User login
Could that back pain be caused by ankylosing spondylitis?
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
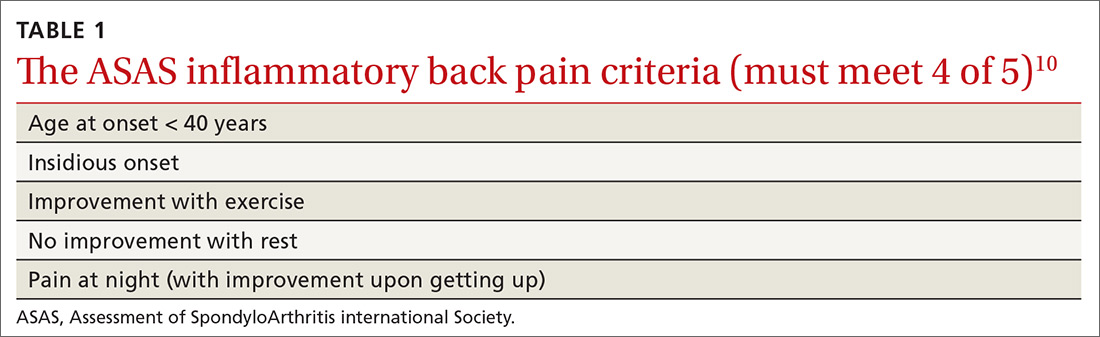
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
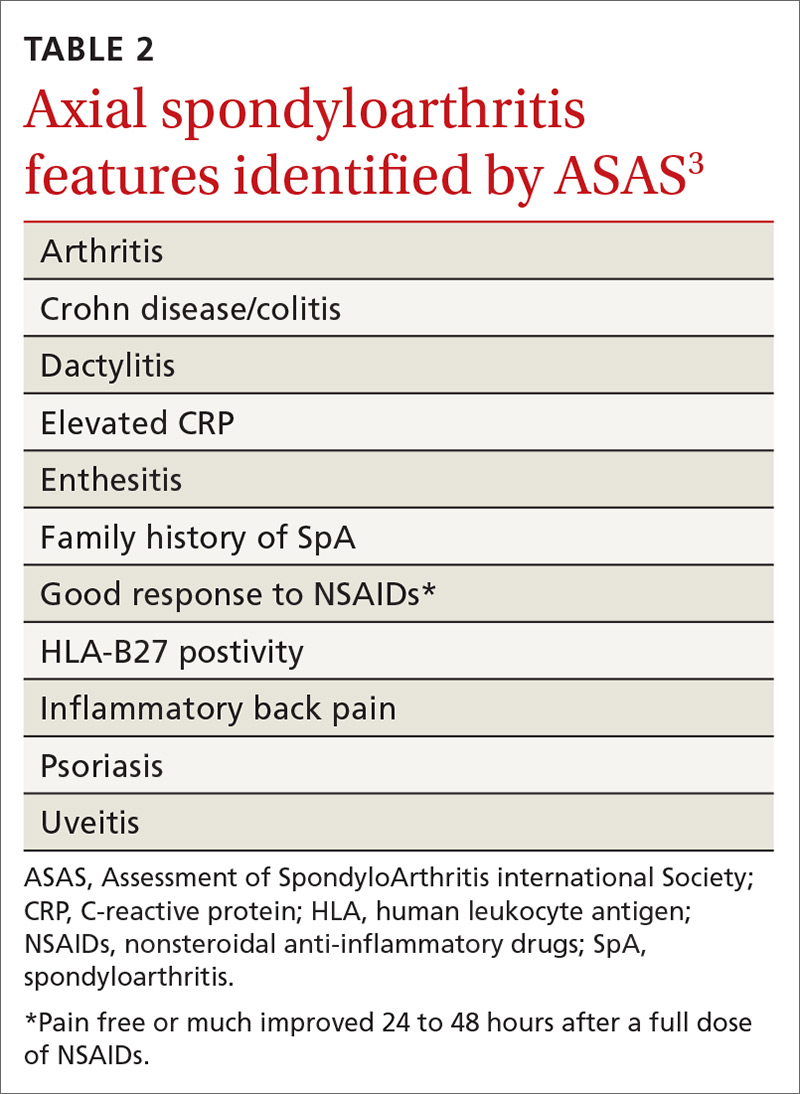
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
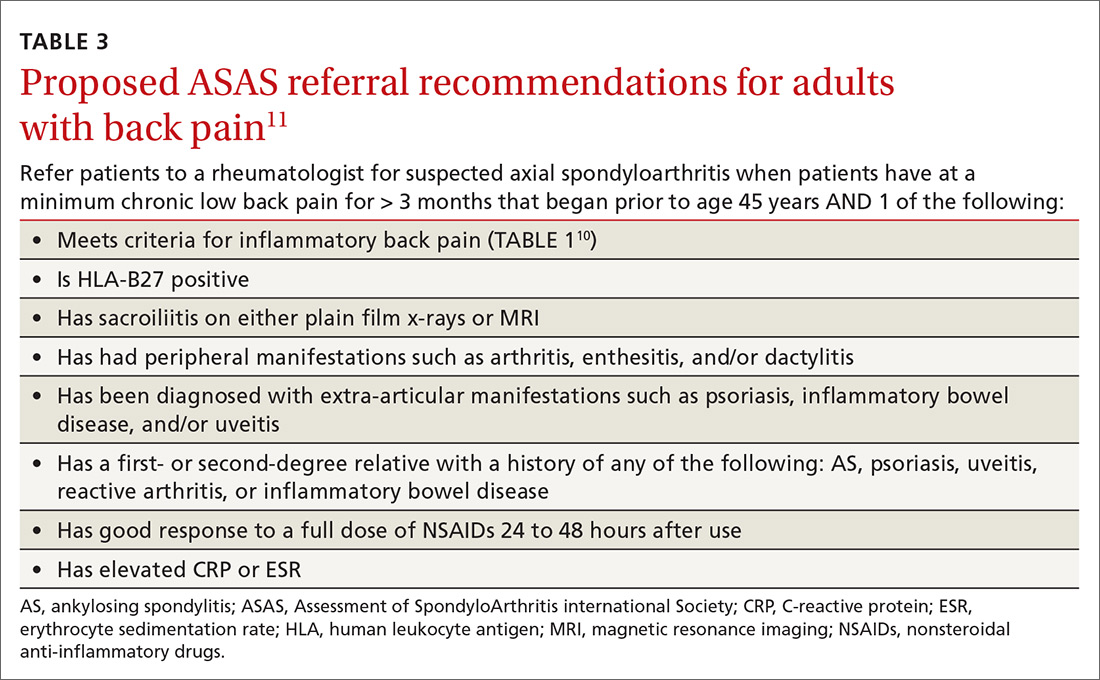
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10

A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful

No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.

Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10

A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful

No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.

Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
PRACTICE RECOMMENDATIONS
› Evaluate all patients with back pain lasting > 3 months for inflammatory back pain features. C
› Treat all patients with confirmed or suspected axial spondyloarthritis with a trial of nonsteroidal anti-inflammatory drugs. A
› Recommend that all patients with back pain—including those with suspected axial spondyloarthritis—start an exercise program that includes both strength and aerobic activities. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Prolotherapy: Can it help your patient?
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
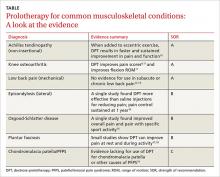
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
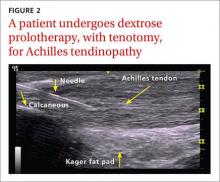
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
Nontraumatic Knee Pain: A Diagnostic & Treatment Guide
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Nontraumatic Knee Pain: A Diagnostic & Treatment Guide
› Consider radiography for
a patient with patellofemoral pain syndrome if examination reveals an effusion, the patient is age
50 years or older, or the condition does not improve after 8 to 12 weeks of treatment. C
› Order plain radiography
for all patients with patellofemoral instability to assess for osseous trauma/deformity; consider magnetic resonance imaging if you suspect significant soft tissue damage or the patient does not respond to conservative therapy. C
› Perform joint aspiration with synovial fluid analysis for patients with painful knee effusion, and provide an orthopedic referral without delay when an infectious joint is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Jane T, age 42, comes to see you because of right knee pain that she’s had for about 6 months. She denies any trauma. Ms. T describes the pain as vague and poorly localized, but worse with activity. She says she started a walking/running program 9 months ago, when she was told she was overweight (body mass index, 29). She has lost 10 pounds since then, Ms. T says, and hopes to lose more by continuing to exercise. upon further review, you find that Ms. T has had increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Ms. T were your patient, what would you include in a physical examination and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain like that of Ms. T. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. In the pages that follow, we provide general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (TABLE).1-31
Continue for anterior knee pain >>
Anterior knee pain
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS), the most common cause of anterior knee pain, is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion, and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with <20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis, but may be considered if examination reveals an effusion, the patient is age 50 years or older, or no improvement occurs after 8 to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a 6-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of nonsteroidal anti-inflammatory drugs (NSAIDs), but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are non-conclusive. There is a paucity of prospective randomized trials of patellar bracing and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
CASE › When you examine Ms. T, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for 6 weeks of physiotherapy.
