not due to spontaneous resolution. Open surgical drainage is required from the outset because an initial success of pericardiocentesis yields to the recurrence of cardiac tamponade.
PEARL 11: ANTICIPATE WHAT THE FLUID SHOULD LOOK LIKE
Before performing pericardiocentesis, anticipate the appearance of the pericardial fluid on the basis of the presumed etiology, ie:
- Sanguinous—trauma, heart surgery, cardiac perforation from a procedure, anticoagulation, uremia, or malignancy
- Serous—congestive heart failure, acute radiation therapy
- Purulent—infections (natural or postoperative)
- Turbid (like gold paint)—mycobacterial infection, rheumatoid arthritis, myxedema
- Chylous—pericardium fistulized to the thoracic duct by a natural or postsurgical cause.
Sanguinous pericardial effusion encountered during a pericardiocentesis, if not anticipated, can be daunting and can cause the operator to question if it is the result of inadvertent needle placement in a cardiac chamber. If the needle is indeed in the heart, blood often surges out under pressure in pulses, which strongly suggests that the needle is not in the pericardial space and should be removed; but if confirmation of the location is needed before removing the needle, it can be done by injecting 2 mL of agitated sterile saline through the pericardiocentesis needle during echocardiographic imaging.12
Before inserting the needle, the ideal access location and needle angle must be determined by the operator with echocardiographic transducer in hand. The distance from skin to a point just through the parietal pericardium can also be measured at this time.
Once the needle is in the pericardial fluid (and you are confident of its placement), removal of 50 to 100 mL of the fluid with a large syringe can be enough to afford the patient easier breathing, higher blood pressure, and lower pulsus paradoxus—and even the physician will breathe easier. The same syringe can be filled and emptied multiple times. Less traumatic and more complete removal of pericardial fluid requires insertion of a multihole pigtail catheter over a J-tipped guidewire that is introduced through the needle.
PEARL 12: DRAIN SLOWLY TO AVOID PULMONARY EDEMA
Pulmonary edema is an uncommon complication of pericardiocentesis that might be avoidable. Heralded by sudden coughing and pink, frothy sputum, it can rapidly deteriorate into respiratory failure. The mechanism has been attributed to a sudden increase in right ventricular stroke volume and resultant left ventricular filling after the excess pericardial fluid has been removed, before the systemic arteries, which constrict to keep the systemic blood pressure up during cardiac tamponade, have had time to relax.13
To avoid this complication, if the volume of pericardial fluid responsible for cardiac tamponade is large, it should be removed slowly,14 stopping for a several-minute rest after each 250 mL. Catheter removal of pericardial fluid by gravity drainage over 24 hours has been suggested.15 A drawback to this approach is catheter clotting or sludging before all the fluid has been removed. It is helpful to keep the drainage catheter close to the patient’s body temperature to make the fluid less viscous. Output should be monitored hourly.
When the pericardial fluid has been completely drained, one must decide how long to leave the catheter in. One reason to remove the catheter at this time is that it causes pleuritic pain; another is to avoid introducing infection. A reason to leave the catheter in is to observe the effect of medical treatment on the hourly pericardial fluid output. Nonsteroidal anti-inflammatory drugs are the drugs of first choice when treating pericardial inflammation and suppressing production of pericardial fluid.16 In most cases the catheter should not be left in place for more than 3 days.
Laboratory analysis of the pericardial fluid should shed light on its suspected cause. Analysis usually involves chemistry testing, microscopic inspection of blood cell smears, cytology, microbiologic stains and cultures, and immunologic tests. Results often take days. Meyers and colleagues17 expound on this subject.
Cardiac tamponade is a life-threatening condition that can be palliated or cured, depending on its cause and on the timeliness of treatment. Making a timely diagnosis and providing the appropriate treatment can be gratifying for both patient and physician.
