User login
Cognition represents the most important function of the human brain and the essence of the mind. Cognitive functions such as memory, learning, comprehension, processing speed, attention, planning, and problem-solving are the best indicators of the status of brain health.
Many psychiatric brain disorders are associated with cognitive impairments. Decades of extensive research have documented that the most severe cognitive deficits occur in schizophrenia. No wonder Emil Kraepelin coined the term “dementia praecox,” which means premature dementia (in youth)1 for this neuropsychiatric brain disorder. This condition was later renamed schizophrenia by Eugen Bleuler,2 who regarded it primarily as a thought disorder, with splitting of associations (not split personality, as misinterpreted by many in the public). Interestingly, a century ago both of those early masters of psychiatry de-emphasized psychotic symptoms (delusions and hallucinations), regarding them as “supplemental symptoms.”3 Yet for the next 100 years, clinicians overemphasized psychotic symptoms in schizophrenia and overlooked the more disabling cognitive impairment and negative symptoms, referred to as Bleuler’s 4 A’s—Associations disruption, Ambivalence, Affect pathology, and Avolition—symptoms that persist even after the psychotic symptoms are successfully treated.3
Most contemporary researchers regard cognitive impairment as the “core” feature of schizophrenia.4 The justification of this view is that cognitive deficits are detected in childhood and early adolescence (by age 13),5 long before the appearance of psychotic symptoms, and many studies have confirmed that cognitive deficits are the primary cause of functional disability and unemployment of patients with schizophrenia. Cognitive dysfunction is also found in milder forms in the parents and siblings of patients with schizophrenia,6 and is thus considered an “endophenotype” of the illness.
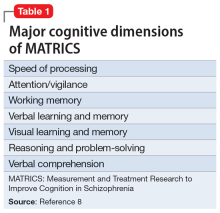
Because of its centrality, cognition has emerged as a major focus of schizophrenia research over the past 20 years. Multiple stakeholders (academic investigators, the National Institute of Mental Health, and the FDA) have collaborated to develop a standard measurement for cognition in schizophrenia. The project culminated in what was labeled MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia).7 The MATRICS settled on a battery of 7 major cognitive functions that are often impaired in individuals with schizophrenia (Table 18). Most contemporary researchers have adopted MATRICS in their studies, which facilitates replication to confirm research findings.
Measuring cognition in patients with schizophrenia is extremely important, as critical as measuring fasting glucose in patients with diabetes or blood pressure in patients with hypertension. Measuring the extent of impairment or nonimpairment across various cognitive tests can help with vocational rehabilitation, to place a patient in a job consistent with their level of cognitive functioning. In addition, once medications are developed and approved for cognitive impairments in schizophrenia, measuring cognition will be necessary to gauge the degree of improvement.
Currently, few psychiatric practitioners measure cognition in their patients. This is perplexing because cognitive measurement is important for confirming the diagnosis of schizophrenia in first-episode psychosis, or distinguishing it from other psychotic disorders (such as drug-induced psychosis, brief reactive psychosis, or delusional disorders) that do not have severe cognitive deficits.
The scores of various cognitive functions in individuals with schizophrenia range from .75 to 2.0 SD below the performance of the general population (matched for age and gender).9 This translates to dismally low percentiles of 2% and 24%. It is essential that all clinicians measure cognition in every patient with psychotic symptoms. It can be argued that cognition should even be measured in other psychiatric patients because cognitive deficits have been well documented in bipolar disorder, major depressive disorder, attention-deficit/hyperactivity disorder, and other disorders, albeit not as severe as in schizophrenia, and these deficits usually correlate with the patient’s vocational and social functioning.
Continue to: So how is cognition measured...
So how is cognition measured, and can clinicians incorporate cognitive batteries in their practices? The most logical answer is to refer the patient to a board-certified neuropsychologist. These specialists are well-trained in assessing cognitive functions, and their evaluations generally are covered by health insurance. They use various validated cognitive batteries. Table 210-18 lists the currently recognized cognitive assessments and how much time they require. Psychiatrists can have nurses or medical assistants administer a brief cognitive test.