Patellar subluxation or chronic dislocation
Patellofemoral instability (PFI) occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable physical exam findings are:
- a positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
- decreased quadriceps (specifically VMO) and hamstring strength and flexibility
- patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
- pain during a patellar tilt test
- a positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/ deformity and to help guide surgical consideration. Magnetic resonance imaging (MRI) can provide additional information when significant soft tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft tissue stabilizer) are clearly shown on imaging.8,11
Next page: Patellar tendinopathy >>
Patellar tendinopathy (jumper’s knee)
Patellar tendinopathy, an overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports like volleyball and basketball, is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury is poorly understood, but is believed to be the result of an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of 4 phases:12 1. pain isolated after activity; 2. pain that occurs during activity but does not impede activity; 3. pain that occurs both during and after the activity and interferes with competition ; 4. a complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle-tendon function should be evaluated by assessing knee mobility and strength of the quads via straight leg raise, decline squat, or single leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but magnetic resonance imaging (MRI) can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training—eg, 3 sets of 15 repetitions performed twice a day for 12 weeks—and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that 3 weekly PRP injections helped 75% of patients—all of whom failed to respond to 4 months of eccentric therapy—return to their pre-symptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk of tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after 3 to 6 months of conservative therapy.14
Continue for lateral knee pain >>
Lateral knee pain
Iliotibial band tendinopathy
Iliotibial band syndrome (ITBS) is a common source of lateral knee pain, particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from micro trauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (FIGURE 1). A positive Ober’s test (FIGURE 2) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the iliotibial band, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to 2 weeks post injection.17,19 When symptoms persist for more than 6 months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
Next: Medial knee pain >>
Medial knee pain
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomical site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed, but may be helpful if significant bony pathology is suspected. Ultrasound, computed tomography (CT), and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and using a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
Posterior knee pain
Popliteal (Baker’s) cyst
The popliteal fossa contains 6 of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for the development of popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt and for patients suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular steroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
What do you do for painful knee effusion? >>
When the problem is painful knee effusion
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. We mention it here because clinical suspicion is paramount in diagnosing a septic joint, a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC >50,000 per mm3, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
CASE › When Ms. T returns for a follow-up visit 8 weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional 8 pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: March 20, 2014.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: Systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41: 19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13: 172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20: 1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21: 469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: December 12, 2013.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21: 486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
› Consider radiography for
a patient with patellofemoral pain syndrome if examination reveals an effusion, the patient is age
50 years or older, or the condition does not improve after 8 to 12 weeks of treatment. C
› Order plain radiography
for all patients with patellofemoral instability to assess for osseous trauma/deformity; consider magnetic resonance imaging if you suspect significant soft tissue damage or the patient does not respond to conservative therapy. C
› Perform joint aspiration with synovial fluid analysis for patients with painful knee effusion, and provide an orthopedic referral without delay when an infectious joint is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Jane T, age 42, comes to see you because of right knee pain that she’s had for about 6 months. She denies any trauma. Ms. T describes the pain as vague and poorly localized, but worse with activity. She says she started a walking/running program 9 months ago, when she was told she was overweight (body mass index, 29). She has lost 10 pounds since then, Ms. T says, and hopes to lose more by continuing to exercise. upon further review, you find that Ms. T has had increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Ms. T were your patient, what would you include in a physical examination and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain like that of Ms. T. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. In the pages that follow, we provide general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (TABLE).1-31
Continue for anterior knee pain >>
Anterior knee pain
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS), the most common cause of anterior knee pain, is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion, and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with <20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis, but may be considered if examination reveals an effusion, the patient is age 50 years or older, or no improvement occurs after 8 to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a 6-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of nonsteroidal anti-inflammatory drugs (NSAIDs), but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are non-conclusive. There is a paucity of prospective randomized trials of patellar bracing and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
CASE › When you examine Ms. T, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for 6 weeks of physiotherapy.