Cardiac tamponade occurs when fluid in the pericardial space reaches a pressure exceeding central venous pressure. This leads to jugular venous distention, visceral organ engorgement, edema, and elevated pulmonary venous pressure that causes dyspnea. Despite compensatory tachycardia, the decrease in cardiac filling leads to a fall in cardiac output and to arterial hypoperfusion of vital organs.
PEARL 1: SLOW ACCUMULATION LEADS TO EDEMA
The rate at which pericardial fluid accumulates influences the clinical presentation of cardiac tamponade, in particular whether or not there is edema. Whereas rapid accumulation is characterized more by hypotension than by edema, the slow accumulation of pericardial fluid affords the patient time to drink enough liquid to keep the central venous pressure higher than the rising pericardial pressure. Thus, edema and dyspnea are more prominent features of cardiac tamponade when there is a slow rise in pericardial pressure.
PEARL 2: EDEMA IS NOT ALWAYS TREATED WITH A DIURETIC
Edema is not always treated with a diuretic. In a patient who has a pericardial effusion that has developed slowly and who has been drinking enough fluid to keep the central venous pressure higher than the pericardial pressure, a diuretic can remove enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure and thus convert a benign pericardial effusion to potentially lethal cardiac tamponade.
One must understand the cause of edema or low urine output before treating it. This underscores the importance of the history and the physical examination. All of the following must be assessed:
- Symptoms and time course of the illness
- Concurrent medical illnesses
- Neck veins
- Blood pressure and its response to inspiration
- Heart sounds
- Heart rate and rhythm
- Abdominal organ engorgement
- Edema (or its absence).
PEARL 3: UNDERSTANDING THE CAUSE IS ESSENTIAL
Understanding the cause of cardiac tamponade is essential.
A trauma patient first encountered in the emergency department may have an underlying disease, but the focus is squarely on the effects of trauma or violent injury. In a patient with multiple trauma, hypotension and tachycardia that do not respond to intravenous volume replacement when there is an obvious rise in central venous pressure should be clues to cardiac tamponade.1
If the patient has recently undergone a cardiac procedure (for example, cardiac surgery, myocardial biopsy, coronary intervention, electrophysiologic study with intracardiac electrodes, transvenous pacemaker placement, pacemaker lead extraction, or radiofrequency ablation), knowing about the procedure narrows the differential diagnosis when hypotension, tachycardia, and jugular venous distention develop.
PEARL 4: CARDIAC OR AORTIC RUPTURE REQUIRES SURGERY
When the etiology of cardiac tamponade is cardiac or aortic rupture, the treatment is surgical.
Painful sudden causes of cardiac tamponade include hemopericardium due to rupture of the free wall after myocardial infarction, and spontaneous or posttraumatic dissection and rupture of the ascending aorta. Prompt diagnosis is necessary, but since these lesions will not close and heal spontaneously, the definitive treatment should be surgery. Moreover, needle removal of intrapericardial blood that has been opposing further bleeding is sure to permit bleeding to recur, often with lethal consequences.2
Causes of cardiac tamponade that have a less-acute onset are likely to be complications of medical problems. Medical illnesses known to be associated with cardiac tamponade include:
- Infectious disease (idiopathic or viral, associated with smallpox vaccination, mycobacterial, purulent bacterial, fungal)
- Metastatic cancer (lung, breast, esophagus, lymphoma, pancreas, liver, leukemia, stomach, melanoma)3
- Connective tissue disease (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, scleroderma, Wegener granulomatosis, acute rheumatic fever)
- Endocrine disease (hypothyroidism)
- Drug side effects (procainamide, isoniazid, hydralazine, minoxidil, phenytoin, anticoagulants, methysergide)
- Inflammatory bowel disease (Crohn disease, ulcerative colitis)
- Congestive heart failure
- Uremia
- Radiation therapy
- Postmyocardial infarction syndrome (Dressler syndrome)
- Postpericardiotomy syndrome.