C-SARS: A self-rated cognition scale
Patient self-rating can provide psychiatric clinicians with valuable information, and is a time-saver. The widely used Patient Health Questionaire-9 (PHQ-9)19 is an excellent example of a self-rating scale for depression that enables patients to recognize and rate their depressive symptoms. It immediately informs the clinician how depressed their patient is and whether the severity of the depression has improved from the previous visit, which can indicate whether the prescribed medication is working. Based on the PHQ-9, which I regularly use—and recognizing that there is no cognition counterpart and that almost all clinicians could use a practical method of measuring their patients’ cognitive function—I developed an instrument called the Cognition Self-Assessment Rating Scale (C-SARS) (Table 3). The C-SARS can be completed online at https://curesz.org/csars/ and patients will be emailed the results within a minute. The C-SARS can be completed by the patient (with the help of their family or caregiver, if necessary, who observe the patient’s daily functioning, which corresponds to their cognition). The main purpose of the C-SARS is to inform the clinician about serious cognitive dysfunction in their patients, which should instigate a referral for formal neurocognitive assessment by a neuropsychology expert.

The items on the C-SARS reflect how well the patient is performing routine daily functions, each of which correlates with one of the cognitive domains of the MATRICS battery. Table 3 shows the 12 items in the C-SARS, their scoring, and their clinical implications (ie, when the results require referral for formal neurocognitive testing). In the future, when the FDA approves medications for addressing cognitive impairment (and several molecules are currently undergoing clinical trials), clinicians will be able to gauge a patient’s response to such treatments using the C-SARS and formal testing as needed. It may take several weeks to detect a significant reversal of cognitive deficits, but doing so would address a major unmet need in schizophrenia and may speed up vocational rehabilitation. The C-SARS also contains 2 items related to social cognition (items 11 and 12), which is also impaired in schizophrenia.20 Future medications that improve social cognition in addition to neurocognition may also lead to improved social functioning among patients with schizophrenia.
In conclusion, the C-SARS, which needs to be validated in controlled studies, is the first cognition self-rating scale for schizophrenia and may be useful for other major psychiatric disorders. It will be a substantial time-saver for clinicians and will facilitate the routine incorporation of the cognitive assessment of patients with psychotic symptoms to help with the differential diagnosis of schizophrenia vs other psychotic disorders. Measuring cognitive functions is a vital step towards the valid diagnosis and treatment of this major clinical challenge in schizophrenia and improving patient outcomes in this serious psychiatric brain syndrome, in which up to 98% of patients have cognitive impairment across several domains.21
1. Kraepelin E. Dementia Praecox and Paraphrenia. Barth; 1904.
2. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
3. Nasrallah HA, Smeltzer DJ. Contemporary Diagnosis and Management of the Patient with Schizophrenia. Handbooks in Health Care Company; 2011.
4. Kahn RS, Keefe RSE. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107-1112.
5. van Oel CJ, Sitskoorn MM, Cremer MPM, et al. School performance as a premorbid marker for schizophrenia: a twin study. Schizophr Bull. 2002;28(3):401-414.
6. Jameson KG, Nasrallah HA, Northern TG, et al. Executive function in first-degree relatives of persons with schizophrenia: a meta-analysis of controlled studies. Asian J Psychiatry 2011;4(2):96-99.
7. Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72(1):5-9.
8. Neuchterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72(1):29-39.
9. Heinrich RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426-445.
10. Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery (MCCB). 3rd ed. MATRICS Assessment Inc.; 2016.
11. Robins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266-281.
12. Pietrzak RH, Olver J, Norman T, et al. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31(7):848-859.
13. Keefe RSE, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-297.
14. Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319.
15. Velligan DI, DiCocco M, Bow-Thomas CC, et al. A brief cognitive assessment for use with schizophrenia patients in community clinics. Schizophr Res. 2004;71(2-3):272-283.
16. Huford IM, Marder SR, Keefe RSE, et al. A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull. 2011;37(3):538-545.
17. Ventura J, Reise SP, Keefe RSE, et al. The Cognitive Assessment Interview (CAI): reliability and validity of a brief interview-based measure of cognition. Schizophr Bull. 2013;39(3):583-591.