Patellar subluxation or chronic dislocation
Patellofemoral instability (PFI) occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable physical exam findings are:
- a positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
- decreased quadriceps (specifically VMO) and hamstring strength and flexibility
- patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
- pain during a patellar tilt test
- a positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/ deformity and to help guide surgical consideration. Magnetic resonance imaging (MRI) can provide additional information when significant soft tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft tissue stabilizer) are clearly shown on imaging.8,11
Next page: Patellar tendinopathy >>
Patellar tendinopathy (jumper’s knee)
Patellar tendinopathy, an overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports like volleyball and basketball, is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury is poorly understood, but is believed to be the result of an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of 4 phases:12 1. pain isolated after activity; 2. pain that occurs during activity but does not impede activity; 3. pain that occurs both during and after the activity and interferes with competition ; 4. a complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle-tendon function should be evaluated by assessing knee mobility and strength of the quads via straight leg raise, decline squat, or single leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but magnetic resonance imaging (MRI) can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training—eg, 3 sets of 15 repetitions performed twice a day for 12 weeks—and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that 3 weekly PRP injections helped 75% of patients—all of whom failed to respond to 4 months of eccentric therapy—return to their pre-symptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk of tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after 3 to 6 months of conservative therapy.14
Continue for lateral knee pain >>
Lateral knee pain
Iliotibial band tendinopathy
Iliotibial band syndrome (ITBS) is a common source of lateral knee pain, particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from micro trauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (FIGURE 1). A positive Ober’s test (FIGURE 2) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the iliotibial band, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to 2 weeks post injection.17,19 When symptoms persist for more than 6 months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
Next: Medial knee pain >>
Medial knee pain
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomical site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed, but may be helpful if significant bony pathology is suspected. Ultrasound, computed tomography (CT), and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and using a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
Posterior knee pain
Popliteal (Baker’s) cyst
The popliteal fossa contains 6 of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for the development of popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt and for patients suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular steroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
What do you do for painful knee effusion? >>
When the problem is painful knee effusion
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. We mention it here because clinical suspicion is paramount in diagnosing a septic joint, a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC >50,000 per mm3, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
CASE › When Ms. T returns for a follow-up visit 8 weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional 8 pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
› Consider radiography for
a patient with patellofemoral pain syndrome if examination reveals an effusion, the patient is age
50 years or older, or the condition does not improve after 8 to 12 weeks of treatment. C
› Order plain radiography
for all patients with patellofemoral instability to assess for osseous trauma/deformity; consider magnetic resonance imaging if you suspect significant soft tissue damage or the patient does not respond to conservative therapy. C
› Perform joint aspiration with synovial fluid analysis for patients with painful knee effusion, and provide an orthopedic referral without delay when an infectious joint is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Jane T, age 42, comes to see you because of right knee pain that she’s had for about 6 months. She denies any trauma. Ms. T describes the pain as vague and poorly localized, but worse with activity. She says she started a walking/running program 9 months ago, when she was told she was overweight (body mass index, 29). She has lost 10 pounds since then, Ms. T says, and hopes to lose more by continuing to exercise. upon further review, you find that Ms. T has had increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Ms. T were your patient, what would you include in a physical examination and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain like that of Ms. T. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. In the pages that follow, we provide general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (TABLE).1-31
Continue for anterior knee pain >>
Anterior knee pain
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS), the most common cause of anterior knee pain, is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion, and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with <20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis, but may be considered if examination reveals an effusion, the patient is age 50 years or older, or no improvement occurs after 8 to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a 6-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of nonsteroidal anti-inflammatory drugs (NSAIDs), but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are non-conclusive. There is a paucity of prospective randomized trials of patellar bracing and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
CASE › When you examine Ms. T, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for 6 weeks of physiotherapy.