PEARL 5: REVIEW IMAGING BEFORE DIAGNOSING
What often brings a patient with cardiac tamponade to the attention of the physician is a finding on echocardiography, computed tomography, or magnetic resonance imaging of the chest.
Always review the imaging studies before making the diagnosis of cardiac tamponade. These tests must be reviewed to assess the anatomy and the size and location of the effusion. Particularly, one must look for atrial and right ventricular collapse and inferior vena caval plethora, which are echocardiographic signs of cardiac tamponade.4 Figures 1, 2, and 3 show imaging studies in a patient who presented with worsening cough 2 weeks after undergoing a cardiac procedure and who was found to have cardiac tamponade.
When the history and these imaging studies place cardiac tamponade high in the differential diagnosis as the cause of edema or dyspnea, it is time to reexamine the patient. The best first step is to measure pulsus paradoxus.
HOW PULSUS PARADOXUS OCCURS
To fully appreciate the subtleties of the next pearls, it is necessary to understand the pathophysiology of cardiac tamponade.
When pericardial fluid accumulation raises the pericardial pressure above the central venous pressure and pulmonary venous pressure (intravascular pressure), blood will not passively return to the right side of the heart from the venae cavae nor to the left side of the heart from the pulmonary veins unless it is influenced by the effects of respiration on intrathoracic pressure. During respiration, the right and left sides of the heart are alternately filled and deprived of their respective venous return.
During inspiration, as the intrathoracic pressure decreases, blood in the venae cavae empties into the right side of the heart, while blood in the pulmonary veins preferentially remains in the pulmonary veins, underfilling the left side of the heart. Since the right ventricle is more filled than the left ventricle during inspiration, the ventricular septum shifts from right to left, further opposing pulmonary venous return. As a result, during cardiac tamponade, the systemic blood pressure falls with inspiration.
During expiration the opposite occurs. Expiration decreases the intrathoracic volume, so the intrathoracic pressure rises. This tends to oppose vena caval return to the right side of the heart and to favor pulmonary venous return to the left side of the heart. The ventricular septum shifts from left to right, further accommodating left ventricular filling, raising stroke volume, and increasing blood pressure. This exaggerated alternate filling of the right and left sides of the heart during cardiac tamponade is what accounts for pulsus paradoxus, an inspiratory fall in systolic blood pressure of greater than 10 mm Hg.
If intravascular pressure is low (due to hemorrhage, dehydration, or diuretic therapy), the pressure in the pericardial space needed to oppose venous return is much less. In this low-pressure scenario, the results are low cardiac output and hypotension, which are treated by giving intravenous fluids to maintain intravascular volume.
PEARL 6: MEASURE PULSUS PARADOXUS
When cardiac tamponade is considered, one must always measure the pulsus paradoxus.
The term pulsus paradoxus was coined by Adolph Kussmaul in 1873, before physicians could even measure blood pressure. All they could do at that time was palpate the pulse and listen to the heart. Kussmaul described his observation as a conspicuous discrepancy between the cardiac action and the arterial pulse.
Although not described by Kussmaul, another explanation for this finding might be more suited to the use of the word “paradoxical.” When the pulse is palpated in a normal patient, with inspiration the pulse rate will increase via the Bainbridge reflex, and with expiration it will decrease. But in a patient with cardiac tamponade, there is a paradoxical inspiratory slowing of the pulse (because the decreased magnitude of the pulse at times makes it imperceptible) and an expiratory increase in pulse rate as the magnitude of the pulse again makes it palpable.
The magnitude of the fall in systolic blood pressure during inspiration has been used to estimate the level of hemodynamic impairment resulting from pericardial effusion.5 A rapidly accumulating pericardial effusion can have more hemodynamic impact than a much larger one that accumulates slowly. Thus, the intrapericardial pressure must be considered more than the volume of pericardial fluid.