18. Keefe RSE, Poe M, Walker TM, et al. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163(3):426-432.
19. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen Intern Med. 2001;16(9):606-613.
20. Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18(2):146-161.
21. Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
Cognition represents the most important function of the human brain and the essence of the mind. Cognitive functions such as memory, learning, comprehension, processing speed, attention, planning, and problem-solving are the best indicators of the status of brain health.
Many psychiatric brain disorders are associated with cognitive impairments. Decades of extensive research have documented that the most severe cognitive deficits occur in schizophrenia. No wonder Emil Kraepelin coined the term “dementia praecox,” which means premature dementia (in youth)1 for this neuropsychiatric brain disorder. This condition was later renamed schizophrenia by Eugen Bleuler,2 who regarded it primarily as a thought disorder, with splitting of associations (not split personality, as misinterpreted by many in the public). Interestingly, a century ago both of those early masters of psychiatry de-emphasized psychotic symptoms (delusions and hallucinations), regarding them as “supplemental symptoms.”3 Yet for the next 100 years, clinicians overemphasized psychotic symptoms in schizophrenia and overlooked the more disabling cognitive impairment and negative symptoms, referred to as Bleuler’s 4 A’s—Associations disruption, Ambivalence, Affect pathology, and Avolition—symptoms that persist even after the psychotic symptoms are successfully treated.3
Most contemporary researchers regard cognitive impairment as the “core” feature of schizophrenia.4 The justification of this view is that cognitive deficits are detected in childhood and early adolescence (by age 13),5 long before the appearance of psychotic symptoms, and many studies have confirmed that cognitive deficits are the primary cause of functional disability and unemployment of patients with schizophrenia. Cognitive dysfunction is also found in milder forms in the parents and siblings of patients with schizophrenia,6 and is thus considered an “endophenotype” of the illness.
Because of its centrality, cognition has emerged as a major focus of schizophrenia research over the past 20 years. Multiple stakeholders (academic investigators, the National Institute of Mental Health, and the FDA) have collaborated to develop a standard measurement for cognition in schizophrenia. The project culminated in what was labeled MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia).7 The MATRICS settled on a battery of 7 major cognitive functions that are often impaired in individuals with schizophrenia (Table 18). Most contemporary researchers have adopted MATRICS in their studies, which facilitates replication to confirm research findings.
Measuring cognition in patients with schizophrenia is extremely important, as critical as measuring fasting glucose in patients with diabetes or blood pressure in patients with hypertension. Measuring the extent of impairment or nonimpairment across various cognitive tests can help with vocational rehabilitation, to place a patient in a job consistent with their level of cognitive functioning. In addition, once medications are developed and approved for cognitive impairments in schizophrenia, measuring cognition will be necessary to gauge the degree of improvement.
Currently, few psychiatric practitioners measure cognition in their patients. This is perplexing because cognitive measurement is important for confirming the diagnosis of schizophrenia in first-episode psychosis, or distinguishing it from other psychotic disorders (such as drug-induced psychosis, brief reactive psychosis, or delusional disorders) that do not have severe cognitive deficits.
The scores of various cognitive functions in individuals with schizophrenia range from .75 to 2.0 SD below the performance of the general population (matched for age and gender).9 This translates to dismally low percentiles of 2% and 24%. It is essential that all clinicians measure cognition in every patient with psychotic symptoms. It can be argued that cognition should even be measured in other psychiatric patients because cognitive deficits have been well documented in bipolar disorder, major depressive disorder, attention-deficit/hyperactivity disorder, and other disorders, albeit not as severe as in schizophrenia, and these deficits usually correlate with the patient’s vocational and social functioning.
Continue to: So how is cognition measured...
So how is cognition measured, and can clinicians incorporate cognitive batteries in their practices? The most logical answer is to refer the patient to a board-certified neuropsychologist. These specialists are well-trained in assessing cognitive functions, and their evaluations generally are covered by health insurance. They use various validated cognitive batteries. Table 210-18 lists the currently recognized cognitive assessments and how much time they require. Psychiatrists can have nurses or medical assistants administer a brief cognitive test.
