User login
Gefitinib Palliates Advanced Esophageal Cancer
VIENNA – Gefitinib failed to improve overall survival in advanced esophageal cancer but could prove beneficial in a setting in which palliative care is the standard of care.
Gefitinib (Iressa) significantly improved progression-free survival and provided some dramatic and durable responses in palliation in the first-ever randomized trial of second-line therapy for esophageal cancer, Dr. David Ferry said at the European Society for Medical Oncology Congress.
In addition, the Cancer Oesophagus Gefitinib (COG) trial demonstrated a striking effect of performance status on survival in 450 patients treated with the epidermal growth factor receptor inhibitor.
Median overall survival increased significantly from 1.97 months in patients with a performance status (PS) of 2 to 3.93 months and 6.03 months, respectively, in those with PS 1 and PS 0 (P value less than .0001; hazard ratios, 1.00, 1.40, 2.98, respectively).
"We can now look at performance status as a prognostic factor for patients with this disease," said Dr. Ferry, of New Cross Hospital, Wolverhampton, England.
The next step lies in the ongoing, companion translational research project, TRANS-COG, analyzing predictive biomarkers in more than 300 of the 450 patients’ biopsies in an effort to identify a molecularly defined subgroup of patients most likely to benefit from gefitinib.
"If we can identify a subgroup of patients – and I think we’re fairly optimistic we can – then we will move on to another trial where placebo will be the control arm and a different targeted agent will be the experimental arm," Dr. Ferry said at a press briefing held at the congress.
He would not say what targeted agent that might be and added that there’s not sufficient benefit in an unselected population for clinicians to begin using gefitinib in their patients with advanced esophageal cancer, "no matter how tempted you might be."
More Study Details
The Cancer Oesophagus Gefitinib was prompted by a round of promising single-agent phase II trials involving the epidermal growth factor receptor inhibitors gefitinib and erlotinib reporting responses in the second-line setting for esophageal cancer. Roughly 50%-70% of esophageal cancers overexpress epidermal growth factor receptor.
At the moment, there is no systemic therapy that has significantly altered the natural history of metastatic esophageal cancer progressing after chemotherapy.
Investigators at 51 centers in the United Kingdom evenly randomized 450 patients with metastatic esophageal cancers and type I/II junctional tumors to once-daily oral gefitinib 500 mg or placebo until progression.
Patients with stable brain metastases who had received prior cranial irradiation were not excluded from the trial.
Three-fourths of patients had adenocarcinoma, 80% had esophageal involvement, and one-third had received at least two prior treatments.
PS was balanced between the placebo and gefitinib arms, with 25% and 26% at PS 0, 55% and 52% at PS 1 and 20% and 22% at PS 2. They were a median age of 64 years.
Progression-free survival was 1.17 months in the placebo arm and 1.60 months in the gefitinib arm, which was statistically significant (P = .017; HR, 0.79), Dr. Ferry said.
Exploratory subgroup analyses showed that all patients, regardless of cytology, disease site, age, sex, and time since diagnosis, benefited equally.
This positive effect on progression did not translate into an overall survival benefit, with a median overall survival of 3.60 months for placebo and 3.73 months for gefitinib (P = .285; HR, 0.90).
At 4 weeks, patients receiving gefitinib reported significant improvements in difficulty swallowing (P = .004), but not in global health quality of life, dysphagia, or difficulty eating, the three other prespecified health-related quality of life outcomes.
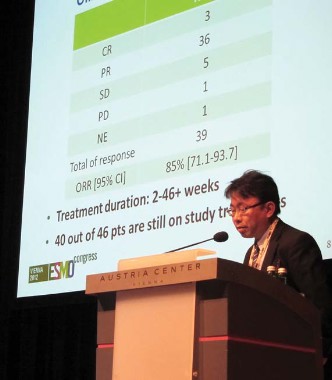
Disease control rates at 8 weeks, however, significantly favored gefitinib over placebo (26% vs. 16%, P = .014), he said. True partial responses occurred in 3.1% vs. 0.4%, respectively.
Dr. Ferry presented images illustrating rapid and durable radiologic responses that were associated with palliation in patients with adenocarcinoma and squamous cancers. Patient receiving gefitinib experienced weight gain, increased appetite, and reduced chest wall pain, with responses lasting 18 months in one patient.
There were no new safety signals with gefitinib, although there was an excess of diarrhea and skin toxicity, he said.
Discussant Arnaud D. Roth of Geneva University Hospital told the audience it would be easy to write COG off as another boring, negative study in esophageal cancer but pointed out that deeper analyses of molecular and clinical factors turned the initially negative PETACC-3 trial in colon cancer into a success story.
"A study of this size is a fantastic opportunity to learn more about esophageal cancer biology," he said, remarking that gefitinib in esophageal cancer is "just the beginning of a spicy story," said Dr. Roth, chief of oncosurgery at the hospital.
Cancer Research UK sponsored the trial. Dr. Ferry reported grant support, honoraria, and an advisory relationship with AstraZeneca in the development of gefitinib.
VIENNA – Gefitinib failed to improve overall survival in advanced esophageal cancer but could prove beneficial in a setting in which palliative care is the standard of care.
Gefitinib (Iressa) significantly improved progression-free survival and provided some dramatic and durable responses in palliation in the first-ever randomized trial of second-line therapy for esophageal cancer, Dr. David Ferry said at the European Society for Medical Oncology Congress.
In addition, the Cancer Oesophagus Gefitinib (COG) trial demonstrated a striking effect of performance status on survival in 450 patients treated with the epidermal growth factor receptor inhibitor.
Median overall survival increased significantly from 1.97 months in patients with a performance status (PS) of 2 to 3.93 months and 6.03 months, respectively, in those with PS 1 and PS 0 (P value less than .0001; hazard ratios, 1.00, 1.40, 2.98, respectively).
"We can now look at performance status as a prognostic factor for patients with this disease," said Dr. Ferry, of New Cross Hospital, Wolverhampton, England.
The next step lies in the ongoing, companion translational research project, TRANS-COG, analyzing predictive biomarkers in more than 300 of the 450 patients’ biopsies in an effort to identify a molecularly defined subgroup of patients most likely to benefit from gefitinib.
"If we can identify a subgroup of patients – and I think we’re fairly optimistic we can – then we will move on to another trial where placebo will be the control arm and a different targeted agent will be the experimental arm," Dr. Ferry said at a press briefing held at the congress.
He would not say what targeted agent that might be and added that there’s not sufficient benefit in an unselected population for clinicians to begin using gefitinib in their patients with advanced esophageal cancer, "no matter how tempted you might be."
More Study Details
The Cancer Oesophagus Gefitinib was prompted by a round of promising single-agent phase II trials involving the epidermal growth factor receptor inhibitors gefitinib and erlotinib reporting responses in the second-line setting for esophageal cancer. Roughly 50%-70% of esophageal cancers overexpress epidermal growth factor receptor.
At the moment, there is no systemic therapy that has significantly altered the natural history of metastatic esophageal cancer progressing after chemotherapy.
Investigators at 51 centers in the United Kingdom evenly randomized 450 patients with metastatic esophageal cancers and type I/II junctional tumors to once-daily oral gefitinib 500 mg or placebo until progression.
Patients with stable brain metastases who had received prior cranial irradiation were not excluded from the trial.
Three-fourths of patients had adenocarcinoma, 80% had esophageal involvement, and one-third had received at least two prior treatments.
PS was balanced between the placebo and gefitinib arms, with 25% and 26% at PS 0, 55% and 52% at PS 1 and 20% and 22% at PS 2. They were a median age of 64 years.
Progression-free survival was 1.17 months in the placebo arm and 1.60 months in the gefitinib arm, which was statistically significant (P = .017; HR, 0.79), Dr. Ferry said.
Exploratory subgroup analyses showed that all patients, regardless of cytology, disease site, age, sex, and time since diagnosis, benefited equally.
This positive effect on progression did not translate into an overall survival benefit, with a median overall survival of 3.60 months for placebo and 3.73 months for gefitinib (P = .285; HR, 0.90).
At 4 weeks, patients receiving gefitinib reported significant improvements in difficulty swallowing (P = .004), but not in global health quality of life, dysphagia, or difficulty eating, the three other prespecified health-related quality of life outcomes.
Disease control rates at 8 weeks, however, significantly favored gefitinib over placebo (26% vs. 16%, P = .014), he said. True partial responses occurred in 3.1% vs. 0.4%, respectively.
Dr. Ferry presented images illustrating rapid and durable radiologic responses that were associated with palliation in patients with adenocarcinoma and squamous cancers. Patient receiving gefitinib experienced weight gain, increased appetite, and reduced chest wall pain, with responses lasting 18 months in one patient.
There were no new safety signals with gefitinib, although there was an excess of diarrhea and skin toxicity, he said.
Discussant Arnaud D. Roth of Geneva University Hospital told the audience it would be easy to write COG off as another boring, negative study in esophageal cancer but pointed out that deeper analyses of molecular and clinical factors turned the initially negative PETACC-3 trial in colon cancer into a success story.
"A study of this size is a fantastic opportunity to learn more about esophageal cancer biology," he said, remarking that gefitinib in esophageal cancer is "just the beginning of a spicy story," said Dr. Roth, chief of oncosurgery at the hospital.
Cancer Research UK sponsored the trial. Dr. Ferry reported grant support, honoraria, and an advisory relationship with AstraZeneca in the development of gefitinib.
VIENNA – Gefitinib failed to improve overall survival in advanced esophageal cancer but could prove beneficial in a setting in which palliative care is the standard of care.
Gefitinib (Iressa) significantly improved progression-free survival and provided some dramatic and durable responses in palliation in the first-ever randomized trial of second-line therapy for esophageal cancer, Dr. David Ferry said at the European Society for Medical Oncology Congress.
In addition, the Cancer Oesophagus Gefitinib (COG) trial demonstrated a striking effect of performance status on survival in 450 patients treated with the epidermal growth factor receptor inhibitor.
Median overall survival increased significantly from 1.97 months in patients with a performance status (PS) of 2 to 3.93 months and 6.03 months, respectively, in those with PS 1 and PS 0 (P value less than .0001; hazard ratios, 1.00, 1.40, 2.98, respectively).
"We can now look at performance status as a prognostic factor for patients with this disease," said Dr. Ferry, of New Cross Hospital, Wolverhampton, England.
The next step lies in the ongoing, companion translational research project, TRANS-COG, analyzing predictive biomarkers in more than 300 of the 450 patients’ biopsies in an effort to identify a molecularly defined subgroup of patients most likely to benefit from gefitinib.
"If we can identify a subgroup of patients – and I think we’re fairly optimistic we can – then we will move on to another trial where placebo will be the control arm and a different targeted agent will be the experimental arm," Dr. Ferry said at a press briefing held at the congress.
He would not say what targeted agent that might be and added that there’s not sufficient benefit in an unselected population for clinicians to begin using gefitinib in their patients with advanced esophageal cancer, "no matter how tempted you might be."
More Study Details
The Cancer Oesophagus Gefitinib was prompted by a round of promising single-agent phase II trials involving the epidermal growth factor receptor inhibitors gefitinib and erlotinib reporting responses in the second-line setting for esophageal cancer. Roughly 50%-70% of esophageal cancers overexpress epidermal growth factor receptor.
At the moment, there is no systemic therapy that has significantly altered the natural history of metastatic esophageal cancer progressing after chemotherapy.
Investigators at 51 centers in the United Kingdom evenly randomized 450 patients with metastatic esophageal cancers and type I/II junctional tumors to once-daily oral gefitinib 500 mg or placebo until progression.
Patients with stable brain metastases who had received prior cranial irradiation were not excluded from the trial.
Three-fourths of patients had adenocarcinoma, 80% had esophageal involvement, and one-third had received at least two prior treatments.
PS was balanced between the placebo and gefitinib arms, with 25% and 26% at PS 0, 55% and 52% at PS 1 and 20% and 22% at PS 2. They were a median age of 64 years.
Progression-free survival was 1.17 months in the placebo arm and 1.60 months in the gefitinib arm, which was statistically significant (P = .017; HR, 0.79), Dr. Ferry said.
Exploratory subgroup analyses showed that all patients, regardless of cytology, disease site, age, sex, and time since diagnosis, benefited equally.
This positive effect on progression did not translate into an overall survival benefit, with a median overall survival of 3.60 months for placebo and 3.73 months for gefitinib (P = .285; HR, 0.90).
At 4 weeks, patients receiving gefitinib reported significant improvements in difficulty swallowing (P = .004), but not in global health quality of life, dysphagia, or difficulty eating, the three other prespecified health-related quality of life outcomes.
Disease control rates at 8 weeks, however, significantly favored gefitinib over placebo (26% vs. 16%, P = .014), he said. True partial responses occurred in 3.1% vs. 0.4%, respectively.
Dr. Ferry presented images illustrating rapid and durable radiologic responses that were associated with palliation in patients with adenocarcinoma and squamous cancers. Patient receiving gefitinib experienced weight gain, increased appetite, and reduced chest wall pain, with responses lasting 18 months in one patient.
There were no new safety signals with gefitinib, although there was an excess of diarrhea and skin toxicity, he said.
Discussant Arnaud D. Roth of Geneva University Hospital told the audience it would be easy to write COG off as another boring, negative study in esophageal cancer but pointed out that deeper analyses of molecular and clinical factors turned the initially negative PETACC-3 trial in colon cancer into a success story.
"A study of this size is a fantastic opportunity to learn more about esophageal cancer biology," he said, remarking that gefitinib in esophageal cancer is "just the beginning of a spicy story," said Dr. Roth, chief of oncosurgery at the hospital.
Cancer Research UK sponsored the trial. Dr. Ferry reported grant support, honoraria, and an advisory relationship with AstraZeneca in the development of gefitinib.
Major Finding: Median overall survival was 3.73 months with gefitinib vs. 3.60 months with placebo (P = .285).
Data Source: Data are from a phase III randomized trial of gefitinib in patients with esophageal cancer progressing after chemotherapy.
Disclosures: Cancer Research U.K. sponsored the trial and AstraZeneca supplied gefitinib and the matched placebo. Dr. Ferry reported grant support, honoraria, and advising AstraZeneca in the development of gefitinib.
Cetuximab Fails First-Line in Gastric Cancer
VIENNA – Adding cetuximab to first-line chemotherapy for gastric cancer was associated with a worse benefit-to-risk ratio in the open-label, randomized, con-trolled, phase III EXPAND trial.
Investigators reported that progression-free survival was nonsignificantly decreased in patients who had received cetuximab (Erbitux) in addition to capecitabine (Xeloda) and cisplatin treatment vs. those just receiving the chemotherapy doublet. Progression-free survival, which was the primary end point, was 4.4 months versus 5.6 months, respectively, with a hazard ratio (HR) of 1.09 (P = .3158).
There was also no benefit in overall survival or objective response rates. Overall survival was 9.4 months vs. 10.7 months (HR = 1.0; P = .96). The best objective response rates were 30% and 29%, respectively.
"Unfortunately, there was no benefit from adding cetuximab to chemotherapy [consisting of] capecitabine and cisplatin as first-line treatment for advanced gastric cancer," said Dr. Florian Lordick, who presented the findings at the European Society for Medical Oncology Congress.
"The results are consistent across subgroups," added Dr. Lordick, of the University Cancer Center Leipzig in Germany.
This is not the first disappointment seen with the use of an epidermal growth factor receptor (EGFR) inhibitor in gastric cancer. Recently reported results of the REAL-3 trial showed that panitumumab (Vectibix) was also not of benefit when added to standard chemotherapy for esophagogastric cancer.
Cetuximab also failed to show a benefit in patients when added to adjuvant oxaliplatin-containing chemotherapy for advanced metastatic colorectal cancer in the PETACC8 trial.
It is approved by the Food and Drug Administration in combination with irinotecan-containing regimens against EGFR-expressing metastatic colorectal cancer.
In the EXPAND study, 870 patients with unresected, advanced adenocarcinoma of the stomach gastric or gastroesophageal junction were rand-omized to receive cisplatin (80 mg/m2 on day 1) and capecitabine (1,000 mg/m2 twice daily, starting the evening of day 1 until the morning of day 15) with or without additional cetuximab (400 mg/m2 initial dose, then 250 mg/m2 per week).