When there is severe cardiac tamponade and overt pulsus paradoxus, simple palpation of a proximal arterial pulse can detect a marked inspiratory decrease or loss of the pulse, which returns with expiration. Tachycardia is almost always present, unless the cause is hypothyroidism.6
How to measure pulsus paradoxus with a manual sphygmomanometer
A stethoscope and manual sphygmomanometer are all that is needed to measure pulsus paradoxus. A noninvasive blood pressure monitor that averages multiple measurements cannot detect or quantify pulsus paradoxus.
The patient should be supine with the head elevated 30° to 45°, and the examiner should be seated comfortably at the patient’s side. The manometer should be on the opposite side of the patient in plain view of the examiner. Position the cuff on the arm above the elbow and place your stethoscope on the antecubital fossa. Then:
- Inflate the cuff 20 mm Hg above the highest systolic pressure previously auscultated.
- Slowly decrease the manometer pressure by 5 mm Hg and hold it there through two or three respiratory cycles while listening for the first Korotkoff (systolic) sound. Repeat this until you can hear the systolic sound (but only during expiration) and mentally note the pressure.
- Continue to decrease the manometer pressure by 5-mm Hg increments while listening. When the Korotkoff sounds no longer disappear with inspiration, mentally note this second value as well. The pulsus paradoxus is the difference between these values.
- When the Korotkoff sounds disappear as the manometer pressure is decreased, note this final value. This is the diastolic blood pressure.
PEARL 7: THE PLETHYSMOGRAM WAVE-FORM PARALLELS PULSUS PARADOXUS
Manual measurement of blood pressure and pulsus paradoxus can be difficult, especially in an obese patient or one with a fat-distorted arm on which the cuff does not maintain its position. In such patients, increased girth of the neck and abdomen also make it difficult to assess the jugular venous distention and visceral organ engorgement that characterize cardiac tamponade.
When the use of a sphygmomanometer is not possible, an arterial catheter can be inserted to demonstrate pulsus paradoxus. Simpler, however, is the novel use of another noninvasive instrument to detect and coarsely quantify pulsus paradoxus.7 The waveform on finger pulse oximetry can demonstrate pulsus paradoxus. The plethysmogram of the finger pulse oximeter can demonstrate the decrease in magnitude of the waveform with each inspiration (Figure 4).
Caution must be taken when interpreting this waveform, as with any measurement of pulsus paradoxus, to exclude a concomitant arrhythmia.
PEARL 8: PULSUS PARADOXUS WITHOUT CARDIAC TAMPONADE
Pulsus paradoxus can be present in the absence of cardiac tamponade. Once pulsus paradoxus of more than 10 mm Hg is measured, one must be sure the patient does not have a condition that can cause pulsus paradoxus without cardiac tamponade. Most of these are pulmonary conditions that necessitate an exaggerated inspiratory effort that can lower intrathoracic pressure sufficiently to oppose pulmonary venous return and cause a fall in systemic blood pressure:
- Chronic bronchitis
- Emphysema
- Mucus plug
- Pneumothorax
- Pulmonary embolism
- Stridor.
In these, there may be pulsus paradoxus, but not due to cardiac tamponade.
PEARL 9: CARDIAC TAMPONADE CAN BE PRESENT WITHOUT PULSUS PARADOXUS
Cardiac tamponade can be present without pulsus paradoxus. This occurs when certain conditions prevent inspiratory underfilling of the left ventricle relative to the filling of the right ventricle.8
How does this work? In cardiac tamponade, factors that drive the exaggerated fall in arterial pressure with inspiration (pulsus paradoxus) are the augmented right ventricular filling and the decreased left ventricular filling, both due to the lowering of the intrathoracic pressure. As the vena caval emptying is augmented, the right ventricular filling is increased, the ventricular septum shifts to the left, and pulmonary venous return to the heart is decreased.