C-SARS: A self-rated cognition scale
Patient self-rating can provide psychiatric clinicians with valuable information, and is a time-saver. The widely used Patient Health Questionaire-9 (PHQ-9)19 is an excellent example of a self-rating scale for depression that enables patients to recognize and rate their depressive symptoms. It immediately informs the clinician how depressed their patient is and whether the severity of the depression has improved from the previous visit, which can indicate whether the prescribed medication is working. Based on the PHQ-9, which I regularly use—and recognizing that there is no cognition counterpart and that almost all clinicians could use a practical method of measuring their patients’ cognitive function—I developed an instrument called the Cognition Self-Assessment Rating Scale (C-SARS) (Table 3). The C-SARS can be completed online at https://curesz.org/csars/ and patients will be emailed the results within a minute. The C-SARS can be completed by the patient (with the help of their family or caregiver, if necessary, who observe the patient’s daily functioning, which corresponds to their cognition). The main purpose of the C-SARS is to inform the clinician about serious cognitive dysfunction in their patients, which should instigate a referral for formal neurocognitive assessment by a neuropsychology expert.

The items on the C-SARS reflect how well the patient is performing routine daily functions, each of which correlates with one of the cognitive domains of the MATRICS battery. Table 3 shows the 12 items in the C-SARS, their scoring, and their clinical implications (ie, when the results require referral for formal neurocognitive testing). In the future, when the FDA approves medications for addressing cognitive impairment (and several molecules are currently undergoing clinical trials), clinicians will be able to gauge a patient’s response to such treatments using the C-SARS and formal testing as needed. It may take several weeks to detect a significant reversal of cognitive deficits, but doing so would address a major unmet need in schizophrenia and may speed up vocational rehabilitation. The C-SARS also contains 2 items related to social cognition (items 11 and 12), which is also impaired in schizophrenia.20 Future medications that improve social cognition in addition to neurocognition may also lead to improved social functioning among patients with schizophrenia.
In conclusion, the C-SARS, which needs to be validated in controlled studies, is the first cognition self-rating scale for schizophrenia and may be useful for other major psychiatric disorders. It will be a substantial time-saver for clinicians and will facilitate the routine incorporation of the cognitive assessment of patients with psychotic symptoms to help with the differential diagnosis of schizophrenia vs other psychotic disorders. Measuring cognitive functions is a vital step towards the valid diagnosis and treatment of this major clinical challenge in schizophrenia and improving patient outcomes in this serious psychiatric brain syndrome, in which up to 98% of patients have cognitive impairment across several domains.21
Cognition represents the most important function of the human brain and the essence of the mind. Cognitive functions such as memory, learning, comprehension, processing speed, attention, planning, and problem-solving are the best indicators of the status of brain health.
Many psychiatric brain disorders are associated with cognitive impairments. Decades of extensive research have documented that the most severe cognitive deficits occur in schizophrenia. No wonder Emil Kraepelin coined the term “dementia praecox,” which means premature dementia (in youth)1 for this neuropsychiatric brain disorder. This condition was later renamed schizophrenia by Eugen Bleuler,2 who regarded it primarily as a thought disorder, with splitting of associations (not split personality, as misinterpreted by many in the public). Interestingly, a century ago both of those early masters of psychiatry de-emphasized psychotic symptoms (delusions and hallucinations), regarding them as “supplemental symptoms.”3 Yet for the next 100 years, clinicians overemphasized psychotic symptoms in schizophrenia and overlooked the more disabling cognitive impairment and negative symptoms, referred to as Bleuler’s 4 A’s—Associations disruption, Ambivalence, Affect pathology, and Avolition—symptoms that persist even after the psychotic symptoms are successfully treated.3
Most contemporary researchers regard cognitive impairment as the “core” feature of schizophrenia.4 The justification of this view is that cognitive deficits are detected in childhood and early adolescence (by age 13),5 long before the appearance of psychotic symptoms, and many studies have confirmed that cognitive deficits are the primary cause of functional disability and unemployment of patients with schizophrenia. Cognitive dysfunction is also found in milder forms in the parents and siblings of patients with schizophrenia,6 and is thus considered an “endophenotype” of the illness.