The median age of enrolled patients was 59-60 years. Three quarters of participants were men and the main tumor site was the stomach in about a third of the cases with almost all patients having metastatic disease.
"Interestingly, the rate of hematologic adverse events was somewhat lower in the chemotherapy plus cetuximab arm," Dr. Lordick said. Compared with chemotherapy only, the rates of grade 3/4 neutropenia were 22% with cetuximab and 32% without. Grades 3/4 anemia occurred in 9% vs. 11% of patients, respectively.
"This is in contrast to other studies that have been performed in colorectal cancer, head and neck cancer, and lung cancer, where usually an increase of hematologic toxicity is seen with the administration of cetuximab in combination with chemotherapy," Dr. Lordick observed.
Nonhematologic adverse events, however, were more common in the cetuximab-containing vs. cetuximab-free arm, including grade 3 or 4 skin reactions (13% vs. 0%), diarrhea (8% vs. 4%), hand-foot syndrome (7% vs. 2%), hypomagnesemia (11% vs. 1%), and hypokalemia (13% vs. 9%).
Patients given the cetuximab-containing regimen also had a slightly higher rate of grade 3/4 cardiac adverse events (6.7% vs. 4.1 %).
"This study confirms a little bit what we heard at ASCO in the REAL-3 trial," said Dr. Arnaud D. Roth of Geneva University Hospital. Dr. Roth, who was not involved in the EXPAND study, noted that it was a well-designed and -conducted study and although negative, provided an excellent opportunity to conduct translational research.
However, Dr. Roth disagreed that this was the right setting to learn much more about anti-EGFRs and gastric cancer. Nevertheless, gaining access to the data and biospecimens was important and "a moral obligation" considering the patients who had given their consent for their participation in the trial.
"The good news is that tissue is available from 97% of patients and biomarker analysis is ongoing," Dr. Lordick said prior to Dr. Roth’s comments.
The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline, and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an adviser for Merck.
VIENNA – Adding cetuximab to first-line chemotherapy for gastric cancer was associated with a worse benefit-to-risk ratio in the open-label, randomized, con-trolled, phase III EXPAND trial.
Investigators reported that progression-free survival was nonsignificantly decreased in patients who had received cetuximab (Erbitux) in addition to capecitabine (Xeloda) and cisplatin treatment vs. those just receiving the chemotherapy doublet. Progression-free survival, which was the primary end point, was 4.4 months versus 5.6 months, respectively, with a hazard ratio (HR) of 1.09 (P = .3158).
There was also no benefit in overall survival or objective response rates. Overall survival was 9.4 months vs. 10.7 months (HR = 1.0; P = .96). The best objective response rates were 30% and 29%, respectively.
"Unfortunately, there was no benefit from adding cetuximab to chemotherapy [consisting of] capecitabine and cisplatin as first-line treatment for advanced gastric cancer," said Dr. Florian Lordick, who presented the findings at the European Society for Medical Oncology Congress.
"The results are consistent across subgroups," added Dr. Lordick, of the University Cancer Center Leipzig in Germany.
This is not the first disappointment seen with the use of an epidermal growth factor receptor (EGFR) inhibitor in gastric cancer. Recently reported results of the REAL-3 trial showed that panitumumab (Vectibix) was also not of benefit when added to standard chemotherapy for esophagogastric cancer.
Cetuximab also failed to show a benefit in patients when added to adjuvant oxaliplatin-containing chemotherapy for advanced metastatic colorectal cancer in the PETACC8 trial.
It is approved by the Food and Drug Administration in combination with irinotecan-containing regimens against EGFR-expressing metastatic colorectal cancer.
In the EXPAND study, 870 patients with unresected, advanced adenocarcinoma of the stomach gastric or gastroesophageal junction were rand-omized to receive cisplatin (80 mg/m2 on day 1) and capecitabine (1,000 mg/m2 twice daily, starting the evening of day 1 until the morning of day 15) with or without additional cetuximab (400 mg/m2 initial dose, then 250 mg/m2 per week).
The median age of enrolled patients was 59-60 years. Three quarters of participants were men and the main tumor site was the stomach in about a third of the cases with almost all patients having metastatic disease.
"Interestingly, the rate of hematologic adverse events was somewhat lower in the chemotherapy plus cetuximab arm," Dr. Lordick said. Compared with chemotherapy only, the rates of grade 3/4 neutropenia were 22% with cetuximab and 32% without. Grades 3/4 anemia occurred in 9% vs. 11% of patients, respectively.
"This is in contrast to other studies that have been performed in colorectal cancer, head and neck cancer, and lung cancer, where usually an increase of hematologic toxicity is seen with the administration of cetuximab in combination with chemotherapy," Dr. Lordick observed.
Nonhematologic adverse events, however, were more common in the cetuximab-containing vs. cetuximab-free arm, including grade 3 or 4 skin reactions (13% vs. 0%), diarrhea (8% vs. 4%), hand-foot syndrome (7% vs. 2%), hypomagnesemia (11% vs. 1%), and hypokalemia (13% vs. 9%).
Patients given the cetuximab-containing regimen also had a slightly higher rate of grade 3/4 cardiac adverse events (6.7% vs. 4.1 %).
"This study confirms a little bit what we heard at ASCO in the REAL-3 trial," said Dr. Arnaud D. Roth of Geneva University Hospital. Dr. Roth, who was not involved in the EXPAND study, noted that it was a well-designed and -conducted study and although negative, provided an excellent opportunity to conduct translational research.
However, Dr. Roth disagreed that this was the right setting to learn much more about anti-EGFRs and gastric cancer. Nevertheless, gaining access to the data and biospecimens was important and "a moral obligation" considering the patients who had given their consent for their participation in the trial.
"The good news is that tissue is available from 97% of patients and biomarker analysis is ongoing," Dr. Lordick said prior to Dr. Roth’s comments.
The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline, and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an adviser for Merck.
VIENNA – Adding cetuximab to first-line chemotherapy for gastric cancer was associated with a worse benefit-to-risk ratio in the open-label, randomized, con-trolled, phase III EXPAND trial.
Investigators reported that progression-free survival was nonsignificantly decreased in patients who had received cetuximab (Erbitux) in addition to capecitabine (Xeloda) and cisplatin treatment vs. those just receiving the chemotherapy doublet. Progression-free survival, which was the primary end point, was 4.4 months versus 5.6 months, respectively, with a hazard ratio (HR) of 1.09 (P = .3158).
There was also no benefit in overall survival or objective response rates. Overall survival was 9.4 months vs. 10.7 months (HR = 1.0; P = .96). The best objective response rates were 30% and 29%, respectively.
"Unfortunately, there was no benefit from adding cetuximab to chemotherapy [consisting of] capecitabine and cisplatin as first-line treatment for advanced gastric cancer," said Dr. Florian Lordick, who presented the findings at the European Society for Medical Oncology Congress.
"The results are consistent across subgroups," added Dr. Lordick, of the University Cancer Center Leipzig in Germany.
This is not the first disappointment seen with the use of an epidermal growth factor receptor (EGFR) inhibitor in gastric cancer. Recently reported results of the REAL-3 trial showed that panitumumab (Vectibix) was also not of benefit when added to standard chemotherapy for esophagogastric cancer.
Cetuximab also failed to show a benefit in patients when added to adjuvant oxaliplatin-containing chemotherapy for advanced metastatic colorectal cancer in the PETACC8 trial.
It is approved by the Food and Drug Administration in combination with irinotecan-containing regimens against EGFR-expressing metastatic colorectal cancer.
In the EXPAND study, 870 patients with unresected, advanced adenocarcinoma of the stomach gastric or gastroesophageal junction were rand-omized to receive cisplatin (80 mg/m2 on day 1) and capecitabine (1,000 mg/m2 twice daily, starting the evening of day 1 until the morning of day 15) with or without additional cetuximab (400 mg/m2 initial dose, then 250 mg/m2 per week).
The median age of enrolled patients was 59-60 years. Three quarters of participants were men and the main tumor site was the stomach in about a third of the cases with almost all patients having metastatic disease.
"Interestingly, the rate of hematologic adverse events was somewhat lower in the chemotherapy plus cetuximab arm," Dr. Lordick said. Compared with chemotherapy only, the rates of grade 3/4 neutropenia were 22% with cetuximab and 32% without. Grades 3/4 anemia occurred in 9% vs. 11% of patients, respectively.
"This is in contrast to other studies that have been performed in colorectal cancer, head and neck cancer, and lung cancer, where usually an increase of hematologic toxicity is seen with the administration of cetuximab in combination with chemotherapy," Dr. Lordick observed.
Nonhematologic adverse events, however, were more common in the cetuximab-containing vs. cetuximab-free arm, including grade 3 or 4 skin reactions (13% vs. 0%), diarrhea (8% vs. 4%), hand-foot syndrome (7% vs. 2%), hypomagnesemia (11% vs. 1%), and hypokalemia (13% vs. 9%).
Patients given the cetuximab-containing regimen also had a slightly higher rate of grade 3/4 cardiac adverse events (6.7% vs. 4.1 %).
"This study confirms a little bit what we heard at ASCO in the REAL-3 trial," said Dr. Arnaud D. Roth of Geneva University Hospital. Dr. Roth, who was not involved in the EXPAND study, noted that it was a well-designed and -conducted study and although negative, provided an excellent opportunity to conduct translational research.
However, Dr. Roth disagreed that this was the right setting to learn much more about anti-EGFRs and gastric cancer. Nevertheless, gaining access to the data and biospecimens was important and "a moral obligation" considering the patients who had given their consent for their participation in the trial.
"The good news is that tissue is available from 97% of patients and biomarker analysis is ongoing," Dr. Lordick said prior to Dr. Roth’s comments.
The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline, and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an adviser for Merck.
Major Findings: Median progression-free survival was 4.4 vs. 5.6 months (HR = 1.09) when comparing triple treatment with cetuximab, capecitabine, and cisplatin with the chemotherapy doublet alone.
Data Source: EXPAND, an open-label, randomized, controlled phase III trial, enrolled 870 patients with advanced gastric cancer.
Disclosures: The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline, and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an adviser for Merck.
Immunomodulator MGN1703 Cuts Risk of Colorectal Cancer Progressing
VIENNA – Maintenance therapy with the investigational immunomodulator MGN1703 reduced the risk of progression in patients with metastatic colorectal cancer in the phase II/III IMPACT trial.
Median progression-free survival from the start of maintenance therapy was 2.8 months with MGN1703 and 2.6 months with placebo. This was not statistically significant (P = .06) but represented a 47% reduction in the risk of progression (hazard ratio, 0.53).
The benefit was even greater in a subgroup of good-risk patients, where the risk of progression was reduced 61% and the median time to progression more than doubled from 2.7 to 5.8 months with MGN1703 (HR, .39; P = .013).
"The findings indicate a potential new approach in the management of metastatic colorectal cancer patients," investigator Dr. Dirk Arnold said at the European Society for Medical Oncology Congress.
MGN1703 is an immunomodulator and toll-like receptor (TLR) 9 agonist that activates all components of the innate and adaptive immune system.
TLR9 agonists have shown activity in clinical trials in renal cell carcinoma, cutaneous T-cell lymphoma, and non-Hodgkin’s lymphoma. In a prior study, a TLR9 agonist combined with first-line taxane-plus-platinum chemotherapy reduced the risk of death from advanced-stage non–small cell lung cancer by 26% (J. Clin. Oncol. 2008;26:3979-86).
Dr. Jean-Yves Douillard, who was invited to discuss the study, agreed that immunomodulation represents a new approach to metastatic colorectal cancer.
"This is an additional set of data showing activity with TLR9 agonists, but this is the first time in metastatic colorectal cancer, which has never been considered a really immunosensitive type of tumor," he said. "The tolerance profile is excellent and this type of approach certainly deserves further evaluation in a larger sample to be convincing."
IMPACT was stopped early after an interim analysis of 55 patients because of slow accrual and observed benefit with MGN1703 in a subgroup of patients with no signs of tumor progression.
The 55 patients had achieved disease control after standard first-line chemotherapy and were randomized in a 2:1 fashion to subcutaneous 60 mg MGN1703 or placebo twice weekly until disease progression.
The good-risk subgroup was composed of 46 patients with two of three previously identified factors: carcinoembryonic antigen less than 30 times the upper limit of normal (ULN); gamma glutamyl transpeptidase less than two times the ULN; or alkaline phosphatase less than two times the ULN.
First-line therapy was: FOLFOX/XELOX or FOLFIRI/XELIRI chemotherapy combined with a standard dose of bevacizumab or FOLFOX/XELOX alone; these regimens were given over an average of 5 months and completed within 2 weeks before treatment with MGN1703.
Complete or partial responses to induction therapy had been observed in 72% of the 40 patients in the MGN1703 arm and 93% of the placebo arm. "This is very unusual and we are dealing here with a very chemo-sensitive population," remarked Dr. Douillard, professor of medical oncology at the University of Nantes and ICO R. Gauducheau, St. Herblain, France.
The secondary end point of progression-free survival from the start of induction reached a median of 9 months in the experimental arm and 8.6 months in the control arm, representing a significant risk reduction of 50% (HR, 0.50; P = .047), reported Dr. Arnold of University Cancer Center Hamburg, Germany.
In all, 33% of patients in both arms reporting a drug-related adverse event. Tolerability was good, with only two patients reporting grade 3 hypertension and two reporting grade 4 ileus, he said.
A further clinical study of MGN1703 is being planning, and the drug is also in development for non–small cell lung cancer.
Based on the data now available, Dr. Douillard said patients can no longer be randomized to placebo and that more effective strategies such as those used in the Treatment Across Multiple Lines (TML) trial could be used as a reference arm to be improved by the immunostimulant.
IMPACT was sponsored by Mologen AG, which is developing MGN1703. Dr. Arnold reported no conflicts, but several of his co-authors reported relationships with Mologen including employment. Dr. Douillard reported serving as a consultant, adviser, or speaker for Roche and Sanofi Aventis, and serving as a consultant, adviser, and data safety monitoring board chair for Bayer.
VIENNA – Maintenance therapy with the investigational immunomodulator MGN1703 reduced the risk of progression in patients with metastatic colorectal cancer in the phase II/III IMPACT trial.
Median progression-free survival from the start of maintenance therapy was 2.8 months with MGN1703 and 2.6 months with placebo. This was not statistically significant (P = .06) but represented a 47% reduction in the risk of progression (hazard ratio, 0.53).
The benefit was even greater in a subgroup of good-risk patients, where the risk of progression was reduced 61% and the median time to progression more than doubled from 2.7 to 5.8 months with MGN1703 (HR, .39; P = .013).
"The findings indicate a potential new approach in the management of metastatic colorectal cancer patients," investigator Dr. Dirk Arnold said at the European Society for Medical Oncology Congress.
MGN1703 is an immunomodulator and toll-like receptor (TLR) 9 agonist that activates all components of the innate and adaptive immune system.
TLR9 agonists have shown activity in clinical trials in renal cell carcinoma, cutaneous T-cell lymphoma, and non-Hodgkin’s lymphoma. In a prior study, a TLR9 agonist combined with first-line taxane-plus-platinum chemotherapy reduced the risk of death from advanced-stage non–small cell lung cancer by 26% (J. Clin. Oncol. 2008;26:3979-86).
Dr. Jean-Yves Douillard, who was invited to discuss the study, agreed that immunomodulation represents a new approach to metastatic colorectal cancer.
"This is an additional set of data showing activity with TLR9 agonists, but this is the first time in metastatic colorectal cancer, which has never been considered a really immunosensitive type of tumor," he said. "The tolerance profile is excellent and this type of approach certainly deserves further evaluation in a larger sample to be convincing."
IMPACT was stopped early after an interim analysis of 55 patients because of slow accrual and observed benefit with MGN1703 in a subgroup of patients with no signs of tumor progression.
