User login
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
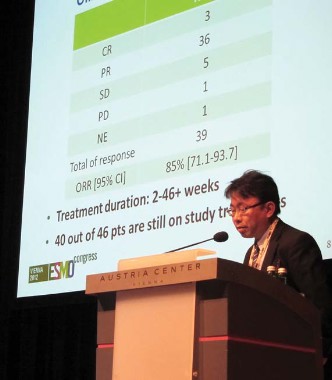
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Response rates reached as high as 85% in patients with advanced tumors that were predominantly non-small cell lung cancer.
Data Source: Investigators reported on early phase I/II trials of novel ALK inhibitors and a second-generation heat shock protein 90 inhibitor.
Disclosures: Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.