Because of its centrality, cognition has emerged as a major focus of schizophrenia research over the past 20 years. Multiple stakeholders (academic investigators, the National Institute of Mental Health, and the FDA) have collaborated to develop a standard measurement for cognition in schizophrenia. The project culminated in what was labeled MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia).7 The MATRICS settled on a battery of 7 major cognitive functions that are often impaired in individuals with schizophrenia (Table 18). Most contemporary researchers have adopted MATRICS in their studies, which facilitates replication to confirm research findings.
Measuring cognition in patients with schizophrenia is extremely important, as critical as measuring fasting glucose in patients with diabetes or blood pressure in patients with hypertension. Measuring the extent of impairment or nonimpairment across various cognitive tests can help with vocational rehabilitation, to place a patient in a job consistent with their level of cognitive functioning. In addition, once medications are developed and approved for cognitive impairments in schizophrenia, measuring cognition will be necessary to gauge the degree of improvement.
Currently, few psychiatric practitioners measure cognition in their patients. This is perplexing because cognitive measurement is important for confirming the diagnosis of schizophrenia in first-episode psychosis, or distinguishing it from other psychotic disorders (such as drug-induced psychosis, brief reactive psychosis, or delusional disorders) that do not have severe cognitive deficits.
The scores of various cognitive functions in individuals with schizophrenia range from .75 to 2.0 SD below the performance of the general population (matched for age and gender).9 This translates to dismally low percentiles of 2% and 24%. It is essential that all clinicians measure cognition in every patient with psychotic symptoms. It can be argued that cognition should even be measured in other psychiatric patients because cognitive deficits have been well documented in bipolar disorder, major depressive disorder, attention-deficit/hyperactivity disorder, and other disorders, albeit not as severe as in schizophrenia, and these deficits usually correlate with the patient’s vocational and social functioning.
Continue to: So how is cognition measured...
So how is cognition measured, and can clinicians incorporate cognitive batteries in their practices? The most logical answer is to refer the patient to a board-certified neuropsychologist. These specialists are well-trained in assessing cognitive functions, and their evaluations generally are covered by health insurance. They use various validated cognitive batteries. Table 210-18 lists the currently recognized cognitive assessments and how much time they require. Psychiatrists can have nurses or medical assistants administer a brief cognitive test.
C-SARS: A self-rated cognition scale
Patient self-rating can provide psychiatric clinicians with valuable information, and is a time-saver. The widely used Patient Health Questionaire-9 (PHQ-9)19 is an excellent example of a self-rating scale for depression that enables patients to recognize and rate their depressive symptoms. It immediately informs the clinician how depressed their patient is and whether the severity of the depression has improved from the previous visit, which can indicate whether the prescribed medication is working. Based on the PHQ-9, which I regularly use—and recognizing that there is no cognition counterpart and that almost all clinicians could use a practical method of measuring their patients’ cognitive function—I developed an instrument called the Cognition Self-Assessment Rating Scale (C-SARS) (Table 3). The C-SARS can be completed online at https://curesz.org/csars/ and patients will be emailed the results within a minute. The C-SARS can be completed by the patient (with the help of their family or caregiver, if necessary, who observe the patient’s daily functioning, which corresponds to their cognition). The main purpose of the C-SARS is to inform the clinician about serious cognitive dysfunction in their patients, which should instigate a referral for formal neurocognitive assessment by a neuropsychology expert.

The items on the C-SARS reflect how well the patient is performing routine daily functions, each of which correlates with one of the cognitive domains of the MATRICS battery. Table 3 shows the 12 items in the C-SARS, their scoring, and their clinical implications (ie, when the results require referral for formal neurocognitive testing). In the future, when the FDA approves medications for addressing cognitive impairment (and several molecules are currently undergoing clinical trials), clinicians will be able to gauge a patient’s response to such treatments using the C-SARS and formal testing as needed. It may take several weeks to detect a significant reversal of cognitive deficits, but doing so would address a major unmet need in schizophrenia and may speed up vocational rehabilitation. The C-SARS also contains 2 items related to social cognition (items 11 and 12), which is also impaired in schizophrenia.20 Future medications that improve social cognition in addition to neurocognition may also lead to improved social functioning among patients with schizophrenia.