The 55 patients had achieved disease control after standard first-line chemotherapy and were randomized in a 2:1 fashion to subcutaneous 60 mg MGN1703 or placebo twice weekly until disease progression.
The good-risk subgroup was composed of 46 patients with two of three previously identified factors: carcinoembryonic antigen less than 30 times the upper limit of normal (ULN); gamma glutamyl transpeptidase less than two times the ULN; or alkaline phosphatase less than two times the ULN.
First-line therapy was: FOLFOX/XELOX or FOLFIRI/XELIRI chemotherapy combined with a standard dose of bevacizumab or FOLFOX/XELOX alone; these regimens were given over an average of 5 months and completed within 2 weeks before treatment with MGN1703.
Complete or partial responses to induction therapy had been observed in 72% of the 40 patients in the MGN1703 arm and 93% of the placebo arm. "This is very unusual and we are dealing here with a very chemo-sensitive population," remarked Dr. Douillard, professor of medical oncology at the University of Nantes and ICO R. Gauducheau, St. Herblain, France.
The secondary end point of progression-free survival from the start of induction reached a median of 9 months in the experimental arm and 8.6 months in the control arm, representing a significant risk reduction of 50% (HR, 0.50; P = .047), reported Dr. Arnold of University Cancer Center Hamburg, Germany.
In all, 33% of patients in both arms reporting a drug-related adverse event. Tolerability was good, with only two patients reporting grade 3 hypertension and two reporting grade 4 ileus, he said.
A further clinical study of MGN1703 is being planning, and the drug is also in development for non–small cell lung cancer.
Based on the data now available, Dr. Douillard said patients can no longer be randomized to placebo and that more effective strategies such as those used in the Treatment Across Multiple Lines (TML) trial could be used as a reference arm to be improved by the immunostimulant.
IMPACT was sponsored by Mologen AG, which is developing MGN1703. Dr. Arnold reported no conflicts, but several of his co-authors reported relationships with Mologen including employment. Dr. Douillard reported serving as a consultant, adviser, or speaker for Roche and Sanofi Aventis, and serving as a consultant, adviser, and data safety monitoring board chair for Bayer.
VIENNA – Maintenance therapy with the investigational immunomodulator MGN1703 reduced the risk of progression in patients with metastatic colorectal cancer in the phase II/III IMPACT trial.
Median progression-free survival from the start of maintenance therapy was 2.8 months with MGN1703 and 2.6 months with placebo. This was not statistically significant (P = .06) but represented a 47% reduction in the risk of progression (hazard ratio, 0.53).
The benefit was even greater in a subgroup of good-risk patients, where the risk of progression was reduced 61% and the median time to progression more than doubled from 2.7 to 5.8 months with MGN1703 (HR, .39; P = .013).
"The findings indicate a potential new approach in the management of metastatic colorectal cancer patients," investigator Dr. Dirk Arnold said at the European Society for Medical Oncology Congress.
MGN1703 is an immunomodulator and toll-like receptor (TLR) 9 agonist that activates all components of the innate and adaptive immune system.
TLR9 agonists have shown activity in clinical trials in renal cell carcinoma, cutaneous T-cell lymphoma, and non-Hodgkin’s lymphoma. In a prior study, a TLR9 agonist combined with first-line taxane-plus-platinum chemotherapy reduced the risk of death from advanced-stage non–small cell lung cancer by 26% (J. Clin. Oncol. 2008;26:3979-86).
Dr. Jean-Yves Douillard, who was invited to discuss the study, agreed that immunomodulation represents a new approach to metastatic colorectal cancer.
"This is an additional set of data showing activity with TLR9 agonists, but this is the first time in metastatic colorectal cancer, which has never been considered a really immunosensitive type of tumor," he said. "The tolerance profile is excellent and this type of approach certainly deserves further evaluation in a larger sample to be convincing."
IMPACT was stopped early after an interim analysis of 55 patients because of slow accrual and observed benefit with MGN1703 in a subgroup of patients with no signs of tumor progression.
The 55 patients had achieved disease control after standard first-line chemotherapy and were randomized in a 2:1 fashion to subcutaneous 60 mg MGN1703 or placebo twice weekly until disease progression.
The good-risk subgroup was composed of 46 patients with two of three previously identified factors: carcinoembryonic antigen less than 30 times the upper limit of normal (ULN); gamma glutamyl transpeptidase less than two times the ULN; or alkaline phosphatase less than two times the ULN.
First-line therapy was: FOLFOX/XELOX or FOLFIRI/XELIRI chemotherapy combined with a standard dose of bevacizumab or FOLFOX/XELOX alone; these regimens were given over an average of 5 months and completed within 2 weeks before treatment with MGN1703.
Complete or partial responses to induction therapy had been observed in 72% of the 40 patients in the MGN1703 arm and 93% of the placebo arm. "This is very unusual and we are dealing here with a very chemo-sensitive population," remarked Dr. Douillard, professor of medical oncology at the University of Nantes and ICO R. Gauducheau, St. Herblain, France.
The secondary end point of progression-free survival from the start of induction reached a median of 9 months in the experimental arm and 8.6 months in the control arm, representing a significant risk reduction of 50% (HR, 0.50; P = .047), reported Dr. Arnold of University Cancer Center Hamburg, Germany.
In all, 33% of patients in both arms reporting a drug-related adverse event. Tolerability was good, with only two patients reporting grade 3 hypertension and two reporting grade 4 ileus, he said.
A further clinical study of MGN1703 is being planning, and the drug is also in development for non–small cell lung cancer.
Based on the data now available, Dr. Douillard said patients can no longer be randomized to placebo and that more effective strategies such as those used in the Treatment Across Multiple Lines (TML) trial could be used as a reference arm to be improved by the immunostimulant.
IMPACT was sponsored by Mologen AG, which is developing MGN1703. Dr. Arnold reported no conflicts, but several of his co-authors reported relationships with Mologen including employment. Dr. Douillard reported serving as a consultant, adviser, or speaker for Roche and Sanofi Aventis, and serving as a consultant, adviser, and data safety monitoring board chair for Bayer.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Maintenance therapy with MGN1703 reduced the risk of progression by 47% vs. placebo (HR, 0.53; P = .06).
Data Source: Phase II/III trial in patients with metastatic colorectal cancer.
Disclosures: IMPACT was funded by Mologen AG, which is developing MGN1703. Dr. Arnold reported no conflicts of interest, but several of his coauthors have relationships with Mologen including employment.Dr. Douillard reported serving as a consultant, adviser, or speaker for Roche and Sanofi Aventis and as a consultant, adviser, and data safety monitoring board chair for Bayer.
Trastuzumab Role May Be Limited in HER2-Positive Bladder Cancer
VIENNA – Trastuzumab appears to have a limited impact on advanced HER2-positive bladder cancer, despite its transformative role in HER2-positive breast cancer.
In a multicenter, randomized phase II trial, no difference was observed in response, survival, or quality of life when gemcitabine (Gemzar) plus platinum-based chemotherapy was compared with the same gemcitabine-plus-platinum regimen plus trastuzumab (Herceptin) in 61 patients with stage IV advanced or metastatic urothelial cancer overexpressing HER2.
There was, however, the intriguing finding that overall survival was related to baseline platinum treatment, which included cisplatin or carboplatin.
Patients receiving cisplatin chemotherapy plus trastuzumab lived a median of 33.1 months, compared with 14.5 months in those receiving cisplatin without trastuzumab and just 9.4 months for patients given carboplatin chemotherapy with trastuzumab, Dr. Stéphane Oudard reported at the European Society for Medical Oncology Congress.
"Trastuzumab could have a synergistic effect with cisplatinum, leading to a longer overall survival," he said.
Urothelial cancer is the fourth most common cancer among men, with about 111,000 new cases reported in 2011. It has proven difficult to target with novel agents, despite the variety of molecular pathways that play a significant role in the formation of these tumors. The incidence of HER2 overexpression and/or amplification remains uncertain, ranging from 23% to 80% in various publications, Dr. Oudard observed.
An earlier phase II trial reported a response rate of 70%, including five complete and 26 partial responses, when trastuzumab was added to paclitaxel (Taxol), carboplatin, and gemcitabine chemotherapy in 44 patients with advanced urothelial cancer not previously treated with chemotherapy for metastasis. Median survival was 14 months, but 22% of patients experienced grades 1-3 cardiac toxicity (J. Clin. Oncol. 2007;25:2218-24).
A more recent phase II double-blind trial reported that adding the oral tyrosine kinase inhibitor vandetanib (Caprelsa) to second-line docetaxel (Taxotere) in advanced urothelial cancer did not significantly improve the overall response rate, progression-free survival, or overall survival, which reached a median of just 7 months (J. Clin. Oncol. 2012;30:507-512).
Treatment in the current trial included gemcitabine 1,000 mg/m2 on days 1 and 8 every 21 days plus cisplatin 70 mg/m2 on day 1 if creatinine clearance was more than 60 mL/min or carboplatin area under the curve (AUC) 5 on day 1 every 21 days if creatinine clearance was 60 mL/min or less, or the same chemotherapy regimen plus trastuzumab at a loading dose of 8 mg/kg then 6 mg/kg every 21 days until progression.
Prior neoadjuvant or adjuvant chemotherapy was allowed if it occurred at least 6 months prior to study entry. Prior trastuzumab therapy was not permitted.
Of the 563 patients tested for HER2 status, only 75 patients (13.3%) were positive on immunohistochemistry, and 14 of these were excluded from the trial for various reasons. The remaining 61 patients had an ECOG performance status of 2 or less, three-fourths had metastatic disease, and half received cisplatin-based chemotherapy.
The 32 patients in the trastuzumab arm received a median of eight chemotherapy cycles vs. a median of six cycles for the 29 patients in the control group, and their median time on therapy was longer, at 5.3 months vs. 3.9 months. Trastuzumab was given for a median of 10 months. Median follow-up was 33.3 months.
The median time to progression was 10.5 months in the control group and 8.3 months with trastuzumab (P = .33), and median overall survival was 15.7 months vs. 14.2 months (P = .56), said Dr. Oudard, of the Georges Pompidou European Hospital, Paris.
Using a Cox regression analysis, baseline serum HER2 was found to be predictive of progression-free survival, whatever the treatment arm (P = .03).
In multivariate Cox regression analysis, the main predictive factors for progression-free survival were ECOG performance status 0-1 vs. 2 (hazard ratio, 3.66) and baseline serum HER2 (HR, 1.01). Trastuzumab was not predictive (P = .49), nor was platinum analogue cisplatin vs. carboplatin (P = .77), he said.
Patients treated with trastuzumab did not have improved quality of life scores, and experienced more grade 3/4 febrile neutropenia (6.5% vs. 0%), dyspnea (3.2% vs. 0%), and decreased left ventricular ejection fraction (3.2% vs. 0%).
Future urothelial cancer trials with HER2-targeting therapy should be performed, but it is unclear whether a trastuzumab combination should be proposed for patients who can only receive cisplatin chemotherapy, whether patient selection should be based on baseline serum HER2, or whether a more powerful drug such as T-DM1 should be used, Dr. Oudard said.
Dr. Oudard reported honoraria from Sanofi-Aventis, Bayer, and other companies.
VIENNA – Trastuzumab appears to have a limited impact on advanced HER2-positive bladder cancer, despite its transformative role in HER2-positive breast cancer.
In a multicenter, randomized phase II trial, no difference was observed in response, survival, or quality of life when gemcitabine (Gemzar) plus platinum-based chemotherapy was compared with the same gemcitabine-plus-platinum regimen plus trastuzumab (Herceptin) in 61 patients with stage IV advanced or metastatic urothelial cancer overexpressing HER2.
There was, however, the intriguing finding that overall survival was related to baseline platinum treatment, which included cisplatin or carboplatin.
Patients receiving cisplatin chemotherapy plus trastuzumab lived a median of 33.1 months, compared with 14.5 months in those receiving cisplatin without trastuzumab and just 9.4 months for patients given carboplatin chemotherapy with trastuzumab, Dr. Stéphane Oudard reported at the European Society for Medical Oncology Congress.
"Trastuzumab could have a synergistic effect with cisplatinum, leading to a longer overall survival," he said.
Urothelial cancer is the fourth most common cancer among men, with about 111,000 new cases reported in 2011. It has proven difficult to target with novel agents, despite the variety of molecular pathways that play a significant role in the formation of these tumors. The incidence of HER2 overexpression and/or amplification remains uncertain, ranging from 23% to 80% in various publications, Dr. Oudard observed.
An earlier phase II trial reported a response rate of 70%, including five complete and 26 partial responses, when trastuzumab was added to paclitaxel (Taxol), carboplatin, and gemcitabine chemotherapy in 44 patients with advanced urothelial cancer not previously treated with chemotherapy for metastasis. Median survival was 14 months, but 22% of patients experienced grades 1-3 cardiac toxicity (J. Clin. Oncol. 2007;25:2218-24).
A more recent phase II double-blind trial reported that adding the oral tyrosine kinase inhibitor vandetanib (Caprelsa) to second-line docetaxel (Taxotere) in advanced urothelial cancer did not significantly improve the overall response rate, progression-free survival, or overall survival, which reached a median of just 7 months (J. Clin. Oncol. 2012;30:507-512).
Treatment in the current trial included gemcitabine 1,000 mg/m2 on days 1 and 8 every 21 days plus cisplatin 70 mg/m2 on day 1 if creatinine clearance was more than 60 mL/min or carboplatin area under the curve (AUC) 5 on day 1 every 21 days if creatinine clearance was 60 mL/min or less, or the same chemotherapy regimen plus trastuzumab at a loading dose of 8 mg/kg then 6 mg/kg every 21 days until progression.
Prior neoadjuvant or adjuvant chemotherapy was allowed if it occurred at least 6 months prior to study entry. Prior trastuzumab therapy was not permitted.
Of the 563 patients tested for HER2 status, only 75 patients (13.3%) were positive on immunohistochemistry, and 14 of these were excluded from the trial for various reasons. The remaining 61 patients had an ECOG performance status of 2 or less, three-fourths had metastatic disease, and half received cisplatin-based chemotherapy.
The 32 patients in the trastuzumab arm received a median of eight chemotherapy cycles vs. a median of six cycles for the 29 patients in the control group, and their median time on therapy was longer, at 5.3 months vs. 3.9 months. Trastuzumab was given for a median of 10 months. Median follow-up was 33.3 months.
The median time to progression was 10.5 months in the control group and 8.3 months with trastuzumab (P = .33), and median overall survival was 15.7 months vs. 14.2 months (P = .56), said Dr. Oudard, of the Georges Pompidou European Hospital, Paris.
Using a Cox regression analysis, baseline serum HER2 was found to be predictive of progression-free survival, whatever the treatment arm (P = .03).
In multivariate Cox regression analysis, the main predictive factors for progression-free survival were ECOG performance status 0-1 vs. 2 (hazard ratio, 3.66) and baseline serum HER2 (HR, 1.01). Trastuzumab was not predictive (P = .49), nor was platinum analogue cisplatin vs. carboplatin (P = .77), he said.
Patients treated with trastuzumab did not have improved quality of life scores, and experienced more grade 3/4 febrile neutropenia (6.5% vs. 0%), dyspnea (3.2% vs. 0%), and decreased left ventricular ejection fraction (3.2% vs. 0%).
Future urothelial cancer trials with HER2-targeting therapy should be performed, but it is unclear whether a trastuzumab combination should be proposed for patients who can only receive cisplatin chemotherapy, whether patient selection should be based on baseline serum HER2, or whether a more powerful drug such as T-DM1 should be used, Dr. Oudard said.
Dr. Oudard reported honoraria from Sanofi-Aventis, Bayer, and other companies.
VIENNA – Trastuzumab appears to have a limited impact on advanced HER2-positive bladder cancer, despite its transformative role in HER2-positive breast cancer.
In a multicenter, randomized phase II trial, no difference was observed in response, survival, or quality of life when gemcitabine (Gemzar) plus platinum-based chemotherapy was compared with the same gemcitabine-plus-platinum regimen plus trastuzumab (Herceptin) in 61 patients with stage IV advanced or metastatic urothelial cancer overexpressing HER2.