Factors that can oppose pulsus paradoxus:
- Positive pressure ventilation prevents pulsus paradoxus by preventing the fall in intrathoracic pressure.
- Severe aortic regurgitation does not permit underfilling of the left ventricle during inspiration.
- An atrial septal defect will always equalize the right and left atrial pressures, preventing differential right ventricular and left ventricular filling with inspiration.
- Severe left ventricular hypertrophy does not permit the inspiratory shift of the ventricular septum from right to left that would otherwise lead to decreased left ventricular filling.
- Severe left ventricular dysfunction, with its low stroke volume and severe elevation of left ventricular end-diastolic pressure, never permits underfilling of the left ventricle, despite cardiac tamponade and an inspiratory decrease in intrathoracic pressure.
- Intravascular volume depletion due to hemorrhage, hemodialysis, or mistaken use of diuretics to treat edema can cause marked hypotension, making pulsus paradoxus impossible to detect.
Knowledge of underlying medical conditions, the likelihood of their causing cardiac tamponade, and the appearance of the echocardiogram prompt the physician to look further when the presence or absence of pulsus paradoxus does not fit with the working diagnosis.
The echocardiogram can give hints to the etiology of a pericardial effusion, such as clotted blood after trauma or a cardiac-perforating procedure, tumor studding of the epicardium,9 or fibrin strands indicating chronicity or an inflammatory process.10 Diastolic collapse of the right ventricle, more than collapse of the right atrium or left atrium, speaks for the severity of cardiac tamponade. With hemodynamically significant pericardial effusion and cardiac tamponade, the inferior vena cava is distended and does not decrease in size with inspiration unless there is severe intravascular volume depletion, at which time the inferior vena cava is underfilled throughout the respiratory cycle.
PEARL 10: PLAN HOW TO DRAIN
The size and location of the pericardial effusion and the patient’s hemodynamics must be integrated when deciding how to relieve cardiac tamponade. When cardiac tamponade is indeed severe and the patient and physician agree that it must be drained, the options are percutaneous needle aspiration (pericardiocentesis) and surgical pericardiostomy (creation of a pericardial window). Here again, as assessed by echocardiography, the access to the pericardial fluid should influence the choice.
Pericardiocentesis can be safely done if certain criteria are met. The patient must be able to lie still in the supine position, perhaps with the head of the bed elevated 30 degrees. Anticoagulation must be reversed or allowed time to resolve if drainage is not an emergency.
Pericardiocentesis can be risky or unsuccessful if there is not enough pericardial fluid to permit respiratory cardiac motion without perforating the heart with the needle; if the effusion is loculated (confined to a pocket) posteriorly; or if it is too far from the skin to permit precise control and placement of a spinal needle into the pericardial space. In cases of cardiac tamponade in which the anatomy indicates surgical pericardiostomy but severe hypotension prevents the induction of anesthesia and positive-pressure ventilation—which can result in profound, irreversible hypotension—percutaneous needle drainage (pericardiocentesis) should be performed in the operating room to relieve the tamponade before the induction of anesthesia and the surgical drainage.11
To reiterate, a suspected cardiac or aortic rupture that causes cardiac tamponade is usually large and not apt to self-seal. In such cases, the halt in the accumulation of pericardial blood is due to hypotension and not due to spontaneous resolution. Open surgical drainage is required from the outset because an initial success of pericardiocentesis yields to the recurrence of cardiac tamponade.
PEARL 11: ANTICIPATE WHAT THE FLUID SHOULD LOOK LIKE
Before performing pericardiocentesis, anticipate the appearance of the pericardial fluid on the basis of the presumed etiology, ie:
- Sanguinous—trauma, heart surgery, cardiac perforation from a procedure, anticoagulation, uremia, or malignancy
- Serous—congestive heart failure, acute radiation therapy
- Purulent—infections (natural or postoperative)
- Turbid (like gold paint)—mycobacterial infection, rheumatoid arthritis, myxedema
- Chylous—pericardium fistulized to the thoracic duct by a natural or postsurgical cause.
