User login
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
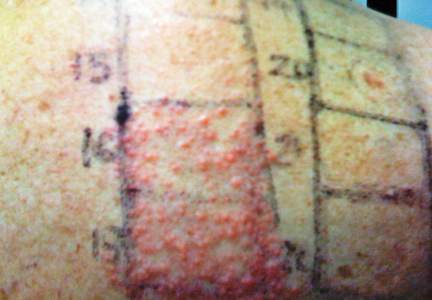
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.
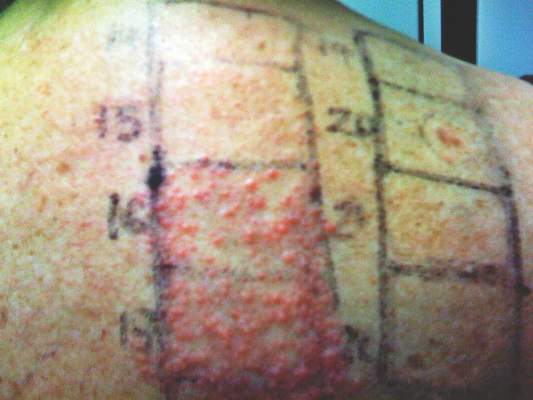
Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8

In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8

In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8

In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
Practice Points
- When lesions develop in the oral cavity during treatment with inhaled corticosteroids, delayed contact allergy should be considered in the differential diagnosis along with fungal infection.
- Although it generally is not considered to be allergenic due to its poor intestinal absorption, oral nystatin may induce systemic allergic disorders.
- All drugs involved in a presumed allergic reaction must be evaluated since concomitant sensitization to multiple drugs could be present. Patch and challenge testing should be conducted to diagnose allergic contact dermatitis and assess drug cross-reactivity.