There was, however, the intriguing finding that overall survival was related to baseline platinum treatment, which included cisplatin or carboplatin.
Patients receiving cisplatin chemotherapy plus trastuzumab lived a median of 33.1 months, compared with 14.5 months in those receiving cisplatin without trastuzumab and just 9.4 months for patients given carboplatin chemotherapy with trastuzumab, Dr. Stéphane Oudard reported at the European Society for Medical Oncology Congress.
"Trastuzumab could have a synergistic effect with cisplatinum, leading to a longer overall survival," he said.
Urothelial cancer is the fourth most common cancer among men, with about 111,000 new cases reported in 2011. It has proven difficult to target with novel agents, despite the variety of molecular pathways that play a significant role in the formation of these tumors. The incidence of HER2 overexpression and/or amplification remains uncertain, ranging from 23% to 80% in various publications, Dr. Oudard observed.
An earlier phase II trial reported a response rate of 70%, including five complete and 26 partial responses, when trastuzumab was added to paclitaxel (Taxol), carboplatin, and gemcitabine chemotherapy in 44 patients with advanced urothelial cancer not previously treated with chemotherapy for metastasis. Median survival was 14 months, but 22% of patients experienced grades 1-3 cardiac toxicity (J. Clin. Oncol. 2007;25:2218-24).
A more recent phase II double-blind trial reported that adding the oral tyrosine kinase inhibitor vandetanib (Caprelsa) to second-line docetaxel (Taxotere) in advanced urothelial cancer did not significantly improve the overall response rate, progression-free survival, or overall survival, which reached a median of just 7 months (J. Clin. Oncol. 2012;30:507-512).
Treatment in the current trial included gemcitabine 1,000 mg/m2 on days 1 and 8 every 21 days plus cisplatin 70 mg/m2 on day 1 if creatinine clearance was more than 60 mL/min or carboplatin area under the curve (AUC) 5 on day 1 every 21 days if creatinine clearance was 60 mL/min or less, or the same chemotherapy regimen plus trastuzumab at a loading dose of 8 mg/kg then 6 mg/kg every 21 days until progression.
Prior neoadjuvant or adjuvant chemotherapy was allowed if it occurred at least 6 months prior to study entry. Prior trastuzumab therapy was not permitted.
Of the 563 patients tested for HER2 status, only 75 patients (13.3%) were positive on immunohistochemistry, and 14 of these were excluded from the trial for various reasons. The remaining 61 patients had an ECOG performance status of 2 or less, three-fourths had metastatic disease, and half received cisplatin-based chemotherapy.
The 32 patients in the trastuzumab arm received a median of eight chemotherapy cycles vs. a median of six cycles for the 29 patients in the control group, and their median time on therapy was longer, at 5.3 months vs. 3.9 months. Trastuzumab was given for a median of 10 months. Median follow-up was 33.3 months.
The median time to progression was 10.5 months in the control group and 8.3 months with trastuzumab (P = .33), and median overall survival was 15.7 months vs. 14.2 months (P = .56), said Dr. Oudard, of the Georges Pompidou European Hospital, Paris.
Using a Cox regression analysis, baseline serum HER2 was found to be predictive of progression-free survival, whatever the treatment arm (P = .03).
In multivariate Cox regression analysis, the main predictive factors for progression-free survival were ECOG performance status 0-1 vs. 2 (hazard ratio, 3.66) and baseline serum HER2 (HR, 1.01). Trastuzumab was not predictive (P = .49), nor was platinum analogue cisplatin vs. carboplatin (P = .77), he said.
Patients treated with trastuzumab did not have improved quality of life scores, and experienced more grade 3/4 febrile neutropenia (6.5% vs. 0%), dyspnea (3.2% vs. 0%), and decreased left ventricular ejection fraction (3.2% vs. 0%).
Future urothelial cancer trials with HER2-targeting therapy should be performed, but it is unclear whether a trastuzumab combination should be proposed for patients who can only receive cisplatin chemotherapy, whether patient selection should be based on baseline serum HER2, or whether a more powerful drug such as T-DM1 should be used, Dr. Oudard said.
Dr. Oudard reported honoraria from Sanofi-Aventis, Bayer, and other companies.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Median progression-free survival was 10.5 months without trastuzumab and 8.3 months with trastuzumab (P = .33).
Data Source: Data are from a multicenter, randomized phase II trial of 61 patients who had advanced or metastatic urothelial carcinoma with HER2 overexpression.
Disclosures: Dr. Oudard reported honoraria from Sanofi-Aventis, Bayer, and other companies.
mTOR Inhibitors Tank in Three Advanced Kidney Cancer Trials
VIENNA – The mammalian target of rapamycin inhibitors temsirolimus and everolimus have racked up a trio of negative trials in advanced renal cell carcinoma.
The phase IIIb INTORACT and phase II RECORD-2 trials show that combinations of bevacizumab (Avastin) and temsirolimus (Torisel) or everolimus (Afinitor) offer no response or survival advantage over existing first-line treatments. In addition, temsirolimus was not superior to sorafenib (Nexavar) as second-line therapy in the phase III INTORSECT trial.
All three trials were reported at the European Society for Medical Oncology Congress.
INTORACT: Temsirolimus
INTORACT randomly assigned 791 treatment naive patients with advanced, predominantly clear cell renal cell carcinoma (RCC) to bevacizumab 10 mg/kg every two weeks plus temsirolimus 25 mg IV weekly or bevacizumab plus interferon-alfa 9 MU three times per week until progression or unacceptable toxicity. Two-thirds of patients were Memorial Sloan-Kettering Cancer Center (MSKCC) intermediate risk, three-fourths were male, and about 85% had undergone a prior nephrectomy.
In INTORACT, the primary end point of progression free-survival by independent review reached 9.1 months with temsirolimus and bevacizumab and 9.3 months with interferon and bevacizumab (hazard ratio, 1.07; P = .759).
Median overall survival was nearly identical at 25.8 months with the temsirolimus combination and 25.5 months with interferon (HR, 1.04; P = .638), as were objective response rates at 27% and 28% (P = .965).
Median duration of response was longer in the interferon arm (17 months vs. 11 months), reported Dr. Brian Rini of the Cleveland Clinic.
According to a subset analysis, patients with a prior nephrectomy had a longer time to progression than did those without nephrectomy in the interferon arm (median 10.9 months vs. 6.8 months), but not in the temsirolimus arm (9.1 months vs. 9.2 months). Progression-free survival was similar between arms based on MSKCC risk status.
Temsirolimus is approved for the treatment of advanced RCC.
Patients receiving temsirolimus had significantly more grade 3/4 mucosal inflammation (8% vs. 0.3%), stomatitis (7% vs. 2%), as well as hypercholesterolemia, hyperglycemia and hypophosphatemia (all 6% vs. 1%). Pneumonitis was lower than expected based on previous trials (1% vs. 0%), he said.
Grade 3/4 neutropenia (8% vs. 2%), fatigue (11% vs. 5%), lymphopenia (6% vs. 3%) and asthenia (10% vs. 6%) were significantly more common in the interferon arm.
"Interferon plus bevacizumab remains a valid treatment option in metastatic renal cell carcinoma, but other combination therapies remain investigational," Dr. Rini concluded.
RECORD-2: Everolimus
The phase II, open-label RECORD-2 (Renal Cell Cancer Treatment With Oral RAD001 given daily–2) trial had a similar design, randomizing 365 treatment-naive patients metastatic RCC patients to bevacizumab 10 mg/kg every two weeks plus everolimus 10 mg/day or to bevacizumab plus interferon alfa-2a 3 to 9 MU three times/week. All patients had a prior nephrectomy, and more than 90% had a good to intermediate MSKCC performance status.
There was no significant difference between the everolimus and interferon groups in objective response rates (27% vs. 28%) or median progression-free survival based on central review (9.3 months vs. 10 months; HR, 0.91; P = .485).
Patients receiving bevacizumab with interferon lived for a median of 26 months, while the median was not yet available for those given everolimus and bevacizumab, reported Dr. Alain Ravaud of Bordeaux University Victor Segalen in France.
The combination of everolimus with bevacizumab was generally well tolerated and resulted in significantly better physical functioning on two quality of life measures, although no significant difference was observed between arms in a time to definitive deterioration analysis. Final overall survival and safety updates are expected later this year, he said.
Everolimus is approved for treatment of advanced RCC after failure of sunitinib (Sutent) or sorafenib.
Results Show Feasibility, Not Efficacy
Invited discussant Dr. Christian Kollmannsberger, senior scientist with the British Columbia Cancer Agency in Vancouver, said the two studies confirm phase I data that combining an mTOR inhibitor with bevacizumab is feasible, whereas combining bevacizumab with tyrosine kinase inhibitors or TKIs with mTOR inhibitors has proven too toxic because of overlapping toxicity profiles.
He said the current results clearly, and inexplicably, also contrast with the recently published French phase II TORAVA trial reporting treatment-limiting toxicity with temsirolimus plus bevacizumab and less clinical activity than the benefit expected from sequential use of each targeted therapy (Lancet Oncol. 2011;12:673-80).
Dr. Kollmannsberger said the overall negative results aren’t surprising since the rationale behind the INTORSECT and RECORD-2 combinations was based on very small phase I/II trials without complete responses and an absence of preclinical data showing an additive or synergistic effect with an mTOR inhibitor and bevacizumab.
He said future trials should combine agents with multiple mechanisms, and that vertical blockade of a single signaling pathway, in this case the vascular endothelial growth factor (VEGF) pathway, "is certainly not the way to go" with currently available agents because it is either too toxic or lacks convincing efficacy.
INTORSECT: Striking Finding
INTORSECT, the first head-to-head phase III trial comparing an mTOR inhibitor and a VEGF receptor inhibitor in second-line RCC, provided no easy answers regarding the optimal treatment sequence after sunitinib in advanced RCC.
The trial randomly assigned 512 patients to temsirolimus 25 mg weekly or sorafenib 400 mg twice daily until progressive disease or unacceptable toxicity. All patients had received first-line sunitinib, and about 82% had clear cell histology.
The confirmed objective response rates were 8% for both the temsirolimus and sorafenib groups, with stable disease reported in 61% of all patients.
Although patients receiving temsirolimus had a numerically longer progression-free survival by independent assessment (median 4.2 months vs. 3.9; HR 0.87; P = .193), those given sorafenib lived a median of four months longer (16.64 months vs. 12.27 months; HR 1.31, P = .014), reported Dr. Thomas Hutson, Pharm.D., of the Texas Oncology–Baylor Charles A. Sammons Cancer Center in Dallas.
Dr. Kollmannsberger described this as the most striking finding of the trial, and said it is likely the longest survival time observed in a randomized trial. The difference in survival is unlikely to be explained by patient characteristics, discontinuations because of adverse events with temsirolimus and sorafenib (17% vs. 14%), or subsequent therapies, since few patients had third-line therapy.
One possible explanation may be that prior VEGF therapy alters the biology of the disease. This hypothesis is supported by a recent analysis of sequential tissue samples suggesting that the protein expression in metastatic cell RCC changes with sunitinib therapy, Dr. Kollmannsberger said.
"I think in the end, the significant survival difference remains to be conclusively explained, but it at least gives rise to the hypothesis that the sequence of drugs does actually matter and matters more than we may think at the present time," he said.
INTORACT was sponsored by Pfizer Inc., which also provided funding for editorial support by Peloton Advantage. Dr. Rini reported research funding from and consulting for Pfizer. RECORD-2 was sponsored by Novartis. Dr. Ravaud reported financial ties with several firms including Pfizer and Novartis. Dr. Hutson reported serving as an adviser and investigator for Aveo, Bayer, GlaxoSmithKline, Novartis, and Pfizer, which sponsored INTORSECT. Dr. Kollmannsberger reported being an investigator on the INTORSECT study, and serving as an advisor for and travel support from Pfizer, Novartis and GSK.
VIENNA – The mammalian target of rapamycin inhibitors temsirolimus and everolimus have racked up a trio of negative trials in advanced renal cell carcinoma.
The phase IIIb INTORACT and phase II RECORD-2 trials show that combinations of bevacizumab (Avastin) and temsirolimus (Torisel) or everolimus (Afinitor) offer no response or survival advantage over existing first-line treatments. In addition, temsirolimus was not superior to sorafenib (Nexavar) as second-line therapy in the phase III INTORSECT trial.
All three trials were reported at the European Society for Medical Oncology Congress.
INTORACT: Temsirolimus
INTORACT randomly assigned 791 treatment naive patients with advanced, predominantly clear cell renal cell carcinoma (RCC) to bevacizumab 10 mg/kg every two weeks plus temsirolimus 25 mg IV weekly or bevacizumab plus interferon-alfa 9 MU three times per week until progression or unacceptable toxicity. Two-thirds of patients were Memorial Sloan-Kettering Cancer Center (MSKCC) intermediate risk, three-fourths were male, and about 85% had undergone a prior nephrectomy.
In INTORACT, the primary end point of progression free-survival by independent review reached 9.1 months with temsirolimus and bevacizumab and 9.3 months with interferon and bevacizumab (hazard ratio, 1.07; P = .759).
Median overall survival was nearly identical at 25.8 months with the temsirolimus combination and 25.5 months with interferon (HR, 1.04; P = .638), as were objective response rates at 27% and 28% (P = .965).
Median duration of response was longer in the interferon arm (17 months vs. 11 months), reported Dr. Brian Rini of the Cleveland Clinic.
According to a subset analysis, patients with a prior nephrectomy had a longer time to progression than did those without nephrectomy in the interferon arm (median 10.9 months vs. 6.8 months), but not in the temsirolimus arm (9.1 months vs. 9.2 months). Progression-free survival was similar between arms based on MSKCC risk status.
Temsirolimus is approved for the treatment of advanced RCC.
Patients receiving temsirolimus had significantly more grade 3/4 mucosal inflammation (8% vs. 0.3%), stomatitis (7% vs. 2%), as well as hypercholesterolemia, hyperglycemia and hypophosphatemia (all 6% vs. 1%). Pneumonitis was lower than expected based on previous trials (1% vs. 0%), he said.
Grade 3/4 neutropenia (8% vs. 2%), fatigue (11% vs. 5%), lymphopenia (6% vs. 3%) and asthenia (10% vs. 6%) were significantly more common in the interferon arm.
"Interferon plus bevacizumab remains a valid treatment option in metastatic renal cell carcinoma, but other combination therapies remain investigational," Dr. Rini concluded.
RECORD-2: Everolimus
The phase II, open-label RECORD-2 (Renal Cell Cancer Treatment With Oral RAD001 given daily–2) trial had a similar design, randomizing 365 treatment-naive patients metastatic RCC patients to bevacizumab 10 mg/kg every two weeks plus everolimus 10 mg/day or to bevacizumab plus interferon alfa-2a 3 to 9 MU three times/week. All patients had a prior nephrectomy, and more than 90% had a good to intermediate MSKCC performance status.
There was no significant difference between the everolimus and interferon groups in objective response rates (27% vs. 28%) or median progression-free survival based on central review (9.3 months vs. 10 months; HR, 0.91; P = .485).
Patients receiving bevacizumab with interferon lived for a median of 26 months, while the median was not yet available for those given everolimus and bevacizumab, reported Dr. Alain Ravaud of Bordeaux University Victor Segalen in France.
The combination of everolimus with bevacizumab was generally well tolerated and resulted in significantly better physical functioning on two quality of life measures, although no significant difference was observed between arms in a time to definitive deterioration analysis. Final overall survival and safety updates are expected later this year, he said.
Everolimus is approved for treatment of advanced RCC after failure of sunitinib (Sutent) or sorafenib.
Results Show Feasibility, Not Efficacy
Invited discussant Dr. Christian Kollmannsberger, senior scientist with the British Columbia Cancer Agency in Vancouver, said the two studies confirm phase I data that combining an mTOR inhibitor with bevacizumab is feasible, whereas combining bevacizumab with tyrosine kinase inhibitors or TKIs with mTOR inhibitors has proven too toxic because of overlapping toxicity profiles.