Patellar subluxation or chronic dislocation
Patellofemoral instability (PFI) occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable physical exam findings are:
- a positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
- decreased quadriceps (specifically VMO) and hamstring strength and flexibility
- patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
- pain during a patellar tilt test
- a positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/ deformity and to help guide surgical consideration. Magnetic resonance imaging (MRI) can provide additional information when significant soft tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft tissue stabilizer) are clearly shown on imaging.8,11
Next page: Patellar tendinopathy >>
Patellar tendinopathy (jumper’s knee)
Patellar tendinopathy, an overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports like volleyball and basketball, is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury is poorly understood, but is believed to be the result of an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of 4 phases:12 1. pain isolated after activity; 2. pain that occurs during activity but does not impede activity; 3. pain that occurs both during and after the activity and interferes with competition ; 4. a complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle-tendon function should be evaluated by assessing knee mobility and strength of the quads via straight leg raise, decline squat, or single leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but magnetic resonance imaging (MRI) can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training—eg, 3 sets of 15 repetitions performed twice a day for 12 weeks—and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that 3 weekly PRP injections helped 75% of patients—all of whom failed to respond to 4 months of eccentric therapy—return to their pre-symptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk of tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after 3 to 6 months of conservative therapy.14
Continue for lateral knee pain >>
Lateral knee pain
Iliotibial band tendinopathy
Iliotibial band syndrome (ITBS) is a common source of lateral knee pain, particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from micro trauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (FIGURE 1). A positive Ober’s test (FIGURE 2) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the iliotibial band, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to 2 weeks post injection.17,19 When symptoms persist for more than 6 months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
Next: Medial knee pain >>
Medial knee pain
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomical site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed, but may be helpful if significant bony pathology is suspected. Ultrasound, computed tomography (CT), and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and using a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
Posterior knee pain
Popliteal (Baker’s) cyst
The popliteal fossa contains 6 of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for the development of popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt and for patients suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular steroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
What do you do for painful knee effusion? >>
When the problem is painful knee effusion
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. We mention it here because clinical suspicion is paramount in diagnosing a septic joint, a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC >50,000 per mm3, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
CASE › When Ms. T returns for a follow-up visit 8 weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional 8 pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: March 20, 2014.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: Systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41: 19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13: 172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20: 1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21: 469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: December 12, 2013.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21: 486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: March 20, 2014.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: Systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41: 19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13: 172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20: 1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21: 469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: December 12, 2013.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21: 486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Nontraumatic knee pain: A diagnostic & treatment guide
› Consider radiography for
a patient with patellofemoral pain syndrome if examination reveals an effusion, the patient is age
50 years or older, or the condition does not improve after 8 to 12 weeks of treatment. C
› Order plain radiography
for all patients with patellofemoral instability to assess for osseous trauma/deformity; consider magnetic resonance imaging if you suspect significant soft tissue damage or the patient does not respond to conservative therapy. C
› Perform joint aspiration with synovial fluid analysis for patients with painful knee effusion, and provide an orthopedic referral without delay when an infectious joint is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Jane T, age 42, comes to see you because of right knee pain that she’s had for about 6 months. She denies any trauma. Ms. T describes the pain as vague and poorly localized, but worse with activity. She says she started a walking/running program 9 months ago, when she was told she was overweight (body mass index, 29). She has lost 10 pounds since then, Ms. T says, and hopes to lose more by continuing to exercise. upon further review, you find that Ms. T has had increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Ms. T were your patient, what would you include in a physical examination and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain like that of Ms. T. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. In the pages that follow, we provide general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (TABLE).1-31
Anterior knee pain
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS), the most common cause of anterior knee pain, is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion, and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with <20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis, but may be considered if examination reveals an effusion, the patient is age 50 years or older, or no improvement occurs after 8 to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a 6-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of nonsteroidal anti-inflammatory drugs (NSAIDs), but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are non-conclusive. There is a paucity of prospective randomized trials of patellar bracing and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
CASE › When you examine Ms. T, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for 6 weeks of physiotherapy.
