User login
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
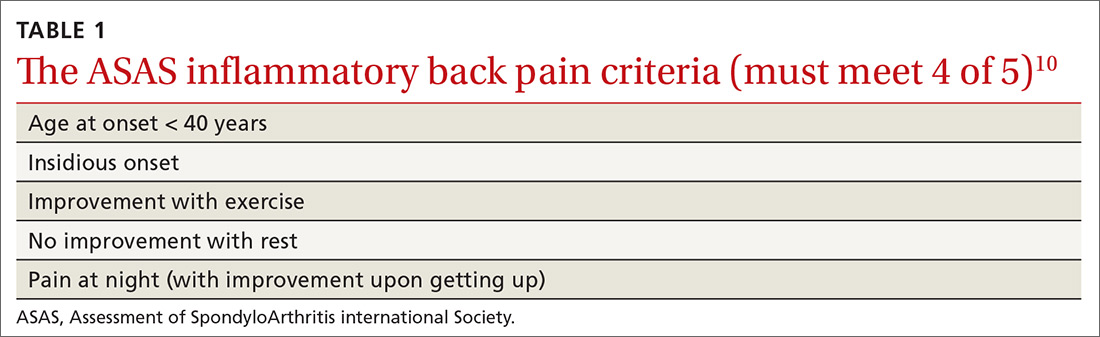
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
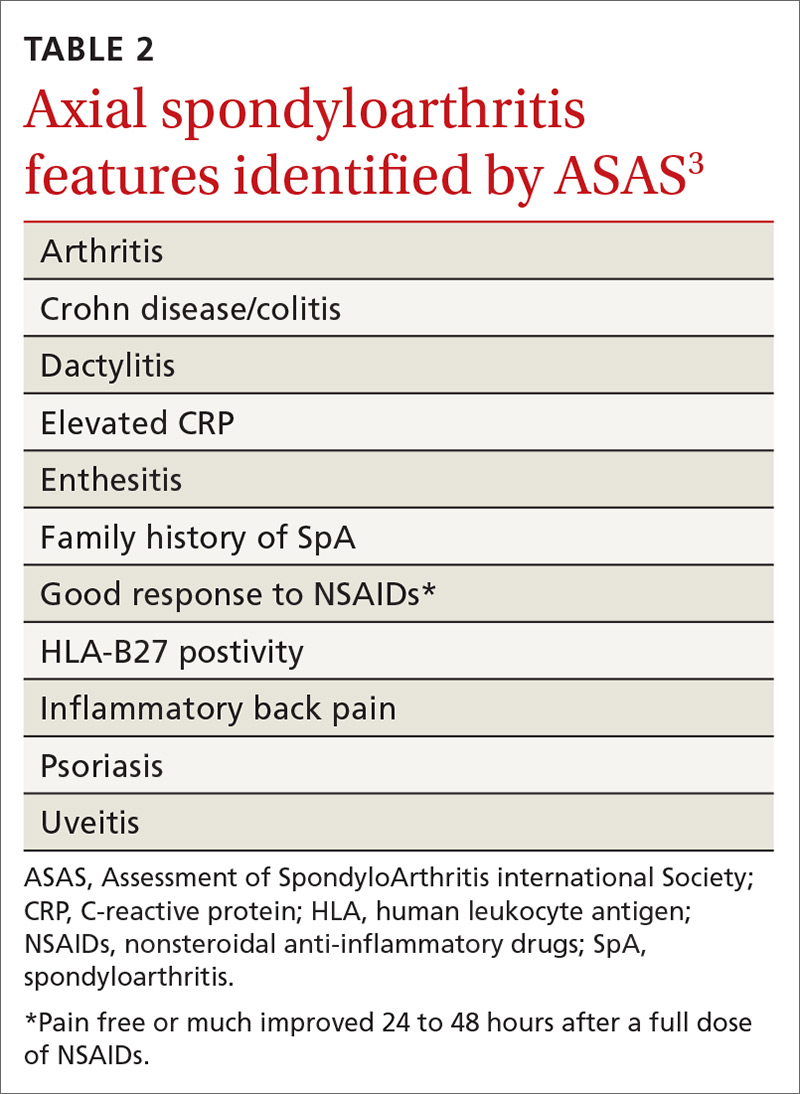
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
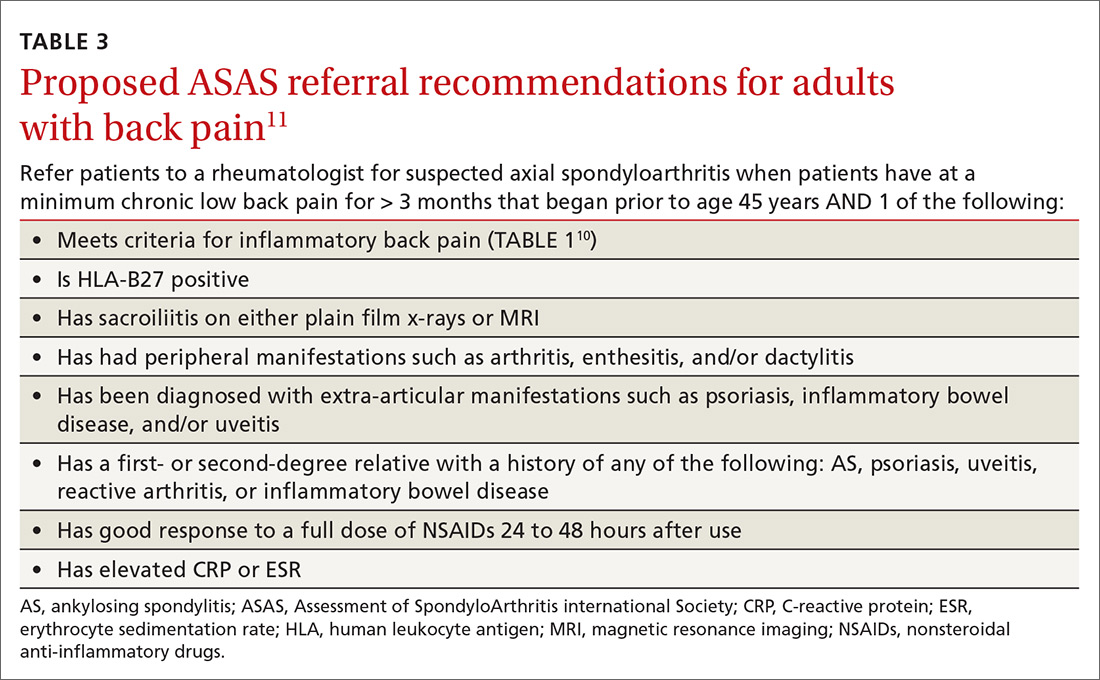
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10

A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful

No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.

Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10

A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful

No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.

Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
PRACTICE RECOMMENDATIONS
› Evaluate all patients with back pain lasting > 3 months for inflammatory back pain features. C
› Treat all patients with confirmed or suspected axial spondyloarthritis with a trial of nonsteroidal anti-inflammatory drugs. A
› Recommend that all patients with back pain—including those with suspected axial spondyloarthritis—start an exercise program that includes both strength and aerobic activities. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