He said the current results clearly, and inexplicably, also contrast with the recently published French phase II TORAVA trial reporting treatment-limiting toxicity with temsirolimus plus bevacizumab and less clinical activity than the benefit expected from sequential use of each targeted therapy (Lancet Oncol. 2011;12:673-80).
Dr. Kollmannsberger said the overall negative results aren’t surprising since the rationale behind the INTORSECT and RECORD-2 combinations was based on very small phase I/II trials without complete responses and an absence of preclinical data showing an additive or synergistic effect with an mTOR inhibitor and bevacizumab.
He said future trials should combine agents with multiple mechanisms, and that vertical blockade of a single signaling pathway, in this case the vascular endothelial growth factor (VEGF) pathway, "is certainly not the way to go" with currently available agents because it is either too toxic or lacks convincing efficacy.
INTORSECT: Striking Finding
INTORSECT, the first head-to-head phase III trial comparing an mTOR inhibitor and a VEGF receptor inhibitor in second-line RCC, provided no easy answers regarding the optimal treatment sequence after sunitinib in advanced RCC.
The trial randomly assigned 512 patients to temsirolimus 25 mg weekly or sorafenib 400 mg twice daily until progressive disease or unacceptable toxicity. All patients had received first-line sunitinib, and about 82% had clear cell histology.
The confirmed objective response rates were 8% for both the temsirolimus and sorafenib groups, with stable disease reported in 61% of all patients.
Although patients receiving temsirolimus had a numerically longer progression-free survival by independent assessment (median 4.2 months vs. 3.9; HR 0.87; P = .193), those given sorafenib lived a median of four months longer (16.64 months vs. 12.27 months; HR 1.31, P = .014), reported Dr. Thomas Hutson, Pharm.D., of the Texas Oncology–Baylor Charles A. Sammons Cancer Center in Dallas.
Dr. Kollmannsberger described this as the most striking finding of the trial, and said it is likely the longest survival time observed in a randomized trial. The difference in survival is unlikely to be explained by patient characteristics, discontinuations because of adverse events with temsirolimus and sorafenib (17% vs. 14%), or subsequent therapies, since few patients had third-line therapy.
One possible explanation may be that prior VEGF therapy alters the biology of the disease. This hypothesis is supported by a recent analysis of sequential tissue samples suggesting that the protein expression in metastatic cell RCC changes with sunitinib therapy, Dr. Kollmannsberger said.
"I think in the end, the significant survival difference remains to be conclusively explained, but it at least gives rise to the hypothesis that the sequence of drugs does actually matter and matters more than we may think at the present time," he said.
INTORACT was sponsored by Pfizer Inc., which also provided funding for editorial support by Peloton Advantage. Dr. Rini reported research funding from and consulting for Pfizer. RECORD-2 was sponsored by Novartis. Dr. Ravaud reported financial ties with several firms including Pfizer and Novartis. Dr. Hutson reported serving as an adviser and investigator for Aveo, Bayer, GlaxoSmithKline, Novartis, and Pfizer, which sponsored INTORSECT. Dr. Kollmannsberger reported being an investigator on the INTORSECT study, and serving as an advisor for and travel support from Pfizer, Novartis and GSK.
VIENNA – The mammalian target of rapamycin inhibitors temsirolimus and everolimus have racked up a trio of negative trials in advanced renal cell carcinoma.
The phase IIIb INTORACT and phase II RECORD-2 trials show that combinations of bevacizumab (Avastin) and temsirolimus (Torisel) or everolimus (Afinitor) offer no response or survival advantage over existing first-line treatments. In addition, temsirolimus was not superior to sorafenib (Nexavar) as second-line therapy in the phase III INTORSECT trial.
All three trials were reported at the European Society for Medical Oncology Congress.
INTORACT: Temsirolimus
INTORACT randomly assigned 791 treatment naive patients with advanced, predominantly clear cell renal cell carcinoma (RCC) to bevacizumab 10 mg/kg every two weeks plus temsirolimus 25 mg IV weekly or bevacizumab plus interferon-alfa 9 MU three times per week until progression or unacceptable toxicity. Two-thirds of patients were Memorial Sloan-Kettering Cancer Center (MSKCC) intermediate risk, three-fourths were male, and about 85% had undergone a prior nephrectomy.
In INTORACT, the primary end point of progression free-survival by independent review reached 9.1 months with temsirolimus and bevacizumab and 9.3 months with interferon and bevacizumab (hazard ratio, 1.07; P = .759).
Median overall survival was nearly identical at 25.8 months with the temsirolimus combination and 25.5 months with interferon (HR, 1.04; P = .638), as were objective response rates at 27% and 28% (P = .965).
Median duration of response was longer in the interferon arm (17 months vs. 11 months), reported Dr. Brian Rini of the Cleveland Clinic.
According to a subset analysis, patients with a prior nephrectomy had a longer time to progression than did those without nephrectomy in the interferon arm (median 10.9 months vs. 6.8 months), but not in the temsirolimus arm (9.1 months vs. 9.2 months). Progression-free survival was similar between arms based on MSKCC risk status.
Temsirolimus is approved for the treatment of advanced RCC.
Patients receiving temsirolimus had significantly more grade 3/4 mucosal inflammation (8% vs. 0.3%), stomatitis (7% vs. 2%), as well as hypercholesterolemia, hyperglycemia and hypophosphatemia (all 6% vs. 1%). Pneumonitis was lower than expected based on previous trials (1% vs. 0%), he said.
Grade 3/4 neutropenia (8% vs. 2%), fatigue (11% vs. 5%), lymphopenia (6% vs. 3%) and asthenia (10% vs. 6%) were significantly more common in the interferon arm.
"Interferon plus bevacizumab remains a valid treatment option in metastatic renal cell carcinoma, but other combination therapies remain investigational," Dr. Rini concluded.
RECORD-2: Everolimus
The phase II, open-label RECORD-2 (Renal Cell Cancer Treatment With Oral RAD001 given daily–2) trial had a similar design, randomizing 365 treatment-naive patients metastatic RCC patients to bevacizumab 10 mg/kg every two weeks plus everolimus 10 mg/day or to bevacizumab plus interferon alfa-2a 3 to 9 MU three times/week. All patients had a prior nephrectomy, and more than 90% had a good to intermediate MSKCC performance status.
There was no significant difference between the everolimus and interferon groups in objective response rates (27% vs. 28%) or median progression-free survival based on central review (9.3 months vs. 10 months; HR, 0.91; P = .485).
Patients receiving bevacizumab with interferon lived for a median of 26 months, while the median was not yet available for those given everolimus and bevacizumab, reported Dr. Alain Ravaud of Bordeaux University Victor Segalen in France.
The combination of everolimus with bevacizumab was generally well tolerated and resulted in significantly better physical functioning on two quality of life measures, although no significant difference was observed between arms in a time to definitive deterioration analysis. Final overall survival and safety updates are expected later this year, he said.
Everolimus is approved for treatment of advanced RCC after failure of sunitinib (Sutent) or sorafenib.
Results Show Feasibility, Not Efficacy
Invited discussant Dr. Christian Kollmannsberger, senior scientist with the British Columbia Cancer Agency in Vancouver, said the two studies confirm phase I data that combining an mTOR inhibitor with bevacizumab is feasible, whereas combining bevacizumab with tyrosine kinase inhibitors or TKIs with mTOR inhibitors has proven too toxic because of overlapping toxicity profiles.
He said the current results clearly, and inexplicably, also contrast with the recently published French phase II TORAVA trial reporting treatment-limiting toxicity with temsirolimus plus bevacizumab and less clinical activity than the benefit expected from sequential use of each targeted therapy (Lancet Oncol. 2011;12:673-80).
Dr. Kollmannsberger said the overall negative results aren’t surprising since the rationale behind the INTORSECT and RECORD-2 combinations was based on very small phase I/II trials without complete responses and an absence of preclinical data showing an additive or synergistic effect with an mTOR inhibitor and bevacizumab.
He said future trials should combine agents with multiple mechanisms, and that vertical blockade of a single signaling pathway, in this case the vascular endothelial growth factor (VEGF) pathway, "is certainly not the way to go" with currently available agents because it is either too toxic or lacks convincing efficacy.
INTORSECT: Striking Finding
INTORSECT, the first head-to-head phase III trial comparing an mTOR inhibitor and a VEGF receptor inhibitor in second-line RCC, provided no easy answers regarding the optimal treatment sequence after sunitinib in advanced RCC.
The trial randomly assigned 512 patients to temsirolimus 25 mg weekly or sorafenib 400 mg twice daily until progressive disease or unacceptable toxicity. All patients had received first-line sunitinib, and about 82% had clear cell histology.
The confirmed objective response rates were 8% for both the temsirolimus and sorafenib groups, with stable disease reported in 61% of all patients.
Although patients receiving temsirolimus had a numerically longer progression-free survival by independent assessment (median 4.2 months vs. 3.9; HR 0.87; P = .193), those given sorafenib lived a median of four months longer (16.64 months vs. 12.27 months; HR 1.31, P = .014), reported Dr. Thomas Hutson, Pharm.D., of the Texas Oncology–Baylor Charles A. Sammons Cancer Center in Dallas.
Dr. Kollmannsberger described this as the most striking finding of the trial, and said it is likely the longest survival time observed in a randomized trial. The difference in survival is unlikely to be explained by patient characteristics, discontinuations because of adverse events with temsirolimus and sorafenib (17% vs. 14%), or subsequent therapies, since few patients had third-line therapy.
One possible explanation may be that prior VEGF therapy alters the biology of the disease. This hypothesis is supported by a recent analysis of sequential tissue samples suggesting that the protein expression in metastatic cell RCC changes with sunitinib therapy, Dr. Kollmannsberger said.
"I think in the end, the significant survival difference remains to be conclusively explained, but it at least gives rise to the hypothesis that the sequence of drugs does actually matter and matters more than we may think at the present time," he said.
INTORACT was sponsored by Pfizer Inc., which also provided funding for editorial support by Peloton Advantage. Dr. Rini reported research funding from and consulting for Pfizer. RECORD-2 was sponsored by Novartis. Dr. Ravaud reported financial ties with several firms including Pfizer and Novartis. Dr. Hutson reported serving as an adviser and investigator for Aveo, Bayer, GlaxoSmithKline, Novartis, and Pfizer, which sponsored INTORSECT. Dr. Kollmannsberger reported being an investigator on the INTORSECT study, and serving as an advisor for and travel support from Pfizer, Novartis and GSK.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: In one trial, the primary end point of progression-free survival by independent review reached 9.1 months with temsirolimus and bevacizumab and 9.3 months with interferon and bevacizumab (hazard ratio, 1.07; P = .759).
Data Source: Investigators reported on two phase III and one phase II trial in advanced renal cell carcinoma.
Disclosures: INTORACT was sponsored by Pfizer Inc., which also provided funding for editorial support by Peloton Advantage. Dr. Rini reported research funding from and consulting for Pfizer. RECORD-2 was sponsored by Novartis. Dr. Ravaud reported financial ties with several firms including Pfizer and Novartis. Dr. Hutson reported serving as an advisor and investigator for Aveo, Bayer, GlaxoSmithKline, Novartis and Pfizer, which sponsored INTORSECT. Dr. Kollmannsberger reported being an investigator on the INTORSECT study, and serving as an advisor for and travel support from Pfizer, Novartis and GSK.
Company for Crizotinib? New ALK Inhibitors Attack Lung Cancer
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Response rates reached as high as 85% in patients with advanced tumors that were predominantly non-small cell lung cancer.
Data Source: Investigators reported on early phase I/II trials of novel ALK inhibitors and a second-generation heat shock protein 90 inhibitor.
Disclosures: Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
TURANDOT Revisits Bevacizumab Partners in Metastatic Breast Cancer
VIENNA – Paclitaxel may be a better partner for bevacizumab than capecitabine for treating chemotherapy-naive women with HER2-negative breast cancer, the first efficacy results from the prospective phase III TURANDOT trial suggest.
The secondary end points of progression-free survival (11 months vs. 8.1 months, hazard ratio 1.36, P = .0052) and objective response rates (44% vs. 27%, P less than .0001) were significantly better with bevacizumab (Avastin) and paclitaxel than with bevacizumab and capecitabine (Xeloda).
The primary end point of overall survival did not show a statistically significant difference between the combinations (HR 1.04), however. Overall survival was 30.5 months with bevacizumab plus paclitaxel and 26 months with bevacizumab plus capecitabine (HR 1.04, P = .059). The respective overall survival rates were 81% versus 79% at 1 year, 68% versus 70% at 2 years, and 60% versus 55% at 3 years.
"Many agents are available for first-line treatment of HER2-negative inoperable locally advanced or metastatic breast cancer, but currently there is no gold standard," Dr. Christoph Zielinski said Sept. 29 at the European Society for Medical Oncology Congress.
Dr. Zielinski, of the Medical University of Vienna, noted that progression-free survival and overall response rates were significantly improved when bevacizumab was combined with first-line weekly paclitaxel in the E2100 trial (J. Clin. Oncol. 2009;27:4966-72) or with capecitabine in the RIBBON-1 trial (J. Clin. Oncol. 2011;29:1252-60), but there was no head-to-head comparison.
Both combinations have been registered for use in Europe, although the National Institute for Health and Clinical Excellence in the United Kingdom has rejected both on the grounds of no clear overall survival benefit and the cost-to-benefit ratio.
In the United States, the Food and Drug Administration withdrew an indication for bevacizumab in metastatic breast cancer after subsequent clinical trials failed to confirm the margin of benefit seen in E2100.
TURANDOT Offers Direct Comparison
TURANDOT (Capecitabine and Bevacizumab Randomized Against Avastin and Taxol) is the first prospective trial directly comparing bevacizumab and paclitaxel versus bevacizumab and capecitabine as first-line treatment for inoperable locally advanced or metastatic breast cancer.
The international, multicenter, double-blind trial involved 564 women (median age 59 years) who had received no prior chemotherapy in the advanced setting. Prior adjuvant or neoadjuvant chemotherapy, radiotherapy, or both were permitted only if the regimens used had been completed at least 6 months before enrollment.
Women were treated until progression or until unacceptable toxicity, with 285 receiving bevacizumab 10 mg/kg on days 1 and 15 plus paclitaxel 90 mg/m2 on days 1, 8 and 14 of a 4-week cycle. The remaining 279 women received bevacizumab 15 mg/kg on day 1 plus capecitabine 1,000 mg/m2 on days 1-14 of a 3-week cycle. The primary objective was to demonstrate that the latter combination was noninferior to bevacizumab plus paclitaxel.
The safety population included 561 women, with 51% versus 64% of patients discontinuing bevacizumab treatment in the paclitaxel and capecitabine arms, respectively. A similar percentage (16% vs. 14%) discontinued bevacizumab because of adverse events, and two and four patients in each group died.
A higher percentage of patients in the bevacizumab-capecitabine arm than in the bevacizumab-paclitaxel arm discontinued chemotherapy due to disease progression (63% vs. 32%), although there were fewer discontinuations due to adverse events (15% vs. 34%). Chemotherapy had to be modified because of toxicity in 46% and 54% of cases, respectively.
Subgroup analysis of overall survival showed no difference between the two treatment arms. Final overall survival results are expected in 2014.
The time to achieve a response was significantly shorter with the paclitaxel-containing regimen (HR 0.58, P = .0002), although the time to treatment failure and duration of response was not significantly different.
Adverse events were consistent with those previously reported with the agents used, with higher rates of hematologic events and neuropathy associated with the paclitaxel-containing regimen and more diarrhea, anemia, hypertension, and hand-foot syndrome with the capecitabine-containing regimen.
"PFS and response rates corroborate results of previous phase III trials," Dr. Zielinski said, adding, "treatment selection depends on each individual’s priorities."
Paclitaxel Might Be More Active
Providing an independent comment on the trial, Dr. Javier Cortes said: "Bevacizumab-based therapy is a good first-line treatment option for patients with HER2-negative metastatic breast cancer."
Dr. Cortes, of the Vall d’Hebron Institute of Oncology in Barcelona, added: "If bevacizumab is used, paclitaxel might be more active than capecitabine.