Patellar subluxation or chronic dislocation
Patellofemoral instability (PFI) occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable physical exam findings are:
- a positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
- decreased quadriceps (specifically VMO) and hamstring strength and flexibility
- patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
- pain during a patellar tilt test
- a positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/ deformity and to help guide surgical consideration. Magnetic resonance imaging (MRI) can provide additional information when significant soft tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy (jumper’s knee)
Patellar tendinopathy, an overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports like volleyball and basketball, is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury is poorly understood, but is believed to be the result of an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of 4 phases:12 1. pain isolated after activity; 2. pain that occurs during activity but does not impede activity; 3. pain that occurs both during and after the activity and interferes with competition ; 4. a complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle-tendon function should be evaluated by assessing knee mobility and strength of the quads via straight leg raise, decline squat, or single leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but magnetic resonance imaging (MRI) can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training—eg, 3 sets of 15 repetitions performed twice a day for 12 weeks—and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that 3 weekly PRP injections helped 75% of patients—all of whom failed to respond to 4 months of eccentric therapy—return to their pre-symptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk of tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after 3 to 6 months of conservative therapy.14
Lateral knee pain
Iliotibial band tendinopathy
Iliotibial band syndrome (ITBS) is a common source of lateral knee pain, particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from micro trauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (FIGURE 1). A positive Ober’s test (FIGURE 2) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the iliotibial band, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to 2 weeks post injection.17,19 When symptoms persist for more than 6 months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
Medial knee pain
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomical site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed, but may be helpful if significant bony pathology is suspected. Ultrasound, computed tomography (CT), and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and using a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
Posterior knee pain
Popliteal (Baker’s) cyst
The popliteal fossa contains 6 of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for the development of popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt and for patients suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular steroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
When the problem is painful knee effusion
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. We mention it here because clinical suspicion is paramount in diagnosing a septic joint, a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC >50,000 per mm3, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
CASE › When Ms. T returns for a follow-up visit 8 weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional 8 pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: March 20, 2014.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: Systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41: 19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13: 172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20: 1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21: 469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: December 12, 2013.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21: 486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
› Consider radiography for
a patient with patellofemoral pain syndrome if examination reveals an effusion, the patient is age
50 years or older, or the condition does not improve after 8 to 12 weeks of treatment. C
› Order plain radiography
for all patients with patellofemoral instability to assess for osseous trauma/deformity; consider magnetic resonance imaging if you suspect significant soft tissue damage or the patient does not respond to conservative therapy. C
› Perform joint aspiration with synovial fluid analysis for patients with painful knee effusion, and provide an orthopedic referral without delay when an infectious joint is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Jane T, age 42, comes to see you because of right knee pain that she’s had for about 6 months. She denies any trauma. Ms. T describes the pain as vague and poorly localized, but worse with activity. She says she started a walking/running program 9 months ago, when she was told she was overweight (body mass index, 29). She has lost 10 pounds since then, Ms. T says, and hopes to lose more by continuing to exercise. upon further review, you find that Ms. T has had increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Ms. T were your patient, what would you include in a physical examination and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain like that of Ms. T. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. In the pages that follow, we provide general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (TABLE).1-31
Anterior knee pain
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS), the most common cause of anterior knee pain, is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion, and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with <20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis, but may be considered if examination reveals an effusion, the patient is age 50 years or older, or no improvement occurs after 8 to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a 6-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of nonsteroidal anti-inflammatory drugs (NSAIDs), but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are non-conclusive. There is a paucity of prospective randomized trials of patellar bracing and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
CASE › When you examine Ms. T, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for 6 weeks of physiotherapy.
Patellar subluxation or chronic dislocation
Patellofemoral instability (PFI) occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable physical exam findings are:
- a positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
- decreased quadriceps (specifically VMO) and hamstring strength and flexibility
- patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
- pain during a patellar tilt test
- a positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/ deformity and to help guide surgical consideration. Magnetic resonance imaging (MRI) can provide additional information when significant soft tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy (jumper’s knee)
Patellar tendinopathy, an overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports like volleyball and basketball, is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury is poorly understood, but is believed to be the result of an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of 4 phases:12 1. pain isolated after activity; 2. pain that occurs during activity but does not impede activity; 3. pain that occurs both during and after the activity and interferes with competition ; 4. a complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle-tendon function should be evaluated by assessing knee mobility and strength of the quads via straight leg raise, decline squat, or single leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but magnetic resonance imaging (MRI) can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training—eg, 3 sets of 15 repetitions performed twice a day for 12 weeks—and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that 3 weekly PRP injections helped 75% of patients—all of whom failed to respond to 4 months of eccentric therapy—return to their pre-symptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk of tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after 3 to 6 months of conservative therapy.14
Lateral knee pain
Iliotibial band tendinopathy