In conclusion, the C-SARS, which needs to be validated in controlled studies, is the first cognition self-rating scale for schizophrenia and may be useful for other major psychiatric disorders. It will be a substantial time-saver for clinicians and will facilitate the routine incorporation of the cognitive assessment of patients with psychotic symptoms to help with the differential diagnosis of schizophrenia vs other psychotic disorders. Measuring cognitive functions is a vital step towards the valid diagnosis and treatment of this major clinical challenge in schizophrenia and improving patient outcomes in this serious psychiatric brain syndrome, in which up to 98% of patients have cognitive impairment across several domains.21
1. Kraepelin E. Dementia Praecox and Paraphrenia. Barth; 1904.
2. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
3. Nasrallah HA, Smeltzer DJ. Contemporary Diagnosis and Management of the Patient with Schizophrenia. Handbooks in Health Care Company; 2011.
4. Kahn RS, Keefe RSE. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107-1112.
5. van Oel CJ, Sitskoorn MM, Cremer MPM, et al. School performance as a premorbid marker for schizophrenia: a twin study. Schizophr Bull. 2002;28(3):401-414.
6. Jameson KG, Nasrallah HA, Northern TG, et al. Executive function in first-degree relatives of persons with schizophrenia: a meta-analysis of controlled studies. Asian J Psychiatry 2011;4(2):96-99.
7. Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72(1):5-9.
8. Neuchterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72(1):29-39.
9. Heinrich RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426-445.
10. Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery (MCCB). 3rd ed. MATRICS Assessment Inc.; 2016.
11. Robins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266-281.
12. Pietrzak RH, Olver J, Norman T, et al. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31(7):848-859.
13. Keefe RSE, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-297.
14. Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319.
15. Velligan DI, DiCocco M, Bow-Thomas CC, et al. A brief cognitive assessment for use with schizophrenia patients in community clinics. Schizophr Res. 2004;71(2-3):272-283.
16. Huford IM, Marder SR, Keefe RSE, et al. A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull. 2011;37(3):538-545.
17. Ventura J, Reise SP, Keefe RSE, et al. The Cognitive Assessment Interview (CAI): reliability and validity of a brief interview-based measure of cognition. Schizophr Bull. 2013;39(3):583-591.
18. Keefe RSE, Poe M, Walker TM, et al. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163(3):426-432.
19. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen Intern Med. 2001;16(9):606-613.
20. Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18(2):146-161.
21. Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
1. Kraepelin E. Dementia Praecox and Paraphrenia. Barth; 1904.
2. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
3. Nasrallah HA, Smeltzer DJ. Contemporary Diagnosis and Management of the Patient with Schizophrenia. Handbooks in Health Care Company; 2011.
4. Kahn RS, Keefe RSE. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107-1112.
5. van Oel CJ, Sitskoorn MM, Cremer MPM, et al. School performance as a premorbid marker for schizophrenia: a twin study. Schizophr Bull. 2002;28(3):401-414.
6. Jameson KG, Nasrallah HA, Northern TG, et al. Executive function in first-degree relatives of persons with schizophrenia: a meta-analysis of controlled studies. Asian J Psychiatry 2011;4(2):96-99.
7. Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72(1):5-9.
8. Neuchterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72(1):29-39.
9. Heinrich RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426-445.
10. Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery (MCCB). 3rd ed. MATRICS Assessment Inc.; 2016.
11. Robins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266-281.
12. Pietrzak RH, Olver J, Norman T, et al. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31(7):848-859.
13. Keefe RSE, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-297.
14. Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319.
15. Velligan DI, DiCocco M, Bow-Thomas CC, et al. A brief cognitive assessment for use with schizophrenia patients in community clinics. Schizophr Res. 2004;71(2-3):272-283.
16. Huford IM, Marder SR, Keefe RSE, et al. A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull. 2011;37(3):538-545.
17. Ventura J, Reise SP, Keefe RSE, et al. The Cognitive Assessment Interview (CAI): reliability and validity of a brief interview-based measure of cognition. Schizophr Bull. 2013;39(3):583-591.
18. Keefe RSE, Poe M, Walker TM, et al. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163(3):426-432.
19. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen Intern Med. 2001;16(9):606-613.
20. Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18(2):146-161.
21. Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.