"Differences in overall survival between paclitaxel and capecitabine when combined with bevacizumab are unlikely to be found, but it does not mean that both schedules are the same."
Roche sponsored the TURANDOT trial. Dr. Zielinski disclosed receiving honoraria from the company. Dr. Javier Cortes has received honoraria from Roche.
VIENNA – Paclitaxel may be a better partner for bevacizumab than capecitabine for treating chemotherapy-naive women with HER2-negative breast cancer, the first efficacy results from the prospective phase III TURANDOT trial suggest.
The secondary end points of progression-free survival (11 months vs. 8.1 months, hazard ratio 1.36, P = .0052) and objective response rates (44% vs. 27%, P less than .0001) were significantly better with bevacizumab (Avastin) and paclitaxel than with bevacizumab and capecitabine (Xeloda).
The primary end point of overall survival did not show a statistically significant difference between the combinations (HR 1.04), however. Overall survival was 30.5 months with bevacizumab plus paclitaxel and 26 months with bevacizumab plus capecitabine (HR 1.04, P = .059). The respective overall survival rates were 81% versus 79% at 1 year, 68% versus 70% at 2 years, and 60% versus 55% at 3 years.
"Many agents are available for first-line treatment of HER2-negative inoperable locally advanced or metastatic breast cancer, but currently there is no gold standard," Dr. Christoph Zielinski said Sept. 29 at the European Society for Medical Oncology Congress.
Dr. Zielinski, of the Medical University of Vienna, noted that progression-free survival and overall response rates were significantly improved when bevacizumab was combined with first-line weekly paclitaxel in the E2100 trial (J. Clin. Oncol. 2009;27:4966-72) or with capecitabine in the RIBBON-1 trial (J. Clin. Oncol. 2011;29:1252-60), but there was no head-to-head comparison.
Both combinations have been registered for use in Europe, although the National Institute for Health and Clinical Excellence in the United Kingdom has rejected both on the grounds of no clear overall survival benefit and the cost-to-benefit ratio.
In the United States, the Food and Drug Administration withdrew an indication for bevacizumab in metastatic breast cancer after subsequent clinical trials failed to confirm the margin of benefit seen in E2100.
TURANDOT Offers Direct Comparison
TURANDOT (Capecitabine and Bevacizumab Randomized Against Avastin and Taxol) is the first prospective trial directly comparing bevacizumab and paclitaxel versus bevacizumab and capecitabine as first-line treatment for inoperable locally advanced or metastatic breast cancer.
The international, multicenter, double-blind trial involved 564 women (median age 59 years) who had received no prior chemotherapy in the advanced setting. Prior adjuvant or neoadjuvant chemotherapy, radiotherapy, or both were permitted only if the regimens used had been completed at least 6 months before enrollment.
Women were treated until progression or until unacceptable toxicity, with 285 receiving bevacizumab 10 mg/kg on days 1 and 15 plus paclitaxel 90 mg/m2 on days 1, 8 and 14 of a 4-week cycle. The remaining 279 women received bevacizumab 15 mg/kg on day 1 plus capecitabine 1,000 mg/m2 on days 1-14 of a 3-week cycle. The primary objective was to demonstrate that the latter combination was noninferior to bevacizumab plus paclitaxel.
The safety population included 561 women, with 51% versus 64% of patients discontinuing bevacizumab treatment in the paclitaxel and capecitabine arms, respectively. A similar percentage (16% vs. 14%) discontinued bevacizumab because of adverse events, and two and four patients in each group died.
A higher percentage of patients in the bevacizumab-capecitabine arm than in the bevacizumab-paclitaxel arm discontinued chemotherapy due to disease progression (63% vs. 32%), although there were fewer discontinuations due to adverse events (15% vs. 34%). Chemotherapy had to be modified because of toxicity in 46% and 54% of cases, respectively.
Subgroup analysis of overall survival showed no difference between the two treatment arms. Final overall survival results are expected in 2014.
The time to achieve a response was significantly shorter with the paclitaxel-containing regimen (HR 0.58, P = .0002), although the time to treatment failure and duration of response was not significantly different.
Adverse events were consistent with those previously reported with the agents used, with higher rates of hematologic events and neuropathy associated with the paclitaxel-containing regimen and more diarrhea, anemia, hypertension, and hand-foot syndrome with the capecitabine-containing regimen.
"PFS and response rates corroborate results of previous phase III trials," Dr. Zielinski said, adding, "treatment selection depends on each individual’s priorities."
Paclitaxel Might Be More Active
Providing an independent comment on the trial, Dr. Javier Cortes said: "Bevacizumab-based therapy is a good first-line treatment option for patients with HER2-negative metastatic breast cancer."
Dr. Cortes, of the Vall d’Hebron Institute of Oncology in Barcelona, added: "If bevacizumab is used, paclitaxel might be more active than capecitabine.
"Differences in overall survival between paclitaxel and capecitabine when combined with bevacizumab are unlikely to be found, but it does not mean that both schedules are the same."
Roche sponsored the TURANDOT trial. Dr. Zielinski disclosed receiving honoraria from the company. Dr. Javier Cortes has received honoraria from Roche.
VIENNA – Paclitaxel may be a better partner for bevacizumab than capecitabine for treating chemotherapy-naive women with HER2-negative breast cancer, the first efficacy results from the prospective phase III TURANDOT trial suggest.
The secondary end points of progression-free survival (11 months vs. 8.1 months, hazard ratio 1.36, P = .0052) and objective response rates (44% vs. 27%, P less than .0001) were significantly better with bevacizumab (Avastin) and paclitaxel than with bevacizumab and capecitabine (Xeloda).
The primary end point of overall survival did not show a statistically significant difference between the combinations (HR 1.04), however. Overall survival was 30.5 months with bevacizumab plus paclitaxel and 26 months with bevacizumab plus capecitabine (HR 1.04, P = .059). The respective overall survival rates were 81% versus 79% at 1 year, 68% versus 70% at 2 years, and 60% versus 55% at 3 years.
"Many agents are available for first-line treatment of HER2-negative inoperable locally advanced or metastatic breast cancer, but currently there is no gold standard," Dr. Christoph Zielinski said Sept. 29 at the European Society for Medical Oncology Congress.
Dr. Zielinski, of the Medical University of Vienna, noted that progression-free survival and overall response rates were significantly improved when bevacizumab was combined with first-line weekly paclitaxel in the E2100 trial (J. Clin. Oncol. 2009;27:4966-72) or with capecitabine in the RIBBON-1 trial (J. Clin. Oncol. 2011;29:1252-60), but there was no head-to-head comparison.
Both combinations have been registered for use in Europe, although the National Institute for Health and Clinical Excellence in the United Kingdom has rejected both on the grounds of no clear overall survival benefit and the cost-to-benefit ratio.
In the United States, the Food and Drug Administration withdrew an indication for bevacizumab in metastatic breast cancer after subsequent clinical trials failed to confirm the margin of benefit seen in E2100.
TURANDOT Offers Direct Comparison
TURANDOT (Capecitabine and Bevacizumab Randomized Against Avastin and Taxol) is the first prospective trial directly comparing bevacizumab and paclitaxel versus bevacizumab and capecitabine as first-line treatment for inoperable locally advanced or metastatic breast cancer.
The international, multicenter, double-blind trial involved 564 women (median age 59 years) who had received no prior chemotherapy in the advanced setting. Prior adjuvant or neoadjuvant chemotherapy, radiotherapy, or both were permitted only if the regimens used had been completed at least 6 months before enrollment.
Women were treated until progression or until unacceptable toxicity, with 285 receiving bevacizumab 10 mg/kg on days 1 and 15 plus paclitaxel 90 mg/m2 on days 1, 8 and 14 of a 4-week cycle. The remaining 279 women received bevacizumab 15 mg/kg on day 1 plus capecitabine 1,000 mg/m2 on days 1-14 of a 3-week cycle. The primary objective was to demonstrate that the latter combination was noninferior to bevacizumab plus paclitaxel.
The safety population included 561 women, with 51% versus 64% of patients discontinuing bevacizumab treatment in the paclitaxel and capecitabine arms, respectively. A similar percentage (16% vs. 14%) discontinued bevacizumab because of adverse events, and two and four patients in each group died.
A higher percentage of patients in the bevacizumab-capecitabine arm than in the bevacizumab-paclitaxel arm discontinued chemotherapy due to disease progression (63% vs. 32%), although there were fewer discontinuations due to adverse events (15% vs. 34%). Chemotherapy had to be modified because of toxicity in 46% and 54% of cases, respectively.
Subgroup analysis of overall survival showed no difference between the two treatment arms. Final overall survival results are expected in 2014.
The time to achieve a response was significantly shorter with the paclitaxel-containing regimen (HR 0.58, P = .0002), although the time to treatment failure and duration of response was not significantly different.
Adverse events were consistent with those previously reported with the agents used, with higher rates of hematologic events and neuropathy associated with the paclitaxel-containing regimen and more diarrhea, anemia, hypertension, and hand-foot syndrome with the capecitabine-containing regimen.
"PFS and response rates corroborate results of previous phase III trials," Dr. Zielinski said, adding, "treatment selection depends on each individual’s priorities."
Paclitaxel Might Be More Active
Providing an independent comment on the trial, Dr. Javier Cortes said: "Bevacizumab-based therapy is a good first-line treatment option for patients with HER2-negative metastatic breast cancer."
Dr. Cortes, of the Vall d’Hebron Institute of Oncology in Barcelona, added: "If bevacizumab is used, paclitaxel might be more active than capecitabine.
"Differences in overall survival between paclitaxel and capecitabine when combined with bevacizumab are unlikely to be found, but it does not mean that both schedules are the same."
Roche sponsored the TURANDOT trial. Dr. Zielinski disclosed receiving honoraria from the company. Dr. Javier Cortes has received honoraria from Roche.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Secondary end points of progression-free survival (HR 1.36, P = .005) and objective response rates (P less than .0001) were significantly better with bevacizumab-paclitaxel than with bevacizumab-capecitabine.
Data Source: Investigators presented first results from the phase III TURANDOT noninferiority trial of bevacizumab in combination with paclitaxel (n = 285) or capecitabine (n = 279).
Disclosures: Roche sponsored the TURANDOT trial. Dr. Zielinski disclosed receiving honoraria from the company. Dr. Javier Cortes has received honoraria from Roche.
Baseline Factors Trump Response to Therapy in Mastectomy Decisions
VIENNA – The decision to undergo breast-conserving surgery depends on baseline characteristics rather than response to therapy, according to a new analysis of data from a phase III trial comparing neoadjuvant regimens for early HER2-positive breast cancer.
Baseline multicentricity or multifocality of the tumor, receptor status, and the type of surgery planned at diagnosis all were found to influence the decision to undergo breast-conserving surgery (BCS) or mastectomy, investigators reported Sept. 30 at the European Society for Medical Oncology Congress.
"These results call for a clear consensus on the role of breast-conserving surgery in patients responding to neoadjuvant therapy," Dr. Carmen Criscitiello told attendees.
"This will ultimately translate the progress in neoadjuvant therapies into improved breast conservation rates," suggested Dr. Criscitiello of the European Institute of Oncology in Milan.
Puzzled by Low BCS Rates
Neoadjuvant therapy has several possible roles, including testing sensitivity to systemic therapy, downstaging tumors to make them operable, eliminating micrometastases, and increasing breast conservation rates, Dr. Criscitiello explained. It was therefore expected that BCS rates would increase significantly in the Neo ALLTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial.
Half of all patients treated with a dual HER2 blockade of lapatinib (Tykerb) and trastuzumab (Herceptin) in addition to paclitaxel achieved a pathological complete response (pCR) in the Neo ALLTO trial. Yet the majority (58.6%) went on to have a mastectomy rather than BCS.
This puzzled investigators, who saw similar BCS rates in patients treated with other regimens in the study. All told, a pCR was achieved by 51.3% of patients treated with lapatinib, trastuzumab, and paclitaxel; 29.5% of those treated with trastuzumab and paclitaxel; and 24.7% given lapatinib and paclitaxel. Yet the respective rates of BCS were 41.4%, 38.9%, and 42.9%.
To investigate why more women did not opt for BCS, the authors examined data on 429 women who had participated in the trial, excluding 26 who did not undergo breast surgery. The records were examined for baseline characteristics including age, multicentricity/multifocality, planned surgery at diagnosis, physical examination before surgery, imaging before surgery, tumor characteristics, and geographic region.
Women were more likely to have BCS than mastectomy if breast-conserving treatment was already planned, they had a lower (T2 vs. T4) tumor grade, or their nodal (N0 vs. N+) status was lower. Geographic location was also important, with women treated in developed countries more likely to have more conservative treatment than those in developing regions.
Conversely, women were more likely to have a mastectomy than BCS if they had more diffuse disease, were estrogen receptor (ER)-negative, and had residual palpable disease.
"Our study suggests that the decision of surgical treatment post neoadjuvant therapy is mainly based on baseline characteristics," Dr. Criscitiello said. The lower rate of BCS in developing countries could be due to the lack of available radiotherapy, she observed.
Identifying pCR Could Be Hitch
"It is disappointing that we get high remission rates and yet a lot of women still have mastectomy," Dr. Ian Smith, commented after hearing these data.
Dr. Smith, a consultant medical oncologist and head of the Breast Unit at the Royal Marsden NHS Foundation Trust in London, noted that a key problem is identifying whether or not a pCR has occurred.
"The fundamental problem is that the surgeon, same as everybody else, doesn’t know if it is a pCR or not until surgery, and I think we have to look at different approaches to guide us," Dr. Smith suggested.
One approach is the use of molecular markers, such as Ki67. Data from the Royal Marsden have shown that Ki67 measured before and after neoadjuvant chemotherapy might help identify patients who could undergo a more conservative surgical approach.
Dr. Smith suggested: "We should be doing a biopsy again after the first course of neoadjuvant treatment and using molecular markers to predict for us who is going to get a pCR, and if they do they may need minimal surgery or they may need no surgery, but that’s another story."
The Neo ALTTO trial was supported by GlaxoSmithKline, but the company had no involvement in the current analysis. Dr. Criscitiello had no disclosures.
VIENNA – The decision to undergo breast-conserving surgery depends on baseline characteristics rather than response to therapy, according to a new analysis of data from a phase III trial comparing neoadjuvant regimens for early HER2-positive breast cancer.
Baseline multicentricity or multifocality of the tumor, receptor status, and the type of surgery planned at diagnosis all were found to influence the decision to undergo breast-conserving surgery (BCS) or mastectomy, investigators reported Sept. 30 at the European Society for Medical Oncology Congress.
"These results call for a clear consensus on the role of breast-conserving surgery in patients responding to neoadjuvant therapy," Dr. Carmen Criscitiello told attendees.
"This will ultimately translate the progress in neoadjuvant therapies into improved breast conservation rates," suggested Dr. Criscitiello of the European Institute of Oncology in Milan.
Puzzled by Low BCS Rates
Neoadjuvant therapy has several possible roles, including testing sensitivity to systemic therapy, downstaging tumors to make them operable, eliminating micrometastases, and increasing breast conservation rates, Dr. Criscitiello explained. It was therefore expected that BCS rates would increase significantly in the Neo ALLTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial.
Half of all patients treated with a dual HER2 blockade of lapatinib (Tykerb) and trastuzumab (Herceptin) in addition to paclitaxel achieved a pathological complete response (pCR) in the Neo ALLTO trial. Yet the majority (58.6%) went on to have a mastectomy rather than BCS.
This puzzled investigators, who saw similar BCS rates in patients treated with other regimens in the study. All told, a pCR was achieved by 51.3% of patients treated with lapatinib, trastuzumab, and paclitaxel; 29.5% of those treated with trastuzumab and paclitaxel; and 24.7% given lapatinib and paclitaxel. Yet the respective rates of BCS were 41.4%, 38.9%, and 42.9%.
To investigate why more women did not opt for BCS, the authors examined data on 429 women who had participated in the trial, excluding 26 who did not undergo breast surgery. The records were examined for baseline characteristics including age, multicentricity/multifocality, planned surgery at diagnosis, physical examination before surgery, imaging before surgery, tumor characteristics, and geographic region.