Iliotibial band syndrome (ITBS) is a common source of lateral knee pain, particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from micro trauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (FIGURE 1). A positive Ober’s test (FIGURE 2) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the iliotibial band, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to 2 weeks post injection.17,19 When symptoms persist for more than 6 months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
Medial knee pain
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomical site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed, but may be helpful if significant bony pathology is suspected. Ultrasound, computed tomography (CT), and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and using a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
Posterior knee pain
Popliteal (Baker’s) cyst
The popliteal fossa contains 6 of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for the development of popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt and for patients suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular steroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
When the problem is painful knee effusion
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. We mention it here because clinical suspicion is paramount in diagnosing a septic joint, a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC >50,000 per mm3, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
CASE › When Ms. T returns for a follow-up visit 8 weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional 8 pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
› Consider radiography for
a patient with patellofemoral pain syndrome if examination reveals an effusion, the patient is age
50 years or older, or the condition does not improve after 8 to 12 weeks of treatment. C
› Order plain radiography
for all patients with patellofemoral instability to assess for osseous trauma/deformity; consider magnetic resonance imaging if you suspect significant soft tissue damage or the patient does not respond to conservative therapy. C
› Perform joint aspiration with synovial fluid analysis for patients with painful knee effusion, and provide an orthopedic referral without delay when an infectious joint is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Jane T, age 42, comes to see you because of right knee pain that she’s had for about 6 months. She denies any trauma. Ms. T describes the pain as vague and poorly localized, but worse with activity. She says she started a walking/running program 9 months ago, when she was told she was overweight (body mass index, 29). She has lost 10 pounds since then, Ms. T says, and hopes to lose more by continuing to exercise. upon further review, you find that Ms. T has had increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Ms. T were your patient, what would you include in a physical examination and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain like that of Ms. T. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. In the pages that follow, we provide general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (TABLE).1-31
Anterior knee pain
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS), the most common cause of anterior knee pain, is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion, and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with <20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis, but may be considered if examination reveals an effusion, the patient is age 50 years or older, or no improvement occurs after 8 to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a 6-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of nonsteroidal anti-inflammatory drugs (NSAIDs), but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are non-conclusive. There is a paucity of prospective randomized trials of patellar bracing and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
CASE › When you examine Ms. T, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for 6 weeks of physiotherapy.
Patellar subluxation or chronic dislocation
Patellofemoral instability (PFI) occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable physical exam findings are:
- a positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
- decreased quadriceps (specifically VMO) and hamstring strength and flexibility
- patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
- pain during a patellar tilt test
- a positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/ deformity and to help guide surgical consideration. Magnetic resonance imaging (MRI) can provide additional information when significant soft tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy (jumper’s knee)
Patellar tendinopathy, an overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports like volleyball and basketball, is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury is poorly understood, but is believed to be the result of an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of 4 phases:12 1. pain isolated after activity; 2. pain that occurs during activity but does not impede activity; 3. pain that occurs both during and after the activity and interferes with competition ; 4. a complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle-tendon function should be evaluated by assessing knee mobility and strength of the quads via straight leg raise, decline squat, or single leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but magnetic resonance imaging (MRI) can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training—eg, 3 sets of 15 repetitions performed twice a day for 12 weeks—and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that 3 weekly PRP injections helped 75% of patients—all of whom failed to respond to 4 months of eccentric therapy—return to their pre-symptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk of tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after 3 to 6 months of conservative therapy.14
Lateral knee pain
Iliotibial band tendinopathy
Iliotibial band syndrome (ITBS) is a common source of lateral knee pain, particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from micro trauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (FIGURE 1). A positive Ober’s test (FIGURE 2) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the iliotibial band, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to 2 weeks post injection.17,19 When symptoms persist for more than 6 months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
Medial knee pain
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomical site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed, but may be helpful if significant bony pathology is suspected. Ultrasound, computed tomography (CT), and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and using a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
Posterior knee pain
Popliteal (Baker’s) cyst
The popliteal fossa contains 6 of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for the development of popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt and for patients suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular steroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
When the problem is painful knee effusion
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. We mention it here because clinical suspicion is paramount in diagnosing a septic joint, a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC >50,000 per mm3, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
CASE › When Ms. T returns for a follow-up visit 8 weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional 8 pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: March 20, 2014.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: Systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41: 19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13: 172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20: 1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21: 469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: December 12, 2013.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21: 486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: March 20, 2014.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: Systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41: 19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13: 172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20: 1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21: 469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus Web site. Available at: http://www.essentialevidenceplus.com. Accessed: December 12, 2013.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21: 486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.