User login
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
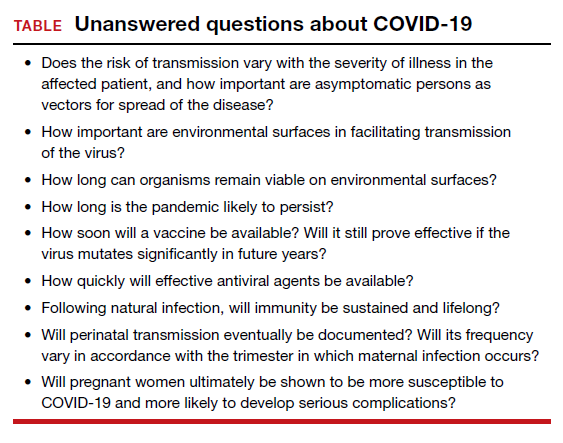
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).
Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.