Women were more likely to have BCS than mastectomy if breast-conserving treatment was already planned, they had a lower (T2 vs. T4) tumor grade, or their nodal (N0 vs. N+) status was lower. Geographic location was also important, with women treated in developed countries more likely to have more conservative treatment than those in developing regions.
Conversely, women were more likely to have a mastectomy than BCS if they had more diffuse disease, were estrogen receptor (ER)-negative, and had residual palpable disease.
"Our study suggests that the decision of surgical treatment post neoadjuvant therapy is mainly based on baseline characteristics," Dr. Criscitiello said. The lower rate of BCS in developing countries could be due to the lack of available radiotherapy, she observed.
Identifying pCR Could Be Hitch
"It is disappointing that we get high remission rates and yet a lot of women still have mastectomy," Dr. Ian Smith, commented after hearing these data.
Dr. Smith, a consultant medical oncologist and head of the Breast Unit at the Royal Marsden NHS Foundation Trust in London, noted that a key problem is identifying whether or not a pCR has occurred.
"The fundamental problem is that the surgeon, same as everybody else, doesn’t know if it is a pCR or not until surgery, and I think we have to look at different approaches to guide us," Dr. Smith suggested.
One approach is the use of molecular markers, such as Ki67. Data from the Royal Marsden have shown that Ki67 measured before and after neoadjuvant chemotherapy might help identify patients who could undergo a more conservative surgical approach.
Dr. Smith suggested: "We should be doing a biopsy again after the first course of neoadjuvant treatment and using molecular markers to predict for us who is going to get a pCR, and if they do they may need minimal surgery or they may need no surgery, but that’s another story."
The Neo ALTTO trial was supported by GlaxoSmithKline, but the company had no involvement in the current analysis. Dr. Criscitiello had no disclosures.
VIENNA – The decision to undergo breast-conserving surgery depends on baseline characteristics rather than response to therapy, according to a new analysis of data from a phase III trial comparing neoadjuvant regimens for early HER2-positive breast cancer.
Baseline multicentricity or multifocality of the tumor, receptor status, and the type of surgery planned at diagnosis all were found to influence the decision to undergo breast-conserving surgery (BCS) or mastectomy, investigators reported Sept. 30 at the European Society for Medical Oncology Congress.
"These results call for a clear consensus on the role of breast-conserving surgery in patients responding to neoadjuvant therapy," Dr. Carmen Criscitiello told attendees.
"This will ultimately translate the progress in neoadjuvant therapies into improved breast conservation rates," suggested Dr. Criscitiello of the European Institute of Oncology in Milan.
Puzzled by Low BCS Rates
Neoadjuvant therapy has several possible roles, including testing sensitivity to systemic therapy, downstaging tumors to make them operable, eliminating micrometastases, and increasing breast conservation rates, Dr. Criscitiello explained. It was therefore expected that BCS rates would increase significantly in the Neo ALLTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial.
Half of all patients treated with a dual HER2 blockade of lapatinib (Tykerb) and trastuzumab (Herceptin) in addition to paclitaxel achieved a pathological complete response (pCR) in the Neo ALLTO trial. Yet the majority (58.6%) went on to have a mastectomy rather than BCS.
This puzzled investigators, who saw similar BCS rates in patients treated with other regimens in the study. All told, a pCR was achieved by 51.3% of patients treated with lapatinib, trastuzumab, and paclitaxel; 29.5% of those treated with trastuzumab and paclitaxel; and 24.7% given lapatinib and paclitaxel. Yet the respective rates of BCS were 41.4%, 38.9%, and 42.9%.
To investigate why more women did not opt for BCS, the authors examined data on 429 women who had participated in the trial, excluding 26 who did not undergo breast surgery. The records were examined for baseline characteristics including age, multicentricity/multifocality, planned surgery at diagnosis, physical examination before surgery, imaging before surgery, tumor characteristics, and geographic region.
Women were more likely to have BCS than mastectomy if breast-conserving treatment was already planned, they had a lower (T2 vs. T4) tumor grade, or their nodal (N0 vs. N+) status was lower. Geographic location was also important, with women treated in developed countries more likely to have more conservative treatment than those in developing regions.
Conversely, women were more likely to have a mastectomy than BCS if they had more diffuse disease, were estrogen receptor (ER)-negative, and had residual palpable disease.
"Our study suggests that the decision of surgical treatment post neoadjuvant therapy is mainly based on baseline characteristics," Dr. Criscitiello said. The lower rate of BCS in developing countries could be due to the lack of available radiotherapy, she observed.
Identifying pCR Could Be Hitch
"It is disappointing that we get high remission rates and yet a lot of women still have mastectomy," Dr. Ian Smith, commented after hearing these data.
Dr. Smith, a consultant medical oncologist and head of the Breast Unit at the Royal Marsden NHS Foundation Trust in London, noted that a key problem is identifying whether or not a pCR has occurred.
"The fundamental problem is that the surgeon, same as everybody else, doesn’t know if it is a pCR or not until surgery, and I think we have to look at different approaches to guide us," Dr. Smith suggested.
One approach is the use of molecular markers, such as Ki67. Data from the Royal Marsden have shown that Ki67 measured before and after neoadjuvant chemotherapy might help identify patients who could undergo a more conservative surgical approach.
Dr. Smith suggested: "We should be doing a biopsy again after the first course of neoadjuvant treatment and using molecular markers to predict for us who is going to get a pCR, and if they do they may need minimal surgery or they may need no surgery, but that’s another story."
The Neo ALTTO trial was supported by GlaxoSmithKline, but the company had no involvement in the current analysis. Dr. Criscitiello had no disclosures.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Findings: Half of all patients treated with a dual HER2 blockade and paclitaxel achieved a pathological complete response, but 58.6% went on to mastectomy rather than breast-conserving surgery.
Data Source: Investigators analyzed data from the phase III Neo ALTTO trial of 520 women with HER2-positive early breast cancer randomized to lapatinib, trastuzumab, or both, in addition to paclitaxel before surgery.
Disclosures: The Neo ALTTO trial was supported by GlaxoSmithKline, but the company had no involvement in the current analysis. Dr. Criscitiello had no disclosures.
Doxorubicin Remains First-Line Standard for Metastatic Soft-Tissue Sarcomas
VIENNA – A more aggressive combination of doxorubicin and ifosfamide provided no significant overall survival advantage over single-agent doxorubicin as first-line chemotherapy in advanced soft-tissue sarcoma in a phase III trial.
The European Organisation for Research and Treatment of Cancer (EORTC) 62012 study’s primary end point of median overall survival reached 14.3 months with the combination and 12.8 months with doxorubicin alone (hazard ratio, 0.83; P = .076). One-year survival rates were 60% and 51%, respectively.
As previously observed, the combination of doxorubicin (Adriamycin) and ifosfamide (Ifex) increased response rates and progression-free survival, but at a cost of considerably more toxicity. "The standard treatment remains single-agent doxorubicin," Dr. Winette van der Graaf said during a Presidential Symposium at the European Society for Medical Oncology Congress.
Combination therapy, she added, can be considered if surgery for unresectable tumors or curative metastasectomy is foreseen, and may be an option for highly symptomatic patients without comorbidity, although the pros and cons should be discussed with the patient.
"The advantage of having the results of this study is that it’s easier now to have this discussion with the patient," said Dr. van der Graaf, of Radboud University Nijmegen (Netherlands) Medical Centre.
The EORTC Soft Tissue and Bone Sarcoma Group designed the trial to answer the long-standing question of whether single-agent doxorubicin or doxorubicin plus ifosfamide is the best first-line treatment for metastatic soft-tissue sarcomas. An earlier EORTC phase III trial (J. Clin. Oncol. 1995;13:1537-45) explored the combination at a lower dose of ifosfamide than is used today, with more recent phase II trials suggesting that a higher dose could increase response rates and progression-free survival.
EORTC 62012 investigators at 38 centers in nine countries randomly assigned 455 patients, aged 18-60 years, with locally advanced unresectable or metastatic soft-tissue sarcoma to receive doxorubicin 75 mg/m2 bolus on day 1 or as a 72-hour continuous IV infusion or doxorubicin 25 mg/m2 on days 1-3 plus ifosfamide 2.5 g/m2 on days 1-4 and pegfilgrastim 6 mg subcutaneous on day 5.
Patients were stratified by age, performance status (0 vs. 1), liver metastases, and histological grade. No prior chemotherapy was allowed for advanced disease.
After a median follow-up of 56 months, median progression-free survival increased significantly from 4.6 months with single-agent doxorubicin to 7.4 months with the addition of ifosfamide (HR 0.74, P = .003), Dr. van der Graaf reported on behalf of lead author Dr. Ian Judson of the Royal Marsden Hospital, London.
Subgroup analyses revealed significantly longer progression-free survival among patients aged 40-49 years, but this significance disappeared when all patients under age 50 were included in the analysis. No subgroups had significantly better overall survival.
The complete response rate was very low, at 0.4% in the doxorubicin arm and 1.8% in the combination arm. Remarkably, partial responses doubled with combination therapy from 13.2% to 24.7%, but overall response rates (13.6% vs. 26.5%) were far less than expected from phase II trials, where they ranged from 52% to 66%, she observed.
At first blush, the difference in progression-free survival with combination therapy may not appear that important, but from the perspective of patients with metastatic soft-tissue sarcoma, who typically have a median survival of only 1 year, this means that they’ll spend about 20% of their lifetime longer with disease control, said discussant Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology at the Dana-Farber Cancer Institute in Boston.
"The question for us as clinicians is can we target patients who truly would get more benefit from this kind of doubling of tumor response and control, despite the sizable toxicities of the more toxic combination chemotherapy," he said.
Dr. Demetri pointed out that significantly more patients discontinued combination therapy than single-agent doxorubicin due to toxicity (40 patients vs. 6 patients). The most common grade 3 or higher adverse events in the combination and doxorubicin arms were febrile neutropenia (46% vs. 13.5%), anemia (35% vs. 4.6%) and thrombocytopenia (33.5% vs. 0.4%).
Dr. Demetri cautioned that the results should not be blindly extrapolated to clinical practice because patients in the trial were younger and healthier than those typically seen in practice. However, the solid data can be used to make informed choices, he said.
If a patient is asymptomatic, as many metastatic sarcoma patients are, then single-agent doxorubicin, which he described as a strong, aggressive, "truck" of a therapy, "may be sufficient" and "more kind, more humane" to the patient, he said. If the patient is symptomatic or tumor shrinkage is required to improve quality of life because of tumor-related pain, "then perhaps combination therapy, although more toxic, may be more beneficial," he added.
The EORTC sponsored the trial. Dr. van der Graaf and Dr. Judson reported no conflicts of interest. Dr. Demetri reported financial relationships with several pharmaceutical companies.
VIENNA – A more aggressive combination of doxorubicin and ifosfamide provided no significant overall survival advantage over single-agent doxorubicin as first-line chemotherapy in advanced soft-tissue sarcoma in a phase III trial.
The European Organisation for Research and Treatment of Cancer (EORTC) 62012 study’s primary end point of median overall survival reached 14.3 months with the combination and 12.8 months with doxorubicin alone (hazard ratio, 0.83; P = .076). One-year survival rates were 60% and 51%, respectively.
As previously observed, the combination of doxorubicin (Adriamycin) and ifosfamide (Ifex) increased response rates and progression-free survival, but at a cost of considerably more toxicity. "The standard treatment remains single-agent doxorubicin," Dr. Winette van der Graaf said during a Presidential Symposium at the European Society for Medical Oncology Congress.
Combination therapy, she added, can be considered if surgery for unresectable tumors or curative metastasectomy is foreseen, and may be an option for highly symptomatic patients without comorbidity, although the pros and cons should be discussed with the patient.
"The advantage of having the results of this study is that it’s easier now to have this discussion with the patient," said Dr. van der Graaf, of Radboud University Nijmegen (Netherlands) Medical Centre.
The EORTC Soft Tissue and Bone Sarcoma Group designed the trial to answer the long-standing question of whether single-agent doxorubicin or doxorubicin plus ifosfamide is the best first-line treatment for metastatic soft-tissue sarcomas. An earlier EORTC phase III trial (J. Clin. Oncol. 1995;13:1537-45) explored the combination at a lower dose of ifosfamide than is used today, with more recent phase II trials suggesting that a higher dose could increase response rates and progression-free survival.
EORTC 62012 investigators at 38 centers in nine countries randomly assigned 455 patients, aged 18-60 years, with locally advanced unresectable or metastatic soft-tissue sarcoma to receive doxorubicin 75 mg/m2 bolus on day 1 or as a 72-hour continuous IV infusion or doxorubicin 25 mg/m2 on days 1-3 plus ifosfamide 2.5 g/m2 on days 1-4 and pegfilgrastim 6 mg subcutaneous on day 5.
Patients were stratified by age, performance status (0 vs. 1), liver metastases, and histological grade. No prior chemotherapy was allowed for advanced disease.
After a median follow-up of 56 months, median progression-free survival increased significantly from 4.6 months with single-agent doxorubicin to 7.4 months with the addition of ifosfamide (HR 0.74, P = .003), Dr. van der Graaf reported on behalf of lead author Dr. Ian Judson of the Royal Marsden Hospital, London.
Subgroup analyses revealed significantly longer progression-free survival among patients aged 40-49 years, but this significance disappeared when all patients under age 50 were included in the analysis. No subgroups had significantly better overall survival.
The complete response rate was very low, at 0.4% in the doxorubicin arm and 1.8% in the combination arm. Remarkably, partial responses doubled with combination therapy from 13.2% to 24.7%, but overall response rates (13.6% vs. 26.5%) were far less than expected from phase II trials, where they ranged from 52% to 66%, she observed.
At first blush, the difference in progression-free survival with combination therapy may not appear that important, but from the perspective of patients with metastatic soft-tissue sarcoma, who typically have a median survival of only 1 year, this means that they’ll spend about 20% of their lifetime longer with disease control, said discussant Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology at the Dana-Farber Cancer Institute in Boston.
"The question for us as clinicians is can we target patients who truly would get more benefit from this kind of doubling of tumor response and control, despite the sizable toxicities of the more toxic combination chemotherapy," he said.
Dr. Demetri pointed out that significantly more patients discontinued combination therapy than single-agent doxorubicin due to toxicity (40 patients vs. 6 patients). The most common grade 3 or higher adverse events in the combination and doxorubicin arms were febrile neutropenia (46% vs. 13.5%), anemia (35% vs. 4.6%) and thrombocytopenia (33.5% vs. 0.4%).
Dr. Demetri cautioned that the results should not be blindly extrapolated to clinical practice because patients in the trial were younger and healthier than those typically seen in practice. However, the solid data can be used to make informed choices, he said.
If a patient is asymptomatic, as many metastatic sarcoma patients are, then single-agent doxorubicin, which he described as a strong, aggressive, "truck" of a therapy, "may be sufficient" and "more kind, more humane" to the patient, he said. If the patient is symptomatic or tumor shrinkage is required to improve quality of life because of tumor-related pain, "then perhaps combination therapy, although more toxic, may be more beneficial," he added.
The EORTC sponsored the trial. Dr. van der Graaf and Dr. Judson reported no conflicts of interest. Dr. Demetri reported financial relationships with several pharmaceutical companies.
VIENNA – A more aggressive combination of doxorubicin and ifosfamide provided no significant overall survival advantage over single-agent doxorubicin as first-line chemotherapy in advanced soft-tissue sarcoma in a phase III trial.
The European Organisation for Research and Treatment of Cancer (EORTC) 62012 study’s primary end point of median overall survival reached 14.3 months with the combination and 12.8 months with doxorubicin alone (hazard ratio, 0.83; P = .076). One-year survival rates were 60% and 51%, respectively.
As previously observed, the combination of doxorubicin (Adriamycin) and ifosfamide (Ifex) increased response rates and progression-free survival, but at a cost of considerably more toxicity. "The standard treatment remains single-agent doxorubicin," Dr. Winette van der Graaf said during a Presidential Symposium at the European Society for Medical Oncology Congress.