Sanguinous pericardial effusion encountered during a pericardiocentesis, if not anticipated, can be daunting and can cause the operator to question if it is the result of inadvertent needle placement in a cardiac chamber. If the needle is indeed in the heart, blood often surges out under pressure in pulses, which strongly suggests that the needle is not in the pericardial space and should be removed; but if confirmation of the location is needed before removing the needle, it can be done by injecting 2 mL of agitated sterile saline through the pericardiocentesis needle during echocardiographic imaging.12
Before inserting the needle, the ideal access location and needle angle must be determined by the operator with echocardiographic transducer in hand. The distance from skin to a point just through the parietal pericardium can also be measured at this time.
Once the needle is in the pericardial fluid (and you are confident of its placement), removal of 50 to 100 mL of the fluid with a large syringe can be enough to afford the patient easier breathing, higher blood pressure, and lower pulsus paradoxus—and even the physician will breathe easier. The same syringe can be filled and emptied multiple times. Less traumatic and more complete removal of pericardial fluid requires insertion of a multihole pigtail catheter over a J-tipped guidewire that is introduced through the needle.
PEARL 12: DRAIN SLOWLY TO AVOID PULMONARY EDEMA
Pulmonary edema is an uncommon complication of pericardiocentesis that might be avoidable. Heralded by sudden coughing and pink, frothy sputum, it can rapidly deteriorate into respiratory failure. The mechanism has been attributed to a sudden increase in right ventricular stroke volume and resultant left ventricular filling after the excess pericardial fluid has been removed, before the systemic arteries, which constrict to keep the systemic blood pressure up during cardiac tamponade, have had time to relax.13
To avoid this complication, if the volume of pericardial fluid responsible for cardiac tamponade is large, it should be removed slowly,14 stopping for a several-minute rest after each 250 mL. Catheter removal of pericardial fluid by gravity drainage over 24 hours has been suggested.15 A drawback to this approach is catheter clotting or sludging before all the fluid has been removed. It is helpful to keep the drainage catheter close to the patient’s body temperature to make the fluid less viscous. Output should be monitored hourly.
When the pericardial fluid has been completely drained, one must decide how long to leave the catheter in. One reason to remove the catheter at this time is that it causes pleuritic pain; another is to avoid introducing infection. A reason to leave the catheter in is to observe the effect of medical treatment on the hourly pericardial fluid output. Nonsteroidal anti-inflammatory drugs are the drugs of first choice when treating pericardial inflammation and suppressing production of pericardial fluid.16 In most cases the catheter should not be left in place for more than 3 days.
Laboratory analysis of the pericardial fluid should shed light on its suspected cause. Analysis usually involves chemistry testing, microscopic inspection of blood cell smears, cytology, microbiologic stains and cultures, and immunologic tests. Results often take days. Meyers and colleagues17 expound on this subject.
- Schiavone WA, Ghumrawi BK, Catalano DR, et al. The use of echocardiography in the emergency management of nonpenetrating traumatic cardiac rupture. Ann Emerg Med 1991; 20:1248–1250.
- Manuchehry A, Fontana GP, Gurudevan S, Marchevsky AM, Siegel RJ. Missed diagnosis of limited ascending aortic dissection by multiple imaging modalities leading to fatal cardiac tamponade and aortic rupture. Echocardiography 2011; 28:E187–E190.
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993; 117:1027–1031.
- Tsang TS, Oh JK, Seward JB, Tajik AJ. Diagnostic value of echocardiography in cardiac tamponade. Herz 2000; 25:734–740.
- Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J 1988; 115:391–398.
- Wang JL, Hsieh MJ, Lee CH, et al. Hypothyroid cardiac tamponade: clinical features, electrocardiography, pericardial fluid and management. Am J Med Sci 2010; 340:276–281.