Combination therapy, she added, can be considered if surgery for unresectable tumors or curative metastasectomy is foreseen, and may be an option for highly symptomatic patients without comorbidity, although the pros and cons should be discussed with the patient.
"The advantage of having the results of this study is that it’s easier now to have this discussion with the patient," said Dr. van der Graaf, of Radboud University Nijmegen (Netherlands) Medical Centre.
The EORTC Soft Tissue and Bone Sarcoma Group designed the trial to answer the long-standing question of whether single-agent doxorubicin or doxorubicin plus ifosfamide is the best first-line treatment for metastatic soft-tissue sarcomas. An earlier EORTC phase III trial (J. Clin. Oncol. 1995;13:1537-45) explored the combination at a lower dose of ifosfamide than is used today, with more recent phase II trials suggesting that a higher dose could increase response rates and progression-free survival.
EORTC 62012 investigators at 38 centers in nine countries randomly assigned 455 patients, aged 18-60 years, with locally advanced unresectable or metastatic soft-tissue sarcoma to receive doxorubicin 75 mg/m2 bolus on day 1 or as a 72-hour continuous IV infusion or doxorubicin 25 mg/m2 on days 1-3 plus ifosfamide 2.5 g/m2 on days 1-4 and pegfilgrastim 6 mg subcutaneous on day 5.
Patients were stratified by age, performance status (0 vs. 1), liver metastases, and histological grade. No prior chemotherapy was allowed for advanced disease.
After a median follow-up of 56 months, median progression-free survival increased significantly from 4.6 months with single-agent doxorubicin to 7.4 months with the addition of ifosfamide (HR 0.74, P = .003), Dr. van der Graaf reported on behalf of lead author Dr. Ian Judson of the Royal Marsden Hospital, London.
Subgroup analyses revealed significantly longer progression-free survival among patients aged 40-49 years, but this significance disappeared when all patients under age 50 were included in the analysis. No subgroups had significantly better overall survival.
The complete response rate was very low, at 0.4% in the doxorubicin arm and 1.8% in the combination arm. Remarkably, partial responses doubled with combination therapy from 13.2% to 24.7%, but overall response rates (13.6% vs. 26.5%) were far less than expected from phase II trials, where they ranged from 52% to 66%, she observed.
At first blush, the difference in progression-free survival with combination therapy may not appear that important, but from the perspective of patients with metastatic soft-tissue sarcoma, who typically have a median survival of only 1 year, this means that they’ll spend about 20% of their lifetime longer with disease control, said discussant Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology at the Dana-Farber Cancer Institute in Boston.
"The question for us as clinicians is can we target patients who truly would get more benefit from this kind of doubling of tumor response and control, despite the sizable toxicities of the more toxic combination chemotherapy," he said.
Dr. Demetri pointed out that significantly more patients discontinued combination therapy than single-agent doxorubicin due to toxicity (40 patients vs. 6 patients). The most common grade 3 or higher adverse events in the combination and doxorubicin arms were febrile neutropenia (46% vs. 13.5%), anemia (35% vs. 4.6%) and thrombocytopenia (33.5% vs. 0.4%).
Dr. Demetri cautioned that the results should not be blindly extrapolated to clinical practice because patients in the trial were younger and healthier than those typically seen in practice. However, the solid data can be used to make informed choices, he said.
If a patient is asymptomatic, as many metastatic sarcoma patients are, then single-agent doxorubicin, which he described as a strong, aggressive, "truck" of a therapy, "may be sufficient" and "more kind, more humane" to the patient, he said. If the patient is symptomatic or tumor shrinkage is required to improve quality of life because of tumor-related pain, "then perhaps combination therapy, although more toxic, may be more beneficial," he added.
The EORTC sponsored the trial. Dr. van der Graaf and Dr. Judson reported no conflicts of interest. Dr. Demetri reported financial relationships with several pharmaceutical companies.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Cetuximab Fails First-Line in Gastric Cancer
VIENNA – Adding cetuximab to first-line chemotherapy for gastric cancer was associated with a worse benefit-to-risk ratio in the open-label, randomized, controlled, phase III EXPAND trial.
Investigators reported that progression-free survival was nonsignificantly decreased in patients who had received cetuximab (Erbitux) in addition to capecitabine (Xeloda) and cisplatin treatment versus those just receiving the chemotherapy doublet. Progression-free survival, which was the primary end point, was 4.4 months versus 5.6 months, respectively, with a hazard ratio (HR) of 1.09 (P = .3158).
There was also no benefit in overall survival or objective response rates. Overall survival was 9.4 months vs. 10.7 months (HR, = 1.0; P = .96). The best objective response rates were 30% and 29%, respectively.
"Unfortunately there was no benefit from adding cetuximab to chemotherapy [consisting of] capecitabine and cisplatin as first-line treatment for advanced gastric cancer," said Dr. Florian Lordick, who presented the findings at the European Society for Medical Oncology Congress.
"The results are consistent across subgroups," added Dr. Lordick, of the University Cancer Center Leipzig in Germany.
This is not the first disappointment seen with the use of an epidermal growth factor receptor inhibitor (EGFR) in gastric cancer. Recently reported results of the REAL-3 trial showed that panitumumab (Vectibix) was also not of benefit when added to standard chemotherapy for esophagogastric cancer.
Cetuximab also failed to show a benefit in patients when added to adjuvant oxaliplatin-containing chemotherapy for advanced metastatic colorectal cancer in the PETACC8 trial.
It is approved by the Food and Drug Administration in combination with irinotecan-containing regimens against EGFR-expressing metastatic colorectal cancer.
In the EXPAND study, 870 patients with unresected, advanced adenocarcinoma of the stomach gastric or gastroesophageal junction were randomized to receive cisplatin (80 mg/m2 on day 1) and capecitabine (1,000 mg/m2 twice daily, starting the evening of day 1 until the morning of day 15) with or without additional cetuximab (400 mg/m2 initial dose, then 250 mg/m2 per week).
The median age of enrolled patients was 59-60 years. Three quarters of participants were men and the main tumor site was the stomach in about a third of the cases with almost all patients having metastatic disease.
"Interestingly, the rate of hematologic adverse events was somewhat lower in the chemotherapy plus cetuximab arm," Dr. Lordick said. Compared with chemotherapy only, the rates of grade 3/4 neutropenia were 22% with cetuximab and 32% without. Grades 3/4 anemia occurred in 9% vs. 11% of patients, respectively.
"This is in contrast to other studies that have been performed in colorectal cancer, head and neck cancer, and lung cancer, where usually an increase of hematologic toxicity is seen with the administration of cetuximab in combination with chemotherapy," Dr. Lordick observed.
Nonhematologic adverse events, however, were more common in the cetuximab-containing vs. cetuximab-free arm, including grade 3 or 4 skin reactions (13% vs. 0%), diarrhea (8% vs. 4%), hand-foot syndrome (7% vs. 2%), hypomagnesemia (11% vs. 1%), and hypokalemia (13% vs. 9%).
Patients given the cetuximab-containing regimen also had a slightly higher rate of grade 3/4 cardiac adverse events (6.7% vs. 4.1 %).
"This study confirms a little bit what we heard at ASCO in the REAL-3 trial," said Dr. Arnaud Roth of Geneva University Hospital. Dr. Roth, who was not involved in the EXPAND study, noted that it was a well designed and conducted study and although negative, provided an excellent opportunity to conduct translational research.
However, Dr. Roth disagreed that this was the right setting to learn much more about anti-EGFRs and gastric cancer. Nevertheless, gaining access to the data and biospecimens was important and "a moral obligation" considering the patients who had given their consent for their participation in the trial.
"The good news is that tissue is available from 97% of patients and biomarker analysis is ongoing," Dr. Lordick said prior to Dr. Roth’s comments.
The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an advisor for Merck.
VIENNA – Adding cetuximab to first-line chemotherapy for gastric cancer was associated with a worse benefit-to-risk ratio in the open-label, randomized, controlled, phase III EXPAND trial.
Investigators reported that progression-free survival was nonsignificantly decreased in patients who had received cetuximab (Erbitux) in addition to capecitabine (Xeloda) and cisplatin treatment versus those just receiving the chemotherapy doublet. Progression-free survival, which was the primary end point, was 4.4 months versus 5.6 months, respectively, with a hazard ratio (HR) of 1.09 (P = .3158).
There was also no benefit in overall survival or objective response rates. Overall survival was 9.4 months vs. 10.7 months (HR, = 1.0; P = .96). The best objective response rates were 30% and 29%, respectively.
"Unfortunately there was no benefit from adding cetuximab to chemotherapy [consisting of] capecitabine and cisplatin as first-line treatment for advanced gastric cancer," said Dr. Florian Lordick, who presented the findings at the European Society for Medical Oncology Congress.
"The results are consistent across subgroups," added Dr. Lordick, of the University Cancer Center Leipzig in Germany.
This is not the first disappointment seen with the use of an epidermal growth factor receptor inhibitor (EGFR) in gastric cancer. Recently reported results of the REAL-3 trial showed that panitumumab (Vectibix) was also not of benefit when added to standard chemotherapy for esophagogastric cancer.
Cetuximab also failed to show a benefit in patients when added to adjuvant oxaliplatin-containing chemotherapy for advanced metastatic colorectal cancer in the PETACC8 trial.
It is approved by the Food and Drug Administration in combination with irinotecan-containing regimens against EGFR-expressing metastatic colorectal cancer.
In the EXPAND study, 870 patients with unresected, advanced adenocarcinoma of the stomach gastric or gastroesophageal junction were randomized to receive cisplatin (80 mg/m2 on day 1) and capecitabine (1,000 mg/m2 twice daily, starting the evening of day 1 until the morning of day 15) with or without additional cetuximab (400 mg/m2 initial dose, then 250 mg/m2 per week).
The median age of enrolled patients was 59-60 years. Three quarters of participants were men and the main tumor site was the stomach in about a third of the cases with almost all patients having metastatic disease.
"Interestingly, the rate of hematologic adverse events was somewhat lower in the chemotherapy plus cetuximab arm," Dr. Lordick said. Compared with chemotherapy only, the rates of grade 3/4 neutropenia were 22% with cetuximab and 32% without. Grades 3/4 anemia occurred in 9% vs. 11% of patients, respectively.
"This is in contrast to other studies that have been performed in colorectal cancer, head and neck cancer, and lung cancer, where usually an increase of hematologic toxicity is seen with the administration of cetuximab in combination with chemotherapy," Dr. Lordick observed.
Nonhematologic adverse events, however, were more common in the cetuximab-containing vs. cetuximab-free arm, including grade 3 or 4 skin reactions (13% vs. 0%), diarrhea (8% vs. 4%), hand-foot syndrome (7% vs. 2%), hypomagnesemia (11% vs. 1%), and hypokalemia (13% vs. 9%).
Patients given the cetuximab-containing regimen also had a slightly higher rate of grade 3/4 cardiac adverse events (6.7% vs. 4.1 %).
"This study confirms a little bit what we heard at ASCO in the REAL-3 trial," said Dr. Arnaud Roth of Geneva University Hospital. Dr. Roth, who was not involved in the EXPAND study, noted that it was a well designed and conducted study and although negative, provided an excellent opportunity to conduct translational research.
However, Dr. Roth disagreed that this was the right setting to learn much more about anti-EGFRs and gastric cancer. Nevertheless, gaining access to the data and biospecimens was important and "a moral obligation" considering the patients who had given their consent for their participation in the trial.
"The good news is that tissue is available from 97% of patients and biomarker analysis is ongoing," Dr. Lordick said prior to Dr. Roth’s comments.
The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an advisor for Merck.
VIENNA – Adding cetuximab to first-line chemotherapy for gastric cancer was associated with a worse benefit-to-risk ratio in the open-label, randomized, controlled, phase III EXPAND trial.
Investigators reported that progression-free survival was nonsignificantly decreased in patients who had received cetuximab (Erbitux) in addition to capecitabine (Xeloda) and cisplatin treatment versus those just receiving the chemotherapy doublet. Progression-free survival, which was the primary end point, was 4.4 months versus 5.6 months, respectively, with a hazard ratio (HR) of 1.09 (P = .3158).
There was also no benefit in overall survival or objective response rates. Overall survival was 9.4 months vs. 10.7 months (HR, = 1.0; P = .96). The best objective response rates were 30% and 29%, respectively.
"Unfortunately there was no benefit from adding cetuximab to chemotherapy [consisting of] capecitabine and cisplatin as first-line treatment for advanced gastric cancer," said Dr. Florian Lordick, who presented the findings at the European Society for Medical Oncology Congress.
"The results are consistent across subgroups," added Dr. Lordick, of the University Cancer Center Leipzig in Germany.
This is not the first disappointment seen with the use of an epidermal growth factor receptor inhibitor (EGFR) in gastric cancer. Recently reported results of the REAL-3 trial showed that panitumumab (Vectibix) was also not of benefit when added to standard chemotherapy for esophagogastric cancer.
Cetuximab also failed to show a benefit in patients when added to adjuvant oxaliplatin-containing chemotherapy for advanced metastatic colorectal cancer in the PETACC8 trial.
It is approved by the Food and Drug Administration in combination with irinotecan-containing regimens against EGFR-expressing metastatic colorectal cancer.
In the EXPAND study, 870 patients with unresected, advanced adenocarcinoma of the stomach gastric or gastroesophageal junction were randomized to receive cisplatin (80 mg/m2 on day 1) and capecitabine (1,000 mg/m2 twice daily, starting the evening of day 1 until the morning of day 15) with or without additional cetuximab (400 mg/m2 initial dose, then 250 mg/m2 per week).
The median age of enrolled patients was 59-60 years. Three quarters of participants were men and the main tumor site was the stomach in about a third of the cases with almost all patients having metastatic disease.
"Interestingly, the rate of hematologic adverse events was somewhat lower in the chemotherapy plus cetuximab arm," Dr. Lordick said. Compared with chemotherapy only, the rates of grade 3/4 neutropenia were 22% with cetuximab and 32% without. Grades 3/4 anemia occurred in 9% vs. 11% of patients, respectively.
"This is in contrast to other studies that have been performed in colorectal cancer, head and neck cancer, and lung cancer, where usually an increase of hematologic toxicity is seen with the administration of cetuximab in combination with chemotherapy," Dr. Lordick observed.
Nonhematologic adverse events, however, were more common in the cetuximab-containing vs. cetuximab-free arm, including grade 3 or 4 skin reactions (13% vs. 0%), diarrhea (8% vs. 4%), hand-foot syndrome (7% vs. 2%), hypomagnesemia (11% vs. 1%), and hypokalemia (13% vs. 9%).
Patients given the cetuximab-containing regimen also had a slightly higher rate of grade 3/4 cardiac adverse events (6.7% vs. 4.1 %).
"This study confirms a little bit what we heard at ASCO in the REAL-3 trial," said Dr. Arnaud Roth of Geneva University Hospital. Dr. Roth, who was not involved in the EXPAND study, noted that it was a well designed and conducted study and although negative, provided an excellent opportunity to conduct translational research.
However, Dr. Roth disagreed that this was the right setting to learn much more about anti-EGFRs and gastric cancer. Nevertheless, gaining access to the data and biospecimens was important and "a moral obligation" considering the patients who had given their consent for their participation in the trial.
"The good news is that tissue is available from 97% of patients and biomarker analysis is ongoing," Dr. Lordick said prior to Dr. Roth’s comments.
The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an advisor for Merck.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Findings: Median progression-free survival was 4.4 vs. 5.6 months (HR = 1.09) when comparing triple treatment with cetuximab, capecitabine and cisplatin with the chemotherapy doublet alone.
Data Source: EXPAND, an open-label, randomized, controlled phase III trial, enrolled 870 patients with advanced gastric cancer.
Disclosures: The study was funded by Merck KGaA. Dr. Lordick has received research funding and honoraria from Merck KGaA, GlaxoSmithKline and Fresenius Biotech, and advisory fees from Amgen, Ganymed GmbH, Ribological GmbH, and Roche. Dr. Roth has acted as an advisor for Merck.