- Tamburro RF, Ring JC, Womback K. Detection of pulsus paradoxus associated with large pericardial effusions in pediatric patients by analysis of the pulse-oximetry waveform. Pediatrics 2002; 109:673–677.
- Spodick DH. Pulsus paradoxus. In:Spodick DH, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:191–199.
- Burke A, Jeudy J, Virmani R. Cardiac tumors. In:Topol EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:710–720.
- Roberts WC. Pericardial heart disease: Its morphologic features and its causes. Proc (Bayl Univ Med Cent) 2005; 18:38–55.
- Stoelting RK, Miller RD, editors. Basics of Anesthesia. 4th ed. New York, NY: Churchill Livingstone; 2000:264–265.
- Ainsworth CD, Salehian O. Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 2011; 123:e210–e211.
- Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587–610.
- Vandyke WH, Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med 1983; 309:595–596.
- Bernal JM, Pradhan J, Li T, Tchokonte R, Afonso L. Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Can J Cardiol 2007; 23:1155–1156.
- Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol 2011; 3:135–143.
- Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997; 111:1213–1221.
- Schiavone WA, Ghumrawi BK, Catalano DR, et al. The use of echocardiography in the emergency management of nonpenetrating traumatic cardiac rupture. Ann Emerg Med 1991; 20:1248–1250.
- Manuchehry A, Fontana GP, Gurudevan S, Marchevsky AM, Siegel RJ. Missed diagnosis of limited ascending aortic dissection by multiple imaging modalities leading to fatal cardiac tamponade and aortic rupture. Echocardiography 2011; 28:E187–E190.
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993; 117:1027–1031.
- Tsang TS, Oh JK, Seward JB, Tajik AJ. Diagnostic value of echocardiography in cardiac tamponade. Herz 2000; 25:734–740.
- Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J 1988; 115:391–398.
- Wang JL, Hsieh MJ, Lee CH, et al. Hypothyroid cardiac tamponade: clinical features, electrocardiography, pericardial fluid and management. Am J Med Sci 2010; 340:276–281.
- Tamburro RF, Ring JC, Womback K. Detection of pulsus paradoxus associated with large pericardial effusions in pediatric patients by analysis of the pulse-oximetry waveform. Pediatrics 2002; 109:673–677.
- Spodick DH. Pulsus paradoxus. In:Spodick DH, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:191–199.
- Burke A, Jeudy J, Virmani R. Cardiac tumors. In:Topol EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:710–720.
- Roberts WC. Pericardial heart disease: Its morphologic features and its causes. Proc (Bayl Univ Med Cent) 2005; 18:38–55.
- Stoelting RK, Miller RD, editors. Basics of Anesthesia. 4th ed. New York, NY: Churchill Livingstone; 2000:264–265.
- Ainsworth CD, Salehian O. Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 2011; 123:e210–e211.
- Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587–610.
- Vandyke WH, Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med 1983; 309:595–596.
- Bernal JM, Pradhan J, Li T, Tchokonte R, Afonso L. Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Can J Cardiol 2007; 23:1155–1156.
- Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol 2011; 3:135–143.
- Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997; 111:1213–1221.
KEY POINTS
- Slow accumulation of pericardial fluid can result in edema, whereas rapid accumulation leads to hypotension.
- Diuretics can worsen tamponade by removing enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure.
- Try to determine why cardiac tamponade has occurred. Cardiac or aortic rupture requires surgery. If the gross appearance of the pericardial fluid does not match the presumed etiology, reconsider your diagnosis.
- Always review imaging studies before making the diagnosis of cardiac tamponade.
- When cardiac tamponade is considered, pulsus paradoxus must be measured, and if present, integrated with other physical findings and the echocardiogram. However, pulsus paradoxus can be present in the absence of cardiac tamponade, and vice versa.
- Consider the size and location of the pericardial effusion and the patient’s hemodynamic status when deciding between surgery and needle aspiration.